Fabrication, Physical–Chemical and Biological Characterization of Retinol-Loaded Poly(vinyl Alcohol) Electrospun Fiber Mats for Wound Healing Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Retinol-Loaded Poly(vinyl Alcohol) Fiber Mats (RPFM) Fabrication
2.3. Characterization
2.3.1. RPFM Chemical Composition and Thermal Stability
2.3.2. RPFM Morphology
2.3.3. RPFM Mechanical Properties
2.4. Retinol Release Quantification
2.5. Cellular Assays
2.5.1. In Vitro Cytotoxicity Assay
2.5.2. In Vitro Cell Proliferation
2.5.3. Wound Healing Assays
2.6. Statistical Analysis
3. Results and Discussions
3.1. Fabrication and Optimization of Retinol-Loaded Poly(vinyl Alcohol) Fiber Mats (RPFM)
3.2. RPFM Scanning Electron Microscopy (SEM)
3.3. RPFM Thermogravimetric Analyses (TGA)
3.4. RPFM Mechanical Properties
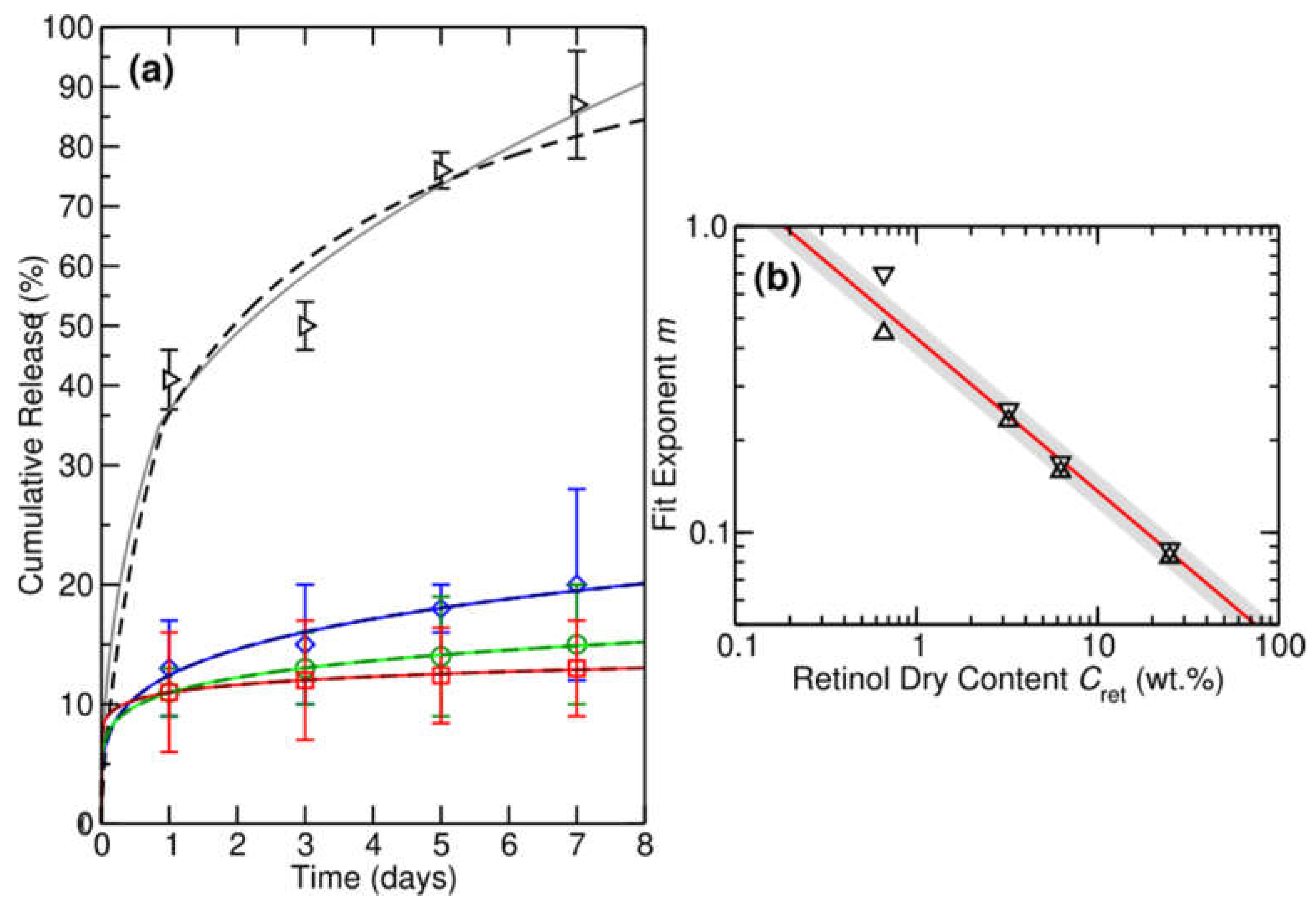
3.5. Retinol Release Quantification
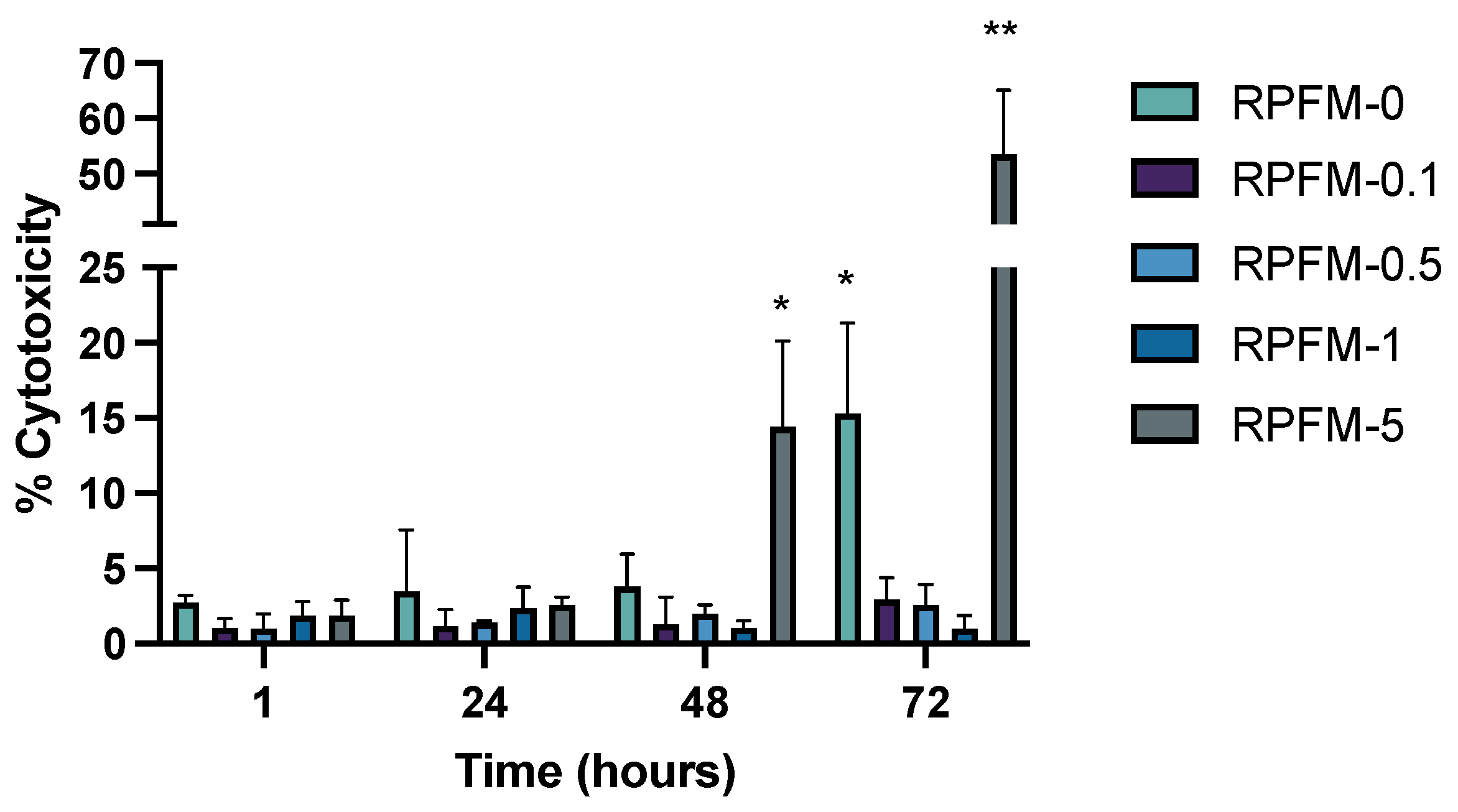
3.6. In Vitro Cytotoxicity Assay
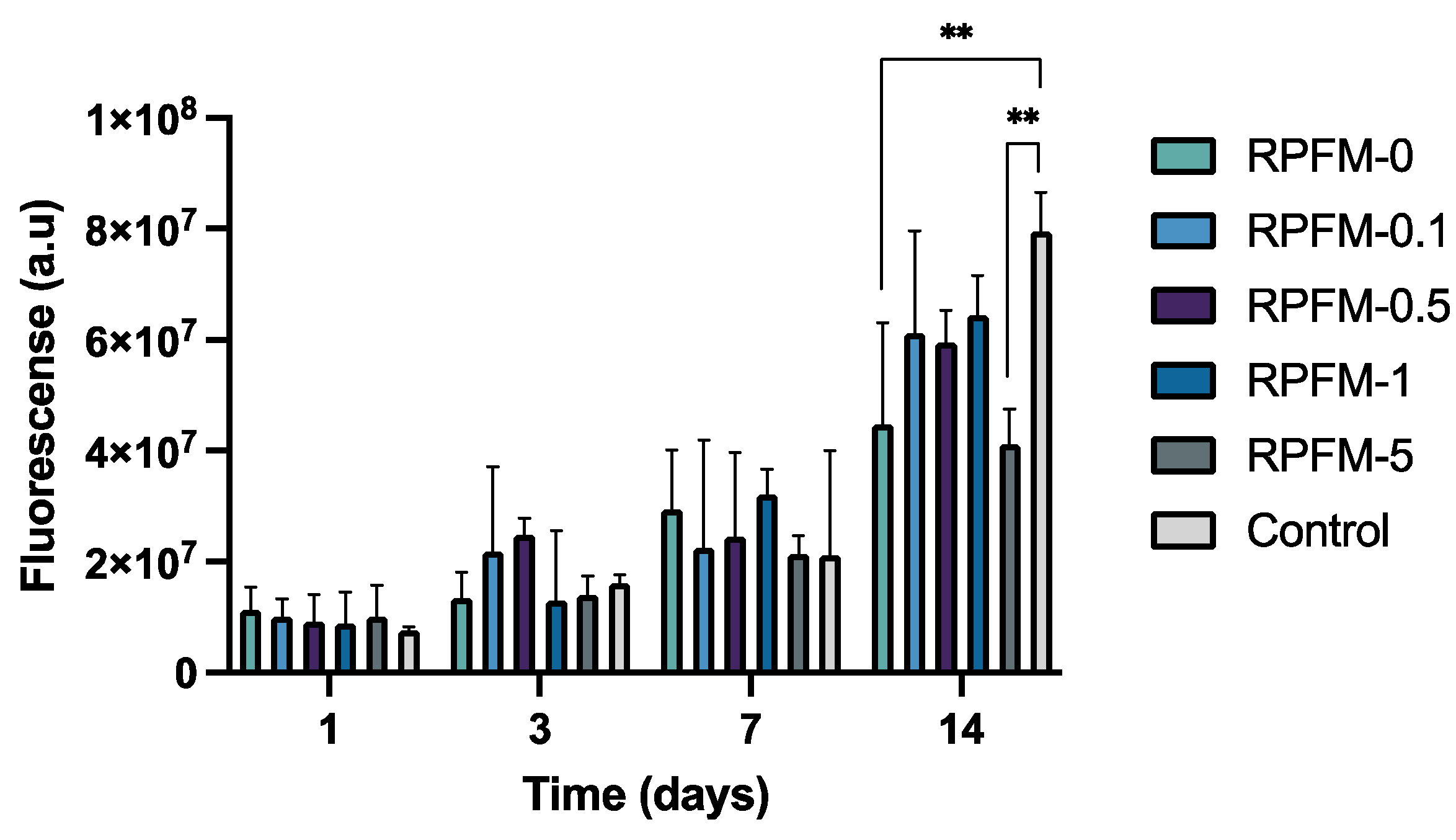
3.7. In Vitro Cell Proliferation
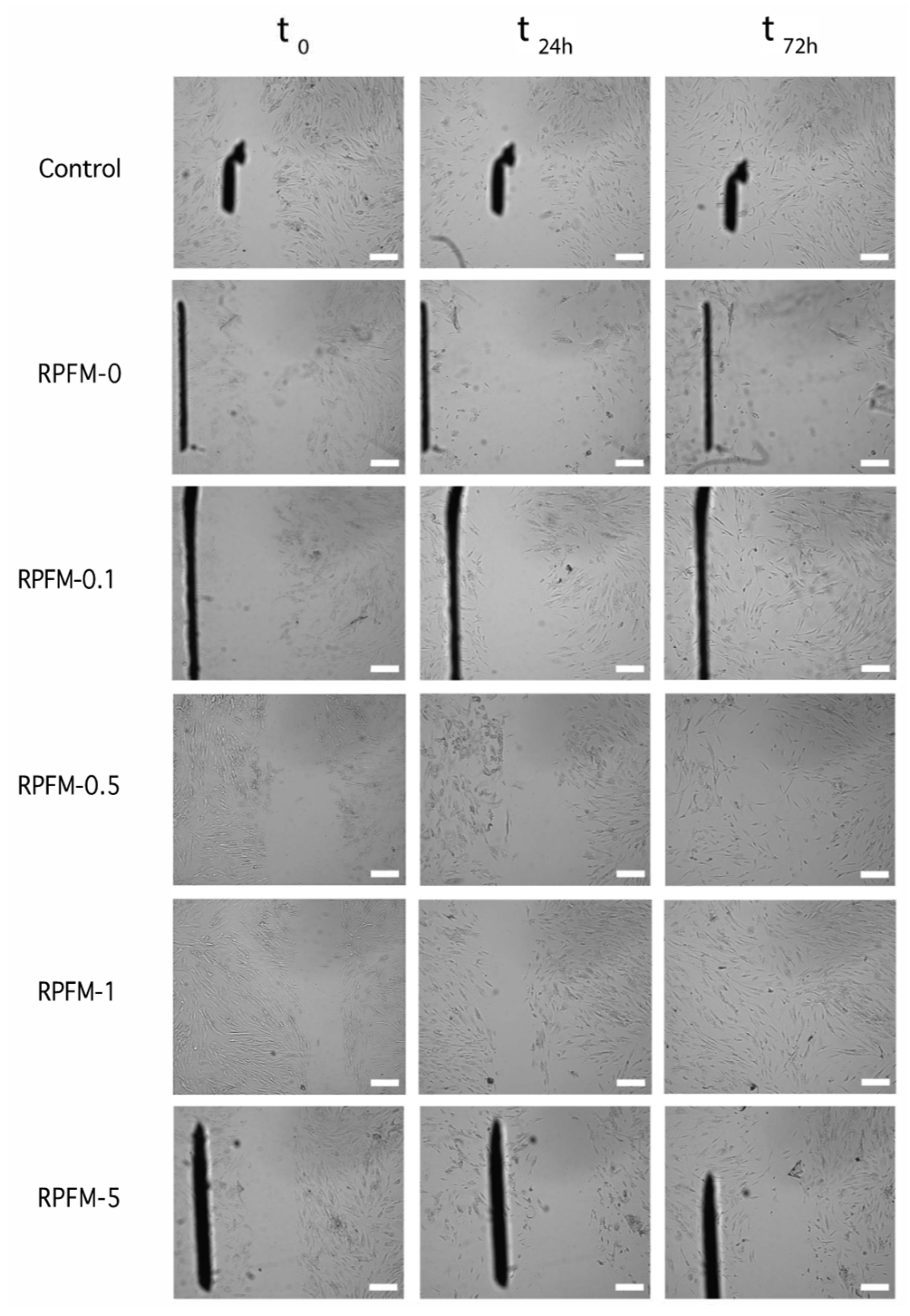
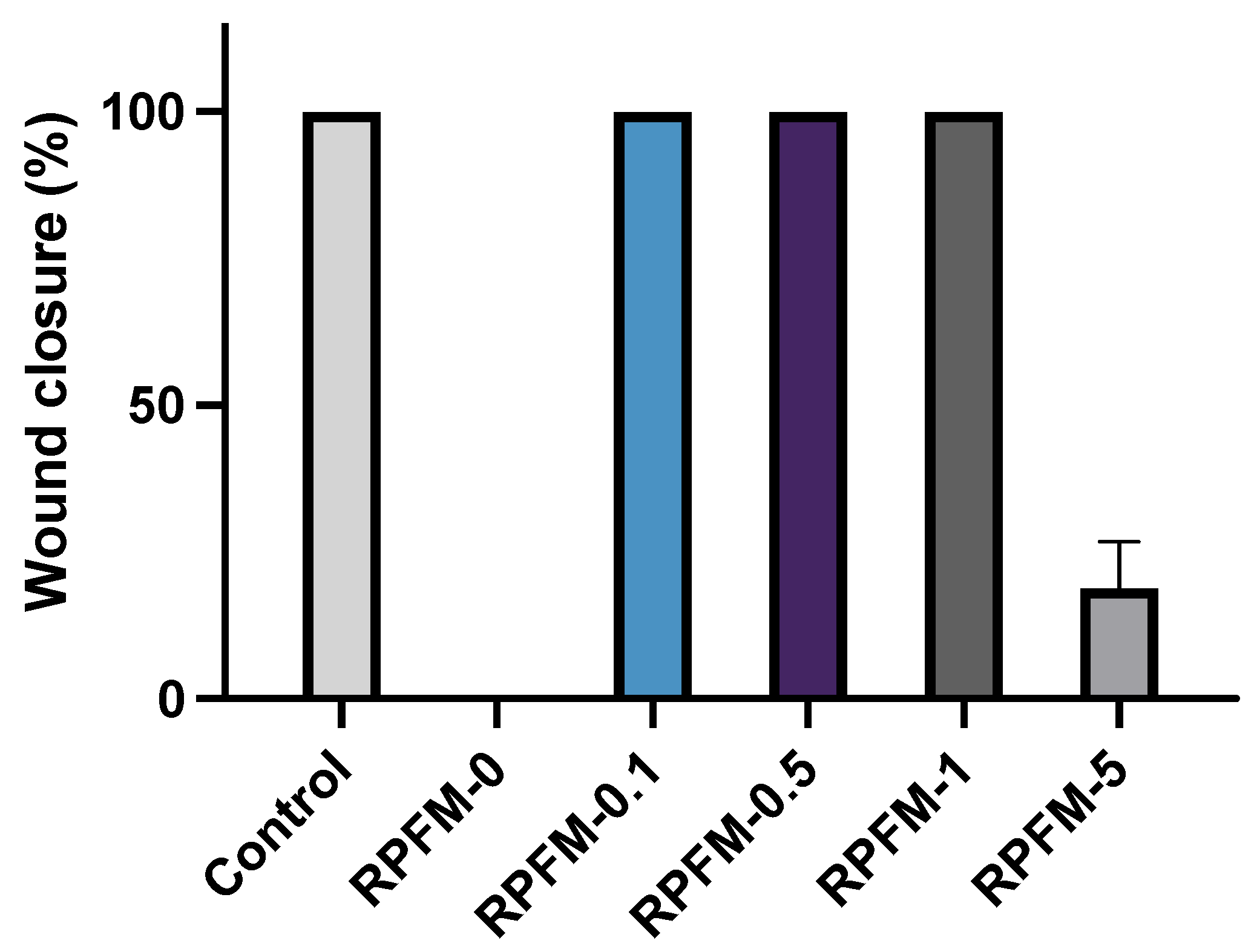
3.8. Wound Healing Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Momoh, F.U.; Boateng, J.S.; Richardson, S.C.W.; Chowdhry, B.Z.; Mitchell, J.C. Development and Functional Characterization of Alginate Dressing as Potential Protein Delivery System for Wound Healing. Int. J. Biol. Macromol. 2015, 81, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.; Vig, K.; Baganizi, D.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.; Pillai, S. Future Prospects for Scaffolding Methods and Biomaterials in Skin Tissue Engineering: A Review. IJMS 2016, 17, 1974. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Gould, M.; Ali, M.A. A Review of Current Advancements for Wound Healing: Biomaterial Applications and Medical Devices. J. Biomed. Mater. Res. 2022, 110, 2542–2573. [Google Scholar] [CrossRef] [PubMed]
- Mirhaj, M.; Labbaf, S.; Tavakoli, M.; Seifalian, A.M. Emerging Treatment Strategies in Wound Care. Int. Wound J. 2022, 19, 1934–1954. [Google Scholar] [CrossRef]
- Shevchenko, R.V.; James, S.L.; James, S.E. A Review of Tissue-Engineered Skin Bioconstructs Available for Skin Reconstruction. J. R. Soc. Interface 2010, 7, 229–258. [Google Scholar] [CrossRef]
- Keirouz, A.; Chung, M.; Kwon, J.; Fortunato, G.; Radacsi, N. 2D and 3D Electrospinning Technologies for the Fabrication of Nanofibrous Scaffolds for Skin Tissue Engineering: A Review. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1626. [Google Scholar] [CrossRef]
- Chopra, H.; Kumar, S.; Singh, I. Biopolymer-Based Scaffolds for Tissue Engineering Applications. CDT 2021, 22, 282–295. [Google Scholar] [CrossRef]
- Elango, J.; Zamora-Ledezma, C.; Negrete-Bolagay, D.; Aza, P.N.D.; Gómez-López, V.M.; López-González, I.; Belén Hernández, A.; De Val, J.E.M.S.; Wu, W. Retinol-Loaded Poly(Vinyl Alcohol)-Based Hydrogels as Suitable Biomaterials with Antimicrobial Properties for the Proliferation of Mesenchymal Stem Cells. IJMS 2022, 23, 15623. [Google Scholar] [CrossRef]
- Guamba, E.; Vispo, N.S.; Whitehead, D.C.; Singh, A.K.; Santos-Oliveira, R.; Niebieskikwiat, D.; Zamora-Ledezma, C.; Alexis, F. Cellulose-Based Hydrogels towards an Antibacterial Wound Dressing. Biomater. Sci. 2022, 11, 3461–3468. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Montaser, A.S.; Jlassi, K.; Ramadan, M.A.; Sleem, A.A.; Attia, M.F. Alginate, Gelatin, and Carboxymethyl Cellulose Coated Nonwoven Fabrics Containing Antimicrobial AgNPs for Skin Wound Healing in Rats. Int. J. Biol. Macromol. 2021, 173, 203–210. [Google Scholar] [CrossRef]
- Attia, M.F.; Montaser, A.S.; Arifuzzaman, M.; Pitz, M.; Jlassi, K.; Alexander-Bryant, A.; Kelly, S.S.; Alexis, F.; Whitehead, D.C. In Situ Photopolymerization of Acrylamide Hydrogel to Coat Cellulose Acetate Nanofibers for Drug Delivery System. Polymers 2021, 13, 1863. [Google Scholar] [CrossRef]
- Abdelrahman, T.; Newton, H. Wound Dressings: Principles and Practice. Surgery 2011, 29, 491–495. [Google Scholar] [CrossRef]
- Han, F.; Dong, Y.; Song, A.; Yin, R.; Li, S. Alginate/Chitosan Based Bi-Layer Composite Membrane as Potential Sustained-Release Wound Dressing Containing Ciprofloxacin Hydrochloride. Appl. Surf. Sci. 2014, 311, 626–634. [Google Scholar] [CrossRef]
- Rivera, A.E.; Spencer, J.M. Clinical Aspects of Full-Thickness Wound Healing. Clin. Dermatol. 2007, 25, 39–48. [Google Scholar] [CrossRef]
- Thu, H.-E.; Zulfakar, M.H.; Ng, S.-F. Alginate Based Bilayer Hydrocolloid Films as Potential Slow-Release Modern Wound Dressing. Int. J. Pharm. 2012, 434, 375–383. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Critical reviews in oral biology & medicine. J. Dent. Res. 2010, 89, 210–229. [Google Scholar] [CrossRef]
- Heydari, P.; Zargar Kharazi, A.; Asgary, S.; Parham, S. Comparing the Wound Healing Effect of a Controlled Release Wound Dressing Containing Curcumin/Ciprofloxacin and Simvastatin/Ciprofloxacin in a Rat Model: A Preclinical Study. J. Biomed. Mater. Res 2022, 110, 341–352. [Google Scholar] [CrossRef]
- Ijaola, A.O.; Akamo, D.O.; Damiri, F.; John, C.; Bamidele, E.A.; Ajiboye, E.G.; Berrada, M.; Onyenokwe, V.O.; Yang, S.-Y.; Asmatulu, E. Polymeric Biomaterials for Wound Healing Applications: A Comprehensive Review. J. Biomater. Sci. Polym. Ed. 2022, 33, 1998–2050. [Google Scholar] [CrossRef]
- López, I.; Zamora, C.; Sanchez, M.I.; Barrenechea, E.T.; Gabaldón, J.A.; Meseguer, L. Modifications in Gene Expression in the Process of Osteoblastic Differentiation of Multipotent Bone Marrow-Derived Human Mesenchymal Stem Cells Induced by a Novel Osteoinductive Porous Medical-Grade 3D-Printed Poly(Ε-caprolactone)/Β-tricalcium Phosphate Composite. Int. J. Mol. Sci. 2021, 22, 11216. [Google Scholar] [CrossRef]
- Pagán, A.; Aznar-Cervantes, S.D.; Pérez-Rigueiro, J.; Meseguer-Olmo, L.; Cenis, J.L. Potential Use of Silkworm Gut Fiber Braids as Scaffolds for Tendon and Ligament Tissue Engineering. J. Biomed. Mater. Res. B 2019, 107, 2209–2215. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Mendes, A.; Bártolo, P. Alginate/Aloe Vera Hydrogel Films for Biomedical Applications. Procedia CIRP 2013, 5, 210–215. [Google Scholar] [CrossRef]
- El Fawal, G. Polymer Nanofibers Electrospinning: A Review. Egypt. J. Chem. 2020, 63, 1279–1303. [Google Scholar] [CrossRef]
- Fahimirad, S.; Ajalloueian, F. Naturally-Derived Electrospun Wound Dressings for Target Delivery of Bio-Active Agents. Int. J. Pharm. 2019, 566, 307–328. [Google Scholar] [CrossRef]
- Miguel, S.P.; Sequeira, R.S.; Moreira, A.F.; Cabral, C.S.D.; Mendonça, A.G.; Ferreira, P.; Correia, I.J. An Overview of Electrospun Membranes Loaded with Bioactive Molecules for Improving the Wound Healing Process. Eur. J. Pharm. Biopharm. 2019, 139, 1–22. [Google Scholar] [CrossRef]
- Schröder, H.C.; Tolba, E.; Diehl-Seifert, B.; Wang, X.; Müller, W.E.G. Electrospinning of Bioactive Wound-Healing Nets. In Blue Biotechnology; Müller, W.E.G., Schröder, H.C., Wang, X., Eds.; Progress in Molecular and Subcellular Biology; Springer International Publishing: Cham, Switzerland, 2017; Volume 55, pp. 259–290. ISBN 978-3-319-51282-2. [Google Scholar]
- Sheng, X.-Y.; Fan, L.-P.; Mo, X.-M.; He, C.-L.; Wang, H.-S. Electrospun Silk Fibroin Composite Nanofibrous Mats Loaded with Vitamin A and E. J. Control. Release 2013, 172, e35–e36. [Google Scholar] [CrossRef]
- Wsoo, M.A.; Shahir, S.; Mohd Bohari, S.P.; Mat Nayan, N.H.; Abd Razak, S.I. A Review on the Properties of Electrospun Cellulose Acetate and Its Application in Drug Delivery Systems: A New Perspective. Carbohydr. Res. 2020, 491, 107978. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; Guo, H.; Liu, L.; Xu, W.; Duan, G. Structural Design toward Functional Materials by Electrospinning: A Review. e-Polymers 2020, 20, 682–712. [Google Scholar] [CrossRef]
- Elkasaby, M.; Hegab, H.A.; Mohany, A.; Rizvi, G.M. Modeling and Optimization of Electrospinning of Polyvinyl Alcohol (PVA). Adv. Polym. Technol. 2018, 37, 2114–2122. [Google Scholar] [CrossRef]
- Narváez-Muñoz, C.; Ryzhakov, P.; Pons-Prats, J. Determination of the Operational Parameters for the Manufacturing of Spherical PVP Particles via Electrospray. Polymers 2021, 13, 529. [Google Scholar] [CrossRef]
- Narváez-Muñoz, C.; Zamora-Ledezma, C.; Carrión-Matamoros, L.M.; Guerrero, I.E.; Debut, A.; Vizuete, K.; Haro, E.E.; López López, A.; Zamora-Ledezma, E. Influence of Ultrasonication on the Properties of Hybrid Electrospun Polyacrylonitrile and Silver Nanoparticles Fibers and Their Potential Use in Water Decontamination. In Applied Technologies; Botto-Tobar, M., Montes León, S., Torres-Carrión, P., Zambrano Vizuete, M., Durakovic, B., Eds.; Communications in Computer and Information Science; Springer International Publishing: Cham, Switzerland, 2022; Volume 1535, pp. 176–188. ISBN 978-3-031-03883-9. [Google Scholar]
- Reshmi, C.R.; Sagitha, P.; Sheeja; Sujith, A. Poly Ɛ-Caprolactone/Nanostarch Composite Nanofibrous Wound Dressing with Antibacterial Property and PH Stimulus Drug Release. Cellulose 2022, 29, 427–443. [Google Scholar] [CrossRef]
- Aznar-Cervantes, S.; Pagán, A.; Martínez, J.G.; Bernabeu-Esclapez, A.; Otero, T.F.; Meseguer-Olmo, L.; Paredes, J.I.; Cenis, J.L. Electrospun Silk Fibroin Scaffolds Coated with Reduced Graphene Promote Neurite Outgrowth of PC-12 Cells under Electrical Stimulation. Mater. Sci. Eng. C 2017, 79, 315–325. [Google Scholar] [CrossRef]
- Patiño Vidal, C.; López de Dicastillo, C.; Rodríguez-Mercado, F.; Guarda, A.; Galotto, M.J.; Muñoz-Shugulí, C. Electrospinning and Cyclodextrin Inclusion Complexes: An Emerging Technological Combination for Developing Novel Active Food Packaging Materials. Crit. Rev. Food Sci. Nutr. 2022, 62, 5495–5510. [Google Scholar] [CrossRef]
- Yue, Y.; Gong, X.; Jiao, W.; Li, Y.; Yin, X.; Si, Y.; Yu, J.; Ding, B. In-Situ Electrospinning of Thymol-Loaded Polyurethane Fibrous Membranes for Waterproof, Breathable, and Antibacterial Wound Dressing Application. J. Colloid Interface Sci. 2021, 592, 310–318. [Google Scholar] [CrossRef]
- Ulag, S.; Ilhan, E.; Sahin, A.; Karademir Yilmaz, B.; Kalaskar, D.M.; Ekren, N.; Kilic, O.; Nuzhet Oktar, F.; Gunduz, O. 3D Printed Artificial Cornea for Corneal Stromal Transplantation. Eur. Polym. J. 2020, 133, 109744. [Google Scholar] [CrossRef]
- Byrne, D.; Boeije, G.; Croft, I.; Hüttmann, G.; Luijkx, G.; Meier, F.; Parulekar, Y.; Stijntjes, G. Biodegradability of Polyvinyl Alcohol Based Film Used for Liquid Detergent Capsules: Biologische Abbaubarkeit Der Für Flüssigwaschmittelkapseln Verwendeten Folie Auf Polyvinylalkoholbasis. Tenside Surfactants Deterg. 2021, 58, 88–96. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Guo, H. Research Progress of Polyvinyl Alcohol Water-Resistant Film Materials. Membranes 2022, 12, 347. [Google Scholar] [CrossRef]
- Moeini, A.; Germann, N.; Malinconico, M.; Santagata, G. Formulation of Secondary Compounds as Additives of Biopolymer-Based Food Packaging: A Review. Trends Food Sci. Technol. 2021, 114, 342–354. [Google Scholar] [CrossRef]
- Sharma, D.; Srivastava, S.; Kumar, S.; Sharma, P.K.; Hassani, R.; Dailah, H.G.; Khalid, A.; Mohan, S. Biodegradable Electrospun Scaffolds as an Emerging Tool for Skin Wound Regeneration: A Comprehensive Review. Pharmaceuticals 2023, 16, 325. [Google Scholar] [CrossRef]
- Serbezeanu, D.; Bargan, A.; Homocianu, M.; Aflori, M.; Rîmbu, C.M.; Enache, A.A.; Vlad-Bubulac, T. Electrospun Polyvinyl Alcohol Loaded with Phytotherapeutic Agents for Wound Healing Applications. Nanomaterials 2021, 11, 3336. [Google Scholar] [CrossRef]
- Sarwar, M.N.; Ali, H.G.; Ullah, S.; Yamashita, K.; Shahbaz, A.; Nisar, U.; Hashmi, M.; Kim, I.-S. Electrospun PVA/CuONPs/Bitter Gourd Nanofibers with Improved Cytocompatibility and Antibacterial Properties: Application as Antibacterial Wound Dressing. Polymers 2022, 14, 1361. [Google Scholar] [CrossRef] [PubMed]
- Parın, F.N.; Yıldırım, K. Preparation and Characterisation of Vitamin-Loaded Electrospun Nanofibres as Promising Transdermal Patches. Fibres Text. East. Eur. 2021, 29, 17–25. [Google Scholar] [CrossRef]
- Allwood, M.C. The Influence of Light on Vitamin A Degradation During Administration. Clin. Nutr. 1982, 1, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Allwood, M.C.; Martin, H.J. The Photodegradation of Vitamins A and E in Parenteral Nutrition Mixtures during Infusion. Clin. Nutr. 2000, 19, 339–342. [Google Scholar] [CrossRef]
- Temova Rakuša, Ž.; Škufca, P.; Kristl, A.; Roškar, R. Retinoid Stability and Degradation Kinetics in Commercial Cosmetic Products. J Cosmet. Dermatol. 2021, 20, 2350–2358. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Zhao, Y.-Q.; Huang, J.-F. Retinoid-Binding Proteins: Similar Protein Architectures Bind Similar Ligands via Completely Different Ways. PLoS ONE 2012, 7, e36772. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Design of Polymer-Free Vitamin-A Acetate/Cyclodextrin Nanofibrous Webs: Antioxidant and Fast-Dissolving Properties. Food Funct. 2020, 11, 7626–7637. [Google Scholar] [CrossRef]
- Polcz, M.E.; Barbul, A. The Role of Vitamin A in Wound Healing. Nutr. Clin. Pract. 2019, 34, 695–700. [Google Scholar] [CrossRef]
- Li, H.; Wang, M.; Williams, G.R.; Wu, J.; Sun, X.; Lv, Y.; Zhu, L.-M. Electrospun Gelatin Nanofibers Loaded with Vitamins A and E as Antibacterial Wound Dressing Materials. RSC Adv. 2016, 6, 50267–50277. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Tolba, E.; Dorweiler, B.; Schröder, H.C.; Diehl-Seifert, B.; Wang, X. Electrospun Bioactive Mats Enriched with Ca-Polyphosphate/Retinol Nanospheres as Potential Wound Dressing. Biochem. Biophys. Rep. 2015, 3, 150–160. [Google Scholar] [CrossRef]
- Ding, B.; Kim, H.-Y.; Lee, S.-C.; Shao, C.-L.; Lee, D.-R.; Park, S.-J.; Kwag, G.-B.; Choi, K.-J. Preparation and Characterization of a Nanoscale Poly(Vinyl Alcohol) Fiber Aggregate Produced by an Electrospinning Method. J. Polym. Sci. B Polym. Phys. 2002, 40, 1261–1268. [Google Scholar] [CrossRef]
- Narváez-Muñoz, C.; Zamora-Ledezma, C.; Ryzhakov, P.; Pons-Prats, J.; Elango, J.; Mena, C.; Navarrete, F.; Morales-Flórez, V.; Cano-Crespo, R.; Segura, L.J. Improving Glass-Fiber Epoxy Composites via Interlayer Toughening with Polyacrylonitrile/Multiwalled Carbon Nanotubes Electrospun Fibers. J. Appl. Polym. Sci. 2022, 140, e53400. [Google Scholar] [CrossRef]
- Rahman, A.; Rahman, M.; Hossain, M.S.; Jahan, S.; Jahan, N.; Bari, L. A Simple and Alternative UV Spectrometric Method for the Estimation of Vitamin D3. Microb. Bioact. 2019, 2, E098–E105. [Google Scholar]
- Fahami, A.; Fathi, M. Development of Cress Seed Mucilage/PVA Nanofibers as a Novel Carrier for Vitamin A Delivery. Food Hydrocoll. 2018, 81, 31–38. [Google Scholar] [CrossRef]
- Tondnevis, F.; Ketabi, M.A.; Fekrazad, R.; Sadeghi, A.; Keshvari, H.; Abolhasani, M.M. In Vitro Characterization of Polyurethane-Carbon Nanotube Drug Eluting Composite Scaffold for Dental Tissue Engineering Application. J. Biomim. Biomater. Biomed. Eng. 2020, 47, 13–24. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Meseguer-Olmo, L.; Aznar-Cervantes, S.; Mazón, P.; De Aza, P.N. “In Vitro” Behaviour of Adult Mesenchymal Stem Cells of Human Bone Marrow Origin Seeded on a Novel Bioactive Ceramics in the Ca2SiO4–Ca3(PO4)2 System. J. Mater. Sci. Mater. Med. 2012, 23, 3003–3014. [Google Scholar] [CrossRef]
- Sethuram, L.; Thomas, J. Therapeutic Applications of Electrospun Nanofibers Impregnated with Various Biological Macromolecules for Effective Wound Healing Strategy—A Review. Biomed. Pharmacother. 2023, 157, 113996. [Google Scholar] [CrossRef]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef]
- Akhmetova, A.; Heinz, A. Electrospinning Proteins for Wound Healing Purposes: Opportunities and Challenges. Pharmaceutics 2020, 13, 4. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Q.; Wang, Y.; Meng, J.; Wu, M.; Xu, H.; Du, L.; Yang, X. Fabrication and Characterization of Electrospun Poly(Caprolactone)/Tannic Acid Scaffold as an Antibacterial Wound Dressing. Polymers 2023, 15, 593. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for Tissue Engineering Applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Electrospun Fibers and Their Application in Drug Controlled Release, Biological Dressings, Tissue Repair, and Enzyme Immobilization. RSC Adv. 2019, 9, 25712–25729. [Google Scholar] [CrossRef] [PubMed]
- Koosha, M.; Mirzadeh, H. Electrospinning, Mechanical Properties, and Cell Behavior Study of Chitosan/PVA Nanofibers: Study of CS/PVA Nanofibers. J. Biomed. Mater. Res. 2015, 103, 3081–3093. [Google Scholar] [CrossRef]
- Ince Yardimci, A.; Kayhan, M.; Tarhan, O. Polyacrylonitrile (PAN)/Polyvinyl Alcohol (PVA) Electrospun Nanofibrous Membranes Synthesis, Characterizations, and Their Air Permeability Properties. J. Macromol. Sci. Part B 2023, 61, 1426–1435. [Google Scholar] [CrossRef]
- Kim, G.-M.; Simon, P.; Kim, J.-S. Electrospun PVA/HAp Nanocomposite Nanofibers: Biomimetics of Mineralized Hard Tissues at a Lower Level of Complexity. Bioinspir. Biomim. 2008, 3, 046003. [Google Scholar] [CrossRef]
- Wang, H.; Fang, P.; Chen, Z.; Wang, S. Synthesis and Characterization of CdS/PVA Nanocomposite Films. Appl. Surf. Sci. 2007, 253, 8495–8499. [Google Scholar] [CrossRef]
- Rootare, H.M.; Craig, R.G. Vapor Phase Adsorption of Water on Hydroxyapatite. J. Dent. Res. 1977, 56, 1437–1448. [Google Scholar] [CrossRef]
- Hong, X.; Zou, L.; Zhao, J.; Li, C.; Cong, L. Dry-Wet Spinning of PVA Fiber with High Strength and High Young’s Modulus. IOP Conf. Ser. Mater. Sci. Eng. 2018, 439, 042011. [Google Scholar] [CrossRef]
- Rajora, A.D.; Bal, T. Evaluation of Cashew Gum-Polyvinyl Alcohol (CG-PVA) Electrospun Nanofiber Mat for Scarless Wound Healing in a Murine Model. Int. J. Biol. Macromol. 2023, 240, 124417. [Google Scholar] [CrossRef]
- Tian, H.; Yuan, L.; Zhou, D.; Niu, J.; Cong, H.; Xiang, A. Improved Mechanical Properties of Poly (Vinyl Alcohol) Films with Glycerol Plasticizer by Uniaxial Drawing. Polym. Adv. Technol. 2018, 29, 2612–2618. [Google Scholar] [CrossRef]
- Jeong, J.S.; Moon, J.S.; Jeon, S.Y.; Park, J.H.; Alegaonkar, P.S.; Yoo, J.B. Mechanical Properties of Electrospun PVA/MWNTs Composite Nanofibers. Thin Solid Films 2007, 515, 5136–5141. [Google Scholar] [CrossRef]
- Ngadiman, N.H.A.; Noordin, M.Y.; Idris, A.; Shakir, A.S.A.; Kurniawan, D. Influence of Polyvinyl Alcohol Molecular Weight on the Electrospun Nanofiber Mechanical Properties. Procedia Manuf. 2015, 2, 568–572. [Google Scholar] [CrossRef]
- Huang, S.-M.; Liu, S.-M.; Tseng, H.-Y.; Chen, W.-C. Effect of Citric Acid on Swelling Resistance and Physicochemical Properties of Post-Crosslinked Electrospun Polyvinyl Alcohol Fibrous Membrane. Polymers 2023, 15, 1738. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, L.; Wang, J.; Jin, M.; Tang, Q.; Chen, Z.; Cheng, Y.; Yang, R.; Zhao, G. Electrospun Nanofibers Promote Wound Healing: Theories, Techniques, and Perspectives. J. Mater. Chem. B 2021, 9, 3106–3130. [Google Scholar] [CrossRef]
- Narváez-Muñoz, C.P.; Carrion-Matamoros, L.M.; Vizuete, K.; Debut, A.; Arroyo, C.R.; Guerrero, V.; Almeida-Naranjo, C.E.; Morales-Flórez, V.; Mowbray, D.J.; Zamora-Ledezma, C. Tailoring Organic–Organic Poly(Vinylpyrrolidone) Microparticles and Fibers with Multiwalled Carbon Nanotubes for Reinforced Composites. ACS Appl. Nano Mater. 2019, 2, 4302–4312. [Google Scholar] [CrossRef]
- Goudarzi, J.; Moshtaghi, H.; Shahbazi, Y. Kappa-Carrageenan-Poly(Vinyl Alcohol) Electrospun Fiber Mats Encapsulated with Prunus Domestica Anthocyanins and Epigallocatechin Gallate to Monitor the Freshness and Enhance the Shelf-Life Quality of Minced Beef Meat. Food Packag. Shelf Life 2023, 35, 101017. [Google Scholar] [CrossRef]
- Sharifi, F.; Bai, Z.; Montazami, R.; Hashemi, N. Mechanical and Physical Properties of Poly(Vinyl Alcohol) Microfibers Fabricated by a Microfluidic Approach. RSC Adv. 2016, 6, 55343–55353. [Google Scholar] [CrossRef]
- Furr, H.C. Analysis of Retinoids and Carotenoids: Problems Resolved and Unsolved. J. Nutr. 2004, 134, 281S–285S. [Google Scholar] [CrossRef]
- Morton, R.A.; Heilbron, I.M. The Absorption Spectrum of Vitamin A. Nature 1928, 10, 122. [Google Scholar]
- Lu, T.; ten Hagen, T.L.M. A Novel Kinetic Model to Describe the Ultra-Fast Triggered Release of Thermosensitive Liposomal Drug Delivery Systems. J. Control. Release 2020, 324, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Kao, W.J. Drug Release Kinetics and Transport Mechanisms of Non-Degradable and Degradable Polymeric Delivery Systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Mares-Bou, S.; Serrano, M.-A.; Gómez-Tejedor, J.A. Core–Shell Polyvinyl Alcohol (PVA) Base Electrospinning Microfibers for Drug Delivery. Polymers 2023, 15, 1554. [Google Scholar] [CrossRef] [PubMed]
- Gaydhane, M.K.; Sharma, C.S.; Majumdar, S. Electrospun Nanofibres in Drug Delivery: Advances in Controlled Release Strategies. RSC Adv. 2023, 13, 7312–7328. [Google Scholar] [CrossRef]
- Zdraveva, E.; Mijovic, B. Frontier Electrospun Fibers for Nanomedical Applications. In Biotechnology; Villareal-Gómez, L.J., Ed.; IntechOpen: London, UK, 2023; Ch. 6. [Google Scholar] [CrossRef]
- Taepaiboon, P.; Rungsardthong, U.; Supaphol, P. Vitamin-Loaded Electrospun Cellulose Acetate Nanofiber Mats as Transdermal and Dermal Therapeutic Agents of Vitamin A Acid and Vitamin E. Eur. J. Pharm. Biopharm. 2007, 67, 387–397. [Google Scholar] [CrossRef]
- Cannella, V.; Altomare, R.; Chiaramonte, G.; Di Bella, S.; Mira, F.; Russotto, L.; Pisano, P.; Guercio, A. Cytotoxicity Evaluation of Endodontic Pins on L929 Cell Line. BioMed Res. Int. 2019, 2019, 3469525. [Google Scholar] [CrossRef]
- Pathan, S.G.; Fitzgerald, L.M.; Ali, S.M.; Damrauer, S.M.; Bide, M.J.; Nelson, D.W.; Ferran, C.; Phaneuf, T.M.; Phaneuf, M.D. Cytotoxicity Associated with Electrospun Polyvinyl Alcohol: Cytotoxic Effects of Electrospun PVA. J. Biomed. Mater. Res. 2015, 103, 1652–1662. [Google Scholar] [CrossRef]
- Gao, C.; Gao, Q.; Li, Y.; Rahaman, M.N.; Teramoto, A.; Abe, K. Preparation and in Vitro Characterization of Electrospun PVA Scaffolds Coated with Bioactive Glass for Bone Regeneration. J. Biomed. Mater. Res. 2012, 100A, 1324–1334. [Google Scholar] [CrossRef]
- Madruga, L.Y.C.; Balaban, R.C.; Popat, K.C.; Kipper, M.J. Biocompatible Crosslinked Nanofibers of Poly(Vinyl Alcohol)/Carboxymethyl-Kappa-Carrageenan Produced by a Green Process. Macromol. Biosci. 2021, 21, 2000292. [Google Scholar] [CrossRef]
- Schulte-Hermann, R.; Grasl-Kraupp, B.; Bursch, W. Dose–Response and Threshold Effects in Cytotoxicity and Apoptosis. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2000, 464, 13–18. [Google Scholar] [CrossRef]
- Veeramani, C.; El Newehy, A.S.; Alsaif, M.A.; Al-Numair, K.S. Vitamin A- and C-Rich Pouteria Camito Fru It Derived Superparamagnetic Nanoparticles Synthesis, Characterization, and Their Cytotoxicity. Afr. Health Sci. 2022, 22, 673–680. [Google Scholar] [CrossRef]
- Baghirova, L.; Kaya Tilki, E.; Öztürk, A.A. Evaluation of Cell Proliferation and Wound Healing Effects of Vitamin A Palmitate-Loaded PLGA/Chitosan-Coated PLGA Nanoparticles: Preparation, Characterization, Release, and Release Kinetics. ACS Omega 2023, 8, 2658–2668. [Google Scholar] [CrossRef]
- Scotchford, C.A.; Cascone, M.G.; Downes, S.; Giusti, P. Osteoblast Responses to Collagen-PVA Bioartificial Polymers in Vitro: The Effects of Cross-Linking Method and Collagen Content. Biomaterials 1998, 19, 1–11. [Google Scholar] [CrossRef]
- Shafiee, A.; Soleimani, M.; Chamheidari, G.A.; Seyedjafari, E.; Dodel, M.; Atashi, A.; Gheisari, Y. Electrospun Nanofiber-Based Regeneration of Cartilage Enhanced by Mesenchymal Stem Cells. J. Biomed. Mater. Res. 2011, 99A, 467–478. [Google Scholar] [CrossRef]
- Mombini, S.; Mohammadnejad, J.; Bakhshandeh, B.; Narmani, A.; Nourmohammadi, J.; Vahdat, S.; Zirak, S. Chitosan-PVA-CNT Nanofibers as Electrically Conductive Scaffolds for Cardiovascular Tissue Engineering. Int. J. Biol. Macromol. 2019, 140, 278–287. [Google Scholar] [CrossRef]
- Weyermann, J.; Lochmann, D.; Zimmer, A. A Practical Note on the Use of Cytotoxicity Assays. Int. J. Pharm. 2005, 288, 369–376. [Google Scholar] [CrossRef]
- Adeli, H.; Khorasani, M.T.; Parvazinia, M. Wound Dressing Based on Electrospun PVA/Chitosan/Starch Nanofibrous Mats: Fabrication, Antibacterial and Cytocompatibility Evaluation and in Vitro Healing Assay. Int. J. Biol. Macromol. 2019, 122, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Alavarse, A.C.; de Oliveira Silva, F.W.; Colque, J.T.; da Silva, V.M.; Prieto, T.; Venancio, E.C.; Bonvent, J.-J. Tetracycline Hydrochloride-Loaded Electrospun Nanofibers Mats Based on PVA and Chitosan for Wound Dressing. Mater. Sci. Eng. C 2017, 77, 271–281. [Google Scholar] [CrossRef]

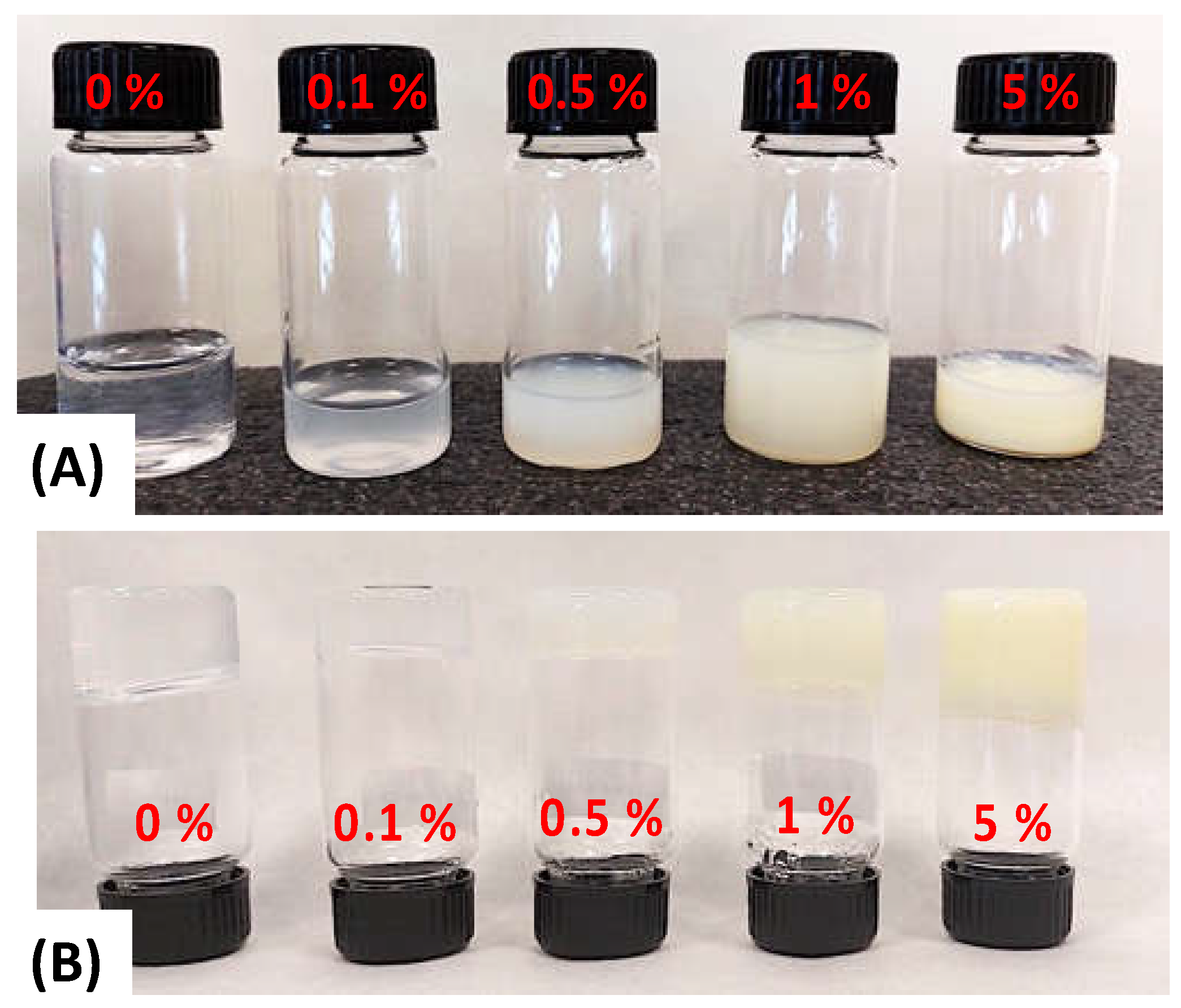
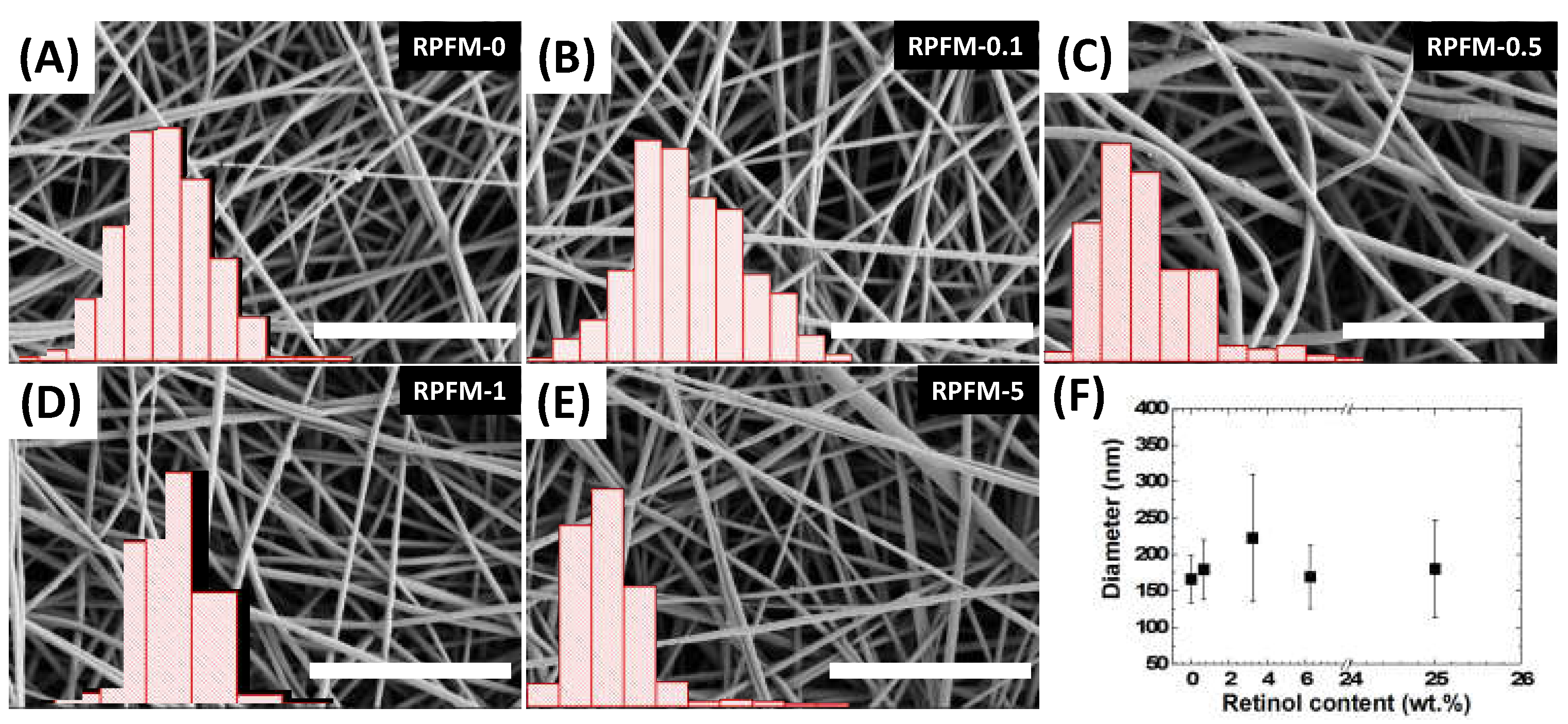

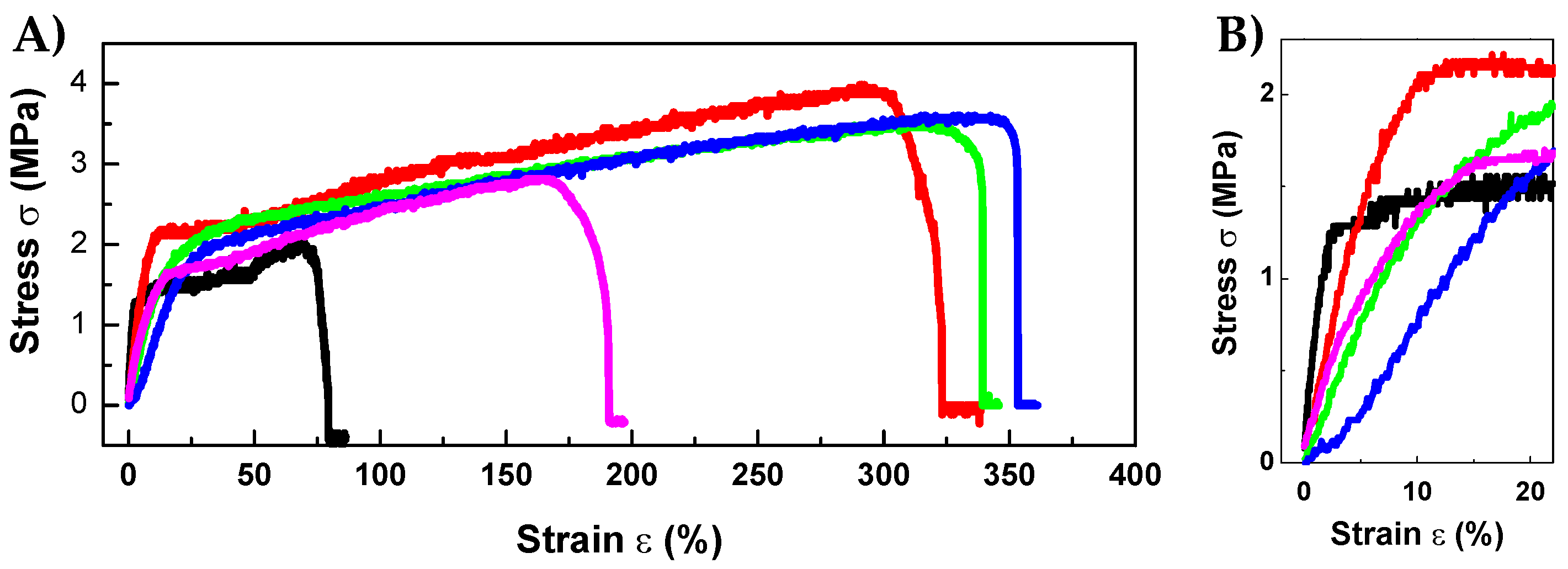

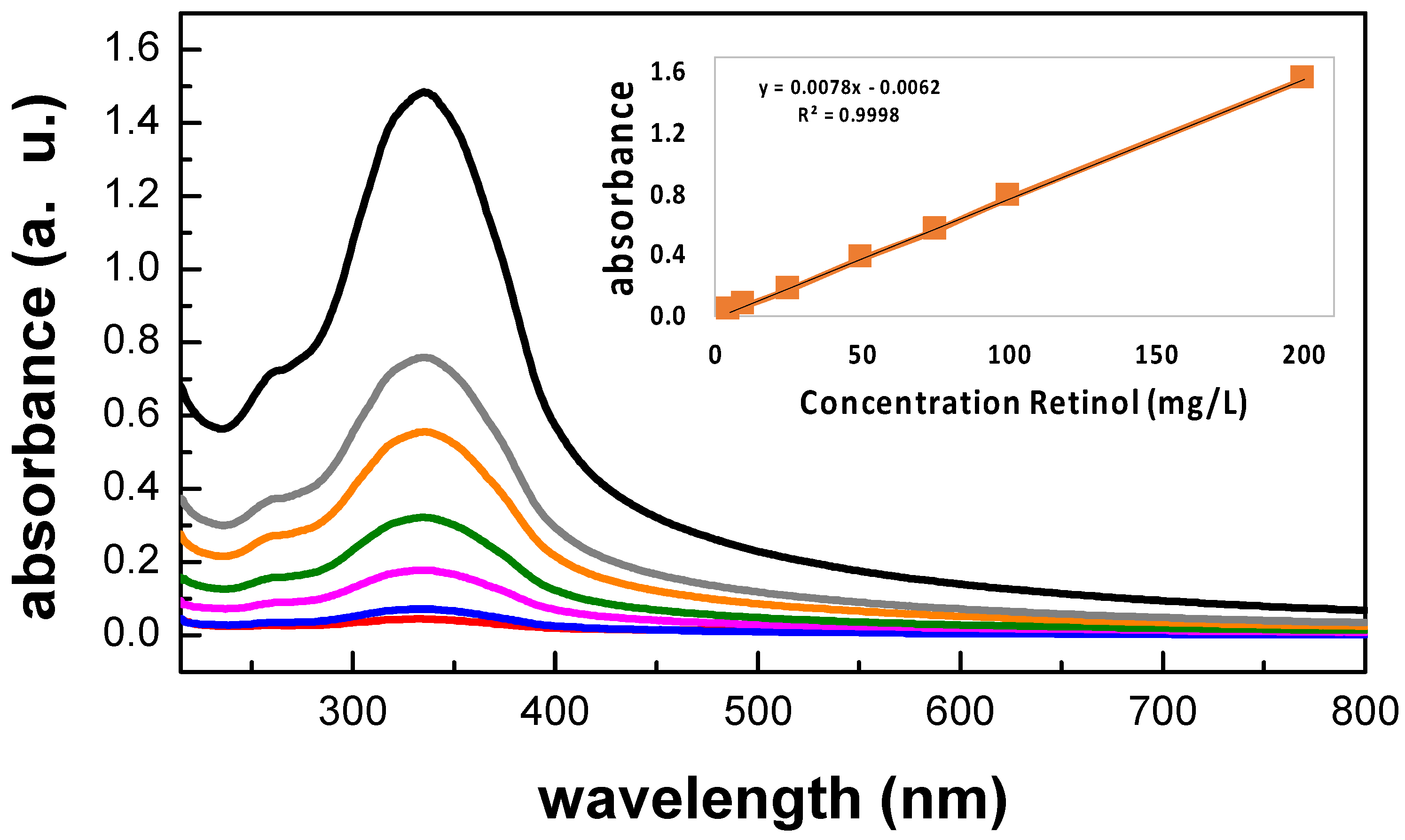
| Sample Name | PVA in Solution (wt.%) | Retinol in Solution (wt.%) | PVA Dry Content (wt.%) | Retinol Dry Content (wt.%) |
|---|---|---|---|---|
| RPFM-0 | 15.00 | 0.00 | 100.00 | 0.00 |
| RPFM-0.1 | 15.00 | 0.10 | 99.34 | 0.66 |
| RPFM-0.5 | 15.00 | 0.50 | 96.77 | 3.23 |
| RPFM-1 | 15.00 | 1.00 | 93.75 | 6.25 |
| RPFM-5 | 15.00 | 5.00 | 75.00 | 25.00 |
| Sample Name | E (MPa) | UTS (MPa) | εmax (%) |
|---|---|---|---|
| RPFM-0 | 44.73 | 2.18 | 79.00 |
| RPFM-0.1 | 22.25 | 3.46 | 219.00 |
| RPFM-0.5 | 9.39 | 2.84 | 233.00 |
| RPFM-1 | 8.13 | 3.69 | 382.00 |
| RPFM-5 | 9.20 | 2.72 | 186.00 |
| Model Name | Mathematical Formula | Coefficients | Year |
|---|---|---|---|
| Higuchi | k indicates the release rate constant | 1963 | |
| Ritger-Peppas | n represents the release (diffusion) exponent | 1987 | |
| Peppas-Sahlin | k1 and k2 represent diffusion and degradation rate constants and m indicates the release exponent | 1989 | |
| Kopcha | A and B indicate diffusion and dissolution terms | 1990 | |
| Fu-Kao Zero Order | k indicates the release rate constant | 2010 | |
| Fu-Kao First Order | k indicates the release rate constant | 2010 | |
| Hixon-Crowell | k indicates the release rate constant | 2011 |
| Sample | m | k (Days−m) | MAE (%) |
|---|---|---|---|
| RPFM-0.1 | 1 | 0.293 | 6.3 |
| RPFM-0.5 | 1 | 0.039 | 4.3 |
| RPFM-1 | 1 | 0.030 | 4.1 |
| RPFM-5 | 1 | 0.026 | 4.2 |
| RPFM-0.1 | ½ | 0.559 | 7.1 |
| RPFM-0.5 | ½ | 0.092 | 1.7 |
| RPFM-1 | ½ | 0.071 | 2.1 |
| RPFM-5 | ½ | 0.063 | 2.4 |
| RPFM-0.1 | 0.7 | 0.435 | 6.0 |
| RPFM-0.5 | 0.252 | 0.133 | 0.5 |
| RPFM-1 | 0.169 | 0.116 | 0.1 |
| RPFM-5 | 0.088 | 0.116 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamora-Ledezma, C.; Hernández, A.B.; López-González, I.; Elango, J.; Paindépice, J.; Alexis, F.; González-Sánchez, M.; Morales-Flórez, V.; Mowbray, D.J.; Meseguer-Olmo, L. Fabrication, Physical–Chemical and Biological Characterization of Retinol-Loaded Poly(vinyl Alcohol) Electrospun Fiber Mats for Wound Healing Applications. Polymers 2023, 15, 2705. https://doi.org/10.3390/polym15122705
Zamora-Ledezma C, Hernández AB, López-González I, Elango J, Paindépice J, Alexis F, González-Sánchez M, Morales-Flórez V, Mowbray DJ, Meseguer-Olmo L. Fabrication, Physical–Chemical and Biological Characterization of Retinol-Loaded Poly(vinyl Alcohol) Electrospun Fiber Mats for Wound Healing Applications. Polymers. 2023; 15(12):2705. https://doi.org/10.3390/polym15122705
Chicago/Turabian StyleZamora-Ledezma, Camilo, Ana Belén Hernández, Ivan López-González, Jeevithan Elango, Janèle Paindépice, Frank Alexis, Manuela González-Sánchez, Víctor Morales-Flórez, Duncan John Mowbray, and Luis Meseguer-Olmo. 2023. "Fabrication, Physical–Chemical and Biological Characterization of Retinol-Loaded Poly(vinyl Alcohol) Electrospun Fiber Mats for Wound Healing Applications" Polymers 15, no. 12: 2705. https://doi.org/10.3390/polym15122705
APA StyleZamora-Ledezma, C., Hernández, A. B., López-González, I., Elango, J., Paindépice, J., Alexis, F., González-Sánchez, M., Morales-Flórez, V., Mowbray, D. J., & Meseguer-Olmo, L. (2023). Fabrication, Physical–Chemical and Biological Characterization of Retinol-Loaded Poly(vinyl Alcohol) Electrospun Fiber Mats for Wound Healing Applications. Polymers, 15(12), 2705. https://doi.org/10.3390/polym15122705













