A pH-Sensitive Lignin-Based Material for Sustained Release of 8-Hydroxyquinoline
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of a pH-Responsive Lignin-Based Polymer
2.3. Acetylation of the pH-Responsive Lignin-Based Polymer
2.4. Calculation of the Content of 8-Hydroxyquinoline
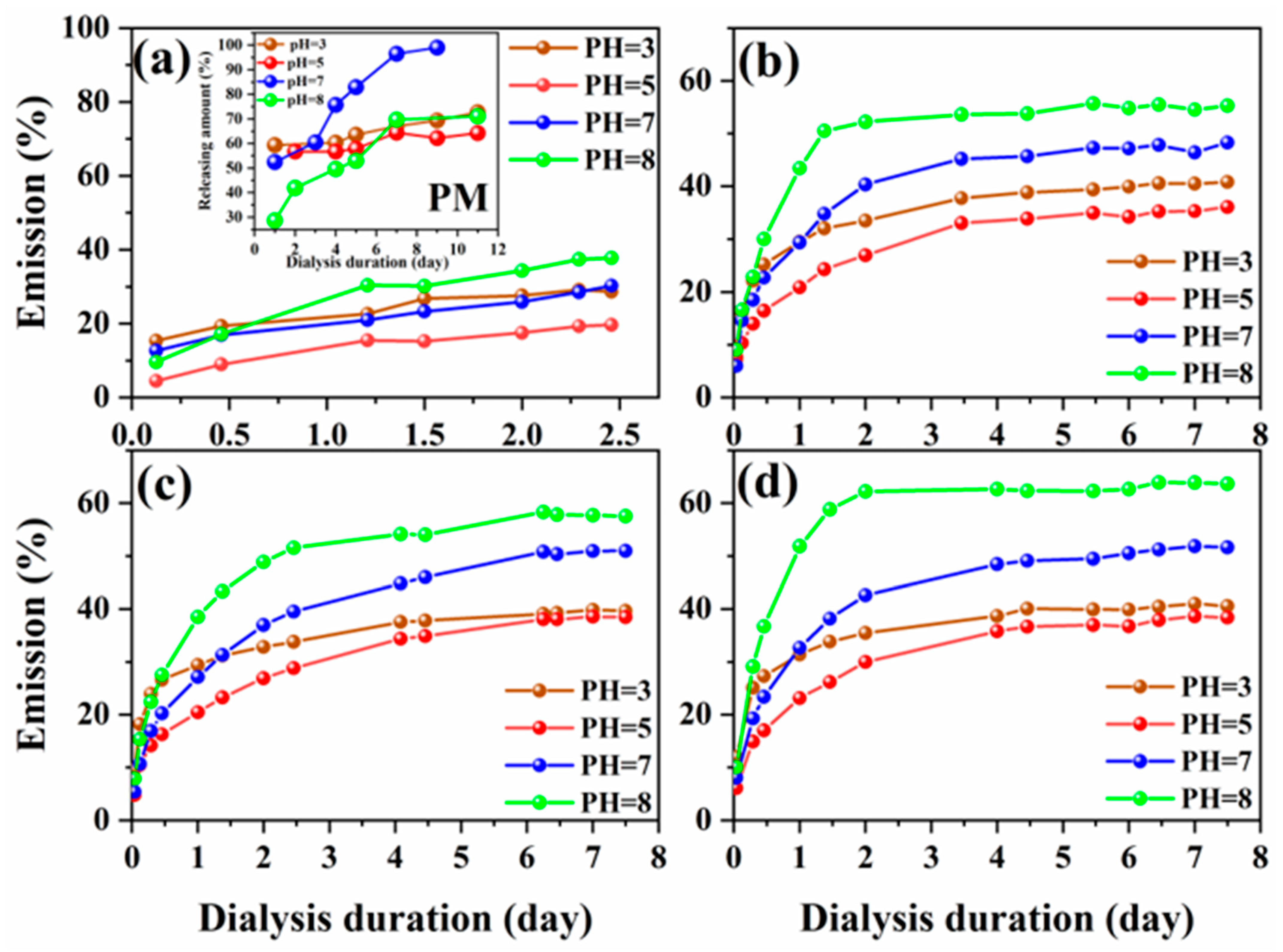
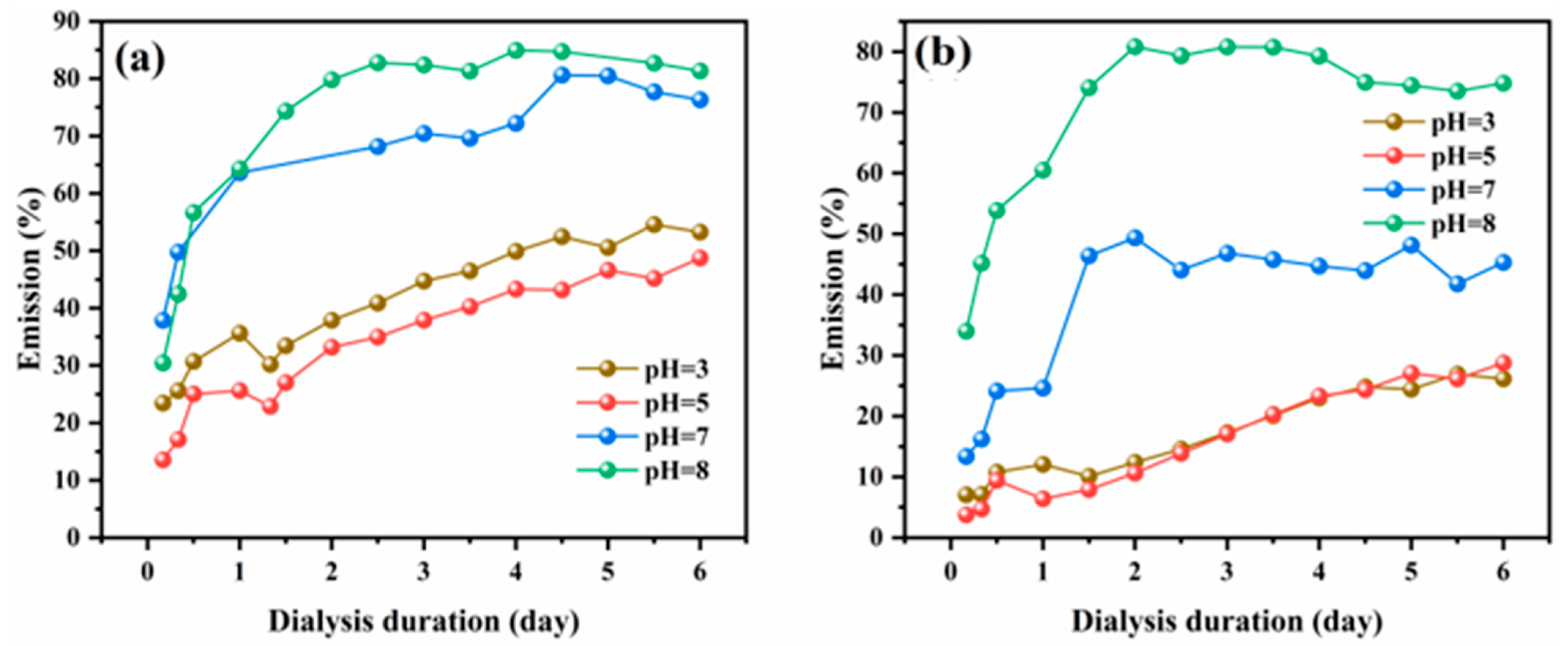
2.5. Release of 8HQ from the Polymer
2.6. Characterization
2.6.1. Ultraviolet Spectrophotometer (UV)
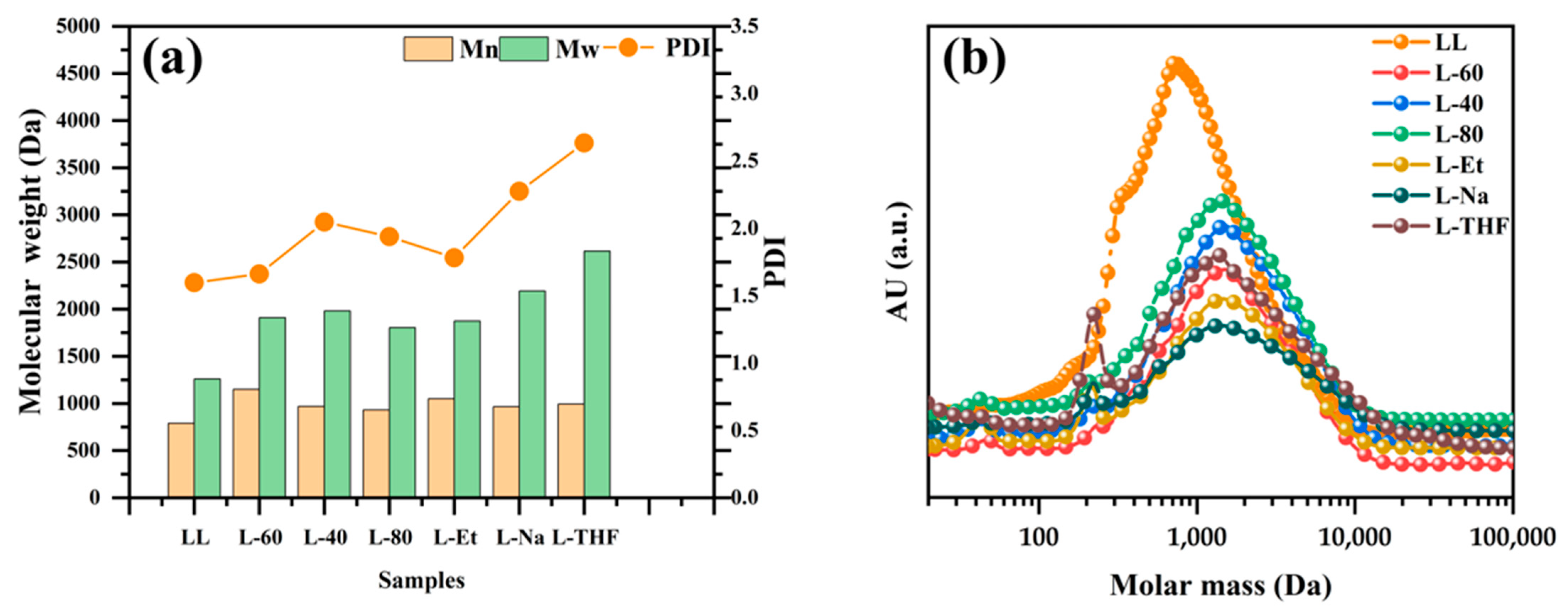
2.6.2. Gel Permeation Chromatography (GPC)
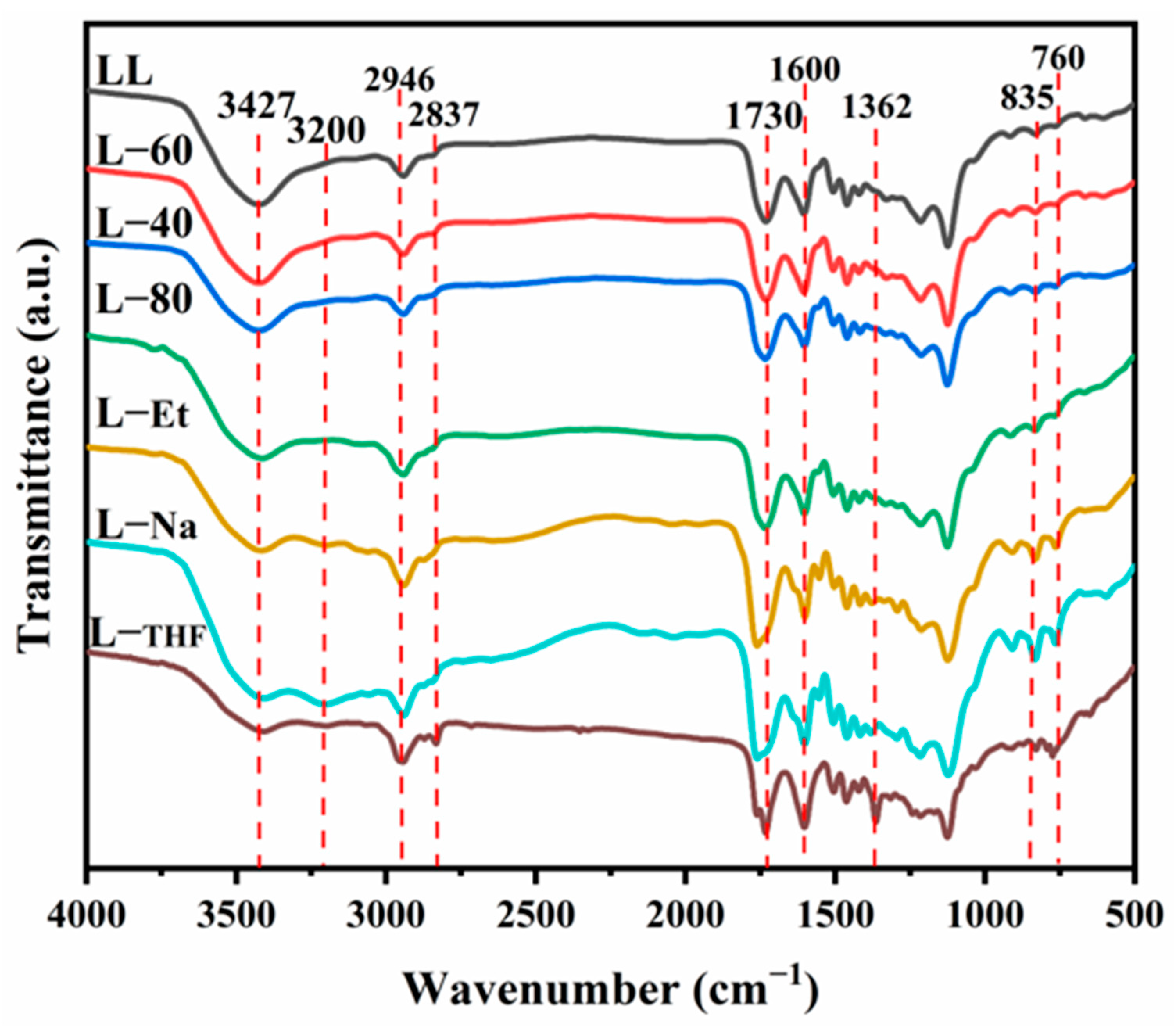
2.6.3. Fourier Transformation Infrared Spectroscopy (FT-IR)
2.6.4. Thermogravimetric Analysis (TGA)
2.6.5. Surface Wettability
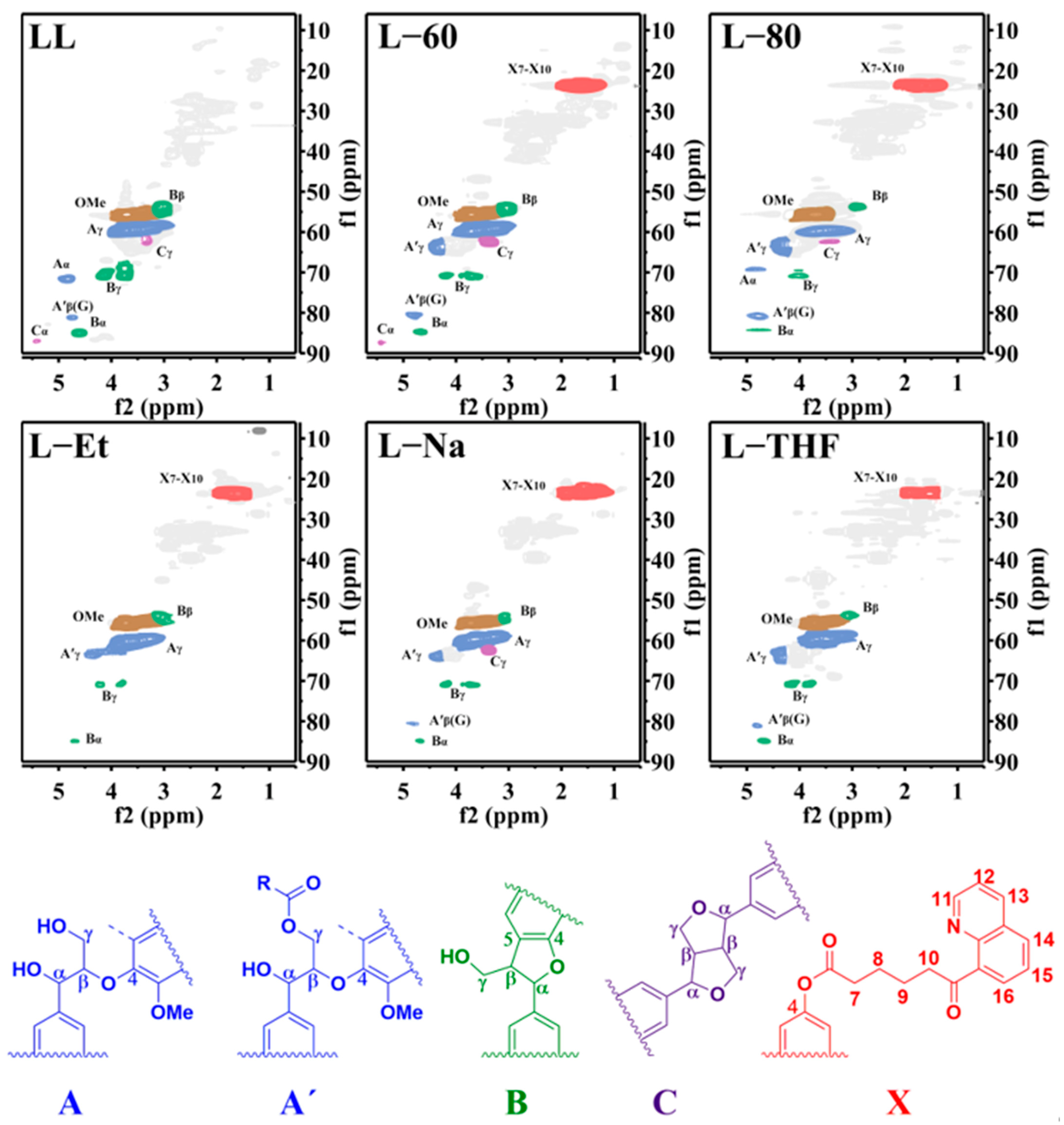
2.6.6. Nuclear Magnetic Resonance (NMR) Spectra
3. Results and Discussion
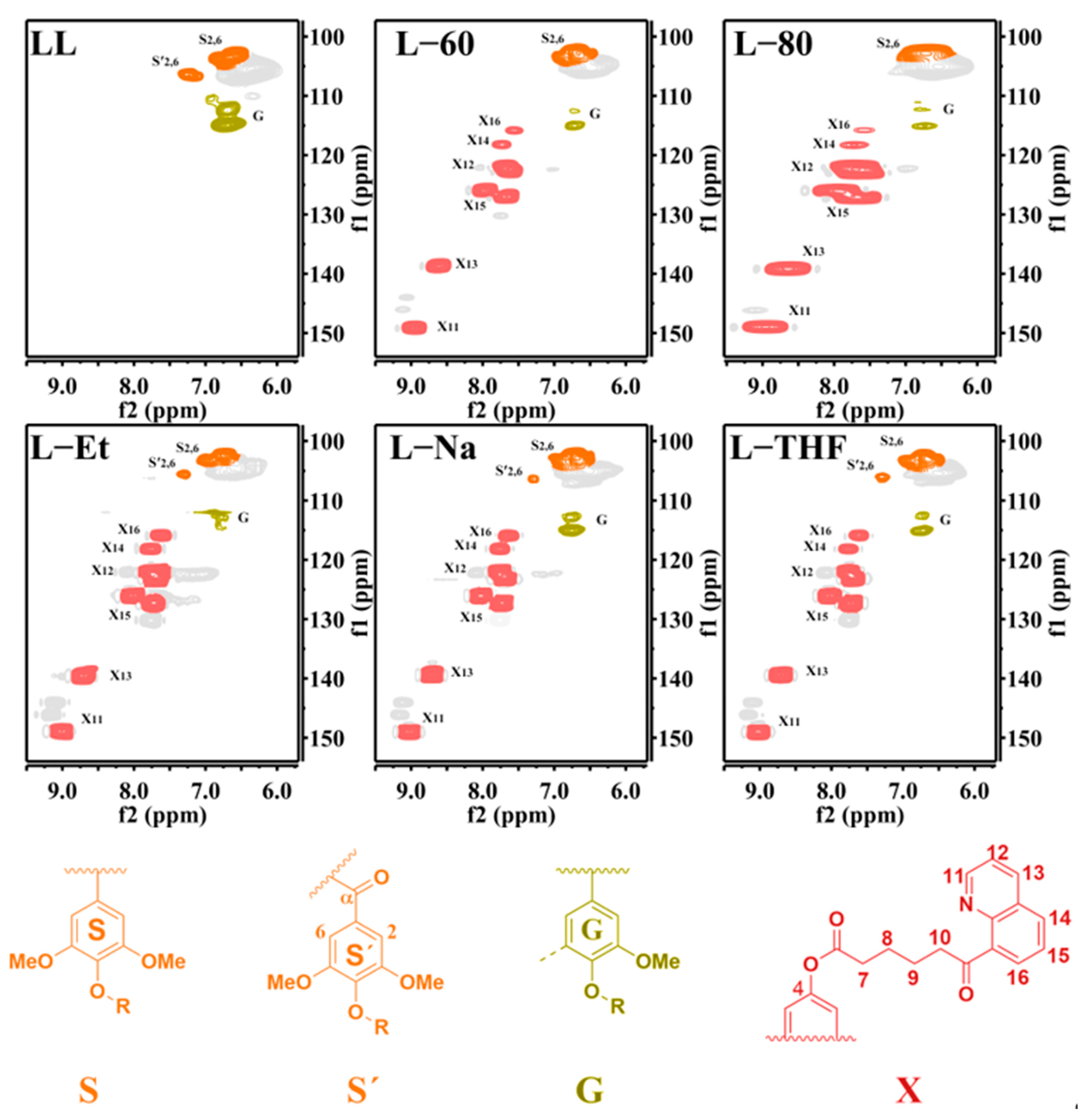
3.1. The Structure of Lignin
3.2. The Substitution Degree (SD) of 8HQ
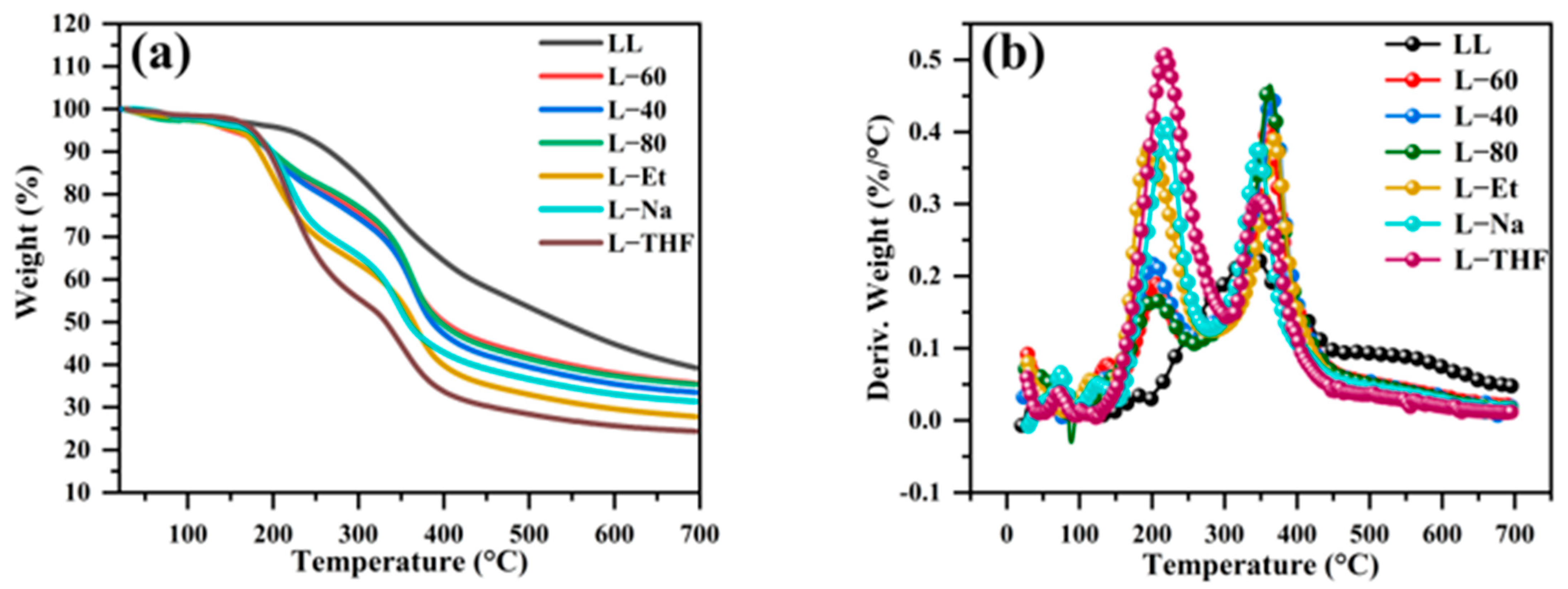
3.3. Thermal Stability of Samples
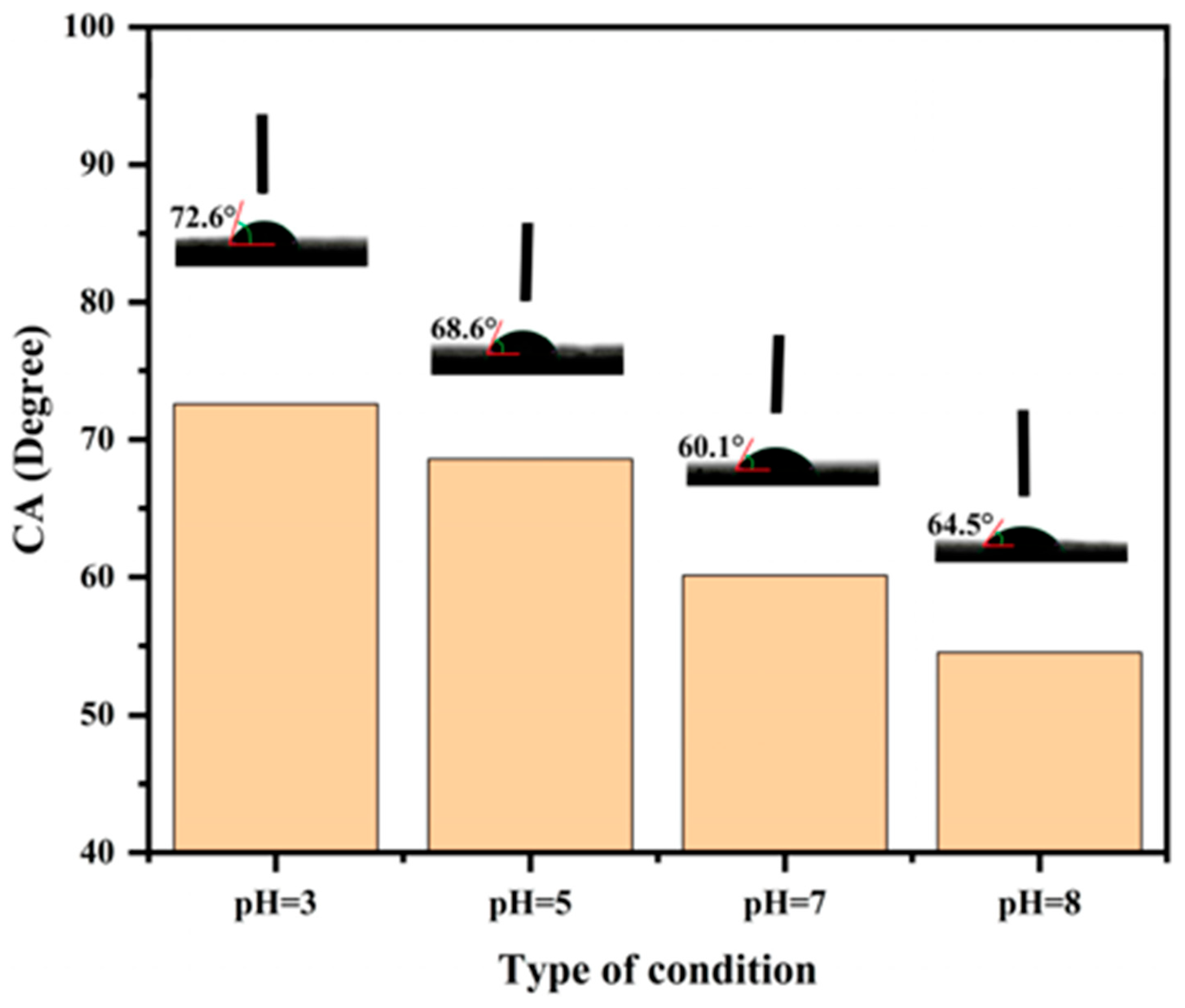
3.4. pH-Responsive Switchable Wettability of L-Na
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seidi, F.; Jenjob, R.; Crespy, D. Designing Smart Polymer Conjugates for Controlled Release of Payloads. Chem. Rev. 2018, 118, 3965–4036. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Ouyang, C.; Yang, K.; Lv, W.; Huang, T.; Li, X.; Zhou, M.; Wu, H.; Xie, M.; Shi, P.; et al. An acid-labile bridged β-CD-based nano-hydrogel with superior anti-tumor drug delivery and release capacity. J. Drug Deliv. Sci. Technol. 2022, 78, 103953. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Huang, Y.; Chen, X.; Jing, X. Biodegradable Block Copolymer-Doxorubicin Conjugates via Different Linkages: Preparation, Characterization, and In Vitro Evaluation. Biomacromolecules 2010, 11, 2094–2102. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tezcan, O.; Li, D.; Beztsinna, N.; Lou, B.; Etrych, T.; Ulbrich, K.; Metselaar, J.M.; Lammers, T.; Hennink, W.E. Overcoming multidrug resistance using folate receptor-targeted and pH-responsive polymeric nanogels containing covalently entrapped doxorubicin. Nanoscale 2017, 9, 10404–10419. [Google Scholar] [CrossRef]
- Domiński, A.; Domińska, M.; Skonieczna, M.; Pastuch-Gawołek, G.; Kurcok, P. Shell-Sheddable Micelles Based on Poly (ethylene glycol)-hydrazone-poly [R, S]-3-hydroxybutyrate Copolymer Loaded with 8-Hydroxyquinoline Glycoconjugates as a Dual Tumor-Targeting Drug Delivery System. Pharmaceutics 2022, 14, 290. [Google Scholar] [CrossRef]
- Zhao, S.; Abu-Omar, M.M. Recyclable and Malleable Epoxy Thermoset Bearing Aromatic Imine Bonds. Macromolecules 2018, 51, 9816–9824. [Google Scholar] [CrossRef]
- Garcia, F.; Pelss, J.; Zuilhof, H.; Smulders, M.M. Multi-responsive coordination polymers utilising metal-stabilised, dynamic covalent imine bonds. Chem. Commun. 2016, 52, 9059–9062. [Google Scholar] [CrossRef]
- Qiu, L.; Hong, C.Y.; Pan, C.Y. Doxorubicin-loaded aromatic imine-contained amphiphilic branched star polymer micelles: Synthesis, self-assembly, and drug delivery. Int. J. Nanomed. 2015, 10, 3623–3640. [Google Scholar]
- Wang, H.; Su, L.; Li, R.; Zhang, S.; Fan, J.; Zhang, F.; Nguyen, T.P.; Wooley, K.L. Polyphosphoramidates That Undergo Acid-Triggered Backbone Degradation. ACS Macro. Lett. 2017, 6, 219–223. [Google Scholar] [CrossRef]
- Murthy, N.; Thng, Y.X.; Schuck, S.; Xu, M.C.; Fréchet, J.M. A Novel Strategy for Encapsulation and Release of Proteins: Hydrogels and Microgels with Acid-Labile Acetal Cross-Linkers. J. Am. Chem. Soc. 2002, 124, 12398–12399. [Google Scholar] [CrossRef]
- Das, M.; Joshi, A.; Devkar, R.; Seshadri, S.; Thakore, S. Vitamin-H Channeled Self-Therapeutic P-gp Inhibitor Curcumin-Derived Nanomicelles for Targeting the Tumor Milieu by pH- and Enzyme-Triggered Hierarchical Disassembly. Bioconjug. Chem. 2022, 33, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Thuaud, F.; Rohrbacher, F.; Zwicky, A.; Bode, J.W. Incorporation of Acid-Labile Masking Groups for the Traceless Synthesis of C-Terminal Peptide alpha-Ketoacids. Org. Lett. 2016, 18, 3670–3673. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Tan, Y.; Li, P.; Luo, Y.; Zhu, Y.; Yu, Y.; Chen, J.; Ning, N.; Zhang, S. Surface charge-convertible quaternary ammonium salt-based micelles for in vivo infection therapy. Chin. Chem. Lett. 2021, 32, 1743–1746. [Google Scholar] [CrossRef]
- Feng, Q.; Xu, J.; Liu, X.; Wang, H.; Xiong, J.; Xiao, K. Targeted delivery by pH-responsive mPEG-S-PBLG micelles significantly enhances the anti-tumor efficacy of doxorubicin with reduced cardiotoxicity. Drug Deliv. 2021, 28, 2495–2509. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Li, J.W.; Hong, C.Y.; Pan, C.Y. Silver Nanoparticles Covered with pH-Sensitive Camptothecin-Loaded Polymer Prodrugs: Switchable Fluorescence "Off" or "On" and Drug Delivery Dynamics in Living Cells. ACS Appl. Mater. Interfaces 2017, 9, 40887–40897. [Google Scholar] [CrossRef]
- Hellberg, P.E.; Bergström, K.; Holmberg, K. Cleavable surfactants. J. Surfactants Deterg. 2000, 3, 81–91. [Google Scholar] [CrossRef]
- Summers, K.L.; Pushie, M.J.; Sopasis, G.J.; James, A.K.; Dolgova, N.V.; Sokaras, D.; Kroll, T.; Harris, H.H.; Pickering, I.J.; George, G.N. Solution Chemistry of Copper (II) Binding to Substituted 8-Hydroxyquinolines. Inorg. Chem. 2020, 59, 13858–13874. [Google Scholar] [CrossRef]
- Shoji, E.; Miyatake, K.; Hlil, A.R.; Hay, A.S.; Maindron, T.; Jousseaume, V.; Dodelet, J.P.; Tao, Y.; D’Iorio, M. Immiscible Polymers in Double Spin-Coated Electroluminescent Devices Containing Phenyl-Substituted Tris (8-hydroxyquinoline) aluminum Derivatives Soluble in a Host Polymer. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3006–3016. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Amira, M.F.; Seleim, S.M.; Mohamed, A.K. Adsorption Isotherm Models, Kinetics Study, and Thermodynamic Parameters of Ni (II) and Zn (II) Removal from Water Using the LbL Technique. J. Chem. Eng. Data 2017, 62, 839–850. [Google Scholar] [CrossRef]
- Auepattana-Aumrung, K.; Crespy, D. Self-healing and anticorrosion coatings based on responsive polymers with metal coordination bonds. Chem. Eng. J. 2023, 452, 139055. [Google Scholar] [CrossRef]
- Vaghefinazari, B.; Lamaka, S.V.; Blawert, C.; Serdechnova, M.; Scharnagl, N.; Karlova, P.; Wieland, D.C.F.; Zheludkevich, M.L. Exploring the corrosion inhibition mechanism of 8-hydroxyquinoline for a PEO-coated magnesium alloy. Corros. Sci. 2022, 203, 110344. [Google Scholar] [CrossRef]
- Motta, M.; Zanocco, M.; Rondinella, A.; Iodice, V.; Sin, A.; Fedrizzi, L.; Andreatta, F. Inhibitive effect of 8-hydroxyquinoline on corrosion of gray cast iron in automotive braking systems. Electrochim. Acta 2023, 449, 142221. [Google Scholar] [CrossRef]
- Dararatana, N.; Seidi, F.; Crespy, D. pH-sensitive polymer conjugates for anticorrosion and corrosion sensing. ACS Appl. Mater. Interfaces 2018, 10, 20876–20883. [Google Scholar] [CrossRef]
- Gupta, R.; Luxami, V.; Paul, K. Insights of 8-hydroxyquinolines: A novel target in medicinal chemistry. Bioorg. Chem. 2021, 108, 104633. [Google Scholar] [CrossRef]
- Kljun, J.; Leon, I.E.; Persic, S.; Cadavid-Vargas, J.F.; Etcheverry, S.B.; He, W.; Bai, Y.; Turel, I. Synthesis and biological characterization of organoruthenium complexes with 8-hydroxyquinolines. J. Inorg. Biochem. 2018, 186, 187–196. [Google Scholar] [CrossRef]
- Chauhan, R.; Chauhan, V.; Sonkar, P.; Dhaked, R.K. Identification of Inhibitors against Botulinum Neurotoxins: 8-Hydroxyquinolines Hold Promise. Mini-Rev. Med. Chem. 2019, 19, 1694–1706. [Google Scholar] [CrossRef]
- Buchler, I.; Akuma, D.; Au, V.; Carr, G.; de Leon, P.; DePasquale, M.; Ernst, G.; Huang, Y.; Kimos, M.; Kolobova, A.; et al. Optimization of 8-Hydroxyquinolines as Inhibitors of Catechol O-Methyltransferase. J. Med. Chem. 2018, 61, 9647–9665. [Google Scholar] [CrossRef]
- Pippi, B.; Lopes, W.; Reginatto, P.; Silva, F.E.K.; Joaquim, A.R.; Alves, R.J.; Silveira, G.P.; Vainstein, M.H.; Andrade, S.F.; Fuentefria, A.M. New insights into the mechanism of antifungal action of 8-hydroxyquinolines. Saudi Pharm. J. 2019, 27, 41–48. [Google Scholar] [CrossRef]
- Odingo, J.O.; Early, J.V.; Smith, J.; Johnson, J.; Bailey, M.A.; Files, M.; Guzman, J.; Ollinger, J.; Korkegian, A.; Kumar, A.; et al. 8-Hydroxyquinolines are bactericidal against Mycobacterium tuberculosis. Drug Dev. Res. 2019, 80, 566–572. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, X.; Feng, X.; Lee, M.J.; Li, Y.; Mao, J.; Weichselbaum, R.R.; Lin, W.A. A Three-in-One Nanoscale Coordination Polymer for Potent Chemo-Immunotherapy. Small Methods 2023, 2201437. [Google Scholar] [CrossRef]
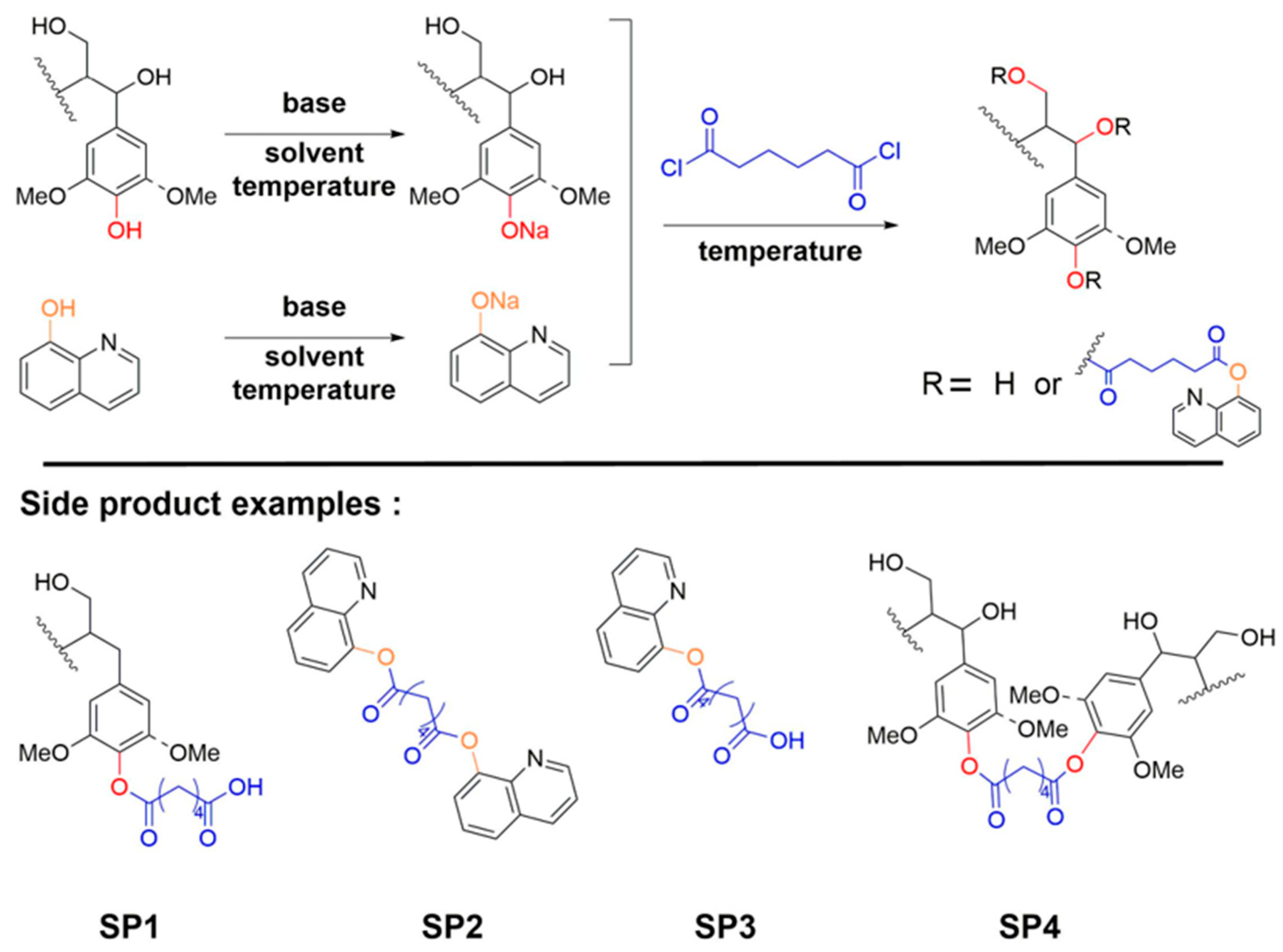

| Entry | Sample | 8HQ (Eq) | Alkali | Solvent | Temperature (°C) |
|---|---|---|---|---|---|
| 1 | L-60 | 2 | NaOH | DMF | 60 |
| 2 | L-40 | 2 | NaOH | DMF | 40 |
| 3 | L-80 | 2 | NaOH | DMF | 80 |
| 4 | L-Et | 2 | Et3N | DMF | 60 |
| 5 | L-Na | 2 | Na2CO3 | DMF | 60 |
| 6 | L-THF | 2 | NaOH | THF | 60 |
| Samples | 1H NMR | Alkaline-Heating Method | ||
|---|---|---|---|---|
| Hydroxyl Content (mmol/g) | Decrease of Hydroxyl Content (%) | 8HQ Content (mmol/g) a | SD of 8HQ b | |
| LL | 4.3 | - | - | - |
| L-60 | 3.7 | 14.0% | 1.4 | 30.0% |
| L-40 | 2.4 | 44.2% | 1.4 | 31.9% |
| L-80 | 2.3 | 46.5% | 1.5 | 32.5% |
| L-Et | 2.2 | 48.8% | 1.5 | 33.0% |
| L-Na | 2.6 | 39.5% | 2.1 (1.6) c | 46.6% (34.7%) c |
| L-THF | 0.6 | 86.0% | 1 | 22.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Q.; Chai, L.; Du, B.; Li, W.; Fu, L.-H.; Chen, X. A pH-Sensitive Lignin-Based Material for Sustained Release of 8-Hydroxyquinoline. Polymers 2023, 15, 1867. https://doi.org/10.3390/polym15081867
Zheng Q, Chai L, Du B, Li W, Fu L-H, Chen X. A pH-Sensitive Lignin-Based Material for Sustained Release of 8-Hydroxyquinoline. Polymers. 2023; 15(8):1867. https://doi.org/10.3390/polym15081867
Chicago/Turabian StyleZheng, Qian, Lanfang Chai, Boyu Du, Wei Li, Lian-Hua Fu, and Xiaohong Chen. 2023. "A pH-Sensitive Lignin-Based Material for Sustained Release of 8-Hydroxyquinoline" Polymers 15, no. 8: 1867. https://doi.org/10.3390/polym15081867
APA StyleZheng, Q., Chai, L., Du, B., Li, W., Fu, L.-H., & Chen, X. (2023). A pH-Sensitive Lignin-Based Material for Sustained Release of 8-Hydroxyquinoline. Polymers, 15(8), 1867. https://doi.org/10.3390/polym15081867






