Lignin Extracted from Various Parts of Castor (Ricinus communis L.) Plant: Structural Characterization and Catalytic Depolymerization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lignin Isolation
2.3. Structural Characterization
2.4. Catalytic Hydrogenolysis of Lignin into Aromatics
3. Results and Discussion
3.1. Chemical Compositional Analysis of Various Castor Parts
3.2. Lignin Isolation
3.3. Thioacidolysis Analysis
3.4. 2D HSQC NMR Analysis
3.5. 31P NMR Spectra
3.6. Molecular Weights Analysis
3.7. Catalytic Hydrogenolysis of Lignin into Aromatics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, R.; Bhaskar, T. Chapter 11-Potential of castor plant (Ricinus communis) for production of biofuels, chemicals, and value-added products. In Waste Biorefinery; Bhaskar, T., Pandey, A., Rene, E.R., Tsang, D.C.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 269–310. [Google Scholar]
- Senthilvel, S.; Manjunatha, T.; Lavanya, C. Castor Breeding. In Fundamentals of Field Crop Breeding; Yadava, D.K., Dikshit, H.K., Mishra, G.P., Tripathi, S., Eds.; Springer Nature: Singapore, 2022; pp. 945–970. [Google Scholar]
- Ogunniyi, D.S. Castor oil: A vital industrial raw material. Bioresour. Technol. 2006, 97, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Severino, L.S.; Auld, D.L. A framework for the study of the growth and development of castor plant. Ind. Crops Prod. 2013, 46, 25–38. [Google Scholar] [CrossRef]
- Mutlu, H.; Meier, M.A.R. Castor oil as a renewable resource for the chemical industry. Eur. J. Lipid Sci. Technol. 2010, 112, 10–30. [Google Scholar] [CrossRef]
- Grigoriou, A.H.; Ntalos, G.A. The potential use of Ricinus communis L. (Castor) stalks as a lignocellulosic resource for particleboards. Ind. Crops Prod. 2001, 13, 209–218. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, C.; Yang, G.; Chen, J.; Xu, F.; Geun Yoo, C.; Lyu, G. Rapid and efficient microwave-assisted guanidine hydrochloride deep eutectic solvent pretreatment for biological conversion of castor stalk. Bioresour. Technol. 2022, 343, 126022. [Google Scholar] [CrossRef]
- Stöcker, M. Biofuels and Biomass-To-Liquid Fuels in the Biorefinery: Catalytic Conversion of Lignocellulosic Biomass using Porous Materials. Angew. Chem. Int. Ed. 2008, 47, 9200–9211. [Google Scholar] [CrossRef]
- Petrus, L.; Noordermeer, M.A. Biomass to biofuels, a chemical perspective. Green Chem. 2006, 8, 861–867. [Google Scholar] [CrossRef]
- Kocaturk, E.; Salan, T.; Ozcelik, O.; Alma, M.H.; Candan, Z. Recent Advances in Lignin-Based Biofuel Production. Energies 2023, 16, 3382. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, L.; Hou, Q.; Mo, Z.; Liu, X.; Li, W. A Review on Catalytic Depolymerization of Lignin towards High-Value Chemicals: Solvent and Catalyst. Fermentation 2023, 9, 386. [Google Scholar] [CrossRef]
- Abdullah, T.; İlyasoğlu, G.; Memić, A. Designing Lignin-Based Biomaterials as Carriers of Bioactive Molecules. Pharmaceutics 2023, 15, 1114. [Google Scholar] [CrossRef]
- del Río, J.C.; Prinsen, P.; Rencoret, J.; Nieto, L.; Jiménez-Barbero, J.; Ralph, J.; Martínez, Á.T.; Gutiérrez, A. Structural Characterization of the Lignin in the Cortex and Pith of Elephant Grass (Pennisetum purpureum) Stems. J. Agric. Food Chem. 2012, 60, 3619–3634. [Google Scholar] [CrossRef]
- Li, M.; Pu, Y.; Ragauskas, A.J. Current Understanding of the Correlation of Lignin Structure with Biomass Recalcitrance. Front. Chem. 2016, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Ma, Y.-X.; Liu, H.-M.; Qin, G.-Y.; Wang, X.-D. Structural elucidation of lignin-carbohydrate complexes (LCCs) from Chinese quince (Chaenomeles sinensis) fruit. Int. J. Biol. Macromol. 2018, 116, 1240–1249. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin Biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Barros, J. Lignin biosynthesis: Old roads revisited and new roads explored. Open Biol. 2019, 9, 190215. [Google Scholar] [CrossRef]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Ralph, S.A.; Christensen, J.H.; et al. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Wang, S.; Shen, Q.; Su, S.; Lin, J.; Song, G. The temptation from homogeneous linear catechyl lignin. Trends Chem. 2022, 4, 948–961. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Mottiar, Y.; Vanholme, R.; Boerjan, W.; Ralph, J.; Mansfield, S.D. Designer lignins: Harnessing the plasticity of lignification. Curr. Opin. Biotechnol. 2016, 37, 190–200. [Google Scholar] [CrossRef]
- Chen, F.; Tobimatsu, Y.; Havkin-Frenkel, D.; Dixon, R.A.; Ralph, J. A polymer of caffeyl alcohol in plant seeds. Proc. Natl. Acad. Sci. USA 2012, 109, 1772–1777. [Google Scholar] [CrossRef]
- Tobimatsu, Y.; Chen, F.; Nakashima, J.; Escamilla-Treviño, L.L.; Jackson, L.; Dixon, R.A.; Ralph, J. Coexistence but Independent Biosynthesis of Catechyl and Guaiacyl/Syringyl Lignin Polymers in Seed Coats. Plant Cell 2013, 25, 2587–2600. [Google Scholar] [CrossRef]
- Chen, F.; Tobimatsu, Y.; Jackson, L.; Nakashima, J.; Ralph, J.; Dixon, R.A. Novel seed coat lignins in the Cactaceae: Structure, distribution and implications for the evolution of lignin diversity. Plant J. 2013, 73, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wang, S.; Song, G. Disassembling catechyl and guaiacyl/syringyl lignins coexisting in Euphorbiaceae seed coats. Green Chem. 2021, 23, 7235–7242. [Google Scholar] [CrossRef]
- Su, S.; Shen, Q.; Wang, S.; Song, G. Discovery, disassembly, depolymerization and derivatization of catechyl lignin in Chinese tallow seed coats. Int. J. Biol. Macromol. 2023, 239, 124256. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Su, S.; Xiao, L.-P.; Wang, B.; Sun, R.-C.; Song, G. Catechyl Lignin Extracted from Castor Seed Coats Using Deep Eutectic Solvents: Characterization and Depolymerization. ACS Sustain. Chem. Eng. 2020, 8, 7031–7038. [Google Scholar] [CrossRef]
- Li, Y.; Shuai, L.; Kim, H.; Motagamwala, A.H.; Mobley, J.K.; Yue, F.; Tobimatsu, Y.; Havkin-Frenkel, D.; Chen, F.; Dixon, R.A.; et al. An “ideal lignin” facilitates full biomass utilization. Sci. Adv. 2018, 4, eaau2968. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, K.; Li, H.; Xiao, L.-P.; Song, G. Selective hydrogenolysis of catechyl lignin into propenylcatechol over an atomically dispersed ruthenium catalyst. Nat. Commun. 2021, 12, 416. [Google Scholar] [CrossRef]
- Rolando, C.; MONTlES, B.; Lapierre, C. Thioacidolysis. In Methods in Lignin Chemistry; Lin, S.Y., Dence, C.W., Eds.; Springer: Heidelberg, Germany, 1992; pp. 334–349. [Google Scholar]
- Wen, J.-L.; Yuan, T.-Q.; Sun, S.-L.; Xu, F.; Sun, R.-C. Understanding the chemical transformations of lignin during ionic liquid pretreatment. Green Chem. 2014, 16, 181–190. [Google Scholar] [CrossRef]
- Wang, S.; Li, W.-X.; Yang, Y.-Q.; Chen, X.; Ma, J.; Chen, C.; Xiao, L.-P.; Sun, R.-C. Unlocking Structure–Reactivity Relationships for Catalytic Hydrogenolysis of Lignin into Phenolic Monomers. ChemSusChem 2020, 13, 4548–4556. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Report NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2012. [Google Scholar]
- Abu-Omar, M.M.; Barta, K.; Beckham, G.T.; Luterbacher, J.S.; Ralph, J.; Rinaldi, R.; Román-Leshkov, Y.; Samec, J.S.M.; Sels, B.F.; Wang, F. Guidelines for performing lignin-first biorefining. Energy Environ. Sci. 2021, 14, 262–292. [Google Scholar] [CrossRef]
- Mansfield, S.D.; Kim, H.; Lu, F.; Ralph, J. Whole plant cell wall characterization using solution-state 2D NMR. Nat. Protoc. 2012, 7, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ralph, J. Solution-state 2D NMR of ball-milled plant cell wall gels in DMSO-d6/pyridine-d5. Org. Biomol. Chem. 2010, 8, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.-L.; Sun, S.-L.; Xue, B.-L.; Sun, R.-C. Recent Advances in Characterization of Lignin Polymer by Solution-State Nuclear Magnetic Resonance (NMR) Methodology. Materials 2013, 6, 359–391. [Google Scholar] [CrossRef] [PubMed]
- del Río, J.C.; Rencoret, J.; Prinsen, P.; Martínez, A.T.; Ralph, J.; Gutiérrez, A. Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. J. Agric. Food Chem. 2012, 60, 5922–5935. [Google Scholar] [CrossRef]
- Prozil, S.O.; Evtuguin, D.V.; Silva, A.M.S.; Lopes, L.P.C. Structural Characterization of Lignin from Grape Stalks (Vitis vinifera L.). J. Agric. Food Chem. 2014, 62, 5420–5428. [Google Scholar] [CrossRef]
- Hatfield, R.D.; Marita, J.M. Enzymatic processes involved in the incorporation of hydroxycinnamates into grass cell walls. Phytochem. Rev. 2010, 9, 35–45. [Google Scholar] [CrossRef]
- del Río, J.C.; Lino, A.G.; Colodette, J.L.; Lima, C.F.; Gutiérrez, A.; Martínez, Á.T.; Lu, F.; Ralph, J.; Rencoret, J. Differences in the chemical structure of the lignins from sugarcane bagasse and straw. Biomass Bioenergy 2015, 81, 322–338. [Google Scholar] [CrossRef]
- Meng, X.; Crestini, C.; Ben, H.; Hao, N.; Pu, Y.; Ragauskas, A.J.; Argyropoulos, D.S. Determination of hydroxyl groups in biorefinery resources via quantitative 31P NMR spectroscopy. Nat. Protoc. 2019, 14, 2627–2647. [Google Scholar] [CrossRef]
- Pu, Y.; Cao, S.; Ragauskas, A.J. Application of quantitative 31P NMR in biomass lignin and biofuel precursors characterization. Energy Environ. Sci. 2011, 4, 3154–3166. [Google Scholar] [CrossRef]
- Crestini, C.; Argyropoulos, D.S. Structural Analysis of Wheat Straw Lignin by Quantitative 31P and 2D NMR Spectroscopy. The Occurrence of Ester Bonds and α-O-4 Substructures. J. Agric. Food Chem. 1997, 45, 1212–1219. [Google Scholar] [CrossRef]
- Yuan, T.-Q.; Sun, S.-N.; Xu, F.; Sun, R.-C. Structural Characterization of Lignin from Triploid of Populus tomentosa Carr. J. Agric. Food Chem. 2011, 59, 6605–6615. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, B.; Qi, Z.; Li, C.; Ji, J.; Dai, T.; Wang, A.; Zhang, T. Valorization of Lignin to Simple Phenolic Compounds over Tungsten Carbide: Impact of Lignin Structure. ChemSusChem 2017, 10, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, H.; Gao, X.; Guo, X.; Wang, S.; Fang, Y.; Song, G. Rational highly dispersed ruthenium for reductive catalytic fractionation of lignocellulose. Nat. Commun. 2022, 13, 4716. [Google Scholar] [CrossRef] [PubMed]
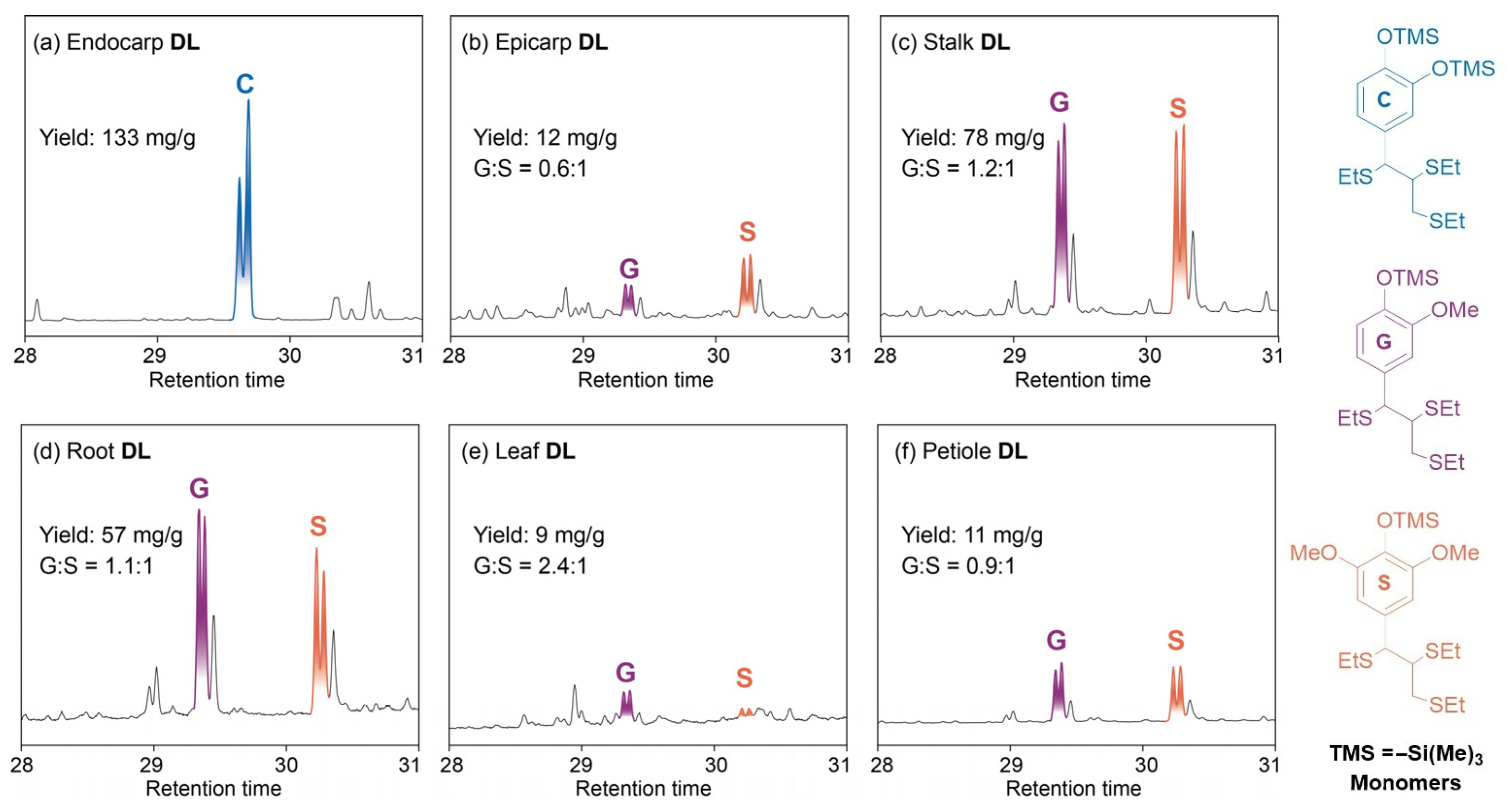
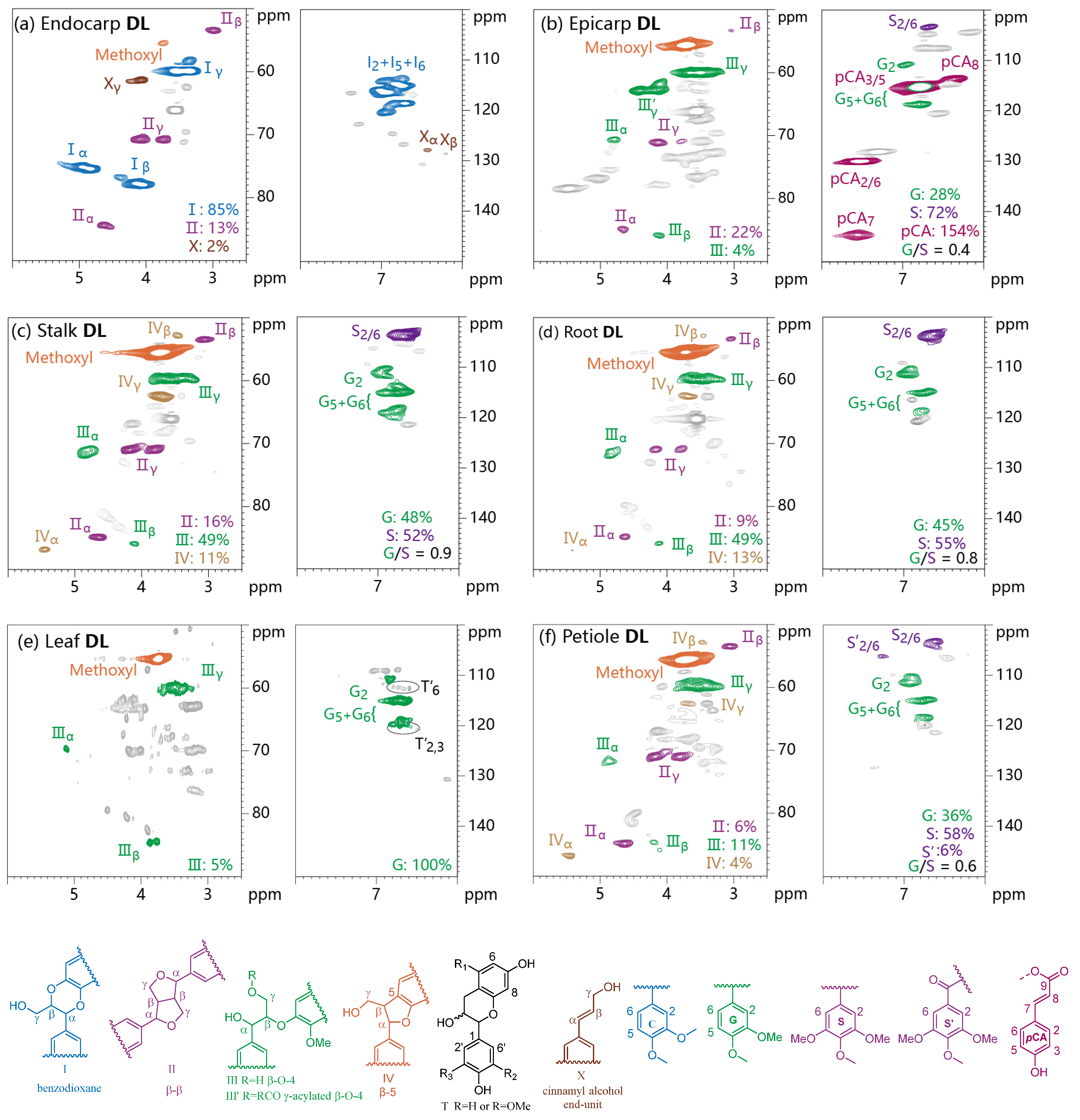
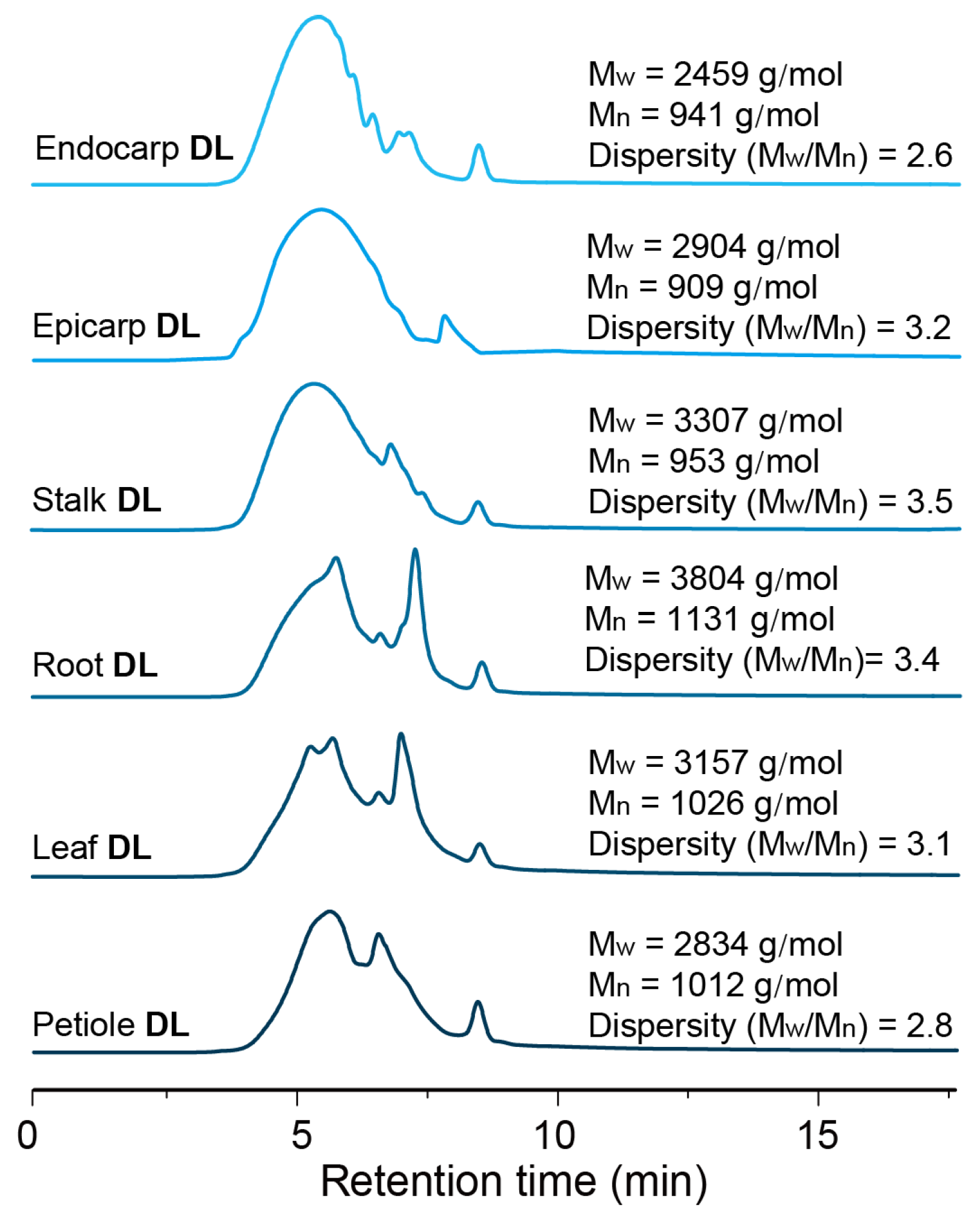
| Entry | Tissues | Cellulose (wt%) | Hemicellulose (wt%) | Lignin (wt%) | Lignin Subunit Distributions (mol%) c | ||
|---|---|---|---|---|---|---|---|
| C | G | S | |||||
| 1 | Endocarp | 19.3 | 10.6 | 13.3 b | 87 | 5 | 8 |
| 2 | Epicarp | 42.1 | 27.2 | 16.8 | − | 29 | 71 |
| 3 | Stalk | 46.2 | 24.0 | 21.0 | − | 47 | 53 |
| 4 | Root | 44.7 | 19.5 | 25.7 | − | 47 | 53 |
| 5 | Leaf | 35.5 | 19.0 | 13.8 | − | 73 | 27 |
| 6 | Petiole | 37.7 | 17.7 | 11.1 | − | 47 | 53 |
| DL Samples | Aliphatic OH | Guaiacyl OH | Syringyl OH | pCA-OH | Catechol OH | Carboxylic Group | Total Phenolic OH | ||
|---|---|---|---|---|---|---|---|---|---|
| Cond. a | Non. Cond. a | Cond. a | Non. Cond. a | ||||||
| Endocarp | 2.38 | − b | − | − | − | − | 2.43 | 0.02 | 2.43 |
| Epicarp | 2.70 | 0.03 | 0.16 | 0.08 | 0.02 | 1.32 | − | 0.02 | 1.61 |
| Stalk | 2.72 | 0.08 | 1.00 | 0.16 | 0.69 | − | − | 0.01 | 1.93 |
| Root | 2.64 | 0.29 | 1.22 | 0.10 | 0.37 | − | − | 0.02 | 1.98 |
| Petiole | 3.32 | 0.19 | 0.53 | 0.45 | 0.53 | − | − | 0.02 | 1.70 |
 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DL Samples | Phenolic Monomers (wt%) a | Total Yields (wt%) a | G/S a | Mw (g/mol) | |||||||
| C1 | C2 | G1 | G2 | S1 | S2 | S3 | H1 | ||||
| Endocarp | 3.2 | 32.4 | − b | − | − | − | − | − | 35.6 | − | 510 |
| Epicarp | − | − | 0.2 | trace | trace | trace | trace | 15.8 | 16.0 | − | 638 |
| Stalk | − | − | 1.2 | 2.0 | 1.6 | 1.8 | 4.8 | − | 11.4 | 0.5 | 699 |
| Root | − | − | 1.0 | 1.8 | 1.0 | 1.4 | 3.2 | − | 8.4 | 0.6 | 633 |
| Leaf | − | − | 0.8 | 0.6 | − | − | − | − | 1.4 | − | 597 |
| Petiole | − | − | 0.4 | 1.0 | 0.8 | 0.2 | 2.0 | − | 4.4 | 1.0 | 530 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Su, S.; Song, G. Lignin Extracted from Various Parts of Castor (Ricinus communis L.) Plant: Structural Characterization and Catalytic Depolymerization. Polymers 2023, 15, 2732. https://doi.org/10.3390/polym15122732
Wang Y, Su S, Song G. Lignin Extracted from Various Parts of Castor (Ricinus communis L.) Plant: Structural Characterization and Catalytic Depolymerization. Polymers. 2023; 15(12):2732. https://doi.org/10.3390/polym15122732
Chicago/Turabian StyleWang, Yihan, Shihao Su, and Guoyong Song. 2023. "Lignin Extracted from Various Parts of Castor (Ricinus communis L.) Plant: Structural Characterization and Catalytic Depolymerization" Polymers 15, no. 12: 2732. https://doi.org/10.3390/polym15122732
APA StyleWang, Y., Su, S., & Song, G. (2023). Lignin Extracted from Various Parts of Castor (Ricinus communis L.) Plant: Structural Characterization and Catalytic Depolymerization. Polymers, 15(12), 2732. https://doi.org/10.3390/polym15122732





