1. Introduction
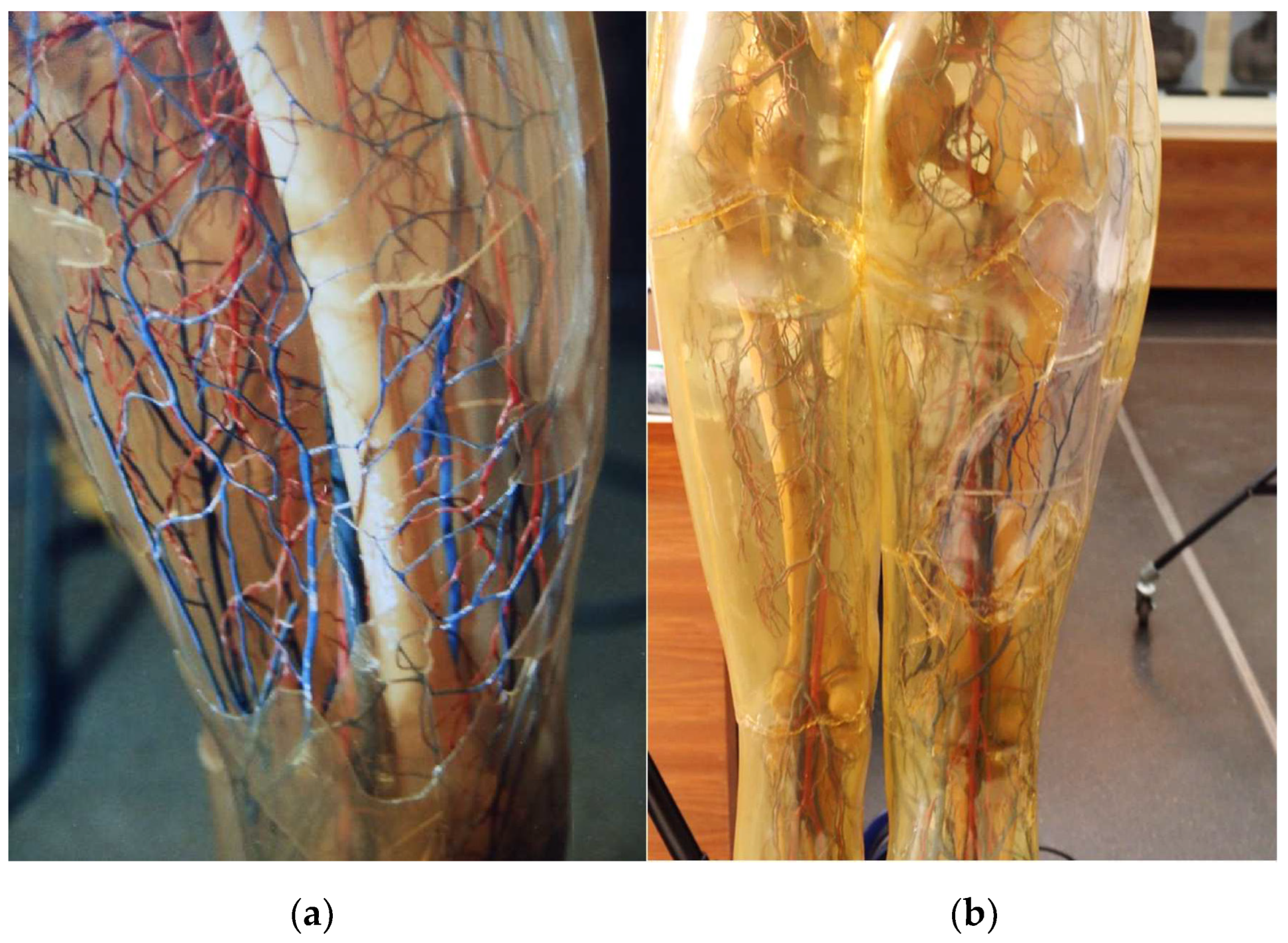
The studies presented here were prepared as part of a project to research the long-term preservation of the so-called Transparent Figures of the Deutsches Hygiene-Museum Dresden (DHMD) [
1]. They are the first life-size teaching models of the human and some animal bodies and were produced at the DHMD for many decades from 1930 onwards [
2]. Their special feature is the transparent outer skin, which allows an extensive view of the inside of the body. The outer skin is made of the then novel polymer cellulose acetate (CA), which is made processable by additives. As is typical for CA, the Transparent Figures show signs of aging in the form of embrittlement, shrinkage and yellowing.
In the following, the general degradation of CA is reviewed, as it can also be observed on the Transparent Figures. During the degradation of CA, acetic acid is released, which in turn induces degradation reactions on other materials in the near environment and acts on the degradation of the CA itself. Furthermore, additives migrate from the CA and can also induce further damage [
3]. Despite this knowledge, the exact reaction mechanisms of the degradation of CA and their dependencies on external agents such as humidity and temperature have not been fully explored. Much research on this has been conducted using animation slides and photographic films [
4,
5,
6,
7,
8,
9]. However, the Transparent Figures are large three-dimensional objects made of CA with a thickness of up to 2 mm, and therefore are distinguished from objects made from thinner foils and films. This present study therefore aims to investigate the degradation of the CA of the Transparent Figures and to develop preventive measures to slow it down as much as possible. For this purpose, it was possible to observe the aging behavior of the CA from different decades, to take samples from selected original figures and to perform specific measurements in and around the objects. These findings are used to perform artificial aging at elevated temperature as a function of external agents such as relative humidity (RH) or acetic acid concentration. The goal is to predict the natural aging behavior [
10]. Due to the access to residual materials from the workshops of the DHMD, original, naturally aged CA was used for the model experiments, so it can be assumed that the results of the analyses and experiments are close to the phenomena of the Transparent Figures. Subsequently, the chemical composition and color of the artificially re-aged CA are analyzed and the effects of the external agents on these are determined. Finally, investigation results on the Transparent Figures are compared with the findings from the model tests. Theoretical considerations of the effects of the external agents on the service life of the CA complement this research. All findings are incorporated into a concept for preventive conservation, which contains suggestions for slowing down the degradation of the CA of the Transparent Figures.
CA gained great importance as a carrier for film materials [
11,
12] and for the production of animation films [
12]. CA is also found in other products such as textile fibers [
13], eyeglass frames, combs or even toys from the beginning of the 20th century [
14,
15,
16]. The wide distribution, good processability, as well as its transparency were among the reasons why CA was used for the Transparent Figures. For the production of CA raw stock, first the cellulose triacetate is synthesized; if desired, the cellulose diacetate is then synthesized by partial hydrolysis [
17,
18]. Even though the latter could be processed better, both variants became attractive for application only after the addition of additives. The properties of the CA, such as solubility and thermal behavior, essentially depend on three factors, which lead to a great diversity of the material [
19]:
degree of substitution (DS), corresponding to the acetyl content
degree of polymerization (DP), corresponding to the chain length
type and content of additives
The choice of additives depends strongly on the intended use of the CA. Common additives are phthalates, esters of aliphatic dicarboxylic acids, phosphoric acid esters, alkyl sulfonic acid esters or polyesters, especially dimethyl phthalate (DMP), diethyl phthalate (DEP) and triphenyl phosphate (TPP) [
20]. They are widely used, for example, in films, injection molding compounds, and even biodegradable products [
21]. The low-cost phthalates serve as plasticizers and TPP as a flame-retardant plasticizer [
19]. Furthermore, triacetin (glycerol triacetate) is frequently used, e.g., in the classic cigarette filter [
21].
Five main processes are responsible for the degradation of CA: hydrolysis, chain scission, additive loss, oxidation and photodegradation [
17]. Hydrolysis of the acetyl groups (deacetylation) is the most characteristic step. In combination with water from the air, acetic acid is formed, which is perceived as a strong vinegar odor. Therefore, this phenomenon is also called “vinegar syndrome”, which often appears on CA objects before any visual change [
22,
23]. Hydrolysis decreases the degree of substitution of CA. Deacetylation reactivity at the three acetylated positions decreases in the order of C2, C6, C3, which is probably related to interactions with neighboring groups [
24,
25]. Various research papers show that increased relative humidity has an influence on CA deacetylation [
26,
27] and therefore is treated experimentally in the present work. The released acetic acid has further negative effects on the CA itself. It catalyzes chiefly deacetylation so that the DS is reduced. As a result, increased moisture absorption is possible due to the higher amount of hydroxy groups [
17]. Furthermore, a volume reduction of the CA is caused by the outgassing of acetic acid. Due to the loss of acetyl groups, the physically bound additives increasingly migrate out of the CA [
7]. The loss of the additives also causes shrinkage of the CA and brittleness. The surface becomes sticky with the remaining additives [
17]. Additives can also themselves contribute to accelerating degradation through reaction products [
28]. Furthermore, the often used TPP recrystallizes in CA after loss of acetic acid and co-plasticizer, sometimes leading to shattering of the polymer [
29]. Oxidation and photodegradation are caused by oxygen and light. Oxidation releases smaller molecular fragments, such as formic acid or oxalic acid [
17]. In photodegradation, the main products are carbon monoxide, carbon dioxide and methane, primarily formed by the radical cleavage of esters [
30,
31]. Oxidations, photodegradation as well as thermal influences contribute to the yellowing of the CA. Presumably, this is favored by the formation of carboxyl and carbonyl groups [
32], while the decomposition of the additives could also contribute to this [
31].
For the preservation of CA, a low temperature and a low relative humidity are generally recommended. As there are no generally valid numerical values for this, many and very different approaches are followed. For example, Allen recommends a dry atmosphere and temperatures below 0 °C [
26]. In March 2006, the Danish National Cultural Heritage Agency recommended a temperature of 2–5 °C and a relative humidity of 20–30% [
28]. For film materials, the Image Permanence Institute (IPI) recommends a temperature between 0 and 20 °C and a relative humidity of 30–50% [
33]. Nevertheless, there is a great need for research on recommendations in the handling of CA because, as mentioned, CA is used for many different applications in a wide variety of forms, thicknesses and combinations with other materials.
Artificial aging attempts to accelerate the course of naturally occurring decomposition processes of materials, whereby the aging process can be better estimated and monitored. Suitable measures derived from this can lead to a significant extension of the service life of the material [
10]. For the aging of CA, temperature and relative humidity are particularly considered in this work. The influence of temperature on the reaction rate constant of chemical reactions can in many cases be described at least approximately by the well-known equation of Svante Arrhenius [
34]. This model was used especially by Adelstein et al. [
4,
5,
6,
8] to explore the effects of relative humidity on a wide variety of materials. Later, this method was standardized in ISO 18924 [
35] and is now a useful way to develop preservation strategies for film and paint materials [
27]. However, the Arrhenius approach also has disadvantages. It assumes that the chemical reactions and physical processes occur at the use temperature in exactly the same way as at the elevated temperature used for accelerated aging. Similarly, the activation energy is assumed to be independent of temperature. Therefore, as soon as the reactions and processes become more complex, the Arrhenius equation reaches its limit [
36]. Therefore, other models exist that complement the thermal approach, such as the so-called isoconversional approach [
37]. In conservation science, the model of Michalski has been introduced [
38,
39]. With this, starting from the Arrhenius equation, calculations can be carried out for the reaction rate or for the service life, taking into account different levels of relative humidity and different activation energies for hydrolytic reactions.
4. Conclusions
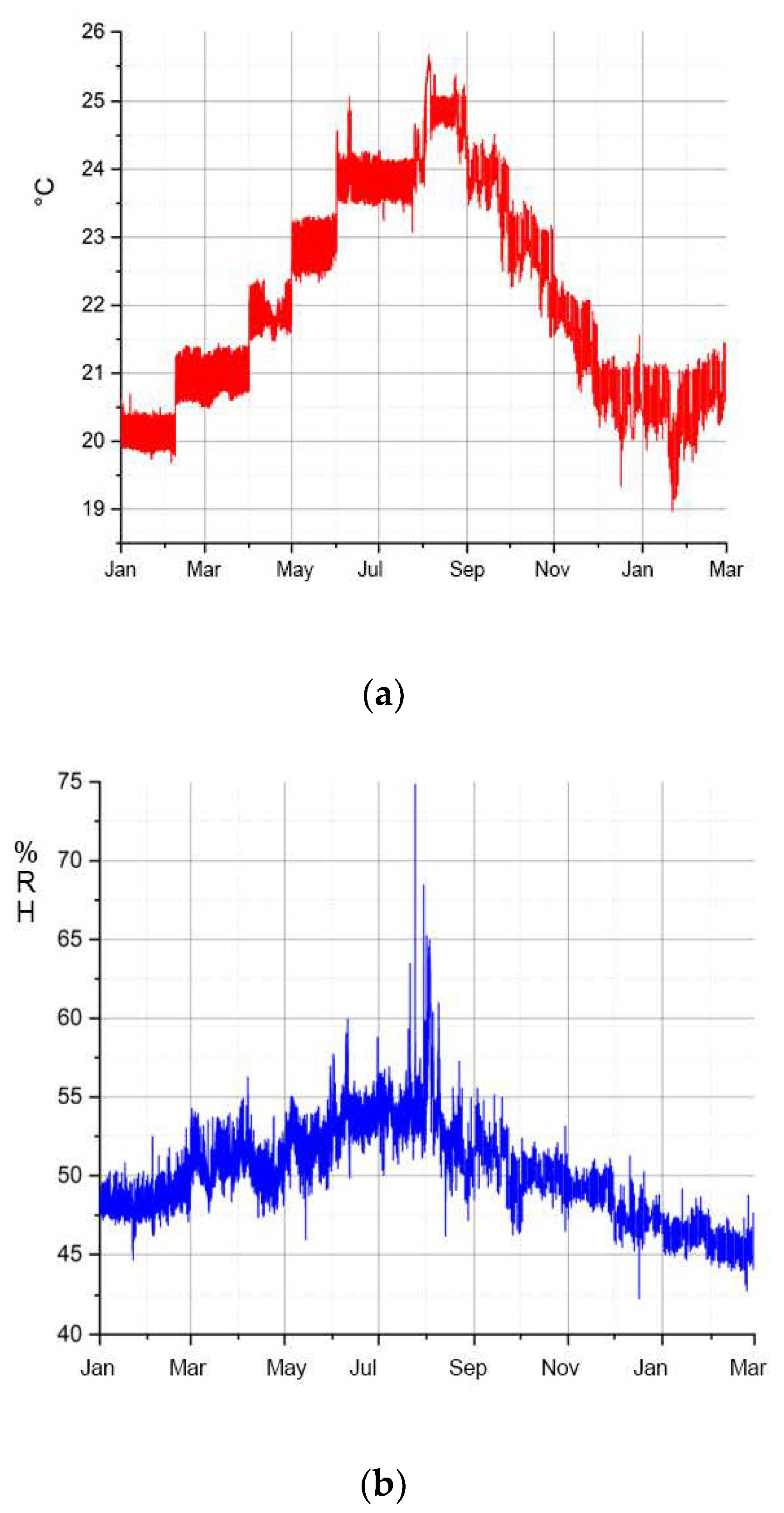
The observations and analyses on the Transparent Cow from 1983 and on the naturally aged CA sheet material from the DHMD workshop show that external agents have significant effects on the degradation of CA, among which relative humidity is the most serious. Temperature had no significant effect in these observations. Accordingly, drier environmental conditions lead to a slowing down of the migration of the additives and thus to a longer preservation of the CA.
Discoloration of the CA specimens during artificial aging is to be expected. The CA of the Transparent Figures also discolors in the course of natural aging over the decades. Nevertheless, these have turned more yellowish-green instead of yellowish-brown-black. One reason for this difference may be the elevated temperature of 70 °C over several weeks during artificial aging. Therefore, the reactions in the CA specimens are not only faster, but presumably also enhanced.
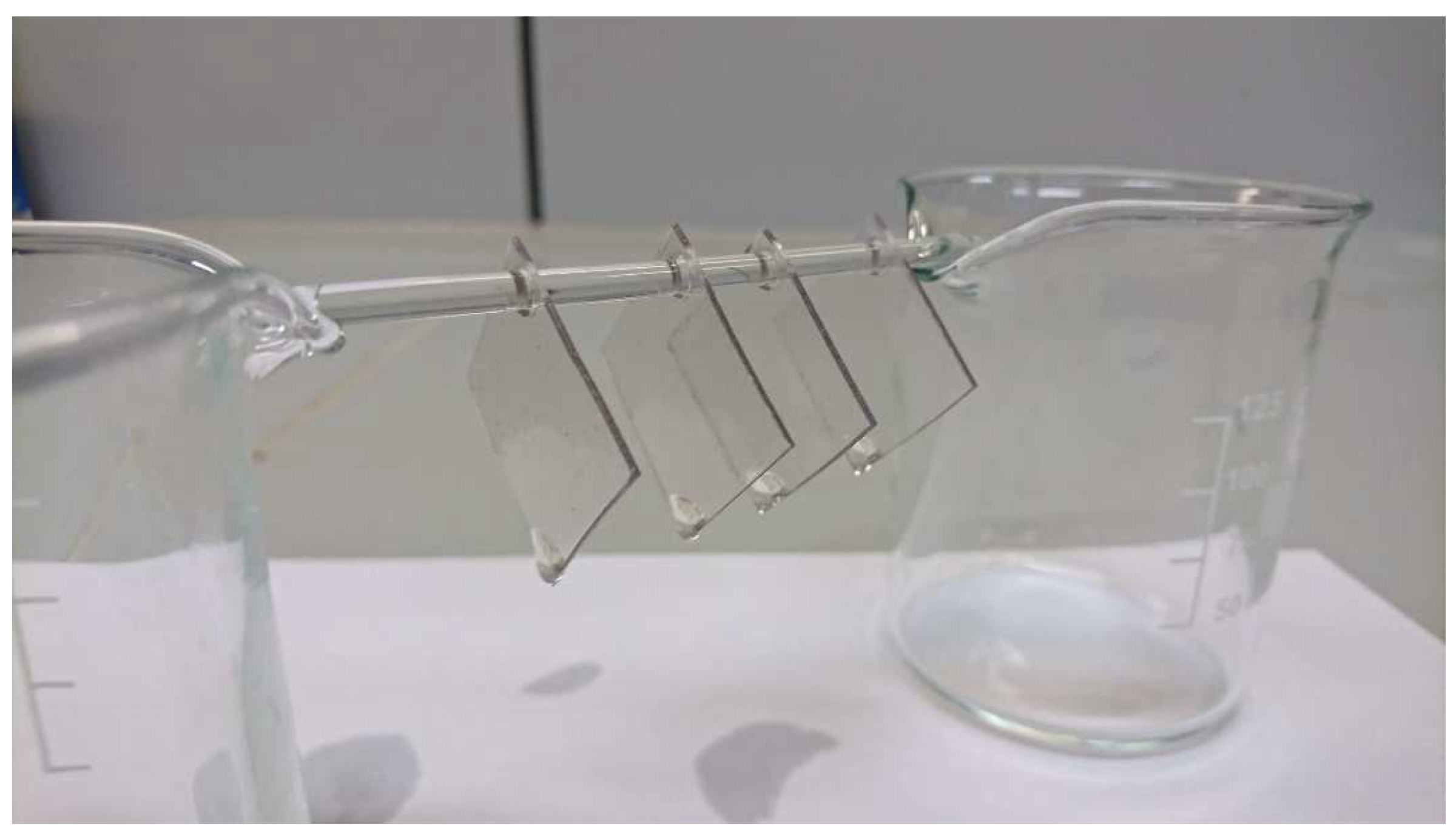
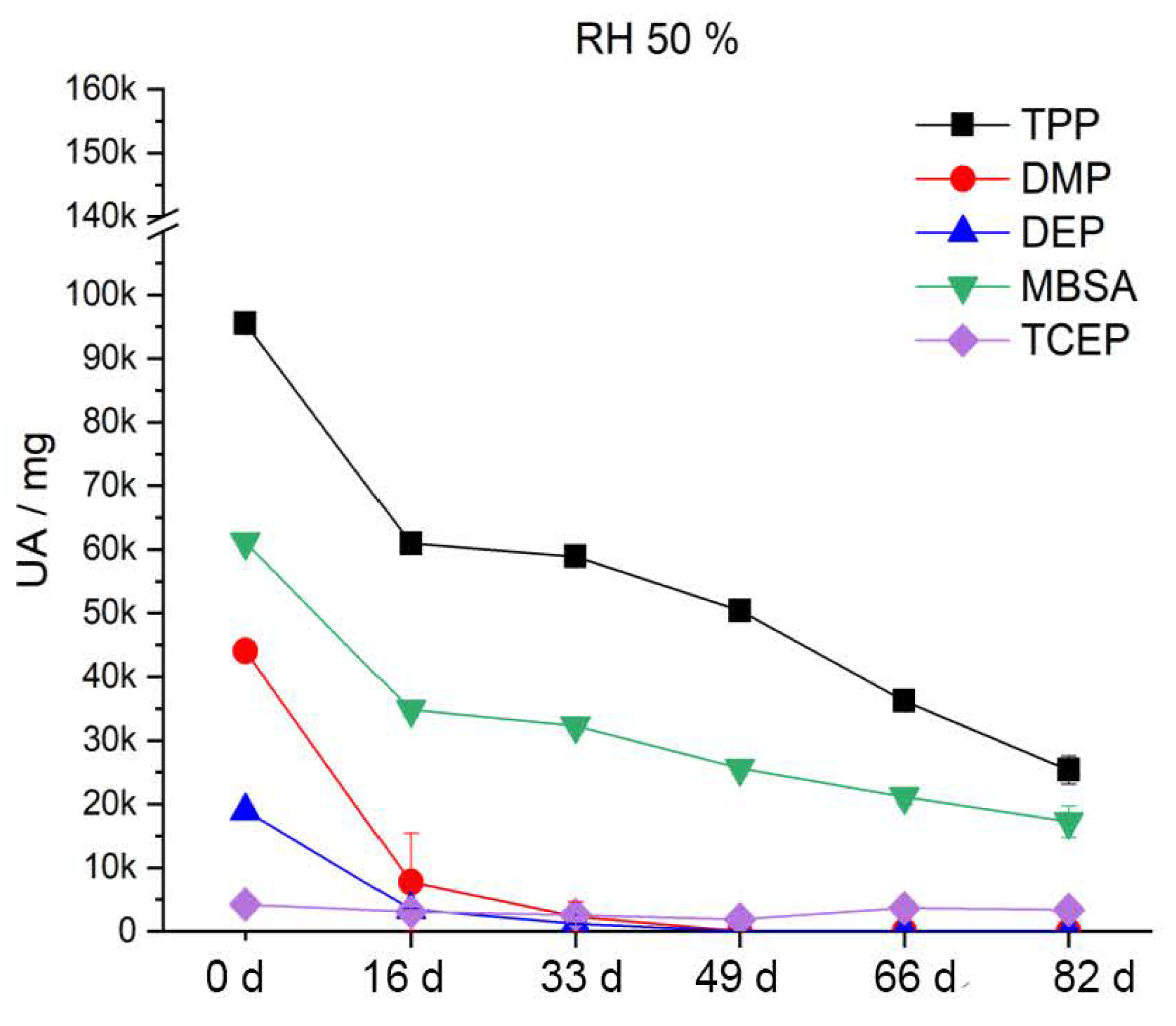
Both under real museum conditions and under laboratory conditions with good ventilation, a visible leakage of additives from the examined CA materials occurs at a relative humidity above 50–60%. Below 50% RH, the additives dry out. Temperature had no significant effect on these observations. This observation is supported by GC–MS analyses on naturally-aged CA sheet materials artificially re-aged under an elevated temperature of 70 °C with variation of relative humidity. The five additives contained (DMP, DEP, TCEP, MBSA, TPP) migrate significantly faster from the CA at elevated relative humidity. The migration rate decreases with 70% RH > 50% RH > 30% RH. The formation of hydroxy groups as a result of deacetylation changes the intermolecular interactions between the additives and the polymer chains. Water can be preferentially incorporated, and the additives migrate out of the polymer to a greater extent. However, the interactions between the additives and the polymer chains can also change due to subsequent reactions of the hydroxyl groups such as oxidation to ketones, aldehydes and carboxylates [
63].
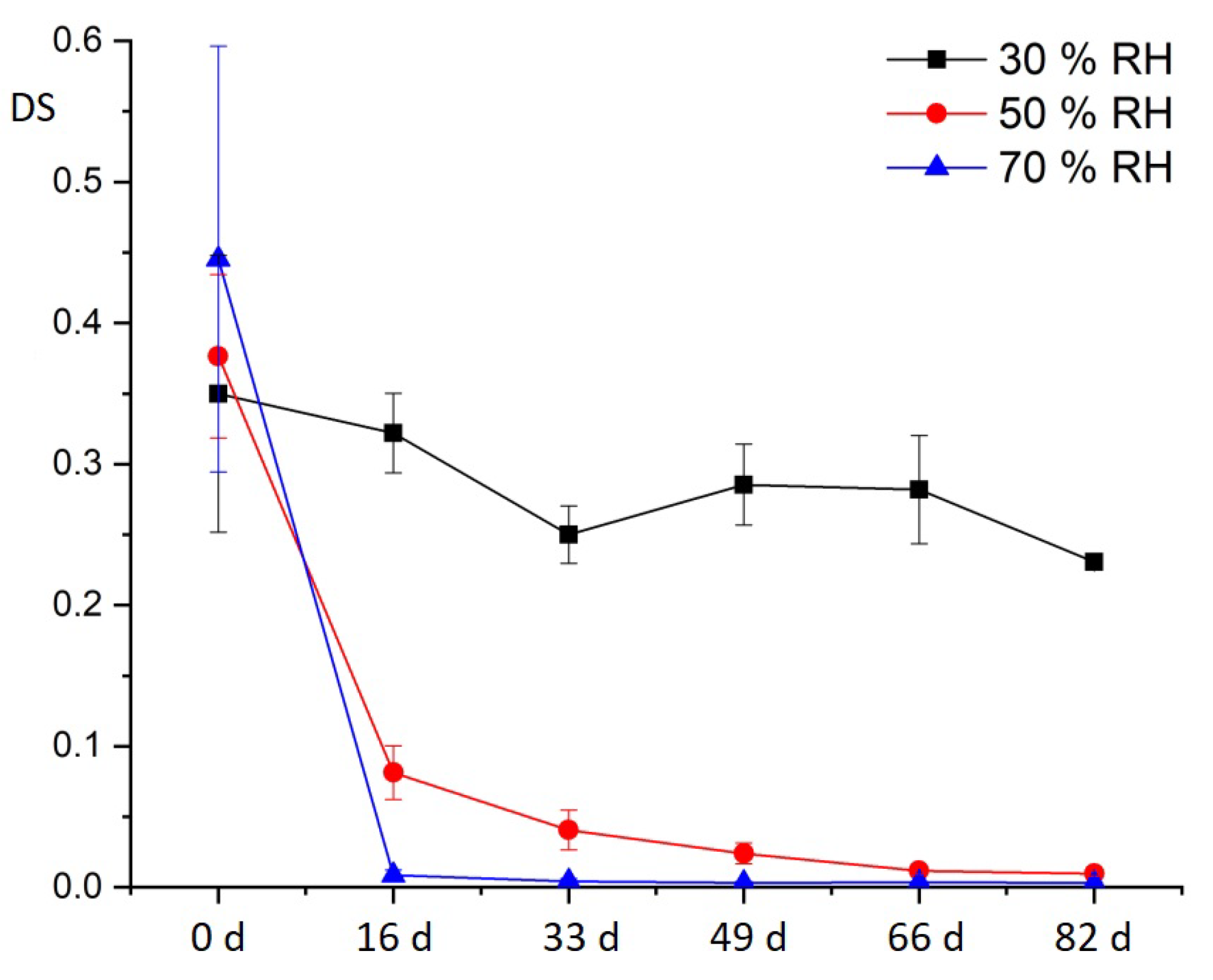
The effect of relative humidity on the CA sheet material has been shown to reduce the degree of substitution (DS) of the CA specimens artificially aged under variation of relative humidity [
24,
25]. This produces acetic acid, which accumulates in the air and can be measured in the Transparent Figures and has further effects. The comparison of the sheet material made of CA from the DHMD workshop with the original CA of the Transparent Figures shows that, with regard to the additive composition, it makes sense to use the plate material made from CA for artificial aging, since very close-to-application results are obtained. Even the CA sheet material with a very low DS of 0.4 gives very clear results in artificial aging with respect to the influence of relative humidity.
In addition to additive migration, deacetylation and discoloration, the relative humidity also has an influence on the degree of polymerization (DP) of the CA test specimens. This decreases faster with increasing relative humidity. The sole influence of acetic acid on the degradation of the CA specimens appears to be small. In contrast to artificial aging under variation of relative humidity, no increased additive migration with increasing acetic acid concentration in the air can be observed. Likewise, no significant decrease in the degree of polymerization can be proven. This is in contrast to descriptions in the literature [
3,
18]. The reason could be that the experimental setup limits the concentration of water molecules in the desiccators as reaction partners for hydrolysis.
For the long-term preservation of the historical Transparent Figures, values for relative humidity (30% RH) and temperature 15 °C are recommended (
Table 9). An overall concept for preservation and, furthermore, restoration of the Transparent Figures is published in a collective volume [
64].