The Synergistic Mechanism and Stability Evaluation of Phosphogypsum and Recycled Fine Powder-Based Multi-Source Solid Waste Geopolymer
Abstract
1. Introduction
2. Raw Materials and Experimental Methods
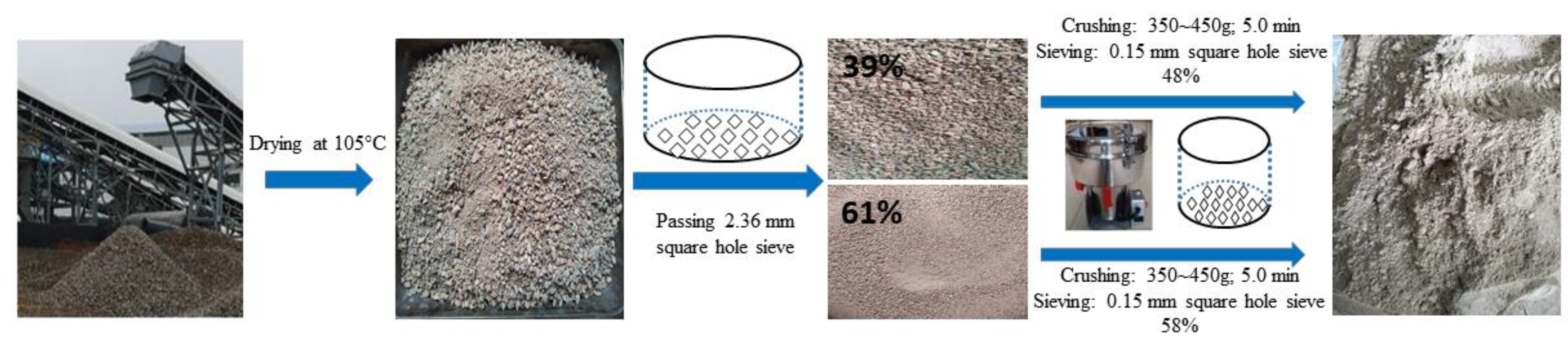
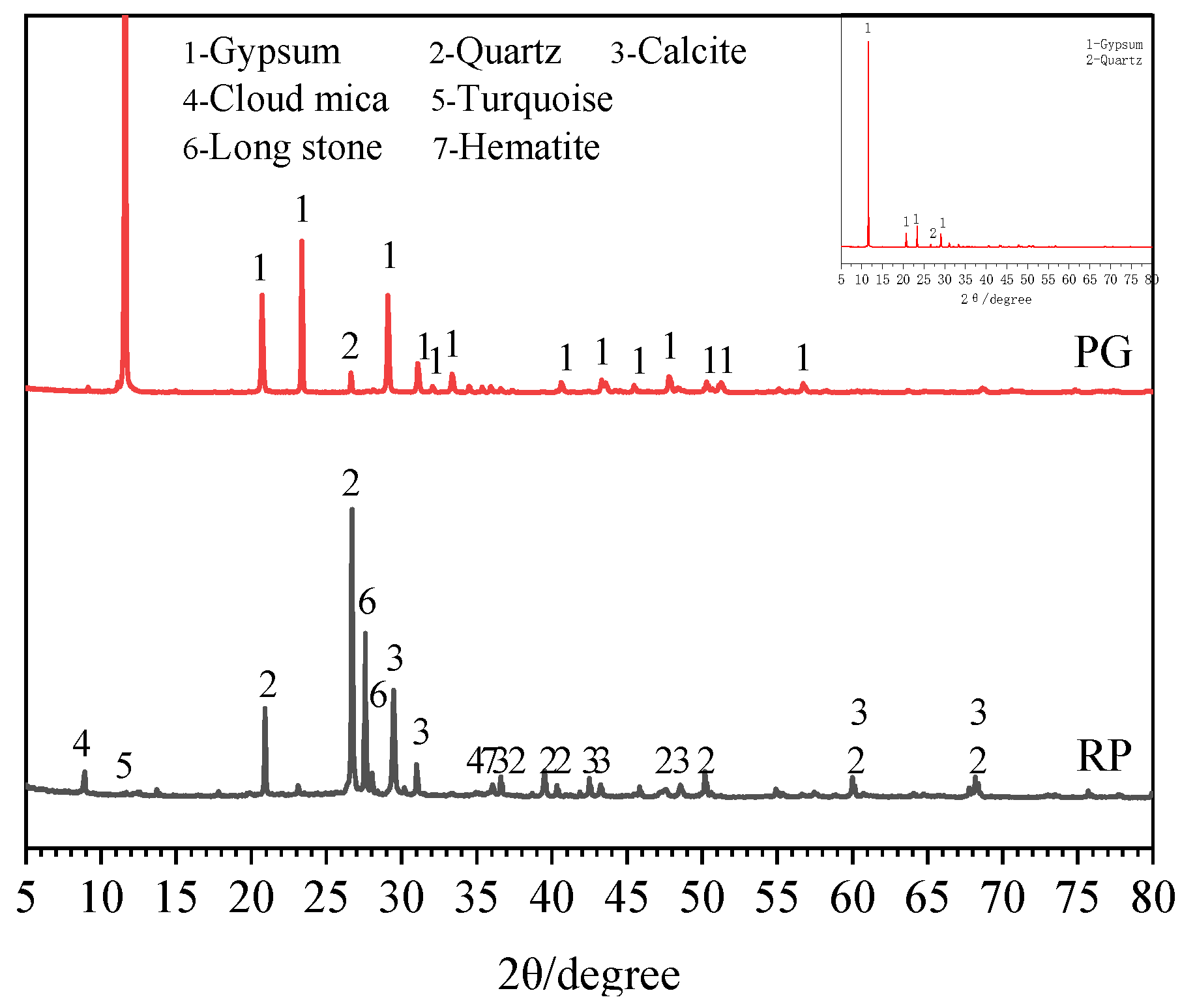
2.1. Raw Materials
2.2. Mix Proportion Design
2.3. Specimen Preparations and Curing Conditions
- (1)
- Forming and curing of geopolymer slurry specimens: First, accurately weighed various powder materials were taken according to the mix proportion in the experiment, mixed and stirred evenly, and then poured together with the prepared modified sodium silicate solution into the mortar mixer. Second, it was first slowly stirred for 1 min, after which the bottom slurry of the pot was scraped manually stirred for 1 min, and then stirred continuously for 2 min to ensure that the powder material and solution were evenly mixed. Then, the inner wall of the mold (25 mm × 25 mm × 280 mm) was cleaned and brushed with engine oil, and then the stirred slurry was poured into the mold and vibrated 60 times, the mold flat was scraped, and the specimens were wrapped and sealed with a cling film. The samples were cured at room temperature (20 3 °C) for 24 h. Finally, the specimen was completely removed from the mold and marked. Then, the specimen was sealed and wrapped with a cling film and transferred to a cement curing box with constant temperature curing until the specified age. It should be noted that some specimens with longer setting times needed to be cured for 48 h before demolding.
- (2)
- Forming and curing of geopolymer mortar specimens: The formation and curing process of the geopolymer mortar specimens was the same as that of the pure slurry specimens. The mortar forming size was 40 mm × 40 mm × 160 mm.
2.4. Tests
- (1)
- Volume change rate test: This test was conducted in accordance with the “Test Method for Dry Shrinkage of Cement Mortar” (JC/T 603-2004) [41]. After 24 h of sealing and curing, the initial length of the specimen was measured using a BC-300 cement length meter (see Figure 5) and then placed in an environment of 20 3 °C and 50 5% relative humidity for 154 days. Measurements were taken every 24 h in the first two weeks; every 48 h from the 3rd to the 4th week; every 7 days from the 5th to the 8th week; every 14 days from the 9th to the 22nd week. The average of the results from each group of 3 specimens was used for the analysis. The volume change rate of the specimen was calculated using Equation (1) as follows:where : rate of volume change, contraction is positive and expansion is negative; %; : initial length of the specimen, mm; : the measured length of the specimen cured at 20 3 °C and 50 5% relative humidity for n days, mm.
- (2)
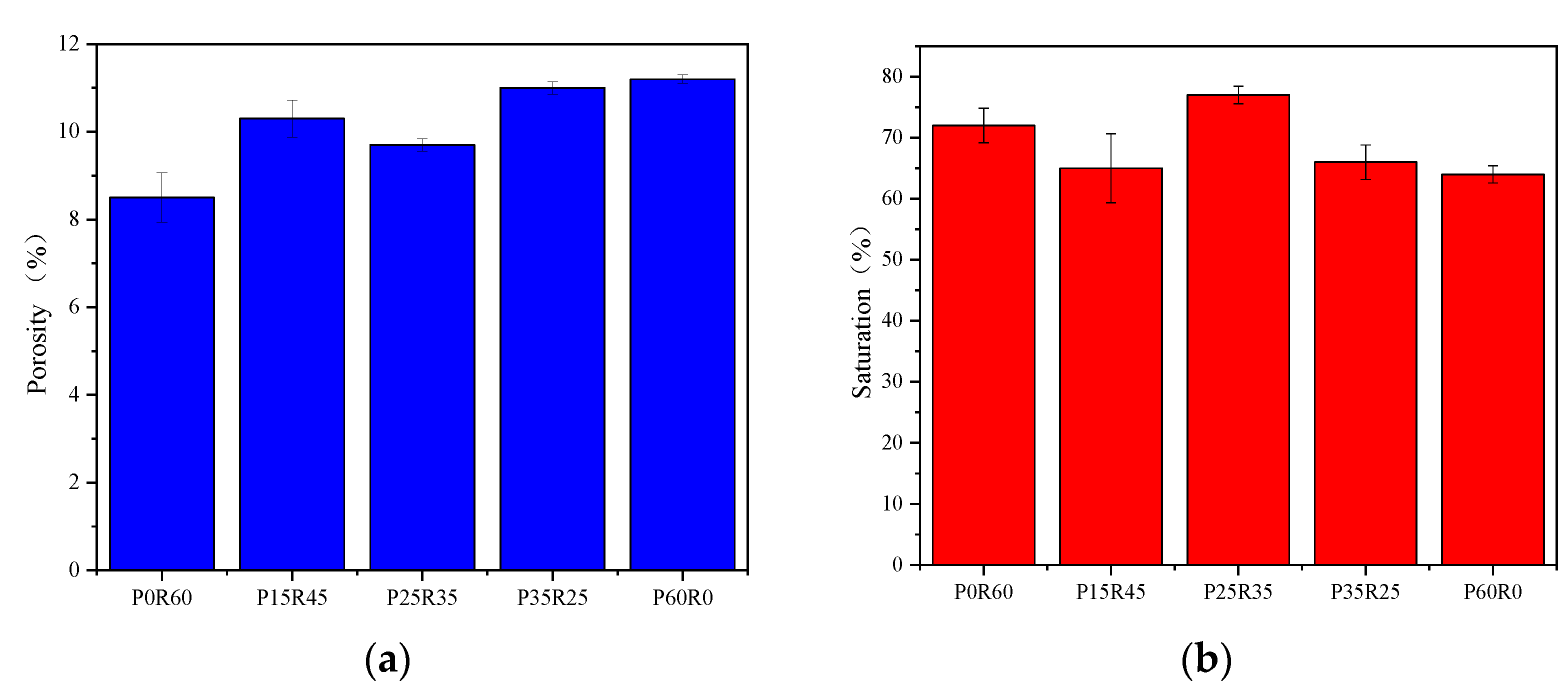
- Porosity and porosity saturation tests: First, the mass of the slurry specimen was weighed and its volume was measured, which were recorded as the mass and volume of the specimen, respectively. The slurry specimen was broken into small pieces and immersed in clean water for 24 h to ensure that the pieces were saturated with water. The surface of the fragments was removed and dried, and the total mass of the fragments was weighed and recorded as the saturated mass of the specimen. Finally, the fragments were placed in an oven (50 °C) for drying, and the total mass of the fragments was weighed at this time and recorded as the dry mass of the test piece. The porosity of the specimen was calculated using Equation (2), and the pore saturation of the specimen was calculated using Equation (3) as follows:where P: the porosity of the specimen, %; S: the saturation of pores, %; M: mass, g; M1: saturation mass, g; M2: dry mass, g; V: volume, cm3; : density of water, 1 g/cm3.
- (3)
- Compressive strength test: The standard “ISO method for testing the strength of cement mortar” (GB/T 17671-1999) was referred to for the testing [42].
- (4)
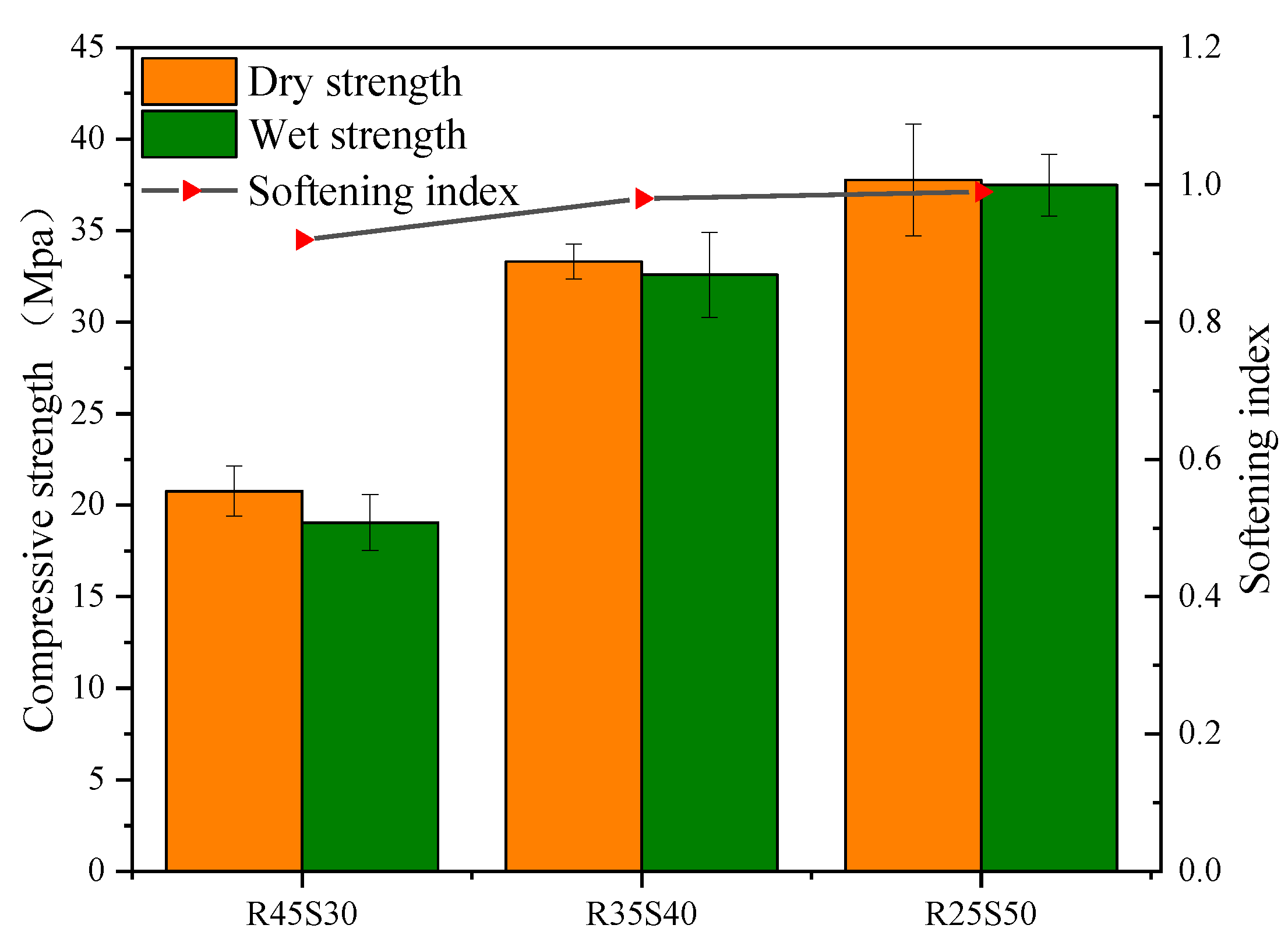
- Softening coefficient test: Reference [43] was referred to for the testing. Two sets of mortar specimens with a curing age of 28 days were taken, and one set was dried in an oven at (45 2) °C to a constant weight. Its compressive strength was measured and recorded as dry the compressive strength. The other group was immersed in warm water at (20 3) °C for 24 h, and then the surface was taken out and wiped dry, and its compressive strength was measured and recorded as the wet compressive strength. The formula for calculating the softening coefficient is shown in Equation (4).where : softening coefficient; : dry compressive strength, Mpa; : wet compressive strength, Mpa.
- (5)
- Mechanical stability performance test: The measurement method of this test was conducted according to reference [44]. Two sets of specimens were taken from the mortar specimens with a curing age of 28 days. One group of specimens was subjected to standard curing at a temperature of (20 3) °C and a relative humidity of (90 ± 5)%, while the other group was subjected to immersion curing in water at a constant temperature of 20 3 °C. After 90 days, the compressive strength of the two sets of specimens was tested separately.
- (6)
- X-ray fluorescence spectrometer (XRF): Powder samples (3 g) were prepared and an X-ray fluorescence spectrometer with Panalytical Axios model was used, and the test mode was in the form of an oxide.
- (7)
- X-ray diffraction (XRD): The specimen at a specified age was first crushed into small pieces. The pieces <1.18 mm were taken and immersed into anhydrous alcohol for 7 days to terminate the hydration process, and then dried at 50 °C to a constant weight. Finally, the samples were ground into a powder and 1 g was selected for the XRD test after being passed through a 200 mesh sieve. Bruke D8 advanced X-ray diffractometer with a scanning speed of 2°/min and scanning angle range of 5~90° was adopted.
- (8)
- Fourier-transform infrared spectroscopy (FTIR): The sample preparation method was the same as that of the XRD. The test mode was a conventional powder tablet. The wavenumber range was 400~4000 cm−1.
- (9)
- Scanning electron microscope (SEM): The block sample with a thickness of less than 1 cm, diameter ≤ 1 cm and relatively flat fracture surface was selected, dried at 50 °C to constant weight, and the Czech Tescan Mira LMS scanning electron microscope was used to test the morphology.
3. Discussion
3.1. Volume Stability Analysis
3.1.1. Effect of Phosphogypsum on Volume Stability
- (1)
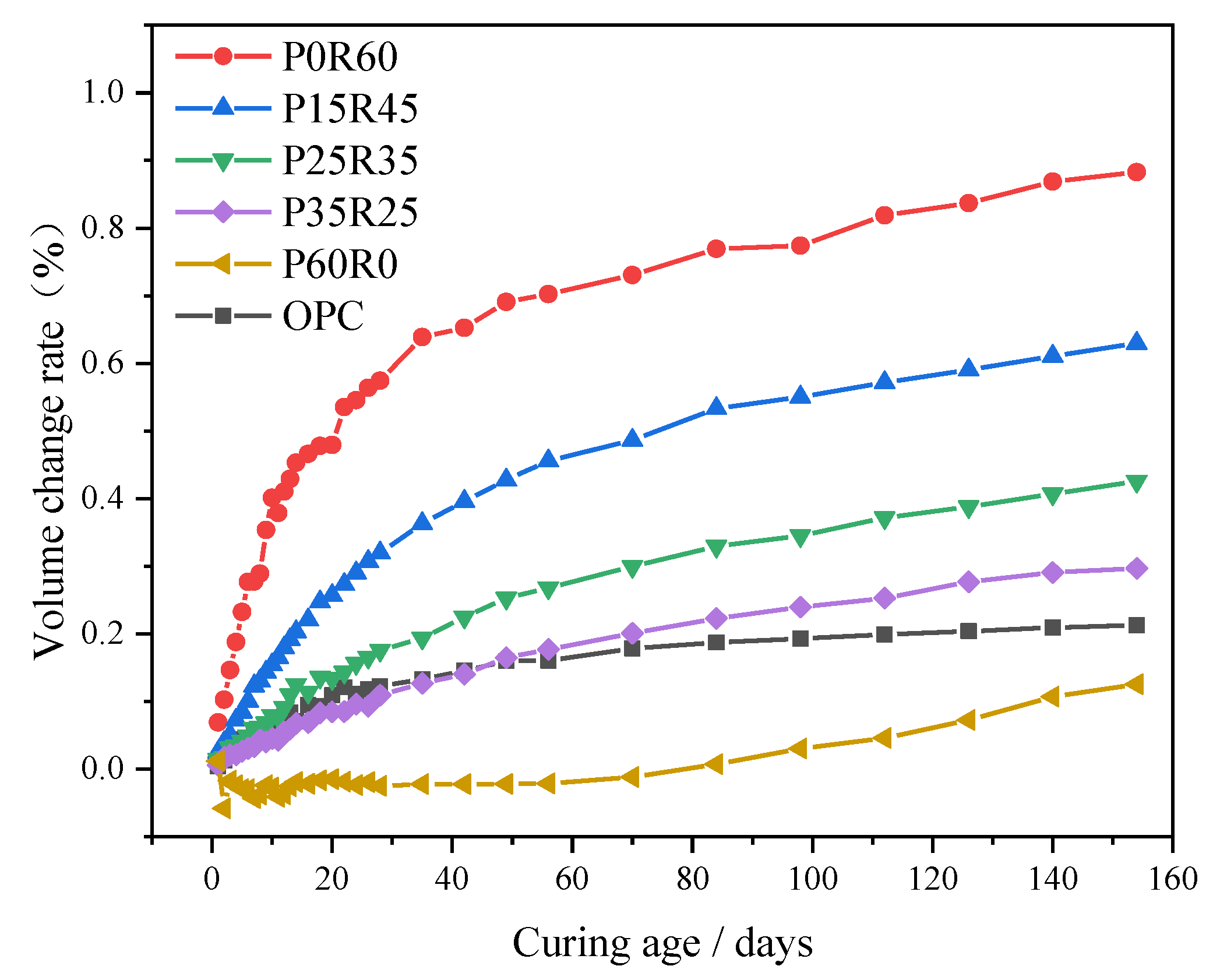
- Volume change rate
- (2)
- Porosity and pore saturation
3.1.2. Effect of Slag on Volume Stability
- (1)
- Volume change rate
- (2)
- Porosity and pore saturation
3.2. Water Stability Analysis
3.2.1. Effect of Recycled Fine Powder on Water Stability
3.2.2. Effect of Slag on Water Stability
3.3. Mechanical Stability Analysis
3.3.1. Effect of Recycled Fine Powder on Mechanical Stability
3.3.2. Effect of the Slag on the Mechanical Stability
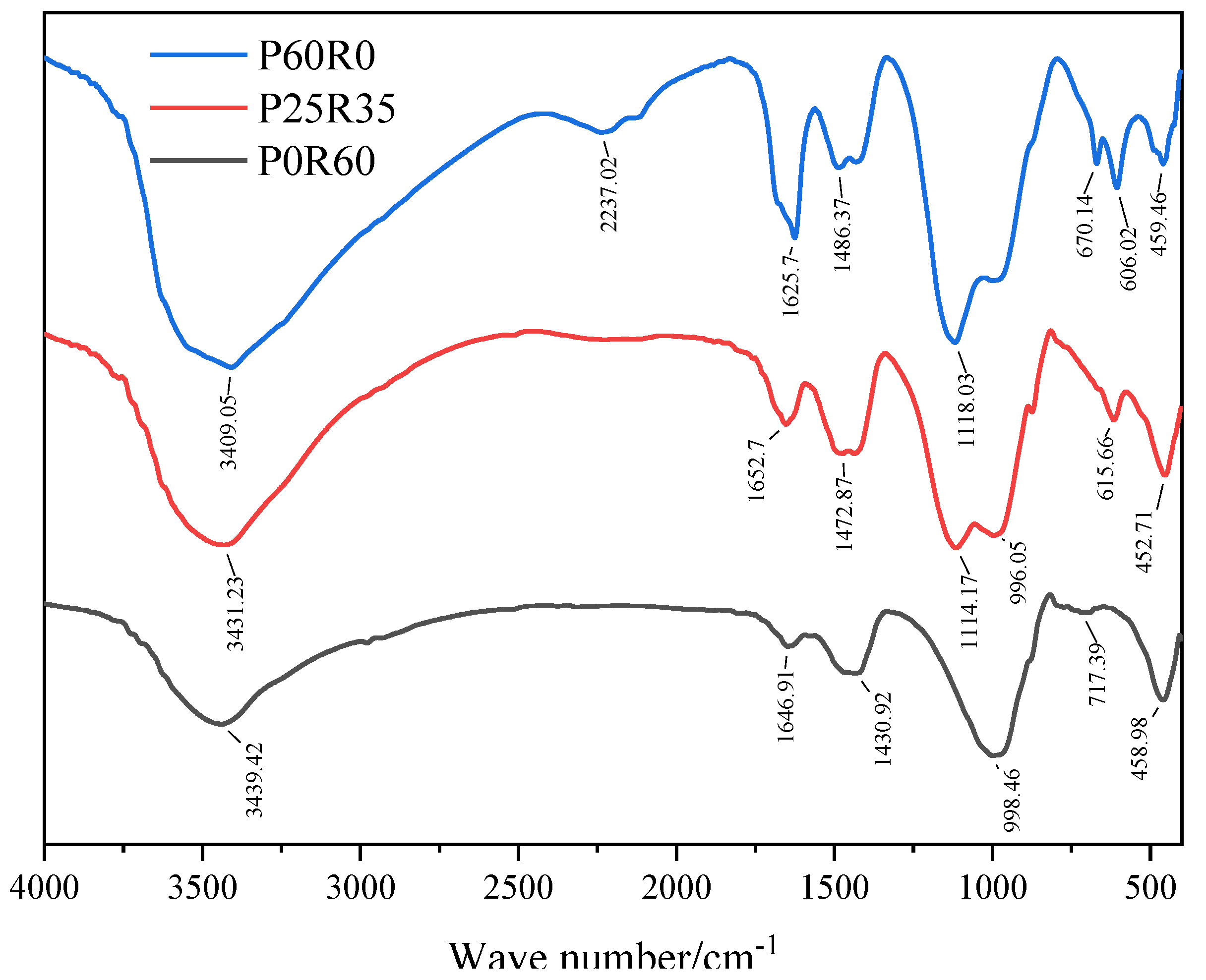
3.4. Microscopic Analysis
3.4.1. XRD
3.4.2. FTIR
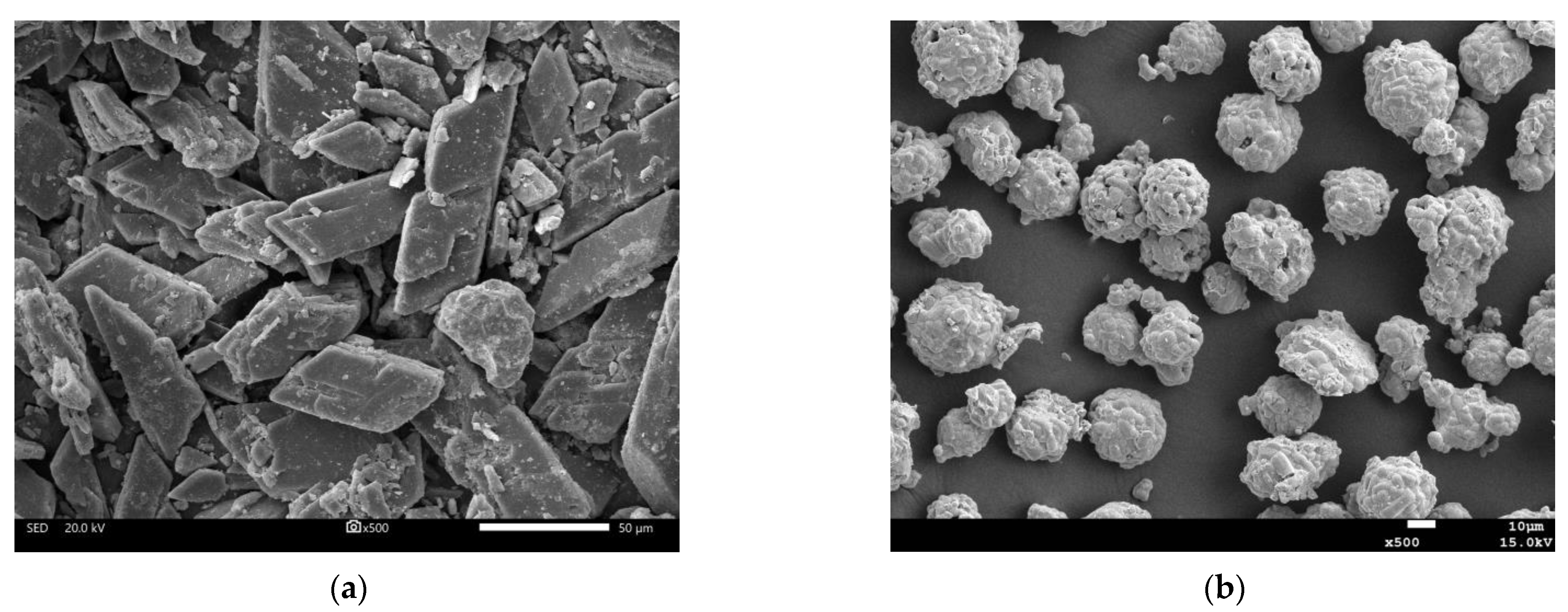
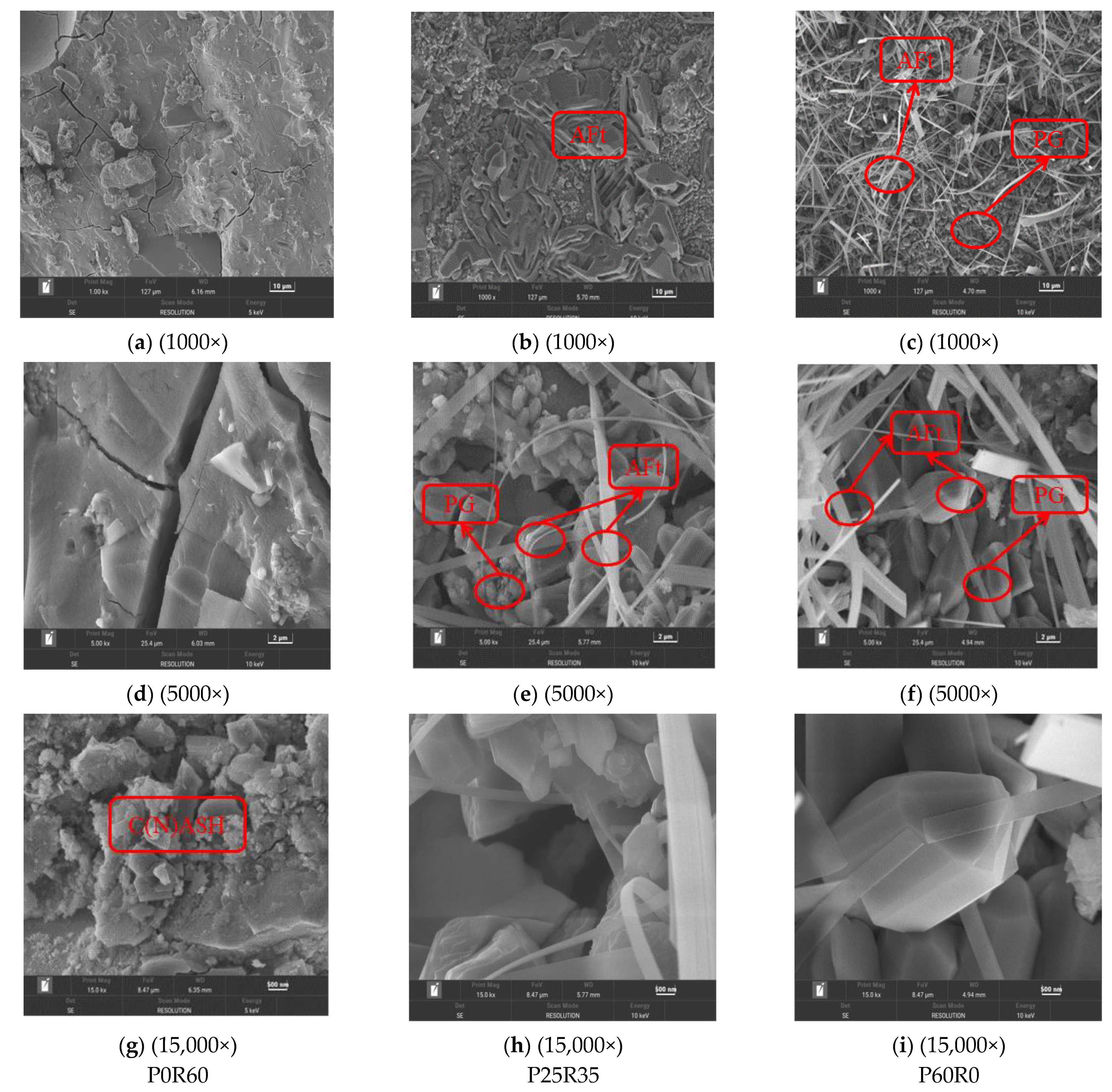
3.4.3. SEM
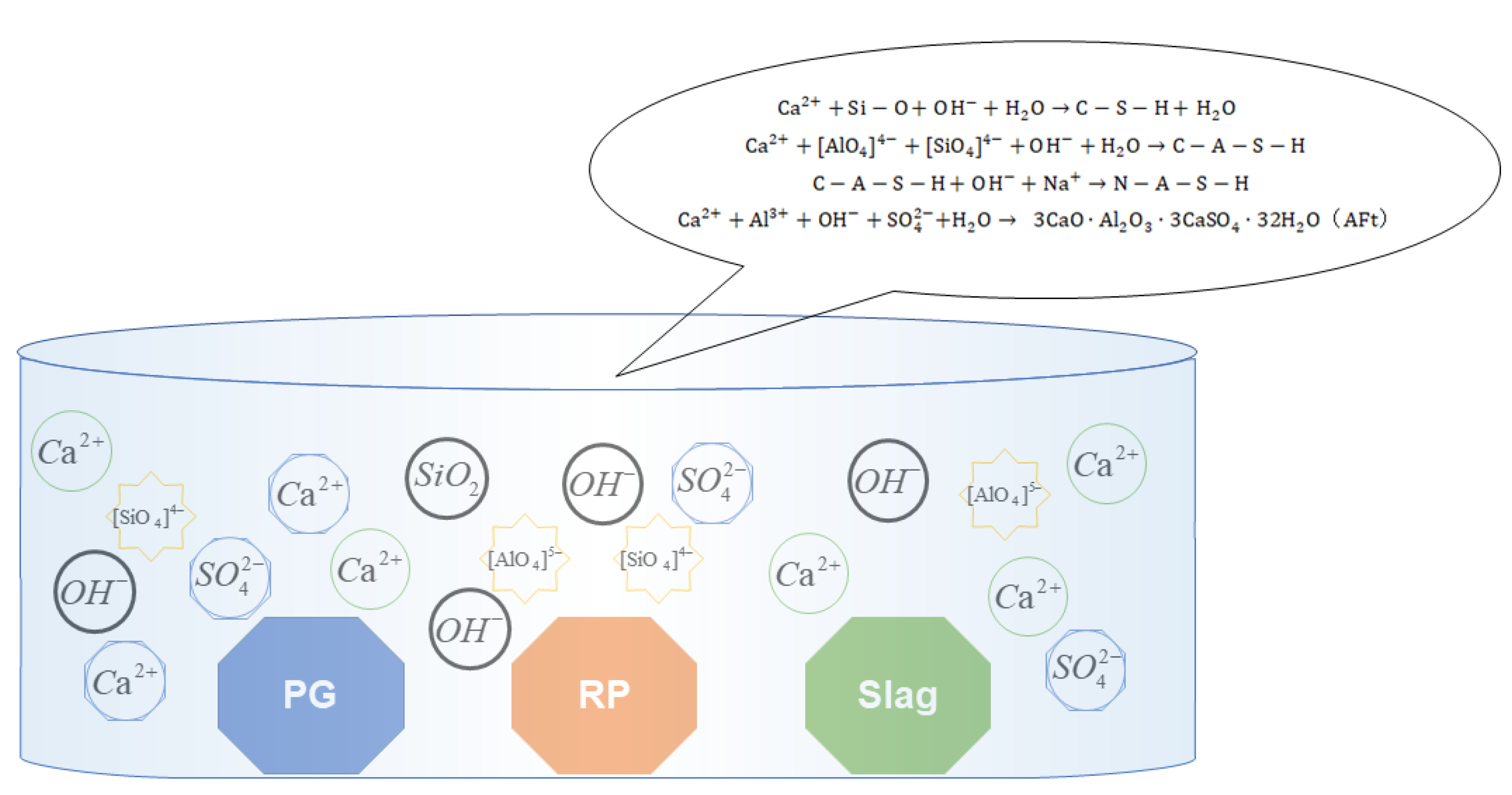
3.5. Stable and Synergistic Mechanism of Multi-Source Solid Waste
4. Conclusions
- (1)
- The combined action of phosphogypsum, recycled fine powder, slag and other multi-source solid wastes can not only control the generation of AFt crystals but also control the capillary stress inside the matrix by adjusting the pore structure of the matrix, thus improving the volume stability of phosphogypsum and recycled fine powder-based multi-source solid waste geopolymers, reducing its volume deformation, and avoiding the expansion and damage of geopolymers. The volume of specimen P35R25 slightly shrinks without any risk of expansion or damage. Meanwhile, the 154-day volume change rate of specimen P35R25 decreased by 66.4% compared to that of specimen P0R60, and its 28-day volume change rate was 10.7% lower than that of specimen OPC.
- (2)
- The synergistic effect of phosphogypsum, recycled fine powder and slag promotes the generation of more CASH and NASH hydration gels, improves the pore structure of the matrix and reduces the negative impact on the matrix, thus improving the water stability of phosphogypsum and recycled fine powder-based multi-source solid waste geopolymer. The water stability of mortar specimen P15R45 is the best, with a softening coefficient of 1.06, which is 26.2% higher than that of mortar specimen P35R25.
- (3)
- The synergistic effect of phosphogypsum, recycled fine powder and slag reduces the negative impact of delayed AFt and improves the mechanical stability of the geopolymer. The 118-day standard curing compressive strength (28 days of sealing curing plus 90 days of standard curing) of mortar specimen P15R45 decreased by only 2.3% compared to its 28-day compressive strength, whereas its 118-day water-curing compressive strength (28 days of sealing curing plus 90 days of immersion curing) increased by 18.2% compared to its 28-day compressive strength.
- (4)
- Microscopic analysis shows that phosphogypsum can promote the generation of more hydration products, especially AFt, which has an important impact on the pore structure and volume change of the slurry. Both phosphogypsum and recycled fine powder in geopolymers do not completely react, and the unreacted phosphogypsum particles and recycled fine powder particles not only have an important impact on the compactness of the matrix structure but also affect its stability. Recycled fine powder can promote the generation of hydration products, such as CASH and NASH, from geopolymers, improve the compactness of the slurry and enhance its stability. When the content of phosphogypsum increases, the content of recycled fine powder decreases, and the compact structure of the geopolymer slurry is destroyed, thereby becoming loose and porous.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Z.; Xiang, L. Review of comprehensive utilization of phosphogypsum. Inorg. Chem. Ind. 2007, 1, 8–10. [Google Scholar]
- Cui, R.; Bai, H.; Gao, Y.; Xiu, X. Current situation of comprehensive utilization of phosphogypsum and development trend of 14th Five-Year Plan. Inorg. Chem. Ind. 2022, 54, 1–4. [Google Scholar]
- Wu, L. Study on the Surface Modified Chopped Fiber Reinforced Phosphorus Building Plaster. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2019. [Google Scholar]
- Antanas, K.; Violeta, L.; Bronius, V.; Zenonas, V. The Study of Neutralization of The Dihydrate Phosphogypsum Impurities. Cearmics Silik. 2006, 50, 178. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Wan, H.; Wang, P.; Tan, D. Experimental Study on the Gypsum Preparation by the Phosphogypsu. J. Wuhan Univ. Technol. 2015, 37, 40–46. [Google Scholar]
- Olmez, H.; Yilmaz, V.T. Infrared study on the refinement of phosphogypsum for cements. Cem. Concr. Res. 1988, 18, 449–454. [Google Scholar] [CrossRef]
- Chen, W.F.; Huang, B.; Yuan, Y.X.; Deng, M. Deterioration Process of Concrete Exposed to Internal Sulfate Attack. Materials 2020, 13, 1336. [Google Scholar] [CrossRef]
- Prince, W.; Espagne, M.; Atcin, P.C. Ettringite formation: A crucial step in cement superplasticizer compatibility. Cem. Concr. Res. 2003, 33, 635–641. [Google Scholar] [CrossRef]
- Zhao, Z. Formation of Ettringite and Its Effect on Shrinkage Properties of Alkali Slag Cement Mortar. Master’s Thesis, Chongqing University, Chongqing, China, 2021. [Google Scholar]
- Wu, F.; Chen, B.; Qu, G.; Liu, S.; Zhao, C.; Ren, Y.; Liu, X. Harmless treatment technology of phosphogypsum: Directional stabilization of toxic and harmful substances. J. Environ. Manag. 2022, 311, 114827. [Google Scholar] [CrossRef]
- Wu, F.H.; He, M.; Qu, G.; Zhang, T.; Ren, Y.; Kuang, L.; Ning, P.; Junyan, L.; Liu, Y. Highly targeted stabilization and release behavior of hazardous substances in phosphogypsum. Miner. Eng. 2022, 189, 107866. [Google Scholar] [CrossRef]
- Wu, H.; Zuo, J.; Zillante, G.; Wang, J.; Yuan, H. Status quo and future directions of construction and demolition waste research: A critical review. J. Clean. Prod. 2019, 240, 118163. [Google Scholar] [CrossRef]
- Ye, T.H.; Xiao, J.; Duan, Z.; Li, S. Geopolymers made of recycled brick and concrete powder—A critical review. Constr. Build. Mater. 2022, 330, 127232. [Google Scholar] [CrossRef]
- Murthi, P.; Krishnamoorthi, S.; Poongodi, K.; Saravanan, R. Development of green masonry mortar using fine recycled aggregate based on the shear bond strength of brick masonry. Mater. Today Proc. 2022, 61, 413–419. [Google Scholar] [CrossRef]
- Premkumar, R.; Hariharan, P.; Rajesh, S. Effect of silica fume and recycled concrete aggregate on the mechanical properties of GGBS based geopolymer concrete. Mater. Today Proc. 2022, 60, 211–215. [Google Scholar] [CrossRef]
- Sahin, F.; Uysal, M.; Canpolat, O.; Aygormez, Y.; Cosgun, T.; Dehghanpour, H. Effect of basalt fiber on metakaolin-based geopolymer mortars containing rilem, basalt and recycled waste concrete aggregates. Constr. Build. Mater. 2021, 301, 124113. [Google Scholar] [CrossRef]
- Zhao, M.Z.; Geng, Y.; Wang, Y.Y.; Hu, J.X. Compounding effect and an expanded theoretical model for recycled coarse and fine aggregate concretes under uniaxial loading. Constr. Build. Mater. 2022, 320, 126226. [Google Scholar] [CrossRef]
- Zhu, Z.; Gu, S.; Tang, Z.; Song, L. Experimental Study on Road Base Material of Geopolymer Recycled Concrete. J. Test. Eval. 2021, 49, 20180100. [Google Scholar] [CrossRef]
- Zhou, C.; Ji, H.; Zhao, L. Application and Research Progress of Recycled Micro-powders in Cement-Based Materials. Bull. Chin. Ceram. Soc. 2019, 38, 8. [Google Scholar] [CrossRef]
- Ding, X.; Xing, W.; Li, H. Effect of regeneration powder on cement mortar performance. Concrete 2018, 11. [Google Scholar] [CrossRef]
- Xiao, J.; Ma, Z.; Sui, T. Mechanical properties of concrete mixed with recycled powder produced from construction and demolition waste. J. Clean. Prod. 2018, 188, 720–731. [Google Scholar] [CrossRef]
- Yang, L. Investigation of Recycled Cementitious Materials with Recycled Concrete Powder. Master’s Thesis, Southeast University, Nanjing, China, 2016. [Google Scholar]
- Davidovits, J. Geopolymer Chemistry and Applications, 5th ed.; Geopolymer Institute: Saint Quentin, France, 2020. [Google Scholar]
- Cong, P.; Cheng, Y. Advances in geopolymer materials: A comprehensive review. J. Traffic Transp. Eng. 2021, 8, 32. [Google Scholar] [CrossRef]
- Alhawat, M.; Ashour, A.; Yildirim, G.; Aldemir, A.; Sahmaran, M. Properties of geopolymer sourced from construction and demolition waste: A review. J. Build. Eng. 2022, 50, 104104. [Google Scholar] [CrossRef]
- Abdulhussein, S.A.; Monower, S.; Patryk, K.; William, A. Future of clay-based construction materials—A review. Constr. Build. Mater. 2019, 210, 172–187. [Google Scholar] [CrossRef]
- Hu, Y.; Tang, Z.; Li, W.G.; Li, Y.N.; Tam, V.W.Y. Physical-mechanical properties of fly ash/GGBFS geopolymer composites with recycled aggregates. Constr. Build. Mater. 2019, 226, 139–151. [Google Scholar] [CrossRef]
- Yang, J.; Xu, L.H.; Wu, H.Q.; Jin, J.; Liu, L. Microstructure and mechanical properties of metakaolin-based geopolymer composites containing high volume of spodumene tailings. Appl. Clay Sci. 2022, 218, 106412. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, K.X.; Wang, J.; Guo, J.J.; Ling, Y.F. Macroscopic and microscopic analyses on mechanical performance of metakaolin/fly ash based geopolymer mortar. J. Clean. Prod. 2021, 294, 126193. [Google Scholar] [CrossRef]
- Frank, C.; Sanjayan, J.G. Effect of pore size distribution on drying shrinking of alkali-activated slag concrete. Cem. Concr. Res. 2000, 30, 1401–1406. [Google Scholar] [CrossRef]
- Thomas, R.J.; Lezama, D.; Peethamparan, S. On drying shrinkage in alkali-activated concrete: Improving dimensional stability by aging or heat-curing. Cem. Concr. Res. 2016, 91, 13–23. [Google Scholar] [CrossRef]
- Shi, C. Strength, pore structure and permeability of alkali-activated slag mortars. Cem. Concr. Res. 1996, 26, 1789–1799. [Google Scholar] [CrossRef]
- Chen, B.; Wang, J.; Zhao, J. Mitigating the Drying Shrinkage and Autogenous Shrinkage of Alkali-Activated Slag by NaAlO2. Materials 2020, 13, 3499. [Google Scholar] [CrossRef]
- Krizan, D.; Zivanovic, B. Effects of dosage and modulus of water glass on early hydration of alkali–slag cements. Cem. Concr. Res. 2002, 32, 1181–1188. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, H.; Cheng, Y.; Huseien, G.F.; Shah, K.W. Shrinkage mechanisms and shrinkage-mitigating strategies of alkali-activated slag composites: A critical review. Constr. Build. Mater. 2022, 318, 125993. [Google Scholar] [CrossRef]
- Lee, N.K.; Abate, S.Y.; Kim, H.K. Use of recycled aggregates as internal curing agent for alkali-activated slag system. Constr. Build. Mater. 2018, 159, 286–296. [Google Scholar] [CrossRef]
- Lee, N.K.; Jang, J.G.; Lee, H.K. Shrinkage characteristics of alkali-activated fly ash/slag paste and mortar at early ages. Cem. Concr. Compos. 2014, 53, 239–248. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Assessing the porosity and shrinkage of alkali activated slag-fly ash composites designed applying a packing model. Constr. Build. Mater. 2016, 119, 175–184. [Google Scholar] [CrossRef]
- Wang, G.; Ma, Y. Drying shrinkage of alkali-activated fly ash/slag blended system. J. Sustain. Cem. Based Mater. 2018, 7, 203–213. [Google Scholar] [CrossRef]
- Huang, D.W.; Chen, P.; Peng, H.; Yang, Y.W.; Yuan, Q.M.; Su, M. A review and comparison study on drying shrinkage prediction between alkali-activated fly ash/slag and ordinary Portland cement. Constr. Build. Mater. 2021, 305, 124760. [Google Scholar] [CrossRef]
- JC/T 603-2004; Standard Test Method for Drying Shinkage of Mortar. Building Material Industry Standards: Beijing, China, 2004.
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). State Administration for Market Regulation; Standardization Administration: Beijing, China, 2021.
- Lin, F.; Peng, J. Water resistance index and test method for gypsum-based materials. China Build. Mater. Sci. Technol. 1995, 4, 5. [Google Scholar]
- Qian, Y. Study on stability and long-term properties of gypsum-based composi cementitious material of high water resistance. Shanxi Archit. 2018, 44, 2. [Google Scholar] [CrossRef]
- Bentz, D.P.; Quenard, D.A.; Garboczi, E.J. Modelling drying shrinkage in reconstructed porous materials: Application to porous Vycor glass. Model. Simul. Mater. Sci. Eng. 1998, 6, 211. [Google Scholar] [CrossRef]
- Fu, Y. Research on the Preparation and Performance of Recycled Brick-Concrete Powder Based Geopolymer. Master’s Thesis, Central South University, Changsha, China, 2022. [Google Scholar]
- Liang, G.; Liu, T.; Li, H.; Wu, K. Shrinkage mitigation, strength enhancement and microstructure improvement of alkali-activated slag/fly ash binders by ultrafine waste concrete powder. Compos. Part B Eng. 2022, 231, 109570. [Google Scholar] [CrossRef]
- Maryam, H.; Aleksandra, R. Shrinkage and strength development of alkali-activated fly ash-slag binary cements. Constr. Build. Mater. 2017, 150, 808–816. [Google Scholar] [CrossRef]
- Li, C.; Zhou, M.; Li, Y.; Zhang, K.; Guo, L. Correlation between Average Elastic Modulus of Solid Waste Coarse Aggregate and Elastic Modulus of Concrete. Mater. Rep. 2023, 1–15. [Google Scholar] [CrossRef]
- Wang, J. Research on the Relationship between Dynamic Elastic Modulus and Compressive Strength of Concrete. Master’s Thesis, Hubei University of Technology, Hubei, China, 2017. [Google Scholar]
- Fang, Y. Study on the Performance of FGD Gypsum Modified by Chemical Admixtures and Inorganic Waterproof Materials. Master’s Thesis, Anhui Jianzhu University, Anhui, China, 2015. [Google Scholar]
- Sun, X. Study on the Properties of Calcined Phosphogypsum-Slag-Alkaline Activator Cementitious Material. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2020. [Google Scholar]
- Shi, C.; Zhang, G.; He, T.; Li, Y. Effects of superplasticizers on the stability and morphology of ettringite. Constr. Build. Mater. 2016, 112, 261–266. [Google Scholar] [CrossRef]
- Das, S.K.; Shrivastava, S. Influence of molarity and alkali mixture ratio on ambient temperature cured waste cement concrete based geopolymer mortar. Constr. Build. Mater. 2021, 301, 124380. [Google Scholar] [CrossRef]
- Ma, H.; Du, E.; Niu, X.; Feng, J. Drying shrinkage characteristics and mechanism primary exploration of MgO-slag mortars. Constr. Build. Mater. 2022, 333, 127416. [Google Scholar] [CrossRef]
- Sanjay, K.; Gábor, M.; Ferenc, K.; Péter, P. Mechanical activation of fly ash and its influence on micro and nano-structural behaviour of resulting geopolymers. Adv. Powder Technol. 2017, 28, 805–813. [Google Scholar] [CrossRef]
- Liu, Y. Study on the State of Phosphorus and Fluorine in Phosphogypsum, Its Migration Pattern and Harmless Treatment. Master’s Thesis, Wuhan Institute of Technology, Wuhan, China, 2022. [Google Scholar]
- Król, M.; Rożek, P.; Chlebda, D.; Mozgawa, W. Influence of alkali metal cations/type of activator on the structure of alkali-activated fly ash—ATR-FTIR studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 33, 33–37. [Google Scholar] [CrossRef]
- Hwang, C.L.; Yehualaw, M.; Vo, D.H.; Huynh, T.P.; Largo, A. Performance evaluation of alkali activated mortar containing high volume of waste brick powder blended with ground granulated blast furnace slag cured at ambient temperature. Constr. Build. Mater. 2019, 223, 657–667. [Google Scholar] [CrossRef]
- Wong, C.L.; Mo, K.H.; Alengaram, U.J.; Poh, Y.S. Mechanical strength and permeation properties of high calcium fly ash-based geopolymer containing recycled brick powder. J. Build. Eng. 2020, 32, 101655. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Liu, X.; Feng, P.; Shen, X.; Wang, H.; Zhao, L.; Mu, S.; Ran, Q.; Miao, C. Advances in the Understanding of Cement Hydrate—Calcium Silicat Hydrate(C-S-H). Mater. Rep. 2021. [Google Scholar] [CrossRef]
- Peng, J.; Lou, Z. Study of the formation mechanism of calcium alumina. J. Chin. Ceram. Soc. 2000, 28, 511–515. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.A.; Valencia, W.G.; Mejia, R. Construction and Demolition Waste (CDW) Recycling—As Both Binder and Aggregates—In Alkali-Activated Materials: A Novel Re-Use Concept. Sustainability 2020, 12, 5775. [Google Scholar] [CrossRef]






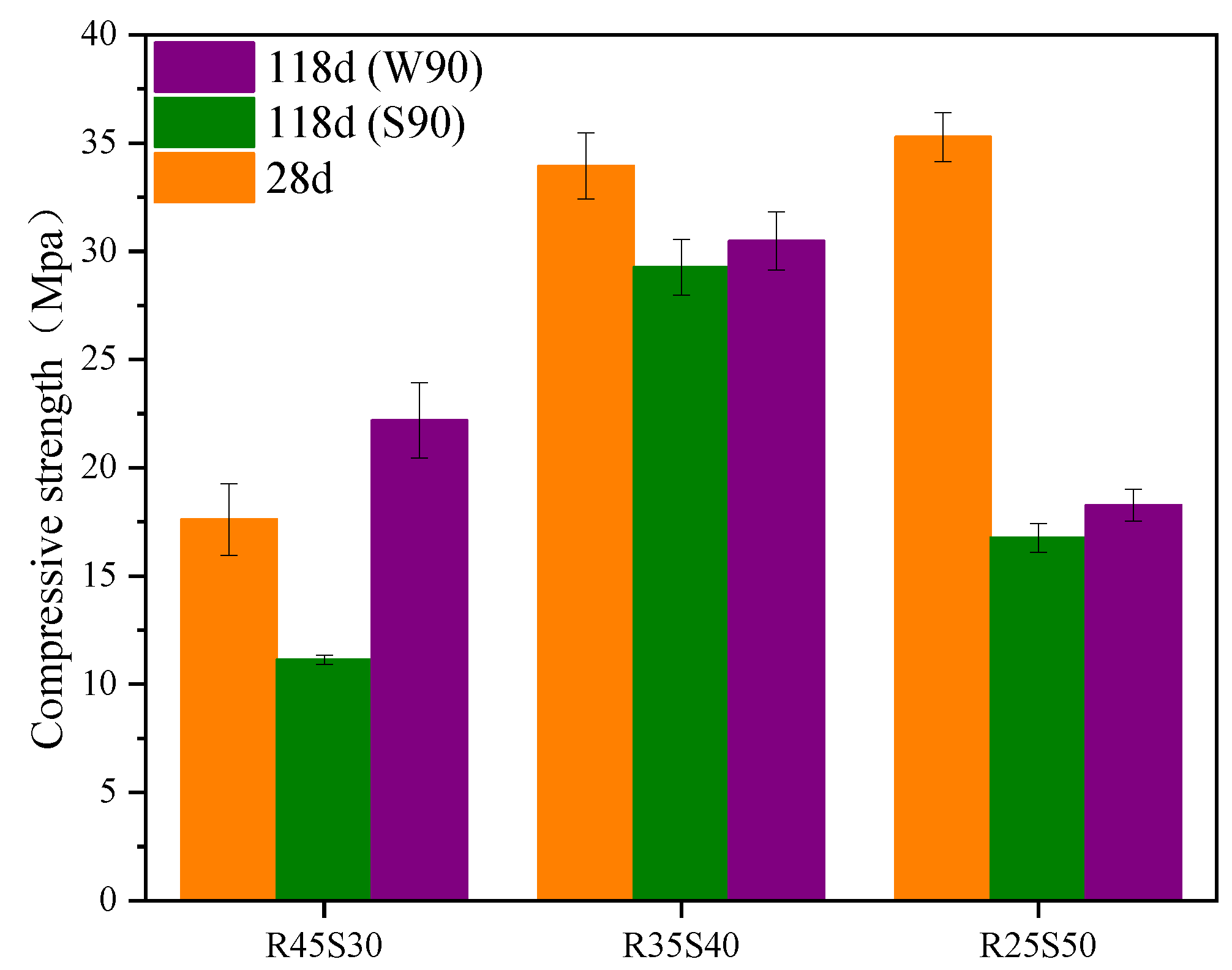


| Others | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4.3 | 0.4 | 33.8 | 41.1 | 1.8 | 0.4 | 1.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 16.7 | |
| 49.4 | 20.2 | 17.3 | 0.9 | / | 4.7 | 0.1 | 1.4 | 1.3 | 0.6 | 0.1 | 2.1 | 1.9 | |
| 31.2 | 8.4 | 38.6 | 0.2 | / | 7.6 | 0.1 | 3.3 | 0.3 | 0.2 | 2.6 | 0.3 | 7.2 |
| Specific Area (m2/kg) | Setting Time (min) | Flexural Strength (Mpa) | Compressive Strength (Mpa) | |||
|---|---|---|---|---|---|---|
| Initial | Final | 3 d | 28 d | 3 d | 28 d | |
| 363 | 161 | 234 | 5.6 | 8.4 | 25.4 | 49.8 |
| Sieve Size (mm) | Residue (g) | Percentage of Residue (%) | Accumulated Residue Percentage (%) | Fineness Modulus |
|---|---|---|---|---|
| 2.36 | 216.82 | 18.8 | 18.8 | 2.96 |
| 1.18 | 157.94 | 13.7 | 32.5 | |
| 0.6 | 331.64 | 28.7 | 61.2 | |
| 0.3 | 317.04 | 27.5 | 88.6 | |
| 0.15 | 73.88 | 6.4 | 95.0 |
| Model | SiO2 Content (wt.%) | Na2O Content (wt.%) | Density (Be °C) | Modulus (M) |
|---|---|---|---|---|
| BE40-6 | 8.5 | 26.5 | 40 | 3.2 |
| Mix ID | Powder Material (wt.%) | Alkali Activator | W c/B d | ||||
|---|---|---|---|---|---|---|---|
| PG | RP | Slag | OPC | Ms a | N b (%) | ||
| OPC | 100 | 0.42 | |||||
| P0R60 | 60 | 40 | 1.3 | 6 | 0.42 | ||
| P15R45 | 15 | 45 | 40 | 1.3 | 6 | 0.42 | |
| P25R35 (R35S40) | 25 | 35 | 40 | 1.3 | 6 | 0.42 | |
| P35R25 | 35 | 25 | 40 | 1.3 | 6 | 0.42 | |
| P60R0 | 60 | 40 | 1.3 | 6 | 0.42 | ||
| R55S20 | 25 | 55 | 20 | 1.3 | 6 | 0.42 | |
| R45S30 | 25 | 45 | 30 | 1.3 | 6 | 0.42 | |
| R25S50 | 25 | 25 | 50 | 1.3 | 6 | 0.42 | |
| Mix ID | Powder Material (wt.%) | a W-R | Alkali Activator | W/B | B/b S | |||
|---|---|---|---|---|---|---|---|---|
| PG | RP | Slag | wt.% | Ms | N (%) | |||
| P35R25 | 35 | 25 | 40 | 1 | 1.3 | 6 | 0.48 | 1:2 |
| P25R35 (R35S40) | 25 | 35 | 40 | 1 | 1.3 | 6 | 0.48 | 1:2 |
| P15R45 | 15 | 45 | 40 | 1 | 1.3 | 6 | 0.48 | 1:2 |
| R45S30 | 25 | 45 | 30 | 1 | 1.3 | 6 | 0.48 | 1:2 |
| R25S50 | 25 | 25 | 50 | 1 | 1.3 | 6 | 0.48 | 1:2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Liu, E. The Synergistic Mechanism and Stability Evaluation of Phosphogypsum and Recycled Fine Powder-Based Multi-Source Solid Waste Geopolymer. Polymers 2023, 15, 2696. https://doi.org/10.3390/polym15122696
Liu X, Liu E. The Synergistic Mechanism and Stability Evaluation of Phosphogypsum and Recycled Fine Powder-Based Multi-Source Solid Waste Geopolymer. Polymers. 2023; 15(12):2696. https://doi.org/10.3390/polym15122696
Chicago/Turabian StyleLiu, Xiaoming, and Erping Liu. 2023. "The Synergistic Mechanism and Stability Evaluation of Phosphogypsum and Recycled Fine Powder-Based Multi-Source Solid Waste Geopolymer" Polymers 15, no. 12: 2696. https://doi.org/10.3390/polym15122696
APA StyleLiu, X., & Liu, E. (2023). The Synergistic Mechanism and Stability Evaluation of Phosphogypsum and Recycled Fine Powder-Based Multi-Source Solid Waste Geopolymer. Polymers, 15(12), 2696. https://doi.org/10.3390/polym15122696







