Edible Film Casting Techniques and Materials and Their Utilization for Meat-Based Product Packaging
Abstract
1. Introduction
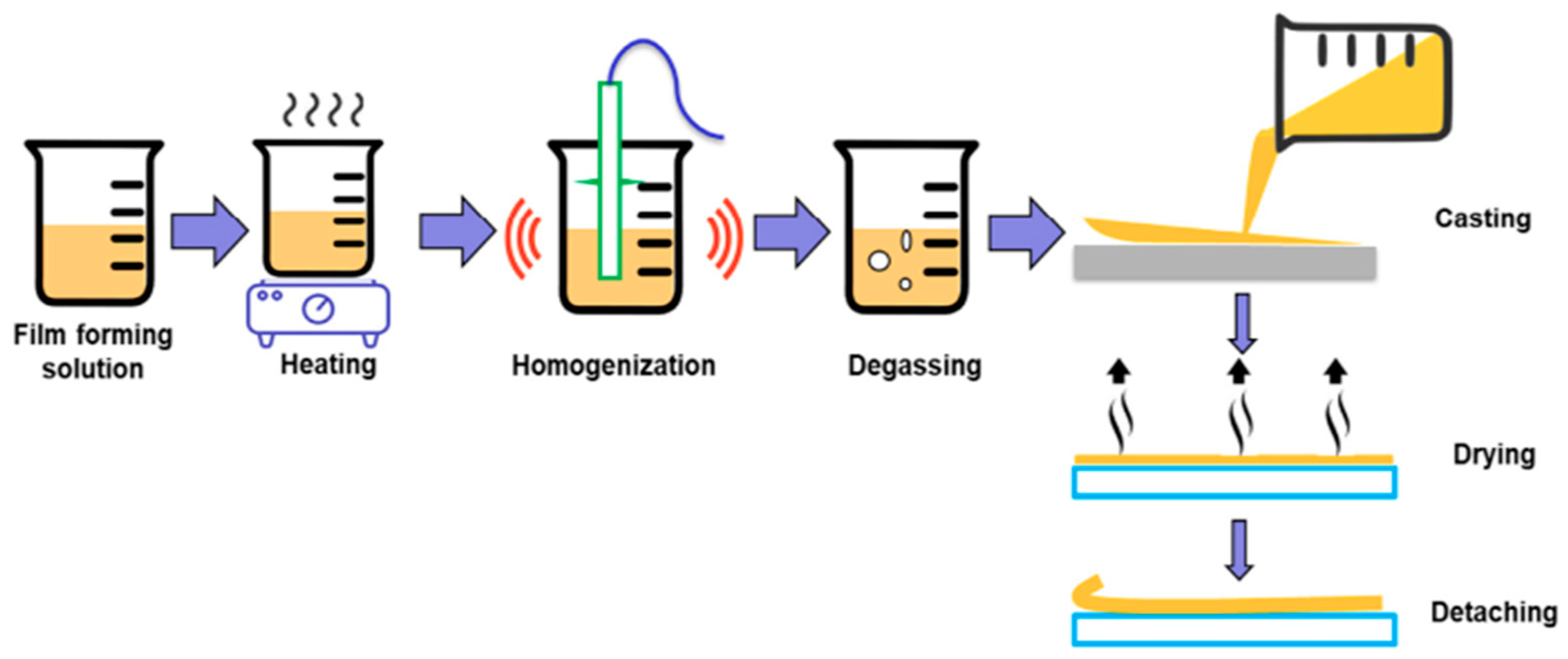
2. Edible Film Casting Techniques
2.1. Wet Formation (Solvent Casting)
2.2. Dry Formation Casting Techniques
3. Biopolymer Materials for Edible Films and Their Application to Meat Products
3.1. Polysaccharides
3.1.1. Starch

| Starch Type | Other Materials | Meat Product | Effects | References |
|---|---|---|---|---|
| Corn starch | Gelatin | Chicken breast fillet | Control microbial growth | [49] |
| Corn starch | Essential oil | Red meat | Antimicrobial and antioxidant effects | [50] |
| Cassava starch | Essential oil | Ground beef | Antimicrobial and antioxidant effects | [51] |
| Potato starch | Essential oil | Pork | Antimicrobial and antioxidant effects | [52] |
| Potato starch | Sea buckthorn | Beef | Reduce oxidation | [53] |
| Cellulose | Essential oil | Beef | Antimicrobial, control pH value | [54] |
| Cellulose | Probiotic | Chicken | Antimicrobial | [55] |
| Cellulose | Alginate | Chicken | Reduce oxidation | [56] |
| Chitosan | Essential oil | Chicken | Reduce microbial activities | [57] |
| Chitosan | Casein | Chicken | Control oxidation | [19] |
| Gum | Soy protein | Chicken breast | Control oxidation | [13] |
3.1.2. Cellulose
3.1.3. Chitosan
3.1.4. Gum
3.2. Lipid
3.2.1. Wax
3.2.2. Vegetables Oils
3.3. Protein
| Protein Type | Other Materials | Meat Product | Effects | References |
|---|---|---|---|---|
| Whey protein isolate | Essential oil | Sausage | Antimicrobial, control sensory quality | [89] |
| Whey | Seaweed extract | Chicken | Antioxidant, reduce lipid oxidation | [90] |
| Whey | Palm oil | Chicken | Antioxidant, reduce lipid oxidation | [91] |
| Gelatin | Corn starch | Chicken breast fillet | Control microbial growth | [49] |
| Gelatin | Henna extract | Beef | Antioxidant, antimicrobial, control sensory quality | [92] |
| Gelatin | Transglutaminase enzyme | Beef | Antioxidant, antimicrobial, control sensory quality | [93] |
| Soy protein | Carboxymethyl cellulose | Pork | Antioxidant, control moisture loss | [94] |
3.3.1. Whey Protein
3.3.2. Gelatin
3.3.3. Soy Protein
4. Advantages and Disadvantages
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pascall, M.A.; DeAngelo, K.; Richards, J.; Arensberg, M.B. Role and Importance of Functional Food Packaging in Specialized Products for Vulnerable Populations: Implications for Innovation and Policy Development for Sustainability. Foods 2022, 11, 3043. [Google Scholar] [CrossRef] [PubMed]
- Topuz, F.; Uyar, T. Antioxidant, Antibacterial and Antifungal Electrospun Nanofibers for Food Packaging Applications. Food Res. Int. 2020, 130, 108927. [Google Scholar] [CrossRef] [PubMed]
- Song, D.H.; Hoa, V.B.; Kim, H.W.; Khang, S.M.; Cho, S.H.; Ham, J.S.; Seol, K.H. Edible Films on Meat and Meat Products. Coatings 2021, 11, 1344. [Google Scholar] [CrossRef]
- Umaraw, P.; Verma, A.K. Comprehensive Review on Application of Edible Film on Meat and Meat Products: An Eco-Friendly Approach. Crit. Rev. Food Sci. Nutr. 2017, 57, 1270–1279. [Google Scholar] [CrossRef]
- Sundqvist-Andberg, H.; Åkerman, M. Sustainability Governance and Contested Plastic Food Packaging—An Integrative Review. J. Clean. Prod. 2021, 306, 127111. [Google Scholar] [CrossRef]
- Kandasamy, S.; Yoo, J.; Yun, J.; Kang, H.B.; Seol, K.H.; Kim, H.W.; Ham, J.S. Application of Whey Protein-Based Edible Films and Coatings in Food Industries: An Updated Overview. Coatings 2021, 11, 1056. [Google Scholar] [CrossRef]
- Shendurse, A. Milk Protein Based Edible Films and Coatings—Preparation, Properties and Food Applications. J. Nutr. Health Food Eng. 2018, 8, 219–226. [Google Scholar] [CrossRef]
- Ulusoy, B.H.; Yildirim, F.K.; Hecer, C. Edible Films and Coatings: A Good Idea from Past to Future Technology. J. Food Technol. Res. 2018, 5, 28–33. [Google Scholar] [CrossRef]
- Sánchez-Ortega, I.; García-Almendárez, B.E.; Santos-López, E.M.; Amaro-Reyes, A.; Barboza-Corona, J.E.; Regalado, C. Antimicrobial Edible Films and Coatings for Meat and Meat Products Preservation. Sci. World J. 2014, 2014, 248935. [Google Scholar] [CrossRef]
- Kumar, L.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Edible Films and Coatings for Food Packaging Applications: A Review. Environ. Chem. Lett. 2022, 20, 875–900. [Google Scholar] [CrossRef]
- Garavito, J.; Moncayo-Martinez, D.; Castellanos, D. Evaluation of antimicrobial coatings on preservation and shelf life of fresh chicken breast fillets under cold storage. Foods 2020, 9, 1203. [Google Scholar] [CrossRef]
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of Broiler Chicken Meat Quality and Factors Affecting Them: A Review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar] [CrossRef]
- Hamann, D.; Maria, B.; Puton, S.; Colet, R.; Steffens, J.; Ceni, G.C.; Cansian, R.L. Active edible films for application in meat products. Res. Soc. Dev. 2021, 10, 1–15. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries Extracts as Natural Antioxidants in Meat Products: A Review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef]
- Yousefi, M.; Azizi, M.; Mohammadifar, M.A.; Ehsani, A. Antimicrobial Coatings and Films on Meats: A Perspective on the Application of Antimicrobial Edible Films or Coatings on Meats from the Past to Future. Bali Med. J. 2018, 7, 87. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Guo, X. Effects of Antimicrobial and Antioxidant Activities of Spice Extracts on Raw Chicken Meat Quality. Food Sci. Hum. Wellness 2016, 5, 39–48. [Google Scholar] [CrossRef]
- Apriliyani, M.W.; Rahayu, P.P.; Manab, A. Stabilitas Daging Ayam Dengan Pelapisan Edible Coating Berbahan Kasein-Kitosan Selama Penyimpanan. J. Ilm. Inov. 2020, 20, 1–6. [Google Scholar] [CrossRef]
- Kupervaser, M.G.; Traffano-Schiffo, M.V.; Dellamea, M.L.; Flores, S.K.; Sosa, C.A. Trends in Starch-Based Edible Films and Coatings Enriched with Tropical Fruits Extracts: A Review. Food Hydrocoll. Health 2023, 100138. [Google Scholar] [CrossRef]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film Formation and Deposition Methods of Edible Coating on Food Products: A Review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef]
- Anjani, F.; Komang Ayu, N.; Putu, S.I. The Effect of Glycerol Concentration on The Characteristic Edible Film Sweet Potato Starch (Ipomoea batatas L.). Media Ilm. Teknol. Pangan 2018, 5, 27–35. [Google Scholar]
- Polnaya, F.J.; Breemer, R.; Augustyn, G.H.; Tuhumury, H.C.D. Karakteristik Sifat Fisiko-Kimia Pati Ubi Jalar, Ubi Kayu, Keladi, Dan Sagu. J. Ilmu Ternak 2015, 5, 37–42. [Google Scholar]
- Ak, K.; Hasan, M.; Mangaraj, S.; Pravitha, M.; Verma, D.K.; Srivastav, P.P. Trends in Edible Packaging Films and Its Prospective Future in Food: A Review. Appl. Food Res. 2022, 2, 100118. [Google Scholar] [CrossRef]
- Velaga, S.P.; Nikjoo, D.; Vuddanda, P.R. Experimental Studies and Modeling of the Drying Kinetics of Multicomponent Polymer Films. AAPS PharmSciTech 2018, 19, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Tharanathan, R.N. Biodegradable Films and Composite Coatings: Past, Present and Future. Trends Food Sci. Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Ricki, M.; Fajar, R.; Raswen, E. Pemanfaatan Kitosan Sebagai Bahan Dasar Edible Film Dari Pati Ubi Jalar Kuning. Electron. Publ. 2017, 4, 1–12. [Google Scholar]
- Li, M.; Liu, P.; Zou, W.; Yu, L.; Xie, F.; Pu, H.; Liu, H.; Chen, L. Extrusion Processing and Characterization of Edible Starch Films with Different Amylose Contents. J. Food Eng. 2011, 106, 95–101. [Google Scholar] [CrossRef]
- Britti Bacalhau, J.; Mumic Cunha, T.; Afonso, C.R.M. Effect of Ni Content on the Hardenability of a Bainitic Steel for Processing of Plastics. In Proceedings of the 24th ABCM International Congress of Mechanical Engineering, Curitiba, Brazil, 3–8 December 2017. [Google Scholar] [CrossRef]
- Chevalier, E.; Chaabani, A.; Assezat, G.; Prochazka, F.; Oulahal, N. Casein/Wax Blend Extrusion for Production of Edible Films as Carriers of Potassium Sorbate—A Comparative Study of Waxes and Potassium Sorbate Effect. Food Packag. Shelf Life 2018, 16, 41–50. [Google Scholar] [CrossRef]
- Dinika, I.; Verma, D.K.; Balia, R.; Utama, G.L.; Patel, A.R. Potential of Cheese Whey Bioactive Proteins and Peptides in the Development of Antimicrobial Edible Film Composite: A Review of Recent Trends. Trends Food Sci. Technol. 2020, 103, 57–67. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-Based Plasticizers and Biopolymer Films: A Review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Jost, V.; Stramm, C. Influence of Plasticizers on the Mechanical and Barrier Properties of Cast Biopolymer Films. J. Appl. Polym. Sci. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Ceballos, R.L.; Ochoa-Yepes, O.; Goyanes, S.; Bernal, C.; Famá, L. Effect of Yerba Mate Extract on the Performance of Starch Films Obtained by Extrusion and Compression Molding as Active and Smart Packaging. Carbohydr. Polym. 2020, 244, 116495. [Google Scholar] [CrossRef]
- Calderón-Castro, A.; Vega-García, M.O.; de Jesús Zazueta-Morales, J.; Fitch-Vargas, P.R.; Carrillo-López, A.; Gutiérrez-Dorado, R.; Limón-Valenzuela, V.; Aguilar-Palazuelos, E. Effect of Extrusion Process on the Functional Properties of High Amylose Corn Starch Edible Films and Its Application in Mango (Mangifera indica L.) Cv. Tommy Atkins. J. Food Sci. Technol. 2018, 55, 905–914. [Google Scholar] [CrossRef]
- Andreuccetti, C.; Carvalho, R.A.; Galicia-García, T.; Martinez-Bustos, F.; González-Nuñez, R.; Grosso, C.R.F. Functional Properties of Gelatin-Based Films Containing Yucca schidigera Extract Produced via Casting, Extrusion and Blown Extrusion Processes: A Preliminary Study. J. Food Eng. 2012, 113, 33–40. [Google Scholar] [CrossRef]
- Krishna, M.; Nindo, C.I.; Min, S.C. Development of Fish Gelatin Edible Films Using Extrusion and Compression Molding. J. Food Eng. 2012, 108, 337–344. [Google Scholar] [CrossRef]
- Vipan, B.; Mahajan, C.; Tandon, R.; Kapoor, S.; Sidhu, M.K. Natural Coatings for Shelf-Life Enhancement and Quality Maintenance of Fresh Fruits and Vegetables—A Review. J. Postharvest Technol. 2018, 6, 12–26. [Google Scholar]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Polysaccharides, Protein and Lipid-Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Regenstein, J.M.; Kafil, H.S. Protection of Foods against Oxidative Deterioration Using Edible Films and Coatings: A Review. Food Biosci. 2019, 32, 100451. [Google Scholar] [CrossRef]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyńska, P. Polysaccharides as Edible Films and Coatings: Characteristics and Influence on Fruit and Vegetable Quality—A Review. Angronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- Mudaffar, R.A. Karakteristik Edible Film Dari Limbah Kulit Singkong Dengan Penambahan Kombinasi Plasticizer Serta Aplikasinya Pada Buah Nanas Terolah Minimal. J. TABARO 2020, 4, 473–483. [Google Scholar] [CrossRef]
- Thakur, R.; Saberi, B.; Pristijono, P.; Golding, J.; Stathopoulos, C.; Scarlett, C.; Bowyer, M.; Vuong, Q. Characterization of Rice Starch-ι-Carrageenan Biodegradable Edible Film. Effect of Stearic Acid on the Film Properties. Int. J. Biol. Macromol. 2016, 93, 952–960. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-Based Films: Major Factors Affecting Their Properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Aisyah, Y.; Irwanda, L.P.; Haryani, S.; Safriani, N. Characterization of Corn Starch-Based Edible Film Incorporated with Nutmeg Oil Nanoemulsion. IOP Conf. Ser. Mater. Sci. Eng. 2018, 352, 012050. [Google Scholar] [CrossRef]
- Majhi, K.C.; Yadav, M. Chapter 5—Synthesis of Inorganic Nanomaterials Using Carbohydrates. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin, Boddula, R., Ahamed, M.I., Asiri, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 109–135. ISBN 978-0-12-821887-7. [Google Scholar]
- Santana, R.F.; Bonomo, R.C.F.; Gandolfi, O.R.R.; Rodrigues, L.B.; Santos, L.S.; dos Santos Pires, A.C.; de Oliveira, C.P.; da Costa Ilhéu Fontan, R.; Veloso, C.M. Characterization of Starch-Based Bioplastics from Jackfruit Seed Plasticized with Glycerol. J. Food Sci. Technol. 2018, 55, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, J.; Li, X.; Wang, Y.; Miao, J.; Mao, X.; Zhao, C.; Gao, W. Effect of Blanching and Drying Temperatures on Starch-Related Physicochemical Properties, Bioactive Components and Antioxidant Activities of Yam Flours. LWT Food Sci. Technol. 2017, 82, 303–310. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. How Glycerol and Water Contents Affect the Structural and Functional Properties of Starch-Based Edible Films. Polymers 2018, 10, 412. [Google Scholar] [CrossRef]
- Biduski, B.; Silva, W.M.F.D.; Colussi, R.; Halal, S.L.D.M.E.; Lim, L.-T.; Dias, Á.R.G.; Zavareze, E.D.R. Starch Hydrogels: The Influence of the Amylose Content and Gelatinization Method. Int. J. Biol. Macromol. 2018, 113, 443–449. [Google Scholar] [CrossRef]
- Moreno, O.; Atarés, L.; Chiralt, A.; Cruz-Romero, M.C.; Kerry, J. Starch-Gelatin Antimicrobial Packaging Materials to Extend the Shelf Life of Chicken Breast Fillets. LWT 2018, 97, 483–490. [Google Scholar] [CrossRef]
- Radha Krishnan, K.; Babuskin, S.; Rakhavan, K.R.; Tharavin, R.; Azhagu Saravana Babu, P.; Sivarajan, M.; Sukumar, M. Potential Application of Corn Starch Edible Films with Spice Essential Oils for the Shelf Life Extension of Red Meat. J. Appl. Microbiol. 2015, 119, 1613–1623. [Google Scholar] [CrossRef]
- dos Caetano, K.S.; Hessel, C.T.; Tondo, E.C.; Flôres, S.H.; Cladera-Olivera, F. Application of Active Cassava Starch Films Incorporated with Oregano Essential Oil and Pumpkin Residue Extract on Ground Beef. J. Food Saf. 2017, 37, e12355. [Google Scholar] [CrossRef]
- Yuan, L.; Feng, W.; Zhang, Z.; Peng, Y.; Xiao, Y.; Chen, J. Effect of Potato Starch-Based Antibacterial Composite Films with Thyme Oil Microemulsion or Microcapsule on Shelf Life of Chilled Meat. LWT 2021, 139, 110462. [Google Scholar] [CrossRef]
- Guo, Z.; Ge, X.; Gou, Q.; Yang, L.; Han, M.; Han, G.; Yu, Q.L.; Han, L. Changes in Chilled Beef Packaged in Starch Film Containing Sea Buckthorn Pomace Extract and Quality Changes in the Film during Super-Chilled Storage. Meat Sci. 2021, 182, 108620. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Han, J.C.; Zhang, Y.G.; Li, S.M.; Li, J. Antimicrobial and Antioxidant Activities of Carboxymethyl Cellulose Edible Films Incorporated with Rosemary Extracts on Fresh Beef during Refrigerated Storage. Adv. Mater. Res. 2012, 554–556, 1187–1194. [Google Scholar] [CrossRef]
- Salimiraad, S.; Safaeian, S.; Basti, A.A.; Khanjari, A.; Nadoushan, R.M. Characterization of Novel Probiotic Nanocomposite Films Based on Nano Chitosan Nano Cellulose/Gelatin for the Preservation of Fresh Chicken Fillets. LWT 2022, 162, 113429. [Google Scholar] [CrossRef]
- Criado, P.; Fraschini, C.; Salmieri, S.; Lacroix, M. Cellulose Nanocrystals (CNCs) Loaded Alginate Films against Lipid Oxidation of Chicken Breast. Food Res. Int. 2020, 132, 109110. [Google Scholar] [CrossRef]
- Karimnezhad, F.; Razavilar, V.; Anvar, A.A.; Dashtgol, S.; Zavareh, A.P. Combined Effect of Chitosan-Based Edible Film Containing Oregano Essential Oil on the Shelf-Life Extension of Fresh Chicken Meat. J. Nutr. Food Secur. 2019, 4, 236–242. [Google Scholar] [CrossRef]
- Siregar, S.H.; Irma, W. Pemanfaatan Kulit Singkong Sebagai Alternatif Bahan Baku Edible Film. Photon J. Sain Dan Kesehat. 2012, 3, 15–21. [Google Scholar] [CrossRef]
- Çakmak, H.; Özselek, Y.; Turan, O.Y.; Fıratlıgil, E.; Karbancioğlu-Güler, F. Whey Protein Isolate Edible Films Incorporated with Essential Oils: Antimicrobial Activity and Barrier Properties. Polym. Degrad. Stab. 2020, 179, 109285. [Google Scholar] [CrossRef]
- Sukhija, S.; Singh, S.; Riar, C.S. Analyzing the Effect of Whey Protein Concentrate and Psyllium Husk on Various Characteristics of Biodegradable Film from Lotus (Nelumbo nucifera) Rhizome Starch. Food Hydrocoll. 2016, 60, 128–137. [Google Scholar] [CrossRef]
- Fu, J.; Alee, M.; Yang, M.; Liu, H.; Li, Y.; Li, Z.; Yu, L. Synergizing Multi-Plasticizers for a Starch-Based Edible Film. Foods 2022, 11, 3254. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. 23—Plasticizers in Edible Films and Coatings. In Innovations in Food Packaging; Han, J.H., Ed.; Food Science and Technology; Academic Press: London, UK, 2005; pp. 403–433. ISBN 978-0-12-311632-1. [Google Scholar]
- Malik, G.K.; Khuntia, A.; Mitra, J. Comparative Effect of Different Plasticizers on Barrier, Mechanical, Optical, and Sorption Properties of Hydroxypropyl Methylcellulose (HPMC)–Based Edible Film. J. Biosyst. Eng. 2022, 47, 93–105. [Google Scholar] [CrossRef]
- Milani, J.M.; Nemati, A. Lipid-Based Edible Films and Coatings: A Review of Recent Advances and Applications. J. Package Technol. Res. 2022, 6, 11–22. [Google Scholar] [CrossRef]
- Da Nóbrega Santos, E.; Cesar De Albuquerque Sousa, T.; Cassiano De Santana Neto, D.; Brandão Grisi, C.V.; Cardoso Da Silva Ferreira, V.; Pereira Da Silva, F.A. Edible Active Film Based on Gelatin and Malpighia Emarginata Waste Extract to Inhibit Lipid and Protein Oxidation in Beef Patties. LWT 2022, 154, 112837. [Google Scholar] [CrossRef]
- Zhao, G.; Lyu, X.; Lee, J.; Cui, X.; Chen, W.N. Biodegradable and Transparent Cellulose Film Prepared Eco-Friendly from Durian Rind for Packaging Application. Food Packag. Shelf Life 2019, 21, 100345. [Google Scholar] [CrossRef]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-Based Active Food Packaging Materials: Sustainable Alternative to Conventional Petrochemical-Based Packaging Materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef]
- Formanek, Z.; Lynch, A.; Galvin, K.; Farkas, J.; Kerry, J.P. Combined Effects of Irradiation and the Use of Natural Antioxidants on the Shelf-Life Stability of Overwrapped Minced Beef. Meat Sci. 2003, 63, 433–440. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. Curr. Med. Chem. 2014, 22, 132–149. [Google Scholar] [CrossRef]
- Vargas, M.; Albors, A.; Chiralt, A. Application of Chitosan-Sunflower Oil Edible Films to Pork Meat Hamburgers. Procedia Food Sci. 2011, 1, 39–43. [Google Scholar] [CrossRef]
- Economou, V.; Tsitsos, A.; Theodoridis, A.; Ambrosiadis, I.; Arsenos, G. Effects of Chitosan Coatings on Controlling Listeria Monocytogenes and Methicillin-Resistant Staphylococcus Aureus in Beef and Mutton Cuts. Appl. Sci. 2022, 12, 11345. [Google Scholar] [CrossRef]
- Desvita, H.; Faisal, M.; Mahidin, M.; Suhendrayatna, S. Edible Coating for Beef Preservation from Chitosan Combined with Liquid Smoke. Int. J. Technol. 2020, 11, 817–829. [Google Scholar] [CrossRef]
- Rakshit, M.; Ramalingam, C. Gum Acacia Coating with Garlic and Cinnamon as an Alternate, Natural Preservative for Meat and Fish. Afr. J. Biotechnol. 2013, 12, 406–413. [Google Scholar] [CrossRef]
- Sheikh, D.M. El Efficiency of Using Arabic Gum and Plantago Seeds Mucilage as Edible Coating for Chicken Boneless Breast. Food Sci. Qual. Manag. 2014, 32, 28–34. [Google Scholar]
- De Pilli, T. Development of a Vegetable Oil and Egg Proteins Edible Film to Replace Preservatives and Primary Packaging of Sweet Baked Goods. Food Control 2020, 114, 107273. [Google Scholar] [CrossRef]
- Cortés-Rodríguez, M.; Villegas-Yépez, C.; Gil González, J.H.; Rodríguez, P.E.; Ortega-Toro, R. Development and Evaluation of Edible Films Based on Cassava Starch, Whey Protein, and Bees Wax. Heliyon 2020, 6, e04884. [Google Scholar] [CrossRef] [PubMed]
- Pashova, S. Application of Plant Waxes in Edible Coatings. Coatings 2023, 13, 911. [Google Scholar] [CrossRef]
- Chiumarelli, M.; Hubinger, M.D. Evaluation of Edible Films and Coatings Formulated with Cassava Starch, Glycerol, Carnauba Wax and Stearic Acid. Food Hydrocoll. 2014, 38, 20–27. [Google Scholar] [CrossRef]
- Tyburcy, A.; Kozyra, D. Effects of Composite Surface Coating and Pre-Drying on the Properties of Kabanosy Dry Sausage. Meat Sci. 2010, 86, 405–410. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food Applications of Emulsion-Based Edible Films and Coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Isfari, D.; Gemilang Lara, U. Cheese Whey as Potential Resource for Antimicrobial Edible Film and Active Packaging Production. Foods Raw Mater. 2019, 7, 229–239. [Google Scholar] [CrossRef]
- Hernalsteens, S. Chapter 24—Edible Films and Coatings Made up of Fruits and Vegetables. In Biopolymer Membranes and Films; de Moraes, M.A., da Silva, C.F., Vieira, R.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 575–588. ISBN 978-0-12-818134-8. [Google Scholar]
- Esfarjani, F.; Khoshtinat, K.; Zargaraan, A.; Mohammadi-Nasrabadi, F.; Salmani, Y.; Saghafi, Z.; Hosseini, H.; Bahmaei, M. Evaluating the Rancidity and Quality of Discarded Oils in Fast Food Restaurants. Food Sci. Nutr. 2019, 7, 2302–2311. [Google Scholar] [CrossRef]
- Salehi, F. Edible Coating of Fruits and Vegetables Using Natural Gums: A Review. Int. J. Fruit Sci. 2020, 20, S570–S589. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Li, Y.; Zhu, L.; Fang, Z.; Shi, Q. Physicochemical, Mechanical and Structural Properties of Composite Edible Films Based on Whey Protein Isolate/Psyllium Seed Gum. Int. J. Biol. Macromol. 2020, 153, 892–901. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of Protein-Based Films and Coatings for Food Packaging: A Review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef]
- Pérez-Vergara, L.D.; Cifuentes, M.T.; Franco, A.P.; Pérez-Cervera, C.E.; Andrade-Pizarro, R.D. Development and Characterization of Edible Films Based on Native Cassava Starch, Beeswax, and Propolis. NFS J. 2020, 21, 39–49. [Google Scholar] [CrossRef]
- Wagh, Y.R.; Pushpadass, H.A.; Emerald, F.M.E.; Nath, B.S. Preparation and Characterization of Milk Protein Films and Their Application for Packaging of Cheddar Cheese. J. Food Sci. Technol. 2014, 51, 3767–3775. [Google Scholar] [CrossRef]
- Kalkan, S.; Erginkaya, Z. Impact of Whey Protein Isolate Coatings Containing Different Antimicrobial Agents on Sliced Bologna-Type Sausage during Refrigerated Storage. Food Sci. Technol. 2020, 40, 136–145. [Google Scholar] [CrossRef]
- Andrade, M.A.; Barbosa, C.H.; Souza, V.G.L.; Coelhoso, I.M.; Reboleira, J.; Bernardino, S.; Ganhão, R.; Mendes, S.; Fernando, A.L.; Vilarinho, F.; et al. Novel Active Food Packaging Films Based on Whey Protein Incorporated with Seaweed Extract: Development, Characterization, and Application in Fresh Poultry Meat. Coatings 2021, 11, 229. [Google Scholar] [CrossRef]
- Wiedyanto, E.; Indah, E.; Khoirul, S.; Esti, W. Pemanfaatan Protein Whey Edible Film Coating Untuk Mempertahankan Kualitas Daing Ayam. Poster ilmiah PIMNAS XIX; Universitas Muhammadiyah: Malang, Indonesia, 24–29 July; 2006; pp. 1–12. [Google Scholar]
- Jridi, M.; Mora, L.; Souissi, N.; Aristoy, M.C.; Nasri, M.; Toldrá, F. Effects of Active Gelatin Coated with Henna (L. inermis) Extract on Beef Meat Quality during Chilled Storage. Food Control 2018, 84, 238–245. [Google Scholar] [CrossRef]
- Battisti, R.; Fronza, N.; Vargas Júnior, Á.; da Silveira, S.M.; Damas, M.S.P.; Quadri, M.G.N. Gelatin-Coated Paper with Antimicrobial and Antioxidant Effect for Beef Packaging. Food Packag. Shelf Life 2017, 11, 115–124. [Google Scholar] [CrossRef]
- Shon, J.; Kim, J.H.; Eo, J.H.; Choi, Y.H. Effect of Soy Protein Isolate Coating on Meat Quality of Pork Fresh Cut during Refrigerated Storage. J. Appl. Biol. Chem. 2012, 55, 27–34. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent Advances on Polysaccharides, Lipids and Protein Based Edible Films and Coatings: A Review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Utama, G.L.; Utba, F.; Cahyana, Y.; Balia, R.L. Mozzarella Whey Indigenous Yeasts and Their Potential in Amino Acid and Peptide Production through Fermentation. Syst. Rev. Pharm. 2021, 12, 1358–1366. [Google Scholar]
- Utba, F.; Balia, R.L.; Utama, L.G. The Presence of Indigenous Yeasts with Proteolytic Activity Isolated from Homemade-Mozzarella Whey. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural Dev. 2018, 18, 507–514. [Google Scholar]
- Korhonen, H.; Pihlanto, A. Technological options for the production of health-promoting proteins and peptides derived from milk and colostrum. Curr. Pharm. Des. 2007, 13, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Seyfzadeh, M.; Motalebi, A.A.; Kakoolaki, S.; Gholipour, H. Chemical, Microbiological and Sensory Evaluation of Gutted Kilka Coated with Whey Protein Based Edible Film Incorporated with Sodium Alginate during Frozen Storage. Iran. J. Fish. Sci. 2013, 12, 140–153. [Google Scholar]
- Kaewprachu, P.; Osako, K.; Benjakul, S.; Rawdkuen, S. Quality Attributes of Minced Pork Wrapped with Catechin-Lysozyme Incorporated Gelatin Film. Food Packag. Shelf Life 2015, 3, 88–96. [Google Scholar] [CrossRef]
- González, A.; Barrera, G.N.; Galimberti, P.I.; Ribotta, P.D.; Alvarez Igarzabal, C.I. Development of Edible Films Prepared by Soy Protein and the Galactomannan Fraction Extracted from Gleditsia triacanthos (Fabaceae) Seed. Food Hydrocoll. 2019, 97, 105227. [Google Scholar] [CrossRef]
- Wulandari, D.; Erwanto, Y.; Pranoto, Y.; Rusman, R. The Properties of Edible Film Derived from Bovine Split Hide Gelatin with Isolated Soy Protein Using Various Levels of Glycerol in The Presence of Transglutaminase. Buletin Peternakan 2017, 41, 319–327. [Google Scholar] [CrossRef]
- Huntrakul, K.; Yoksan, R.; Sane, A.; Harnkarnsujarit, N. Effects of Pea Protein on Properties of Cassava Starch Edible Films Produced by Blown-Film Extrusion for Oil Packaging. Food Packag. Shelf Life 2020, 24, 100480. [Google Scholar] [CrossRef]
- Khwaldia, K.; Ferez, C.; Banon, S.; Desobry, S.; Hardy, J. Milk Proteins for Edible Films and Coatings. Crit. Rev. Food Sci. Nutr. 2004, 44, 239–251. [Google Scholar] [CrossRef]
- Avramescu, S.M.; Butean, C.; Popa, C.V.; Ortan, A.; Moraru, I.; Temocico, G. Edible and Functionalized Films/Coatings-Performances and Perspectives. Coatings 2020, 10, 687. [Google Scholar] [CrossRef]
- Janjarasskul, T.; Rauch, D.J.; McCarthy, K.L.; Krochta, J.M. Barrier and Tensile Properties of Whey Protein-Candelilla Wax Film/Sheet. LWT 2014, 56, 377–382. [Google Scholar] [CrossRef]
- Liu, L.; Kerry, J.F.; Kerry, J.P. Effect of Food Ingredients and Selected Lipids on the Physical Properties of Extruded Edible Films/Casings. Int. J. Food Sci. Technol. 2006, 41, 295–302. [Google Scholar] [CrossRef]
- Bolívar-Monsalve, J.; Ramírez-Toro, C.; Bolívar, G.; Ceballos-González, C. Mechanisms of Action of Novel Ingredients Used in Edible Films to Preserve Microbial Quality and Oxidative Stability in Sausages—A Review. Trends Food Sci. Technol. 2019, 89, 100–109. [Google Scholar] [CrossRef]
- Belyamani, I.; Prochazka, F.; Assezat, G. Production and Characterization of Sodium Caseinate Edible Films Made by Blown-Film Extrusion. J. Food Eng. 2014, 121, 39–47. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible Films/Coating with Tailored Properties for Active Packaging of Meat, Fish and Derived Products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Min, S.; Rumsey, T.R.; Krochta, J.M. Diffusion of the Antimicrobial Lysozyme from a Whey Protein Coating on Smoked Salmon. J. Food Eng. 2008, 84, 39–47. [Google Scholar] [CrossRef]
- Jeevahan, J.; Studies, A.; Govindaraj, M. A Brief Review on Edible Food Packing Materials. J. Glob. Eng. Probl. Solut. 2017, 1, 9–19. [Google Scholar]
- Ruban, S. Biobased Packaging—Application in Meat Industry. Vet. World 2009, 2, 79. [Google Scholar] [CrossRef]
- Yanti, N.A.; Ahmad, S.W.; Ramadhan, L.O.A.N.; Jamili; Muzuni; Walhidayah, T.; Mamangkey, J. Properties and Application of Edible Modified Bacterial Cellulose Film Based Sago Liquid Waste as Food Packaging. Polymers 2021, 13, 3570. [Google Scholar] [CrossRef]
- Carvalho, R.A.; Santos, T.A.; de Oliveira, A.C.S.; de Azevedo, V.M.; Dias, M.V.; Ramos, E.M.; Borges, S.V. Biopolymers of WPI/CNF/TEO in Preventing Oxidation of Ground Meat. J. Food Process. Preserv. 2019, 43, e14269. [Google Scholar] [CrossRef]
- Hossaeini Marashi, S.M.; Hashemi, M.; Berizi, E.; Raeisi, M.; Noori, S.M.A. Elaboration of Whey Protein-Based Films in Food Products: Emphasis on the Addition of Natural Edible Bio-Nanocomposites with Antioxidant and Antimicrobial Activity. Jundishapur J. Nat. Pharm. Prod. 2021, 17, e117046. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakoso, F.A.H.; Indiarto, R.; Utama, G.L. Edible Film Casting Techniques and Materials and Their Utilization for Meat-Based Product Packaging. Polymers 2023, 15, 2800. https://doi.org/10.3390/polym15132800
Prakoso FAH, Indiarto R, Utama GL. Edible Film Casting Techniques and Materials and Their Utilization for Meat-Based Product Packaging. Polymers. 2023; 15(13):2800. https://doi.org/10.3390/polym15132800
Chicago/Turabian StylePrakoso, Fauzi Atsani Harits, Rossi Indiarto, and Gemilang Lara Utama. 2023. "Edible Film Casting Techniques and Materials and Their Utilization for Meat-Based Product Packaging" Polymers 15, no. 13: 2800. https://doi.org/10.3390/polym15132800
APA StylePrakoso, F. A. H., Indiarto, R., & Utama, G. L. (2023). Edible Film Casting Techniques and Materials and Their Utilization for Meat-Based Product Packaging. Polymers, 15(13), 2800. https://doi.org/10.3390/polym15132800






