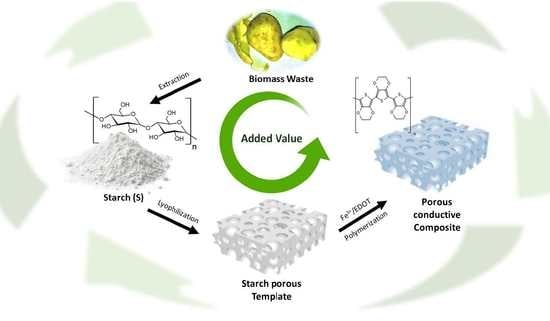
Evaluating the Effect of Iron(III) in the Preparation of a Conductive Porous Composite Using a Biomass Waste-Based Starch Template
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
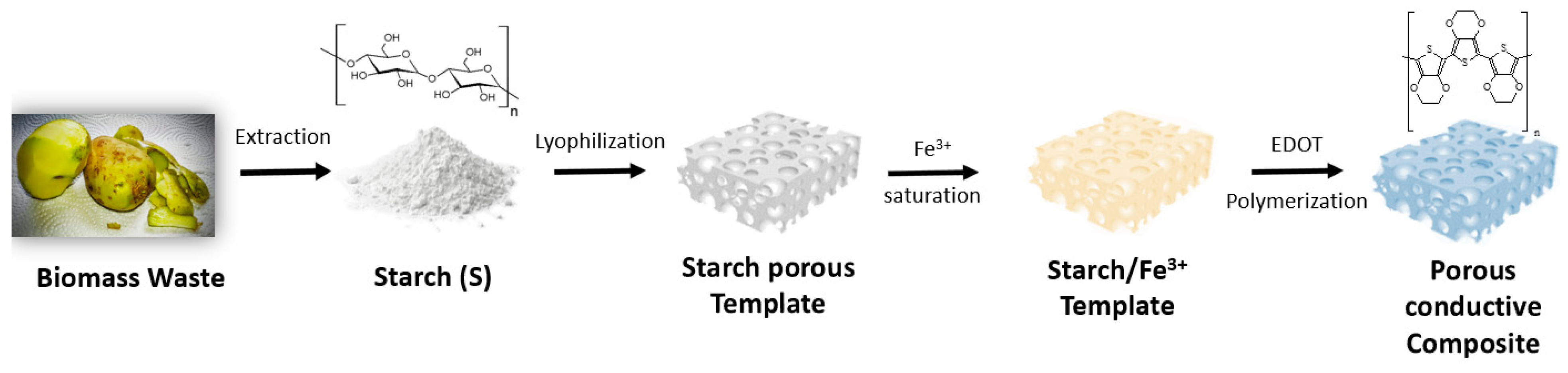
2.2. Starch Isolation from Agro-Industrial Waste Potatoes
2.3. Starch Cryogels Polymerization
2.4. Physical Characterization of the Porous Materials during the Functionalization Process
2.5. Analysis of the Composition of the PEDOT Polymerized in Porous Materials after Different Soak Times in Iron(III)
2.6. Effect of the Starch–Porous Material Iron(III) Soak Time on the Electrical Properties of the PEDOT Polymerized Cryogels
3. Results and Discussion
3.1. Starch Extraction from Biomass Waste and Its Structuration
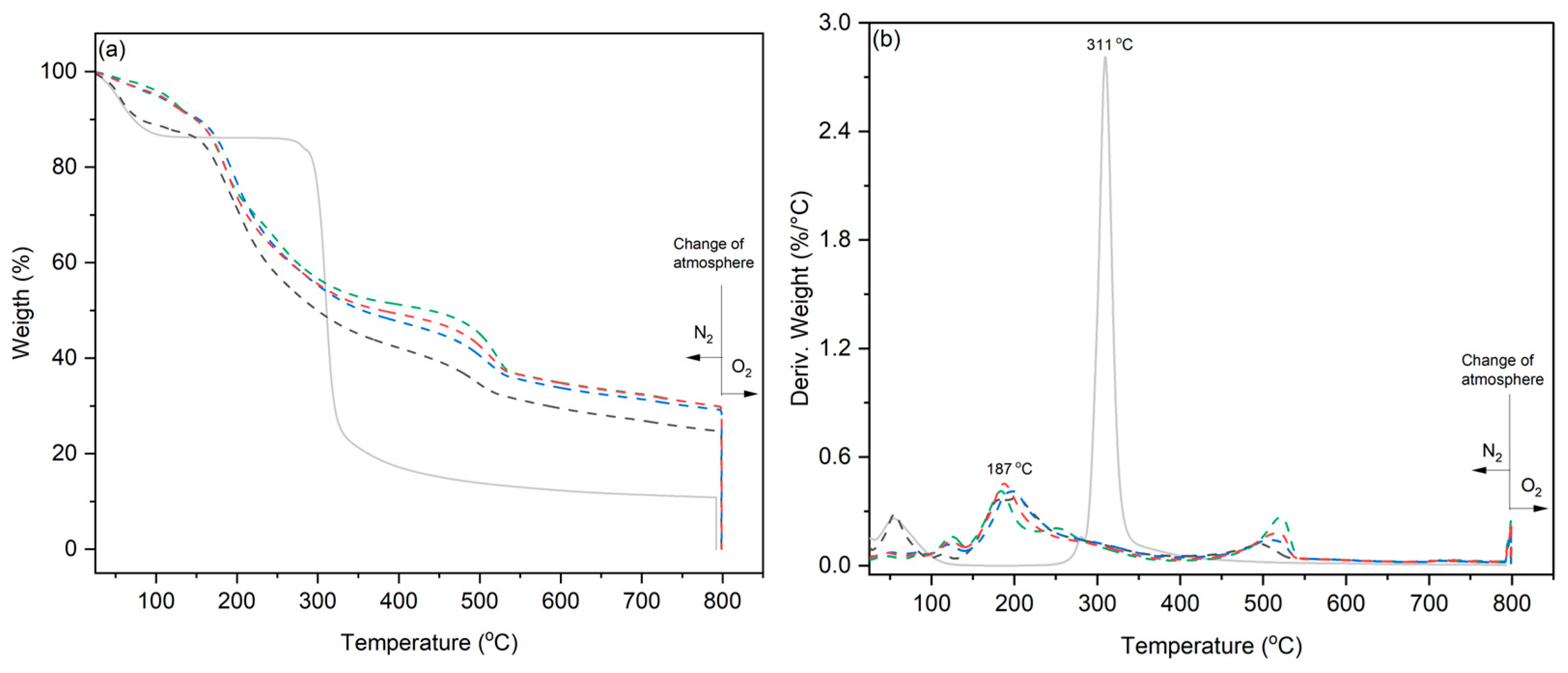
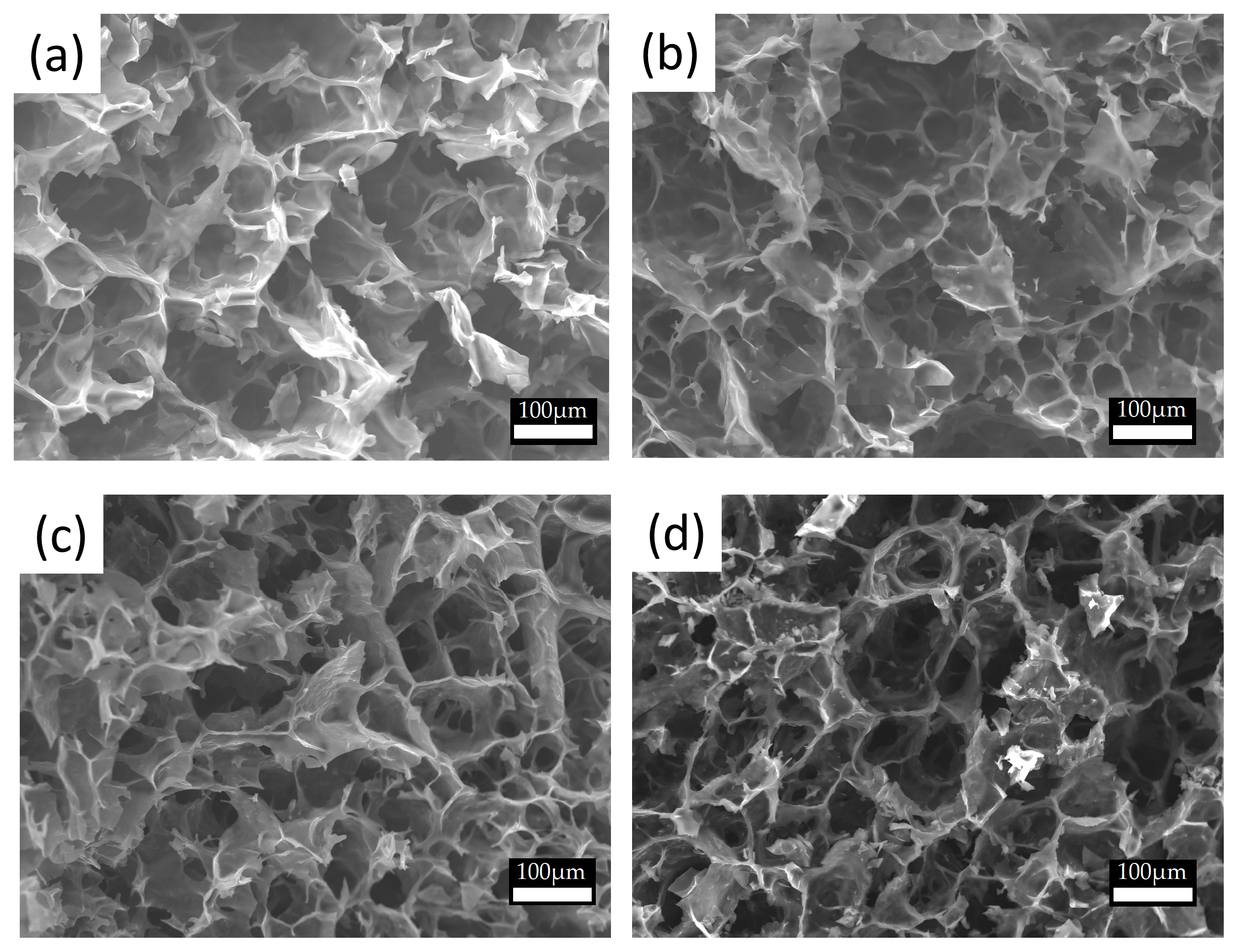
3.2. Characterization of the Starch Template, Iron(III)/Starch, and Conductive Composite Cryogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velvizhi, G.; Balakumar, K.; Shetti, N.P.; Ahmad, E.; Pant, K.K.; Aminabhavi, T.M. Integrated biorefinery processes for conversion of lignocellulosic biomass to value added materials: Paving a path towards circular economy. Bioresour. Technol. 2022, 343, 126151. [Google Scholar] [CrossRef]
- den Hollander, M.C.; Bakker, C.A.; Hultink, E.J. Product Design in a Circular Economy: Development of a Typology of Key Concepts and Terms. J. Ind. Ecol. 2017, 21, 517–525. [Google Scholar] [CrossRef]
- Zhu, F. Starch based aerogels: Production, properties and applications. Trends Food Sci. Technol. 2019, 89, 1–10. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Song, P. Recent advances in polysaccharide-based carbon aerogels for environmental remediation and sustainable energy. Sustain. Mater. Technol. 2021, 27, e00240. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Yan, B.; Sar, T.; Gómez-García, R.; Ren, L.; Sharma, P.; Binod, P.; Sindhu, R.; Kumar, V.; Kumar, D.; et al. Organic waste recycling for carbon smart circular bioeconomy and sustainable development: A review. Bioresour. Technol. 2022, 360, 127620. [Google Scholar] [CrossRef] [PubMed]
- Reyniers, S.; Ooms, N.; Gomand, S.V.; Delcour, J.A. What makes starch from potato (Solanum tuberosum L.) tubers unique: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2588–2612. [Google Scholar] [CrossRef]
- Jagadeesan, S.; Govindaraju, I.; Mazumder, N. An Insight into the Ultrastructural and Physiochemical Characterization of Potato Starch: A Review. Am. J. Potato Res. 2020, 97, 464–476. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y. Recent progress in the conversion of biomass wastes into functional materials for value-added applications. Sci. Technol. Adv. Mater. 2020, 21, 787–804. [Google Scholar] [CrossRef]
- Tharanathan, R.N. Starch-Value addition by modification. Crit. Rev. Food Sci. Nutr. 2005, 45, 371–384. [Google Scholar] [CrossRef]
- Alvarado-Hidalgo, F.; Ramírez-Sánchez, K.; Starbird-Perez, R. Smart porous multi-stimulus polysaccharide-based biomaterials for tissue engineering. Molecules 2020, 25, 5286. [Google Scholar] [CrossRef] [PubMed]
- Quignard, F.; Valentin, R.; Di Renzo, F. Aerogel materials from marine polysaccharides. New J. Chem. 2008, 32, 1300. [Google Scholar] [CrossRef]
- Gizli, N.; Çok, S.S.; Koç, F. Aerogel, xerogel, and cryogel: Synthesis, surface chemistry, and properties—Practical environmental applications and the future developments. In Advanced Materials for Sustainable Environmental Remediation Terrestrial and Aquatic Environments; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–229. [Google Scholar] [CrossRef]
- Starbird, R.; García-González, C.A.; Smirnova, I.; Krautschneider, W.H.; Bauhofer, W. Synthesis of an organic conductive porous material using starch aerogels as template for chronic invasive electrodes. Mater. Sci. Eng. C 2014, 37, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Oetjen, G.W.; Haseley, P. Freeze-Drying: Second, Completely Revised and Extended Edition; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004. [Google Scholar] [CrossRef]
- Baudron, V.; Gurikov, P.; Smirnova, I.; Whitehouse, S. Porous starch materials via supercritical-and freeze-drying. Gels 2019, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.E.; Othman, S.I.; Allam, A.A.; Morsy, O.M. Synthesis, drying process and medical application of polysaccharide-based aerogels. Int. J. Biol. Macromol. 2020, 145, 1115–1128. [Google Scholar] [CrossRef]
- Pham, T.T.H.; Vadanan, S.V.; Lim, S. Enhanced rheological properties and conductivity of bacterial cellulose hydrogels and aerogels through complexation with metal ions and PEDOT/PSS. Cellulose 2020, 27, 8075–8086. [Google Scholar] [CrossRef]
- Ledezma-Espinoza, A.; Rodríguez-Quesada, L.; Araya-Leitón, M.; Avendaño-Soto, E.D.; Starbird-Perez, R. Modified cellulose/poly(3,4-ethylenedioxythiophene) composite as photocatalyst for the removal of sulindac and carbamazepine from water. Environ. Technol. Innov. 2022, 27, 102483. [Google Scholar] [CrossRef]
- Ramírez-Sánchez, K.; Alvarado-Hidalgo, F.; Zamora-Sequeira, R.; Sáenz-Arce, G.; Rojas-Carrillo, O.; Avedaño-Soto, E.; Ruepert, C.; Mena-Torres, F.; Starbird-Pérez, R.; Avendano, E.; et al. Biosensor based on the directly enzyme immobilization into a gold nanotriangles/conductive polymer biocompatible coat for electrochemical detection of Chlorpyrifos in water. Med. Devices Sens. 2019, 2, e10047. [Google Scholar] [CrossRef]
- Zamora-Sequeira, R.; Ardao, I.; Starbird, R.; García-González, C.A.C.A. Conductive nanostructured materials based on poly-(3,4-ethylenedioxythiophene) (PEDOT) and starch/κ-carrageenan for biomedical applications. Carbohydr. Polym. 2018, 189, 304–312. [Google Scholar] [CrossRef]
- Teodor, A.H.; Monge, S.; Aguilar, D.; Tames, A.; Nunez, R.; Gonzalez, E.; Rodríguez, J.J.M.; Bergkamp, J.J.; Starbird, R.; Renugopalakrishnan, V.; et al. PEDOT-Carbon Nanotube Counter Electrodes and Bipyridine Cobalt (II/III) Mediators as Universally Compatible Components in Bio-Sensitized Solar Cells Using Photosystem I and Bacteriorhodopsin. Int. J. Mol. Sci. 2022, 23, 3865. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.M.; Fabretto, M.; Mueller, M.; Molino, P.J.; Griesser, H.J.; Short, R.D.; Wallace, G.G. Cell attachment and proliferation on high conductivity PEDOT-glycol composites produced by vapour phase polymerisation. Biomater. Sci. 2013, 1, 368–378. [Google Scholar] [CrossRef]
- Hernandez-Suarez, P.; Ramirez, K.; Alvarado, F.; Avendano, E.; Starbird, R. Electrochemical characterization of poly(3,4-ethylenedioxythiophene)/κ-carrageenan as a biocompatible conductive coat for biologic applications. MRS Commun. 2019, 9, 218–223. [Google Scholar] [CrossRef]
- Haleem, A.; Chen, S.Q.; Ullah, M.; Siddiq, M.; He, W.D. Highly porous cryogels loaded with bimetallic nanoparticles as an efficient antimicrobial agent and catalyst for rapid reduction of water-soluble organic contaminants. J. Environ. Chem. Eng. 2021, 9, 106510. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Luo, Y. Aligned/unaligned conducting polymer cryogels with three-dimensional macroporous architectures from ice-segregation-induced self-assembly of PEDOT-PSS. Langmuir 2011, 27, 1915–1923. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, T.; Zhou, Y. Recent Advances of Synthesis, Properties, Film Fabrication Methods, Modifications of Poly(3,4-ethylenedioxythiophene), and Applications in Solution-Processed Photovoltaics. Adv. Funct. Mater. 2020, 30, 2006213. [Google Scholar] [CrossRef]
- Maity, S.; Datta, S.; Mishra, M.; Banerjee, S.; Das, S.; Chatterjee, K. Poly(3,4 ethylenedioxythiophene)-tosylate—Its synthesis, properties and various applications. Polym. Adv. Technol. 2021, 32, 1409–1427. [Google Scholar] [CrossRef]
- Rahimzadeh, Z.; Naghib, S.M.; Zare, Y.; Rhee, K.Y. An overview on the synthesis and recent applications of conducting poly(3,4-ethylenedioxythiophene) (PEDOT) in industry and biomedicine. J. Mater. Sci. 2020, 55, 7575–7611. [Google Scholar] [CrossRef]
- Atanasov, S.E.; Losego, M.D.; Gong, B.; Sachet, E.; Maria, J.P.; Williams, P.S.; Parsons, G.N. Highly conductive and conformal poly(3,4-ethylenedioxythiophene) (PEDOT) thin films via oxidative molecular layer deposition. Chem. Mater. 2014, 26, 3471–3478. [Google Scholar] [CrossRef]
- Gharahcheshmeh, M.H.; Robinson, M.T.; Gleason, E.F.; Gleason, K.K. Optimizing the Optoelectronic Properties of Face-On Oriented Poly(3,4-Ethylenedioxythiophene) via Water-Assisted Oxidative Chemical Vapor Deposition. Adv. Funct. Mater. 2021, 31, 2008712. [Google Scholar] [CrossRef]
- Elschner, A.; Kirchmeyer, S.; Lövenich, W.; Merker, U.; Reuter, K. PEDOT: Principles and Applications of an Intrinsically Conductive Polymer; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Cho, M.S.; Kim, S.Y.; Nam, J.D.; Lee, Y. Preparation of PEDOT/Cu composite film by in situ redox reaction between EDOT and copper(II) chloride. Synth. Met. 2008, 158, 865–869. [Google Scholar] [CrossRef]
- Ali, M.A.; Kim, H.H.; Lee, C.Y.; Soh, H.S.; Lee, J.G. Effects of the FeCl3 concentration on the polymerization of conductive poly(3,4-ethylenedioxythiophene) thin films on (3-aminopropyl) trimethoxysilane monolayer-coated SiO2 surfaces. Met. Mater. Int. 2009, 15, 977–981. [Google Scholar] [CrossRef]
- Ali, M.A.; Kim, H.; Lee, C.; Nam, H.; Lee, J. Effects of iron(III) p-toluenesulfonate hexahydrate oxidant on the growth of conductive poly(3,4-ethylenedioxythiophene) (PEDOT) nanofilms by vapor phase polymerization. Synth. Met. 2011, 161, 1347–1352. [Google Scholar] [CrossRef]
- Zhao, Q.; Jamal, R.; Zhang, L.; Wang, M.; Abdiryim, T. The structure and properties of PEDOT synthesized by template-free solution method. Nanoscale Res. Lett. 2014, 9, 557. [Google Scholar] [CrossRef]
- Gyurcsik, B.; Nagy, L. Carbohydrates as ligands: Coordination equilibria and structure of the metal complexes. Coord. Chem. Rev. 2000, 203, 81–149. [Google Scholar] [CrossRef]
- Veiga, V.; Ryan, D.H.; Sourty, E.; Llanes, F.; Marchessault, R.H. Formation and characterization of superparamagnetic cross-linked high amylose starch. Carbohydr. Polym. 2000, 42, 353–357. [Google Scholar] [CrossRef]
- Somsook, E.; Hinsin, D.; Buakhrong, P.; Teanchai, R.; Mophan, N.; Pohmakotr, M.; Shiowatana, J. Interactions between iron(III) and sucrose, dextran, or starch in complexes. Carbohydr. Polym. 2005, 61, 281–287. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Q.; Gao, L.; Gong, X.; Qu, Y.; Feng, B. Functional and physicochemical properties of flours and starches from different tuber crops. Int. J. Biol. Macromol. 2020, 148, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Akhila, P.P.; Sunooj, K.V.; Aaliya, B.; Navaf, M.; Sudheesh, C.; Yadav, D.N.; Khan, M.A.; Mir, S.A.; George, J. Morphological, physicochemical, functional, pasting, thermal properties and digestibility of hausa potato (Plectranthus rotundifolius) flour and starch. Appl. Food Res. 2022, 2, 100193. [Google Scholar] [CrossRef]
- Santos-Rosales, V.; Alvarez-Rivera, G.; Hillgärtner, M.; Cifuentes, A.; Itskov, M.; García-González, C.A.; Rege, A. Stability Studies of Starch Aerogel Formulations for Biomedical Applications. Biomacromolecules 2020, 21, 5336–5344. [Google Scholar] [CrossRef]
- Altemimi, A.B. Extraction and optimization of potato starch and its application as a stabilizer in yogurt manufacturing. Foods 2018, 7, 14. [Google Scholar] [CrossRef]
- Chen, X.; Huang, G. Synthesis and antioxidant activities of garlic polysaccharide-Fe(III) complex. Int. J. Biol. Macromol. 2020, 145, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Liu, T.T.; Liu, H.; Fan, H.; Chen, B.; Wang, D.; Zhang, Y.; Sun, F. Preparation and Characterization of a Novel Polysaccharide-Iron(III) Complex in Auricularia auricula Potentially Used as an Iron Supplement. Biomed Res. Int. 2019, 2019, 6416941. [Google Scholar] [CrossRef]
- Shivaraju, V.K.; Appukuttan, S.V.; Kumar, S.K.S. The Influence of Bound Water on the FTIR Characteristics of Starch and Starch Nanocrystals Obtained from Selected Natural Sources. Starch/Staerke 2019, 71, 1700026. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, S.; Li, M.; Zhang, R.; Zhang, H.; Zheng, Y.; Zhang, D.; Wu, L. Structural characterization and biological activities of polysaccharide iron complex synthesized by plant polysaccharides: A review. Front. Nutr. 2022, 9, 1013067. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, H.; Wang, Y.; Xing, L. Synthesis and characterization of a new Inonotus obliquus polysaccharide-iron(III) complex. Int. J. Biol. Macromol. 2015, 75, 210–217. [Google Scholar] [CrossRef]
- Gao, X.; Qu, H.; Gao, Z.; Zeng, D.; Wang, J.; Baranenko, D.; Li, Y.; Lu, W. Protective effects of Ulva pertusa polysaccharide and polysaccharide-iron (III) complex on cyclophosphamide induced immunosuppression in mice. Int. J. Biol. Macromol. 2019, 133, 911–919. [Google Scholar] [CrossRef]
- Sánchez, K.R.; Ledezma-Espinoza, A.; Sánchez-Kopper, A.; Avendaño-Soto, E.; Prado, M.; Perez, R.S. Polysaccharide κ-carrageenan as doping agent in conductive coatings for electrochemical controlled release of dexamethasone at therapeutic doses. Molecules 2020, 25, 2139. [Google Scholar] [CrossRef]
- García-González, C.A.; Smirnova, I. Use of supercritical fluid technology for the production of tailor-made aerogel particles for delivery systems. J. Supercrit. Fluids 2013, 79, 152–158. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, Y.; Tong, Z.; Zhu, Y.; Cao, K.; Chen, W.; Zhao, D.; Yu, H. Micro-interfacial polymerization of porous PEDOT for printable electronic devices. EcoMat 2022, 5, e12288. [Google Scholar] [CrossRef]
- Imberty, A.; Chanzy, H.; Pérez, S.; Bulèon, A.; Tran, V. The double-helical nature of the crystalline part of A-starch. J. Mol. Biol. 1988, 201, 365–378. [Google Scholar] [CrossRef]
- Niu, L.; Kvarnström, C.; Fröberg, K.; Ivaska, A. Electrochemically controlled surface morphology and crystallinity in poly(3,4-ethylenedioxythiophene) films. Synth. Met. 2001, 122, 425–429. [Google Scholar] [CrossRef]
- Liu, P.; Ma, C.; Li, Y.; Wang, L.; Wei, L.; Yan, Y.; Xie, F. Facile Preparation of Eco-Friendly, Flexible Starch-Based Materials with Ionic Conductivity and Strain-Responsiveness. ACS Sustain. Chem. Eng. 2020, 8, 19117–19128. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Z.; Wang, Y.; Guo, L.; Yin, C.; Zhang, X.; Hao, J.; Zhang, G.; Chen, L. Eco-Friendly, Self-Healing Hydrogels for Adhesive and Elastic Strain Sensors, Circuit Repairing, and Flexible Electronic Devices. Macromolecules 2019, 52, 2531–2541. [Google Scholar] [CrossRef]
- Chen, C.C.; Huang, B.C.; Lin, M.S.; Lu, Y.J.; Cho, T.Y.; Chang, C.H.; Tien, K.C.; Liu, S.H.; Ke, T.H.; Wu, C.C. Impedance spectroscopy and equivalent circuits of conductively doped organic hole-transport materials. Org. Electron. Phys. Mater. Appl. 2010, 11, 1901–1908. [Google Scholar] [CrossRef]

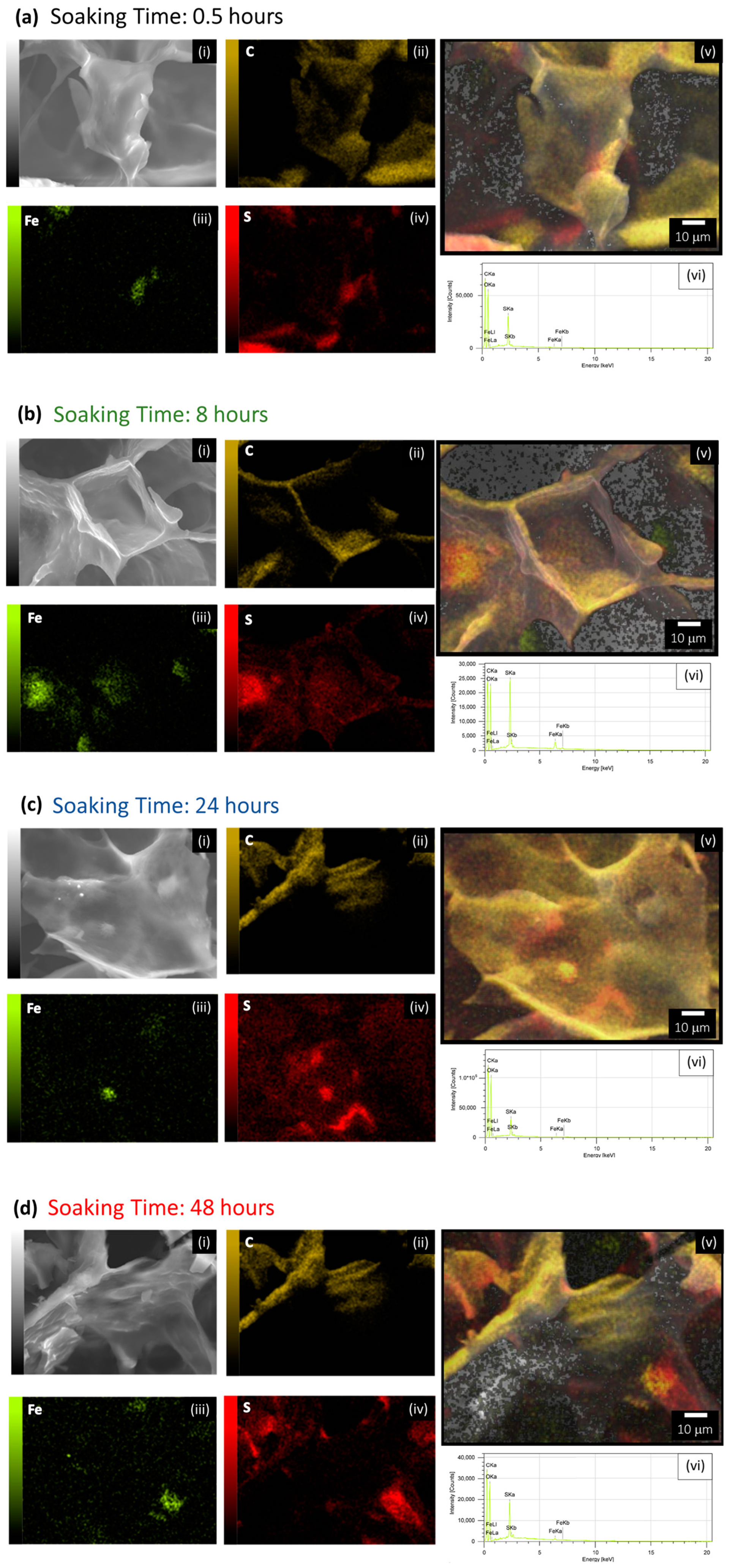
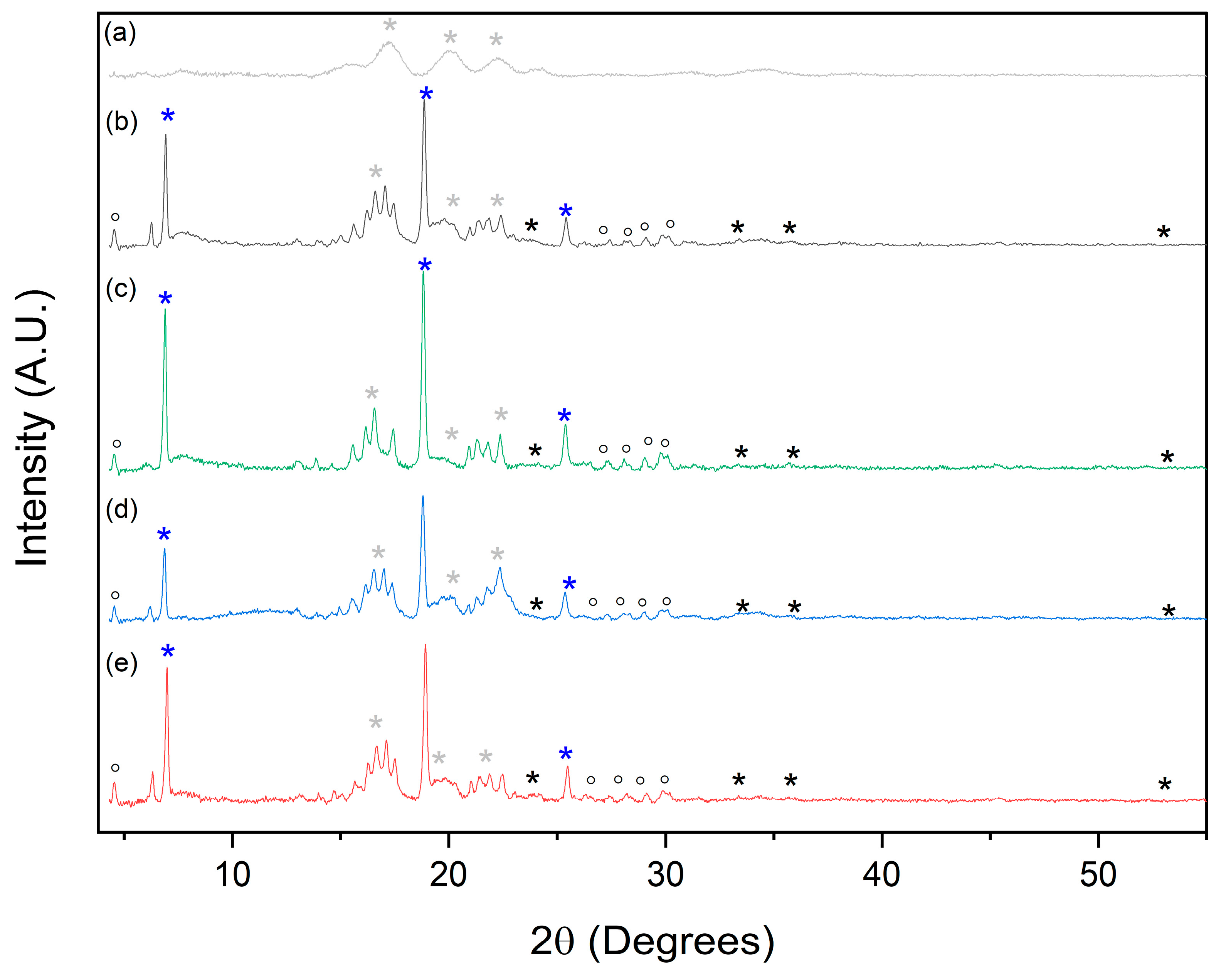
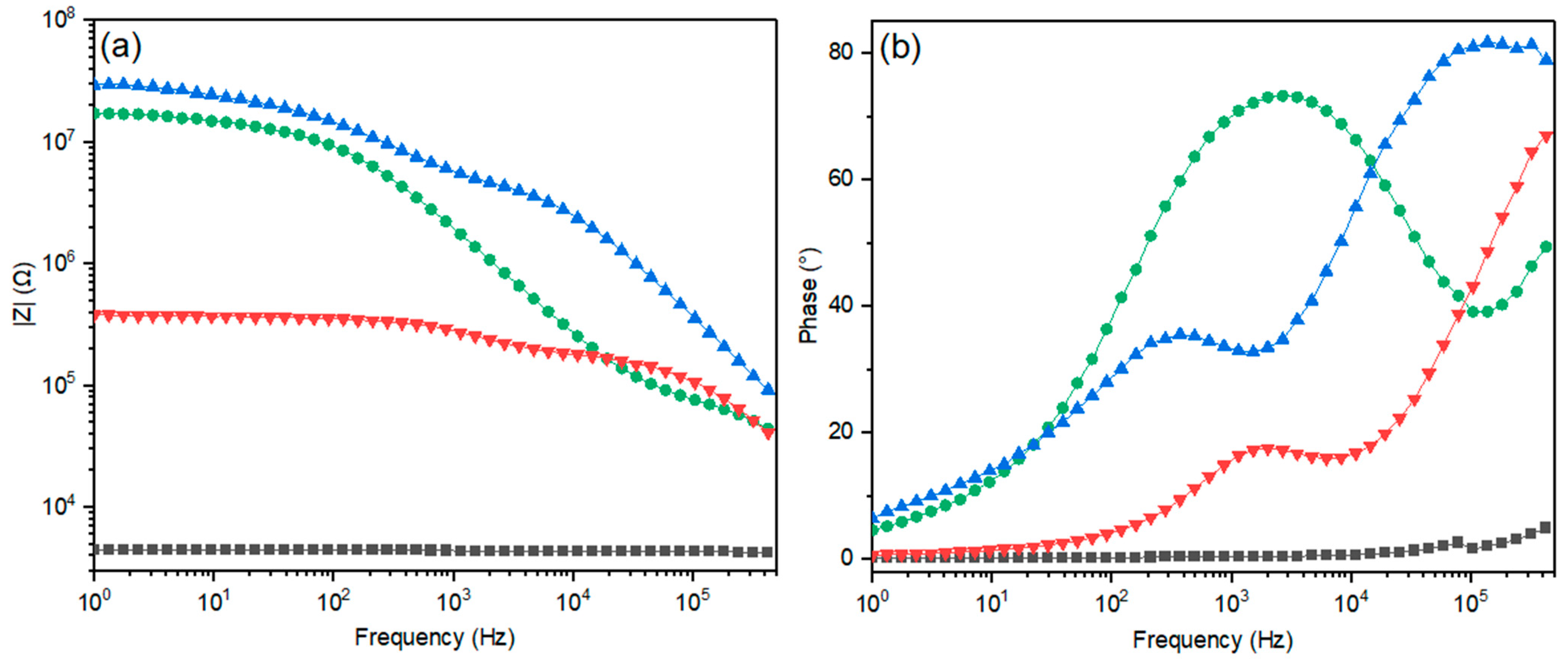
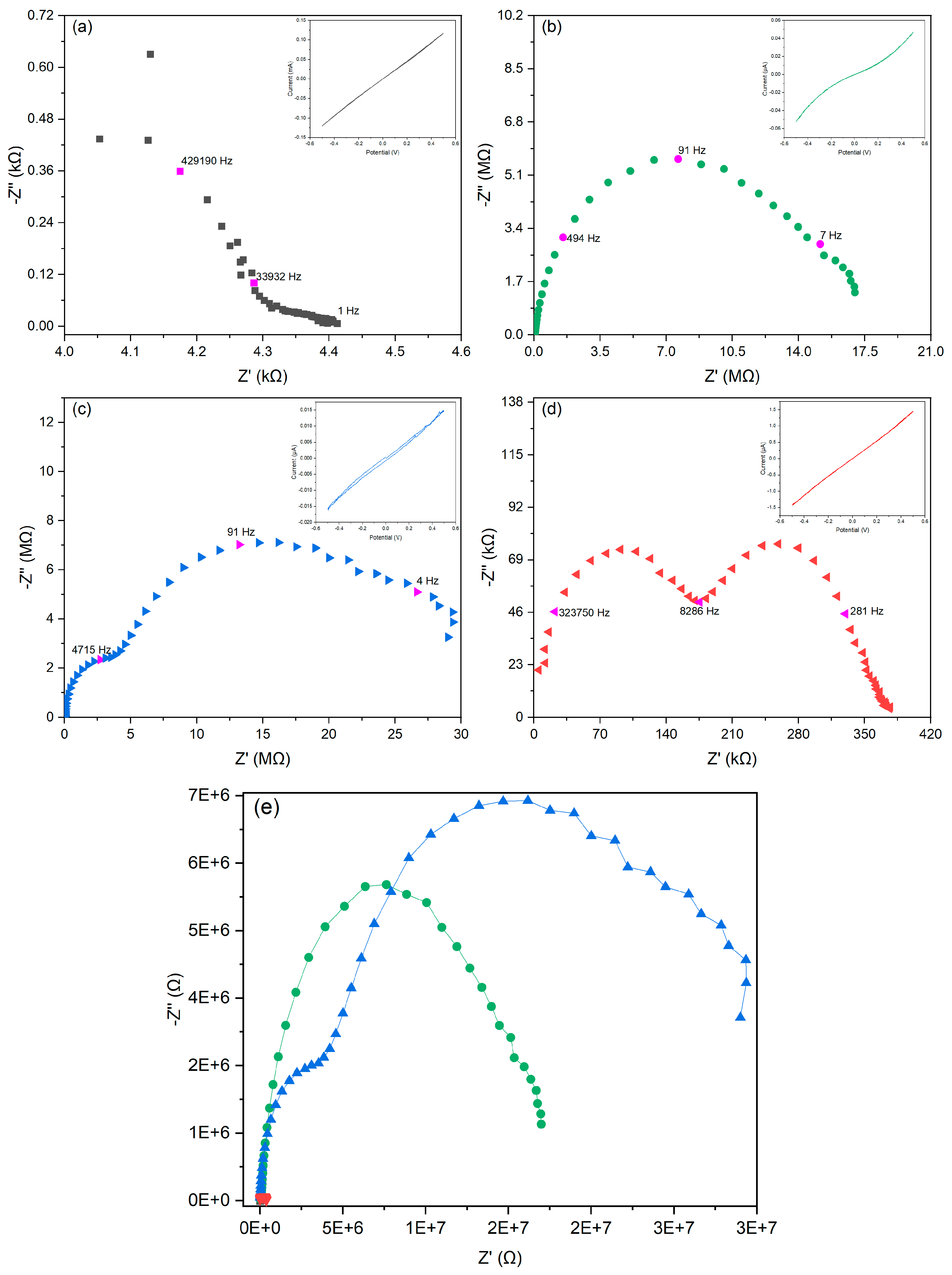
| Conductive Cryogels at Different Soak Time in Iron(III) Solution (h) | Sample Ashes (%) | ||
|---|---|---|---|
| Iron(III) Absorption | Total Residues Obtained after PEDOT Synthesis | PEDOT Obtained in the Structure | |
| 0.5 | 24.24 | 27.13 | 2.89 |
| 8 | 28.22 | 34.04 | 5.82 |
| 24 | 29.12 | 35.70 | 6.58 |
| 48 | 29.57 | 36.12 | 6.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Quesada, L.; Ramírez-Sánchez, K.; León-Carvajal, S.; Sáenz-Arce, G.; Vásquez-Sancho, F.; Avendaño-Soto, E.; Montero-Rodríguez, J.J.; Starbird-Perez, R. Evaluating the Effect of Iron(III) in the Preparation of a Conductive Porous Composite Using a Biomass Waste-Based Starch Template. Polymers 2023, 15, 2560. https://doi.org/10.3390/polym15112560
Rodríguez-Quesada L, Ramírez-Sánchez K, León-Carvajal S, Sáenz-Arce G, Vásquez-Sancho F, Avendaño-Soto E, Montero-Rodríguez JJ, Starbird-Perez R. Evaluating the Effect of Iron(III) in the Preparation of a Conductive Porous Composite Using a Biomass Waste-Based Starch Template. Polymers. 2023; 15(11):2560. https://doi.org/10.3390/polym15112560
Chicago/Turabian StyleRodríguez-Quesada, Laria, Karla Ramírez-Sánchez, Sebastián León-Carvajal, Giovanni Sáenz-Arce, Fabián Vásquez-Sancho, Esteban Avendaño-Soto, Juan José Montero-Rodríguez, and Ricardo Starbird-Perez. 2023. "Evaluating the Effect of Iron(III) in the Preparation of a Conductive Porous Composite Using a Biomass Waste-Based Starch Template" Polymers 15, no. 11: 2560. https://doi.org/10.3390/polym15112560
APA StyleRodríguez-Quesada, L., Ramírez-Sánchez, K., León-Carvajal, S., Sáenz-Arce, G., Vásquez-Sancho, F., Avendaño-Soto, E., Montero-Rodríguez, J. J., & Starbird-Perez, R. (2023). Evaluating the Effect of Iron(III) in the Preparation of a Conductive Porous Composite Using a Biomass Waste-Based Starch Template. Polymers, 15(11), 2560. https://doi.org/10.3390/polym15112560