Efficient and Sustainable Bidentate Amines-Functionalized Resins for Removing Ag+, Cu2+, Pb2+, and Fe3+ from Water
Abstract
1. Introduction
2. Materials and Methods
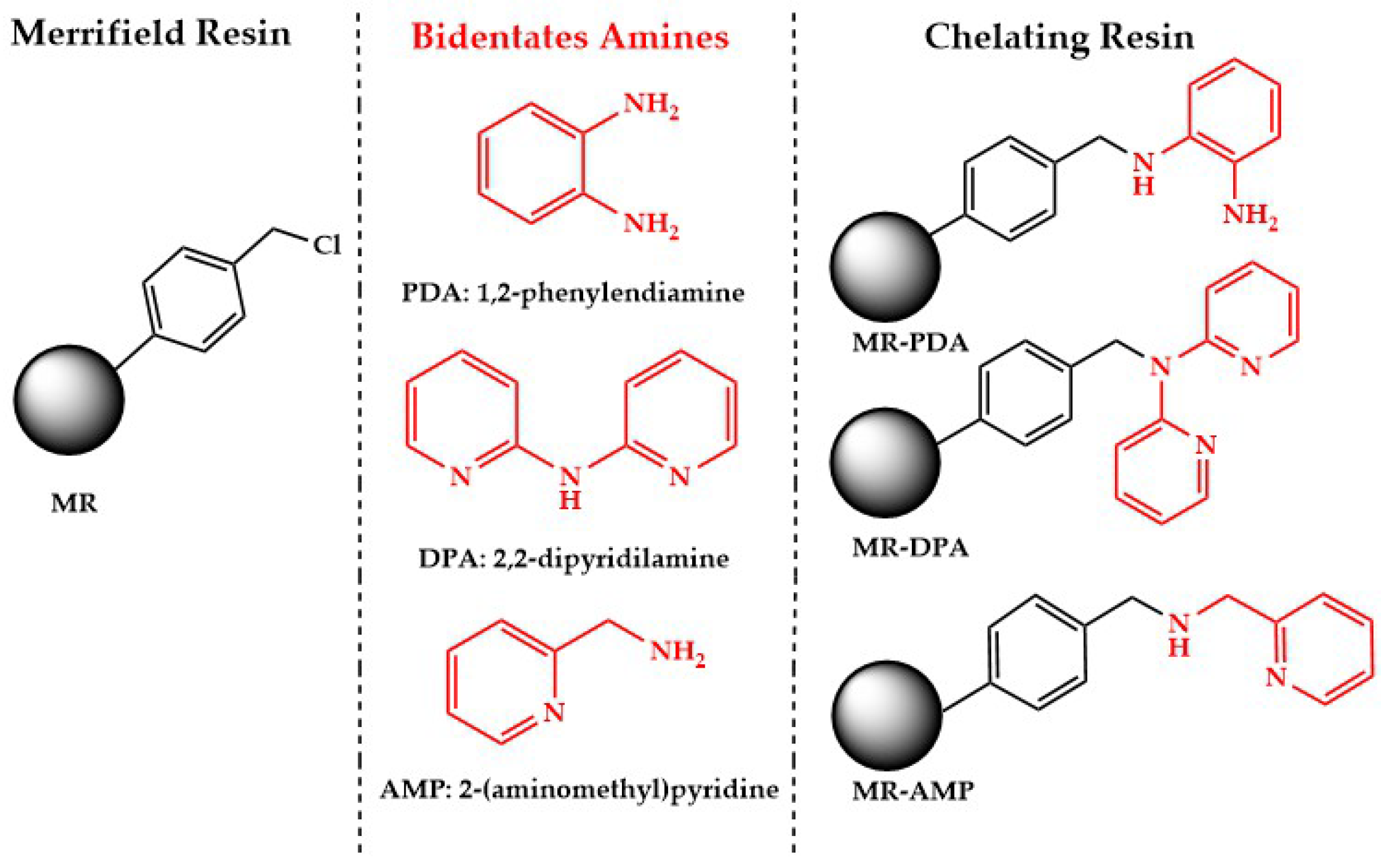
2.1. Synthesis and Characterization of Chelating Resins
2.2. Methods for the Evaluation of Sensing Properties of Chelation Resins
2.2.1. Tests for the Evaluation of the Adsorption Properties of Chelating Resins
2.2.2. Chelating Resin Reusability Test
3. Results and Discussion
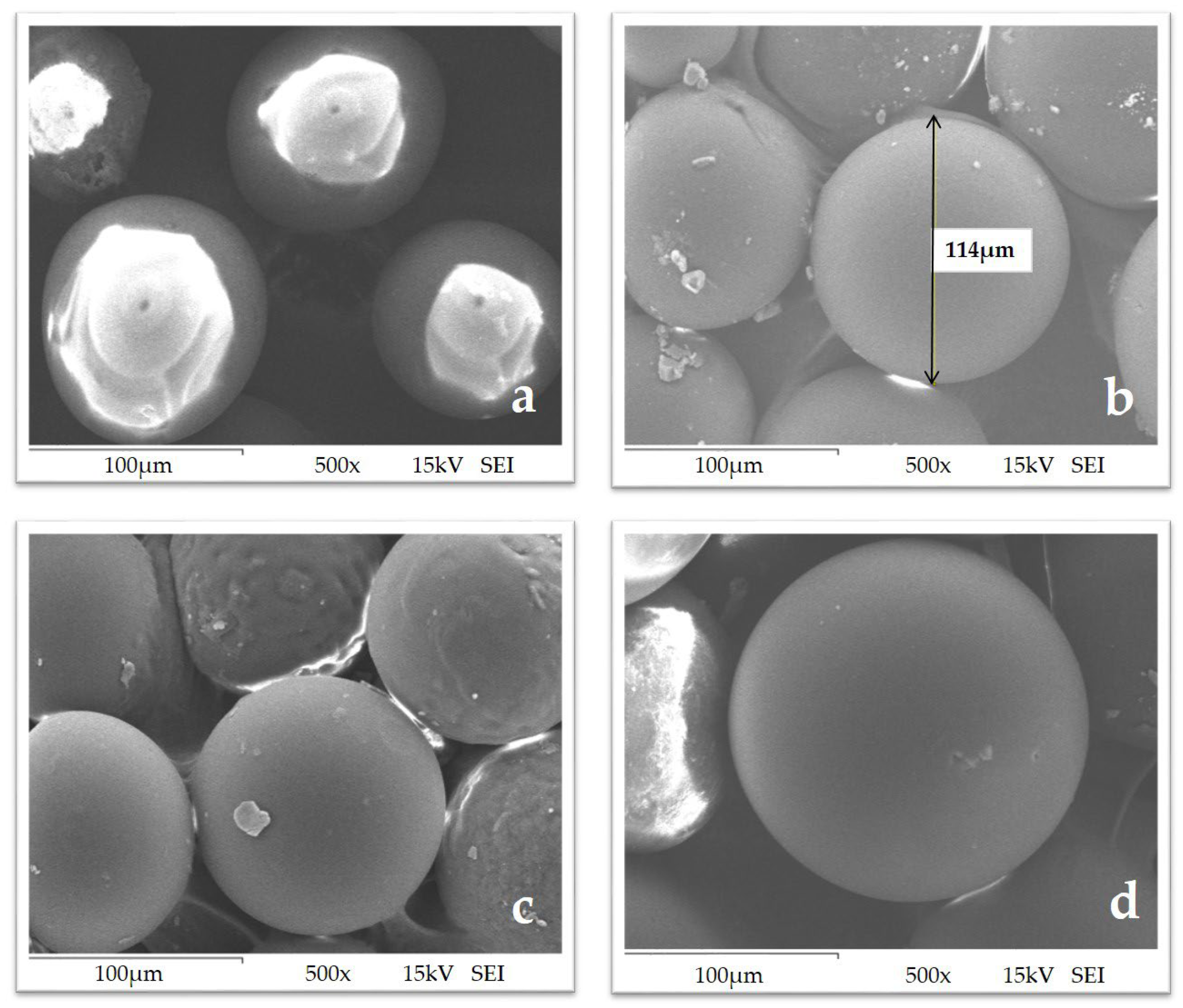
3.1. Synthesis and Characterization of Chelating Resins
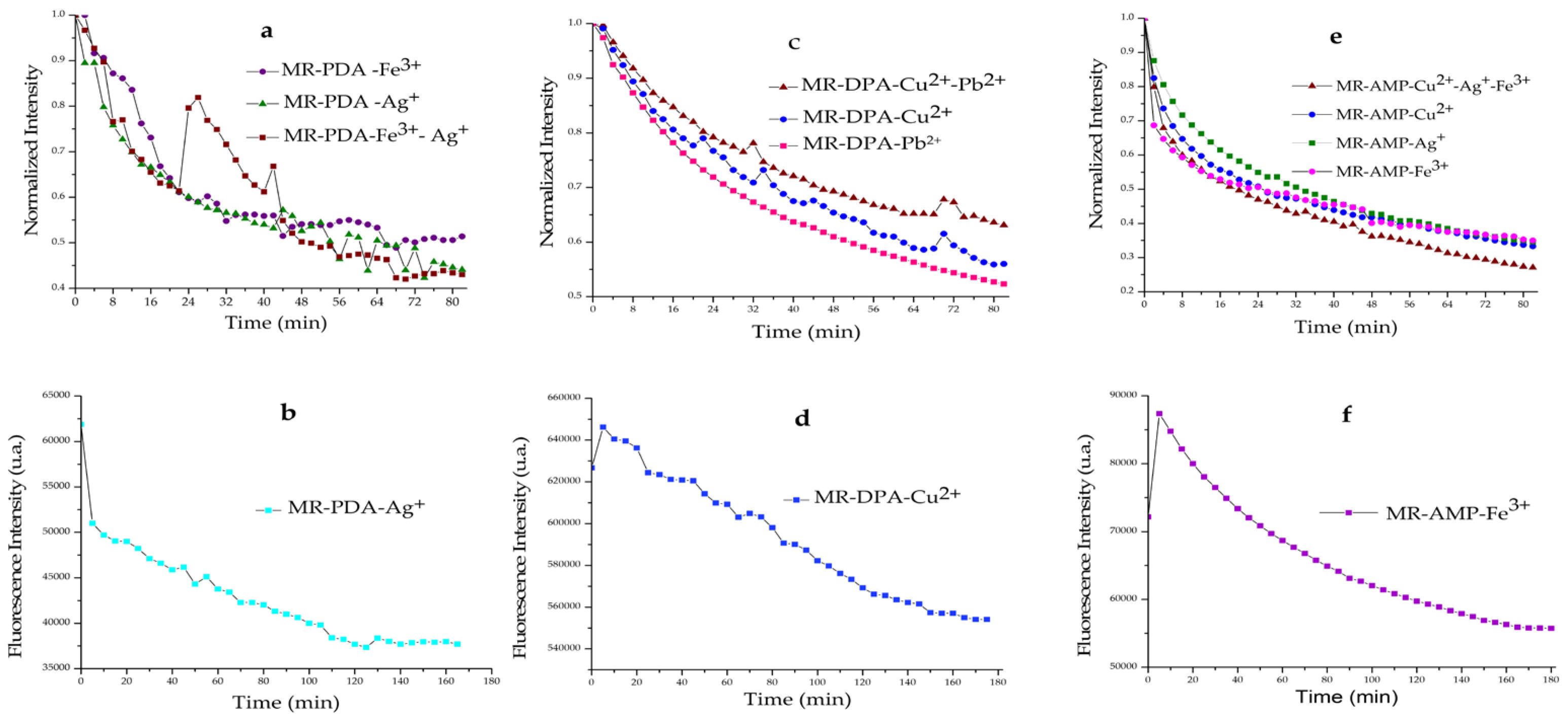
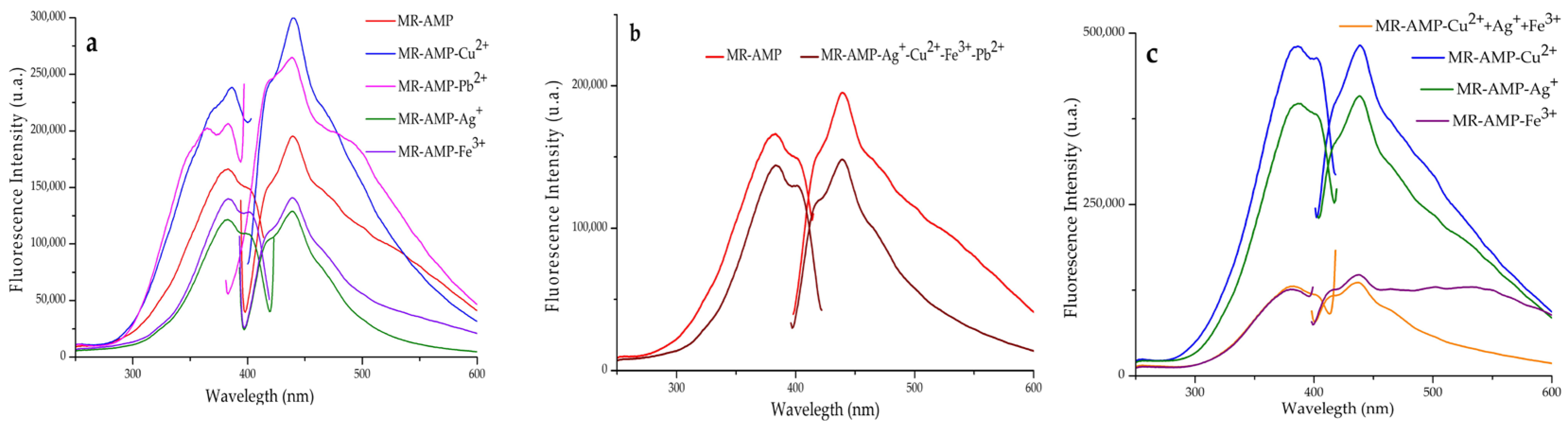
3.2. Sensing Properties of Chelating Resins towards Metal Cations
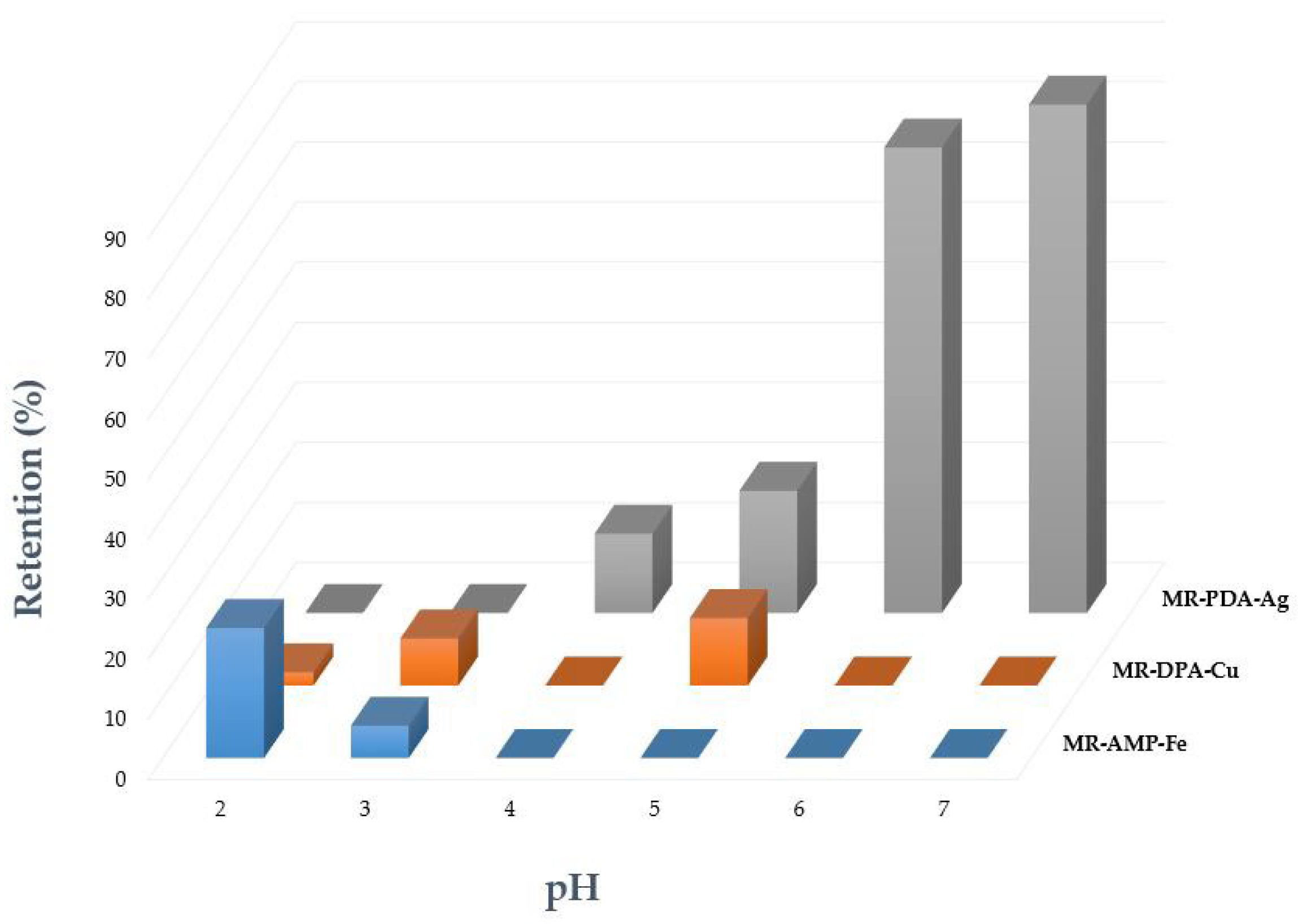
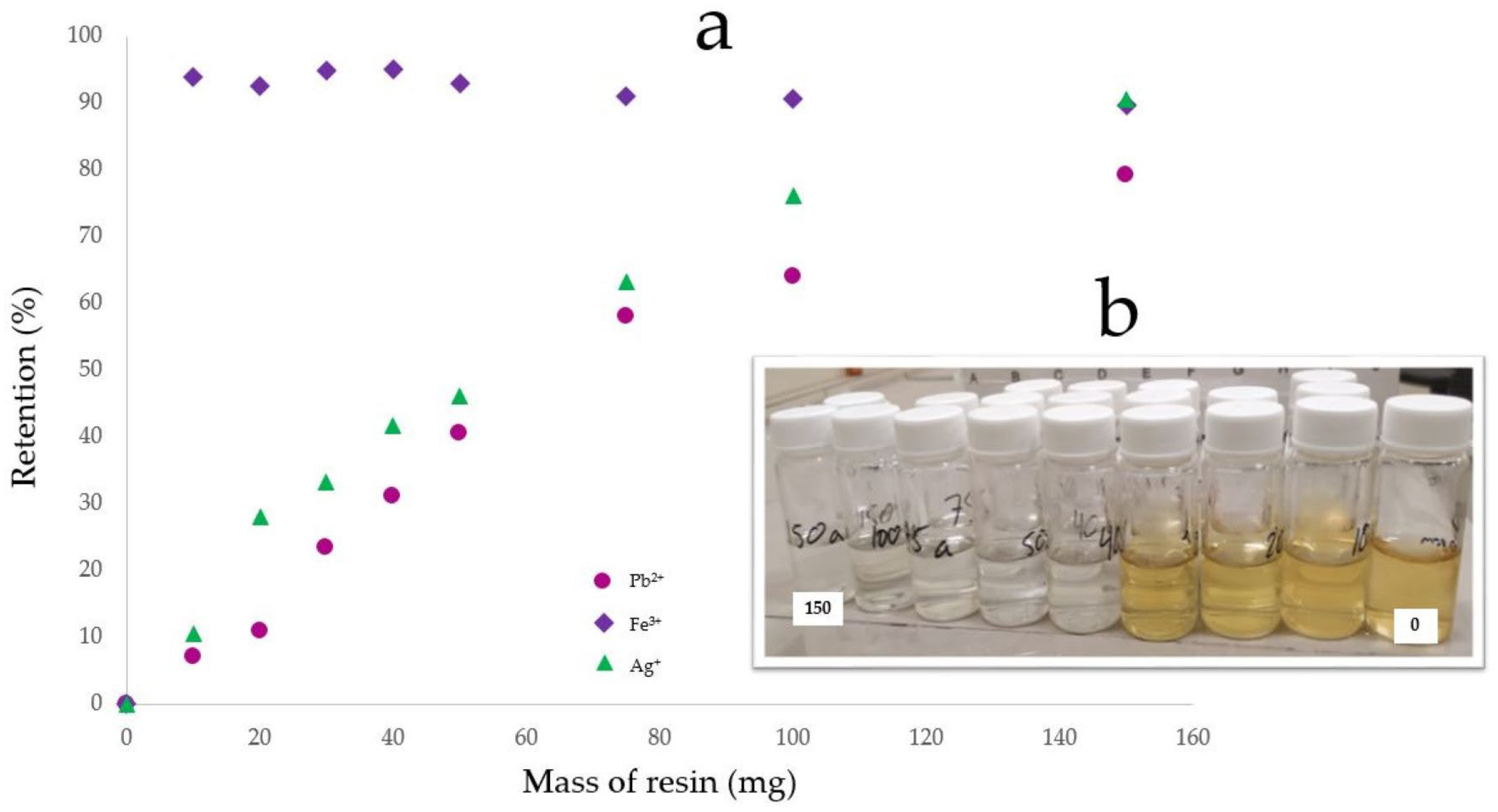
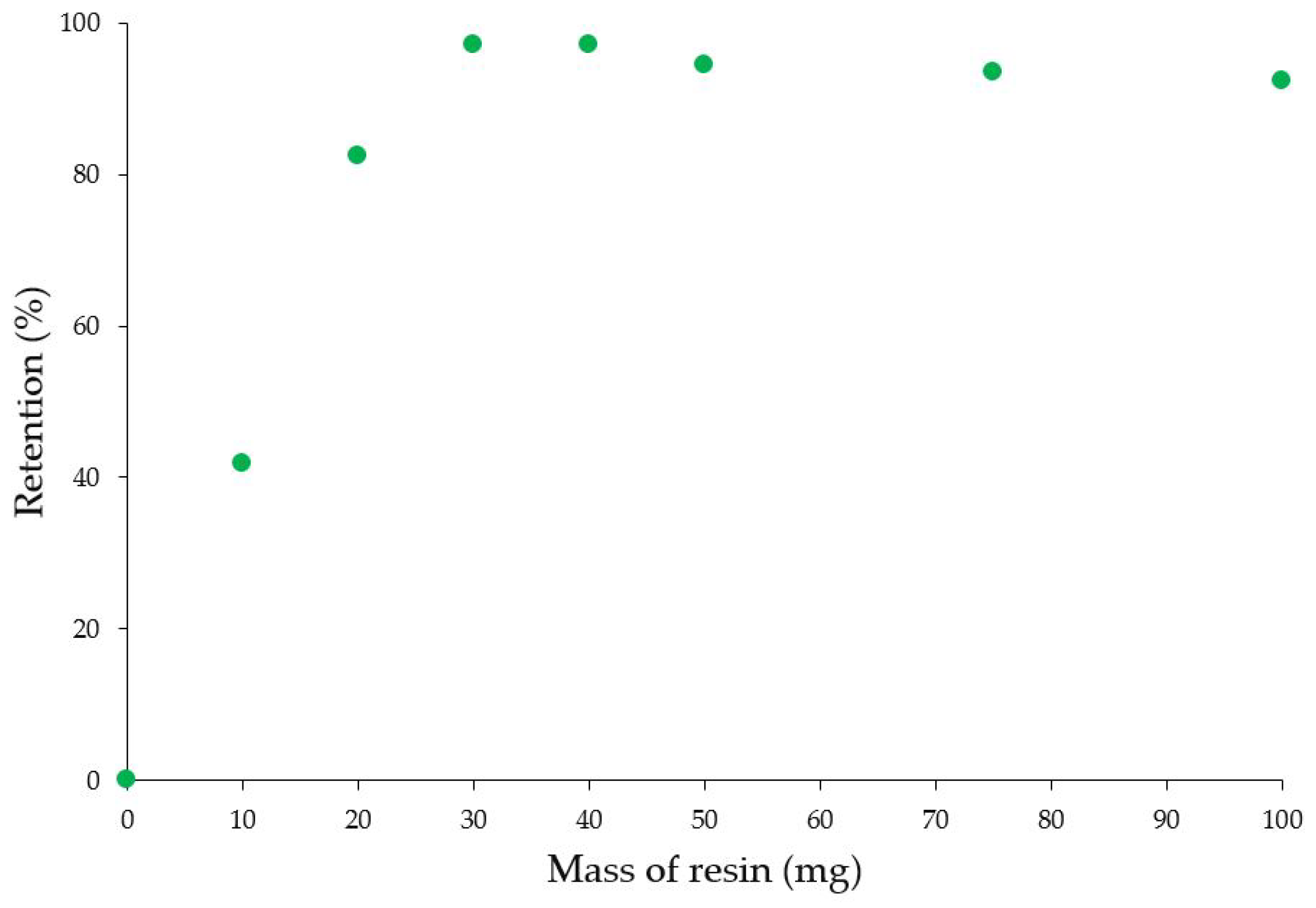
3.3. Adsorption Properties of Chelating Resins
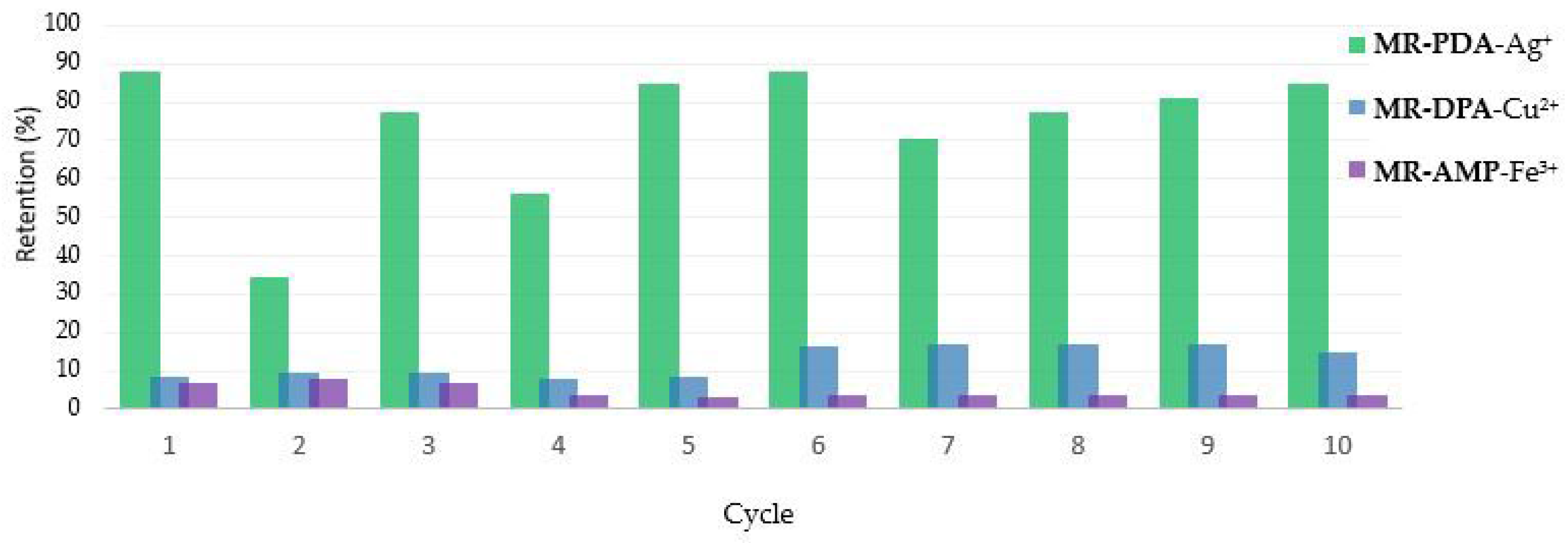
3.4. Chelating Resins Reusability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, R.; Goldsby, K. Química, 12th ed.; McGraw-Hill: Ciudad de México, México, 2017; pp. 930–955. [Google Scholar]
- González, P.A. Química de los Metales. Alambique 1999, 21. Available online: https://www.researchgate.net/publication/259638333_Quimica_de_los_metales (accessed on 22 November 2022).
- Covarrubias, S.A.; Peña Cabriales, J.J. Contaminación ambiental por metales pesados en México: Problemática y estrategias de fitorremediación. Rev. Int. Contam. Ambient. 2017, 33, 7–21. [Google Scholar] [CrossRef]
- Londoño Franco, L.F.; Londoño Muñoz, P.T.; Muñoz Garcia, F.G. Los riesgos de los metales pesados en la salud humana y animal. Biotecnol. Sector Agropecuario Agroind. 2016, 14, 145–153. [Google Scholar] [CrossRef]
- Minería Tóxica, Una lacra que se debe exterminar (Sonora). Available online: https://www.grieta.org.mx/index.php/2019/07/23/mineria-toxica-una-lacra-que-se-debe-exterminar-sonora/ (accessed on 8 June 2023).
- Se derrama combustóleo de refinería de Pemex en Hidalgo; Afecta al Río Tula. Available online: https://www.eluniversal.com.mx/estados/se-derrama-combustoleo-de-refineria-de-pemex-en-hidalgo-afecta-al-rio-tula/ (accessed on 8 June 2023).
- Peñoles: Minería Tóxica. Available online: https://www.jornada.com.mx/2015/01/12/opinion/026o1eco (accessed on 8 June 2023).
- Panorama Minero del Estado de Sonora. Available online: https://www.sgm.gob.mx/Gobmx/productos/panoramas/SONORA_jul2006.pdf (accessed on 8 June 2023).
- Cancer. Available online: https://www.who.int/es/news-room/fact-sheets/detail/cancer (accessed on 8 June 2023).
- Cancer Today. Available online: https://gco.iarc.fr/today/data/factsheets/populations/484-mexico-fact-sheets.pdf (accessed on 8 June 2023).
- Aldaco-Sarvide, F.; Pérez-Pérez, P.; Cervantes-Sánchez, G.; Torrecillas-Torres, L.; Erazo-V, A.E. Mortalidad por cáncer en México 2000–2010: El recuento de los daños. Gamo 2012, 11, 371–379. [Google Scholar]
- Este tipo de cáncer es el más letal para los mexicanos. Available online: https://www.nacion321.com/ciudadanos/este-tipo-de-cancer-es-el-mas-letal-para-los-mexicanos (accessed on 8 June 2023).
- López-Abente, G.; Locutura-Rupérez, J.; Fernández-Navarro, P.; Martín-Méndez, I.; Bel-Lan, A.; Núñez, O. Compositional analysis of topsoil metals and its associations with cancer mortality using spatial misaligned data. Environ. Geochem. Health 2018, 40, 283–294. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Men, Y.; Wang, W.; Zhang, W. Heavy metals interfere with plasma metabolites, including lipids and amino acids, in patients with breast cancer. Oncol. Lett. 2020, 19, 2925–2933. [Google Scholar] [CrossRef]
- Rodríguez-Heredia, D. Occupational poisoning due to heavy metals. Medisan 2017, 21, 3372–3386. [Google Scholar]
- Nava-Ruíz, C.; Méndez-Armenta, M. Neurotoxic effects of heavy metals cadmium, lead arsenic, and thallium. Arch. Neurocien. 2011, 16, 140–147. [Google Scholar]
- Ihsanullah, A.; Adnan, A.; Tahar, M.; Mustafa, N.; Majeda, K.; Muataz, A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2017, 157, 141–161. [Google Scholar] [CrossRef]
- Dinu, M.V.; Dragan, E.S. Heavy metals adsorption on some iminodiacetate chelating resins as a function of the adsorption parameters. React. Funct. Polym. 2008, 68, 1346–1354. [Google Scholar] [CrossRef]
- Parra-García, V. Sensores Químicos Basados en Materiales Moleculares: De la Molécula al Material, del Material al Dispositivo. An. Quím. 2008, 104, 5–14. [Google Scholar]
- Prasanna de Silva, A.; McCaughan, B.; McKinney, B.; Querol, M. Newer optical-based molecular devices from older coordination chemistry. Dalton Trans. 2003, 10, 1902–1913. [Google Scholar] [CrossRef]
- Santacruz-Ortega, H.; Pina-Luis, G.; López, K.; Rivero, I. Preparation of a Library of EDTA Amide x-Amine Naphthalene-and-sulfonic Acid Derivatives on Solid Phase and Their Fluorescence Behavior toward Transition Metals. J. Comb. Chem. 2009, 11, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Merrifield, R.B. Solid Phase Peptide Synthesis, I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Aguilar-Martínez, M.; Vargas-Durazo, J.; Ochoa-Lara, K.; Santacruz-Ortega, H.; Gálvez-Ruiz, J.C. Merrifield and Wang Resins Functionalized with Bidentate Amines: Useful Materials to Support Reducing Complexes and as Alkali Metal Sensors. Z. Anorg. Allg. Chem. 2013, 639, 1166–1172. [Google Scholar] [CrossRef]
- Pina-Luis, G.; Ochoa, A.; Rivero, I.A. Solid Phase Synthesis of N-Alkyl-bis-o-aminobenzamides for Metal Ion Sensing Based on a Fluorescent Dansyl Platform. J. Comb. Chem. 2009, 11, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Pina-Luis, G.; Rosqueta, G.; Váldez, A.; Ochoa, A.; Rivero, I.; Díaz-García, M. Morin functionalized Merrifield´s resin: A new material for the enrichment and sensing heavy metals. React. Funct. Polym. 2010, 72, 61–68. [Google Scholar] [CrossRef]
- Ghassabzadeh, H.; Mohadespour, A.; Torab-Mostaedi, M.; Zaheri, P.; Maragheh, M.; Taheri, H. Adsorption of Ag, Cu, and Hg from aqueous solutions using expanded perlite. J. Hazard. Mater. 2010, 177, 950–955. [Google Scholar] [CrossRef]
- Martínez-Meza, R.; Certucha-Barragán, M.; Zavala-Rivera, P.; Gómez-Álvarez, A.; Almazán-Holguín, L. Remoción de hierro y magnesio de un efluente contaminado utilizando una resina quelante. Rev. Int. Contam. Ambie. 2017, 33, 55–63. [Google Scholar] [CrossRef]
- Zou, B.; Zhang, S.; Sun, P.; Ye, Z.; Zhao, Q.; Zhang, W.; Zhou, L. Preparation of novel poly-chloromethyl styrene chelating resin containing hetero-fluorenone pendant groups for the removal of Cu(II), Pb(II), and Ni (II) from wastewaters. Colloid Interface Sci. Commun. 2021, 40, 100349. [Google Scholar] [CrossRef]
- Duan, G.; Zhanfang, C.; Zhong, H.; Ma, X.; Wang, S. Highly efficient poly(6-acryloylamino-N-hydroxyhexanamide) resin for adsorption of heavy metal ions. J. Environ. Manag. 2022, 308, 114631. [Google Scholar] [CrossRef]
- Marquardt, M.; Eifler-Lima, V. The solid phase organic synthesis and its most used polymeric supports. Quím. Nova 2001, 24, 846–855. [Google Scholar] [CrossRef]
- Rivero, I.; Aceves, R. Síntesis de BOP (acoplador de péptidos) en fase sólida. Rev. Soc. Quím. Méx. 2000, 44, 97–100. [Google Scholar]
- Islam, A.; Ahmad, H.; Zaidi, M.N.; Yadav, S. Selective Separation of Aluminum from Biological and Environmental Samples Using Glyoxal-bis(2-hydroxy anil) Functionalized Amberlite XAD-16 Resin: Kinetics and Equilibrium Studies. Ind. Eng. Chem. Res. 2013, 52, 5213–5220. [Google Scholar] [CrossRef]
- Pina-Luis, G.; Badía, R.; Díaz-García, M.E.; Rivero, I. Fluorometric monitoring of organic reactions on the solid phase. J. Comb. Chem. 2004, 6, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Skoog, D.A.; West, D.M.; Holler, F.J.; Crouch, S.R. Fundamentos de Química Analítica; Cengage Learning: Ciudad de México, México, 2015; pp. 760–766. [Google Scholar]
- Li, X.; Qing, Z.; Li, Y.; Zou, Z.; Yang, S.; Yang, R. Natural Peptide Probe Screened for High-Performance Fluorescent Sensing of Copper Ion: Especially Sensitivity, Rapidity, and Environment-Friendliness. ACS Omega 2019, 4, 793–800. [Google Scholar] [CrossRef]
- Ristroph, K.D.; Prud’homme, R.K. Hydrophobic ion pairing: Encapsulating small molecules, peptides, and proteins into nanocarriers. Nanoscale Adv. 2019, 1, 4207–4237. [Google Scholar] [CrossRef]
- Hubicki, Z.; Kołodyğska, D. Selective Removal of Heavy Metal Ions from Waters and Waste Waters Using Ion Exchange Methods. In Ion Exchange Technologies; Kilislioǵlu, A., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 193–240. [Google Scholar] [CrossRef]
- Kołodyńska, D. Chelating Agents of a New Generation as an Alternative to Conventional Chelators for Heavy Metal Ions Removal from Different Waste Waters. In Expanding Issues in Desalination; Ning, R.Y., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 339–370. [Google Scholar] [CrossRef]
- Simonescu, C.M.; Lavric, V.; Musina, A.; Antonescu, O.A.; Culita, D.C.; Marinescu, V.; Tardei, C.; Oprea, O.; Pandele, A.M. Experimental and modeling of cadmium ions removal by chelating resins. J. Mol. Liq. 2020, 307, 112973. [Google Scholar] [CrossRef]
- Rivas, B.L.; Muñoz, C. Removal of environmentally impacting metal ions using functional resin poly(4-styrene sulfonate-co-4-vinylpyridine): Synthesis and metal ion retention properties. J. Appl. Polym. Sci. 2007, 104, 1769–1774. [Google Scholar] [CrossRef]
- Singh, A.; Chahar, U. Synthesis of novel tamarind 8-hydroxyquinoline-5-sulfonic acid (THQSA) resin and their application in industrial effluent treatment. Int. J. Pharm. Pharm. Sci. 2014, 6, 340–344. [Google Scholar]
- Pérez, F.; García, D.; De Ávila, J. Preparación y Utilización de una Resina Quelante en la Adsorción de Iones Metálicos sobre Desechos Catalíticos y su Posible Reutilización. Rev. ION 1997, 14, 21–36. [Google Scholar]





| Resin | Cation | Maximum Retention % | Adsorbent Dose (mg) for Maximum Retention |
|---|---|---|---|
| MR | Ag+ Pb2+ Fe3+ | 42 26 83 | 150 150 150 |
| MR-PDA | Fe3+ Ag+ Pb2+ | 93 91 80 | 10 150 150 |
| MR-DPA | Ag+ Pb2+ Cu2+ | 91 98 94 | 30 40 100 |
| MR-AMP | Ag+ | 97 | 30 |
| Adsorbent | Metals/Adsorption Capacity | pH | Reference |
|---|---|---|---|
| MR-AMP | Fe3+ (4.3 mg/g) | 2 | This work |
| Dowex-4195 | Fe3+ (7.5 mg/g) | 2 | [27] |
| THQSA | Fe2+ (9.1 × 103 mg/g) | 2 | [41] |
| MR-DPA | Cu2+ (2.2 mg/g) | 5 | This work |
| Bis-(phosphonomethyl)amine resin | Cu2+ (569.5 mg/g) | 5 | [42] |
| Polychloromethylestyrene resin | Cu2+ (161.9 mg/g) | 5 | [28] |
| Poly(6-acryloyl amino-N-hydroxyhexanamide) resin | Cu2+ (238.6 mg/g) | 5 | [29] |
| THQSA | Cu2+ (10.6 × 103 mg/g) | 5 | [41] |
| Expanded perlite | Cu2+ (2.0 mg/g) | 6.5 | [26] |
| Morin and Merrifield resin | Cu2+ (19 × 10−6 mg/g) | 7 | [25] |
| MR-PDA | Ag+ (18.4 mg/g) | 7 | This work |
| Expanded perlite | Ag+ (8.5 mg/g) | 6.5 | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villa-Reyna, A.-L.; Aguilar-Martínez, M.; Ochoa-Terán, A.; Santacruz-Ortega, H.; Leyva-Peralta, M.-A.; Vargas-Durazo, J.-T.; Salazar-Gastelum, M.I.; García-Elías, J.; Gálvez-Ruiz, J.-C. Efficient and Sustainable Bidentate Amines-Functionalized Resins for Removing Ag+, Cu2+, Pb2+, and Fe3+ from Water. Polymers 2023, 15, 2778. https://doi.org/10.3390/polym15132778
Villa-Reyna A-L, Aguilar-Martínez M, Ochoa-Terán A, Santacruz-Ortega H, Leyva-Peralta M-A, Vargas-Durazo J-T, Salazar-Gastelum MI, García-Elías J, Gálvez-Ruiz J-C. Efficient and Sustainable Bidentate Amines-Functionalized Resins for Removing Ag+, Cu2+, Pb2+, and Fe3+ from Water. Polymers. 2023; 15(13):2778. https://doi.org/10.3390/polym15132778
Chicago/Turabian StyleVilla-Reyna, Ana-Laura, Milagros Aguilar-Martínez, Adrián Ochoa-Terán, Hisila Santacruz-Ortega, Mario-Alberto Leyva-Peralta, Judas-Tadeo Vargas-Durazo, Moisés I. Salazar-Gastelum, José García-Elías, and Juan-Carlos Gálvez-Ruiz. 2023. "Efficient and Sustainable Bidentate Amines-Functionalized Resins for Removing Ag+, Cu2+, Pb2+, and Fe3+ from Water" Polymers 15, no. 13: 2778. https://doi.org/10.3390/polym15132778
APA StyleVilla-Reyna, A.-L., Aguilar-Martínez, M., Ochoa-Terán, A., Santacruz-Ortega, H., Leyva-Peralta, M.-A., Vargas-Durazo, J.-T., Salazar-Gastelum, M. I., García-Elías, J., & Gálvez-Ruiz, J.-C. (2023). Efficient and Sustainable Bidentate Amines-Functionalized Resins for Removing Ag+, Cu2+, Pb2+, and Fe3+ from Water. Polymers, 15(13), 2778. https://doi.org/10.3390/polym15132778