Development of a Novel Surfactant-Based Viscoelastic Fluid System as an Alternative Nonpolymeric Fracturing Fluid and Comparative Analysis with Traditional Guar Gum Gel Fluid
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Type 3 Fluids without Nanoparticle Additives
2.2. Preparation of Type 4 Fluid with Nanoparticle Additives
2.3. Preparation of Guar Gum Fluid Gel
3. Rheometric Analysis and Observations
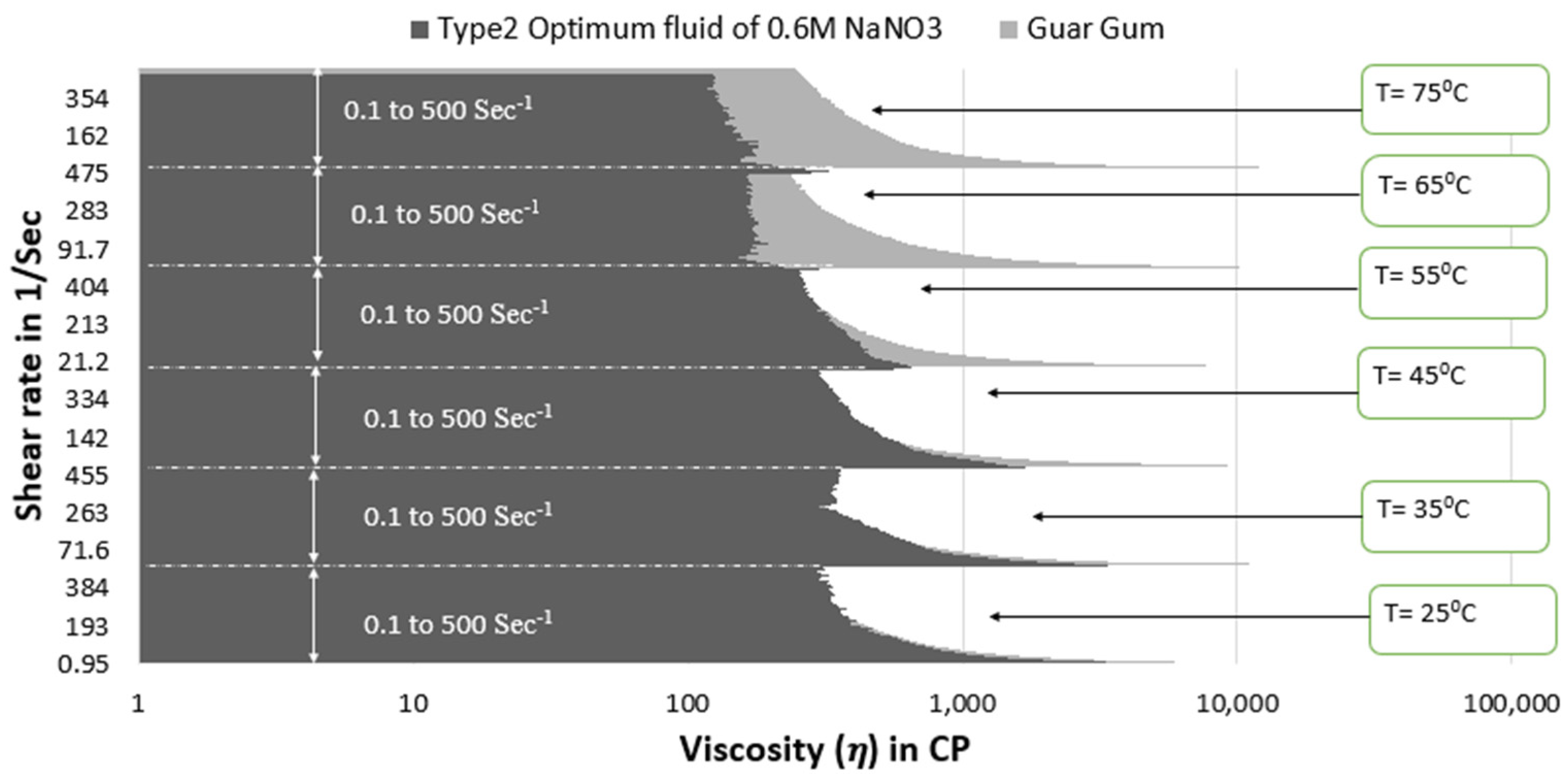
3.1. Rheometric Analysis of Guar Gum Gel Fluid
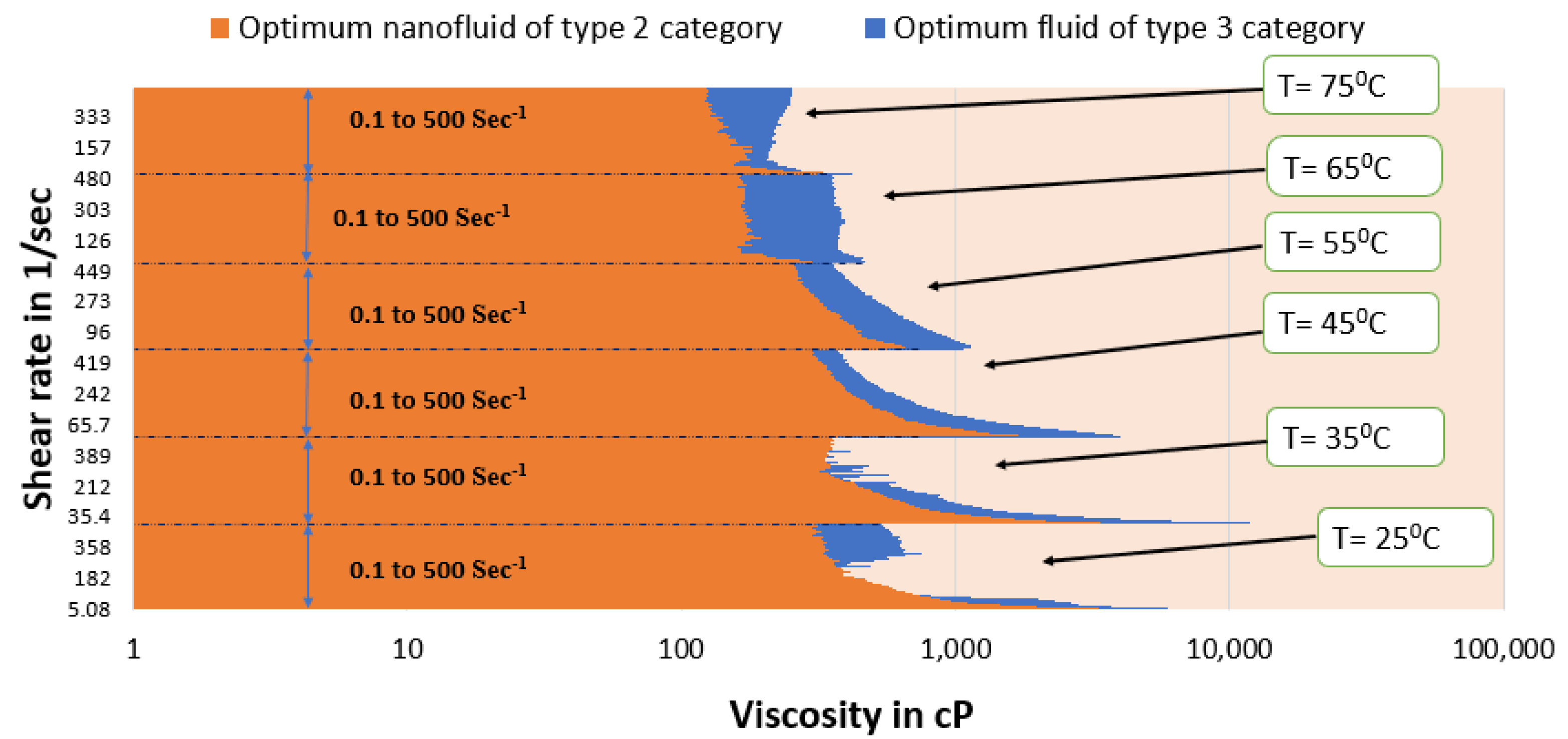
3.2. Rheometric Analysis of Type 3 Fluids and Optimization of Fluid Concentration
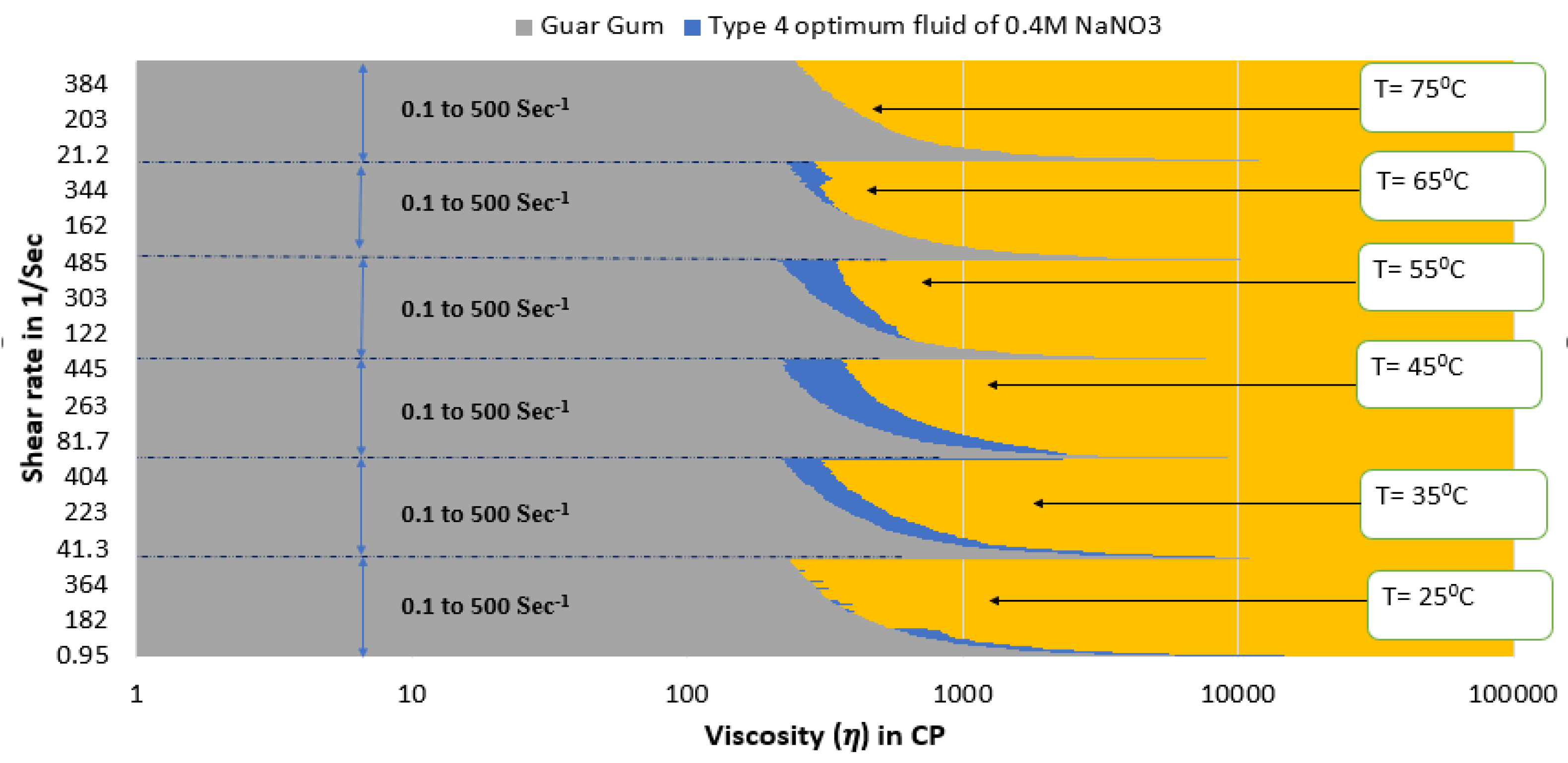
3.3. Rheometric Analysis of Type 4 Fluids and Optimization of Concentration
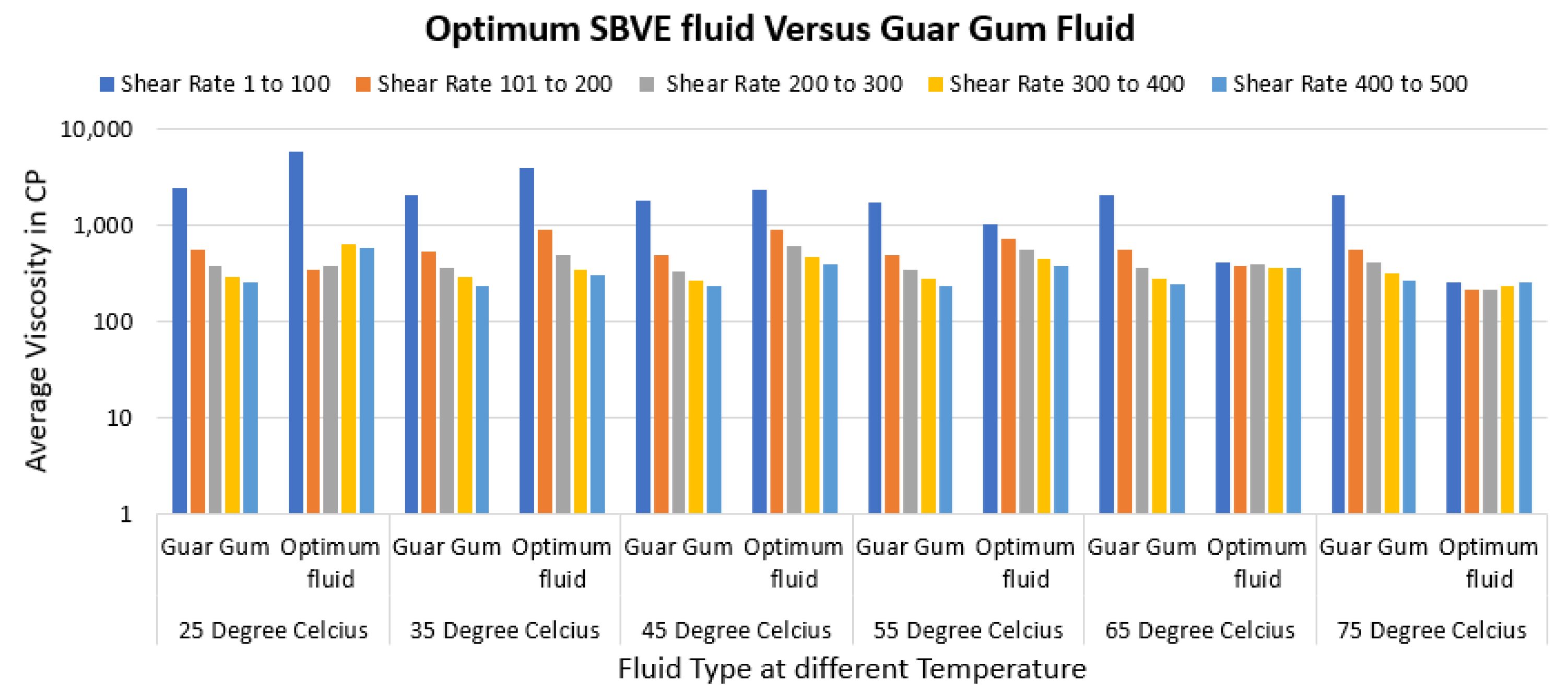
3.4. Comparative Analysis of Optimal SBVE Fluids to Guar Gum Gel Fluid
3.5. Comparative Analysis of All Optimum Fluids to Finding the Overall Optimum Fluid
4. Discussion
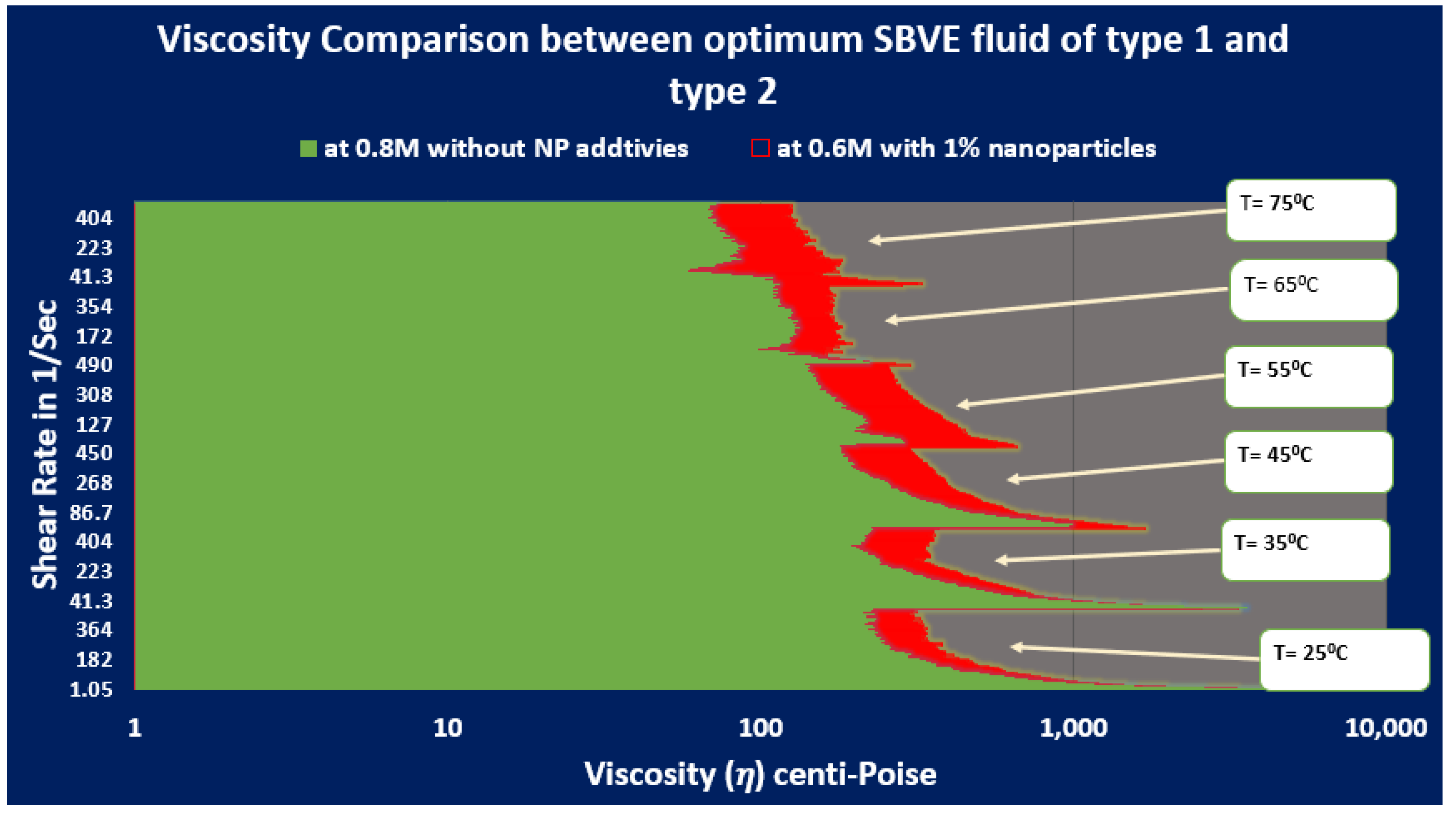
- The low-surfactant optimum SBVE fluids of types 1 and 2 cannot replace guar gum, showing poor rheology under elevated shear rate and temperature (ESRT) conditions.
- The high-surfactant SBVE fluids (type 3) and nanofluids (type 4) of 0.2 M CTAB concentration show optimal rheology when prepared at 1.2 M and 0.4 M counter-ion sodium concentration, respectively.
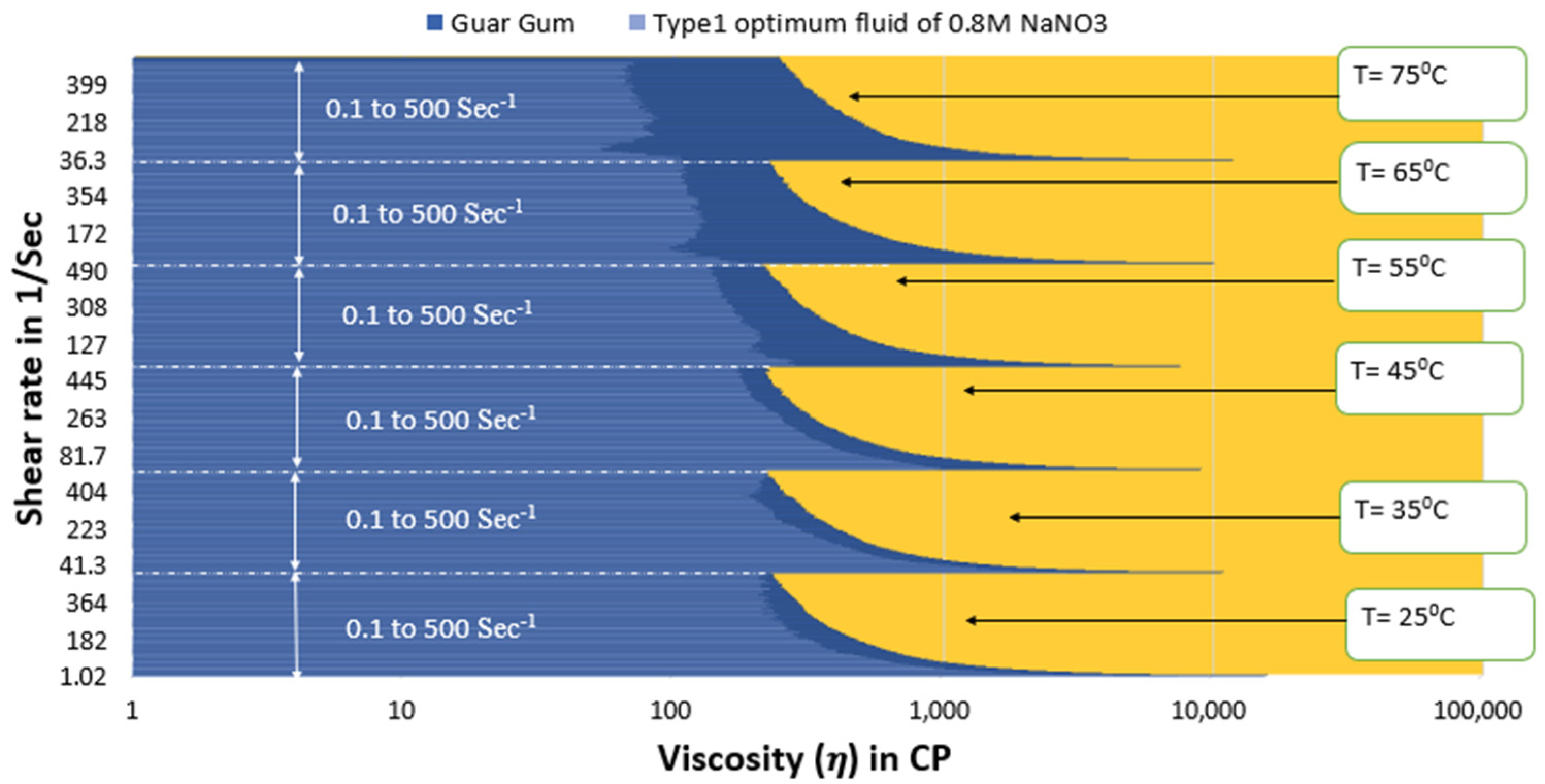
- The optimal type 3 fluid and type 4 nanofluid show better viscosity than guar gum gel fluid up to 55 °C under all conditions.
- The guar gum fluid shows similar viscosity values under initial shear rate conditions (up to 150 s−1 shear rate conditions) at 65 °C to both optimum SBVE fluid and nanofluid. However, the optimum fluid and nanofluid show better viscosity under high shear rate conditions.
- The guar gum fluid shows better viscosities at 75 °C temperature when compared to both the optimum fluid and nanofluid under low shear rate conditions.
- The optimum type 4 nanofluid shows poorer rheology than guar gum fluid under high shear rate conditions; however, the type 3 optimum fluid shows similar viscosities to guar gum at high shear rate conditions at 75 °C.
- The type 3 optimum fluid shows better viscosity for the entire shear rate range, even under high-temperature conditions, than optimum fluids of type 1, type 2, and type 4. Therefore, the synthesized SBVE fluid of 0.2 M cetyltrimethylammonium bromide and 1.2 M sodium nitrate is the best out of all fluids prepared in this study. In other words, it is the overall optimum fluid.
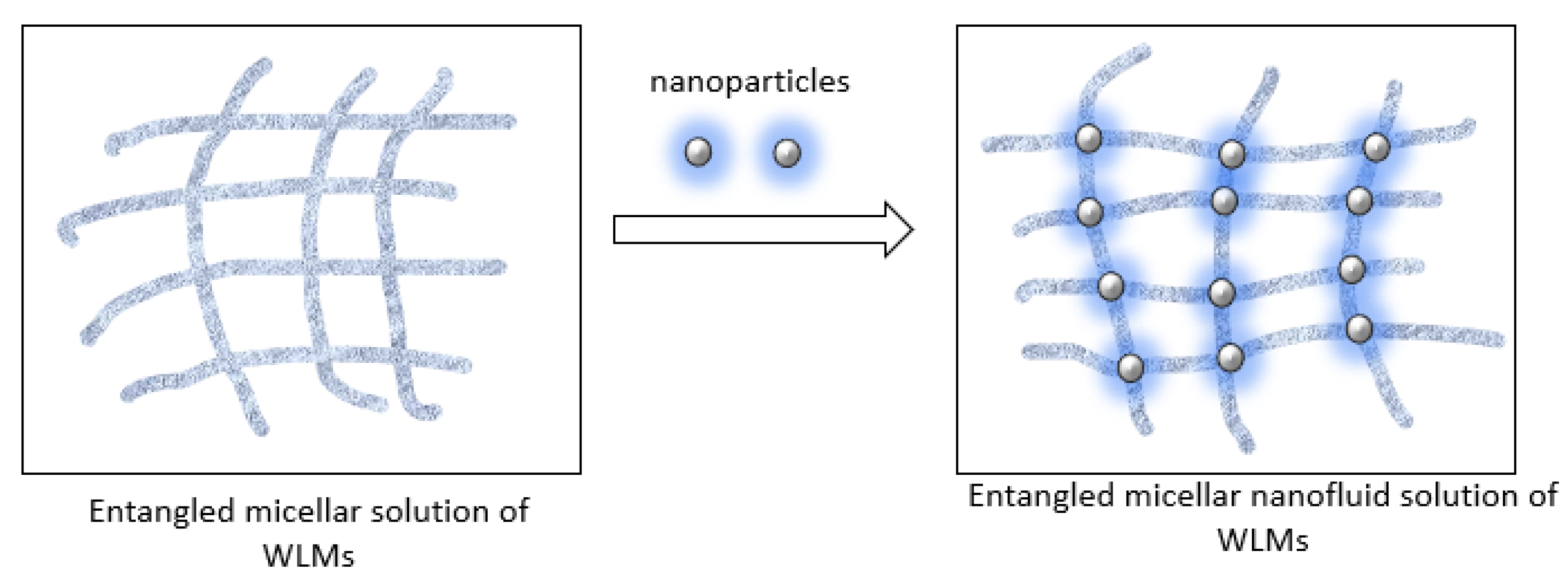
- As reported previously, ZnO NPs additives can improve the rheological characteristics of SBVE fluids of low surfactant concentration. However, this part of the study of high surfactant SBVE fluids indicates that SBVE optimum fluid in the category with no nano-additives (type 3) shows better rheology than the optimum nanofluid prepared with the addition of 1 wt% ZnO nano dispersion (type 4).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, F.; Sayed, M.; Al-Muntasheri, G.A.; Chang, F.F.; Li, L. A comprehensive review on proppant technologies. Petroleum 2016, 2, 26–39. [Google Scholar] [CrossRef]
- Philippova, O.E.; Molchanov, V.S. Enhanced rheological properties and performance of viscoelastic surfactant fluids with embedded nanoparticles. Curr. Opin. Colloid Interface Sci. 2019, 43, 52–62. [Google Scholar] [CrossRef]
- Economides, M.J.; Nolte, K. Reservoir Stimulation, 3rd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2000; Available online: https://www.researchgate.net/file.PostFileLoader.html?id=591b038148954c7bac0eeb2d&assetKey=AS%3A494639238529025%401494942593011 (accessed on 13 April 2023).
- Armstrong, K.; Card, R.; Navarrete, R.; Erik, N. Advanced Fracturing Fluids Improve Well Economics, Schlumberger. January 1995. Available online: https://www.slb.com/resource-library/industry-article/co/advanced-fracturing-fluids-improve-well-economics (accessed on 13 April 2023).
- Gaurina-Međimurec, N.; Brkić, V.; Topolovec, M.; Mijić, P. Fracturing Fluids and Their Application in the Republic of Croatia. Appl. Sci. 2021, 11, 2807. [Google Scholar] [CrossRef]
- Samuel, M.M.; Card, R.J.; Nelson, E.B.; Brown, J.E.; Vinod, P.S.; Temple, H.L.; Qu, Q.; Fu, D.K. Polymer-Free Fluid for Fracturing Applications. SPE Drill. Complet. 1999, 14, 240–246. [Google Scholar] [CrossRef]
- Hasan, A.M.; Abdel-Raouf, M.E. Applications of guar gum and its derivatives in petroleum industry: A review. Egypt. J. Pet. 2018, 27, 1043–1050. [Google Scholar] [CrossRef]
- Asmorowati, D.; Kristanto, D.; Helmy, M.; Yudha, F. Compatibility of Guar Gum-Based Fracturing Fluid and Breaker Due to Residue and Proppant Carrying Performance. Int. J. Oil Gas Coal Eng. 2022, 10, 115–120. [Google Scholar] [CrossRef]
- Patel, M.C.; Singh, A. Near Wellbore Damage and Types of Skin Depending on Mechanism of Damage, SPE-179011-MS. In Proceedings of the SPE International Conference & Exhibition on Formation Damage Control, Lafayette, LA, USA, 24–26 February 2016. [Google Scholar] [CrossRef]
- Ghosh, B.; Abdelrahim, M.; Ghosh, D.; Belhaj, H. Delayed Breaker Systems To Remove Residual Polymer Damage in Hydraulically Fractured Reservoirs. ACS Omega 2021, 6, 31646–31657. [Google Scholar] [CrossRef]
- Chu, Z.; Dreiss, C.A.; Feng, Y. Smart wormlike micelles. Chem. Soc. Rev. 2013, 42, 7174–7203. [Google Scholar] [CrossRef]
- Flood, C.; Dreiss, C.A.; Croce, V.; Cosgrove, T.; Karlsson, G. Wormlike Micelles Mediated by Polyelectrolyte. Langmuir 2005, 21, 7646–7652. [Google Scholar] [CrossRef]
- Wheeler, E.K.; Izu, P.; Fuller, G.G. Structure and rheology of wormlike micelles. Rheol. Acta 1996, 35, 139–149. [Google Scholar] [CrossRef]
- Yang, J. Viscoelastic wormlike micelles and their applications. Curr. Opin. Colloid Interface Sci. 2002, 7, 276–281. [Google Scholar] [CrossRef]
- Candau, S.J.; Khatory, A.; Lequeux, F.; Kern, F. Rheological behaviour of wormlike micelles: Effect of salt content. J. Phys. IV 1993, 3, C1–C197. [Google Scholar] [CrossRef]
- Fanzatovich, I.I.; Aleksandrovich, K.D.; Rinatovich, I.A.; Evna, B.N.Y.; Yarullovna, Z.L.; Valerevich, Z.S.; Rashidovna, A.M.; Evgenevna, K.N. Supramolecular system based on cylindrical micelles of anionic surfactant and silica nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2016, 507, 255–260. [Google Scholar] [CrossRef]
- Cates, M. Dynamics of living polymers and flexible surfactant micelles: Scaling laws for dilution. J. Phys. 1988, 49, 1593–1600. [Google Scholar] [CrossRef]
- Turner, M.S.; Cates, M.E. Linear viscoelasticity of living polymers: A quantitative probe of chemical relaxation times. Langmuir 1991, 7, 1590–1594. [Google Scholar] [CrossRef]
- Kuperkar, K.; Abezgauz, L.; Danino, D.; Verma, G.; Hassan, P.; Aswal, V.; Varade, D.; Bahadur, P. Viscoelastic micellar water/CTAB/NaNO3 solutions: Rheology, SANS and cryo-TEM analysis. J. Colloid Interface Sci. 2008, 323, 403–409. [Google Scholar] [CrossRef]
- Jiang, N.; Li, P.; Wang, Y.; Wang, J.; Yan, H.; Thomas, R.K. Aggregation behavior of hexadecyltrimethylammonium surfactants with various counterions in aqueous solution. J. Colloid Interface Sci. 2005, 286, 755–760. [Google Scholar] [CrossRef]
- Dong, B.; Zhang, J.; Zheng, L.; Wang, S.; Li, X.; Inoue, T. Salt-induced viscoelastic wormlike micelles formed in surface active ionic liquid aqueous solution. J. Colloid Interface Sci. 2008, 319, 338–343. [Google Scholar] [CrossRef]
- Suja, V.C.; Kannan, A.; Kubicka, B.A.; Hadidi, A.; Fuller, G.G. Bubble Coalescence at Wormlike Micellar Solution–Air Interfaces. Langmuir 2020, 36, 11836–11844. [Google Scholar] [CrossRef]
- Dreiss, C.A.; Feng, Y. Wormlike Micelles: Advances in Systems, Characterisation and Applications; Royal Society of Chemistry: Cambridge, UK, 2017; pp. 23–30. [Google Scholar] [CrossRef]
- Perween, S.; Beg, M.; Shankar, R.; Sharma, S.; Ranjan, A. Effect of zinc titanate nanoparticles on rheological and filtration properties of water based drilling fluids. J. Pet. Sci. Eng. 2018, 170, 844–857. [Google Scholar] [CrossRef]
- Fakoya, M.F.; Shah, S.N. SPE 163921 Rheological Properties of Surfactant-Based and Polymeric Nano-Fluids. In Proceedings of the SPE/ICoTA Coiled Tubing & Well Intervention Conference & Exhibition, Houston, TX, USA, 21–22 March 2013. [Google Scholar] [CrossRef]
- Riley, M.; Stamatakis, E.; Young, S.; Hoelsher, K.P.; De Stefano, G.; Ji, L.; Guo, Q.; Friedheim, J. Wellbore Stability in Unconventional Shale—The Design of a Nano-particle Fluid, SPE 153729. In Proceedings of the SPE Oil and Gas India Conference and Exhibition, Mumbai, India, 28–30 March 2012. [Google Scholar] [CrossRef]
- Raj, K.A.; Balikram, A.; Ojha, K. Impact assessment of nanoparticles on microstructure and rheological behaviour of VES fracturing fluid formulated with mixed surfactant system. J. Mol. Liq. 2022, 345, 118241. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Q.; Gao, M.; Luo, W.; Cai, H. Study of Rheological Property and Flow Behavior for Nanoparticles Enhanced VES System in Porous Media. Front. Energy Res. 2021, 9, 598177. [Google Scholar] [CrossRef]
- Fan, Q.; Li, W.; Zhang, Y.; Fan, W.; Li, X.; Dong, J. Nanoparticles induced micellar growth in sodium oleate wormlike micelles solutions. Colloid Polym. Sci. 2015, 293, 2507–2513. [Google Scholar] [CrossRef]
- Nettesheim, F.; Liberatore, M.W.; Hodgdon, T.K.; Wagner, N.J.; Kaler, E.W.; Vethamuthu, M. Influence of Nanoparticle Addition on the Properties of Wormlike Micellar Solutions. Langmuir 2008, 24, 7718–7726. [Google Scholar] [CrossRef]
- Helgeson, M.E.; Hodgdon, T.K.; Kaler, E.W.; Wagner, N.J.; Vethamuthu, M.; Ananthapadmanabhan, K.P. Formation and Rheology of Viscoelastic “Double Networks” in Wormlike Micelle−Nanoparticle Mixtures. Langmuir 2010, 26, 8049–8060. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, Y.; Zou, C.; Dai, C.; Gao, M.; Li, Y.; Lv, W.; Jiang, J.; Wu, Y. Can More Nanoparticles Induce Larger Viscosities of Nanoparticle-Enhanced Wormlike Micellar System (NEWMS)? Materials 2017, 10, 1096. [Google Scholar] [CrossRef]
- Qin, W.; Yue, L.; Liang, G.; Jiang, G.; Yang, J.; Liu, Y. Effect of multi-walled carbon nanotubes on linear viscoelastic behavior and microstructure of zwitterionic wormlike micelle at high temperature. Chem. Eng. Res. Des. 2017, 123, 14–22. [Google Scholar] [CrossRef]
- Bandyopadhyay, R.; Sood, A. Effect of silica colloids on the rheology of viscoelastic gels formed by the surfactant cetyl trimethylammonium tosylate. J. Colloid Interface Sci. 2004, 283, 585–591. [Google Scholar] [CrossRef]
- Fan, H.J.; Luo, M.L.; Jia, Z.L.; Sun, H.T. Effect of Nano-SiO2 on the Rheology of Anionic Viscoelastic Solutions Formed by the Biodegradable Surfactant Fatty Acid Methyl Ester Sulfonate. In Materials Science Forum; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2011; Volume 694, pp. 64–67. [Google Scholar] [CrossRef]
- Luo, M.; Jia, Z.; Sun, H.; Liao, L.; Wen, Q. Rheological behavior and microstructure of an anionic surfactant micelle solution with pyroelectric nanoparticle. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 395, 267–275. [Google Scholar] [CrossRef]
- Patel, M.C.; Ayoub, M.A.; Hassan, A.M.; Idress, M.B. A Novel ZnO Nanoparticles Enhanced Surfactant Based Viscoelastic Fluid Systems for Fracturing under High Temperature and High Shear Rate Conditions: Synthesis, Rheometric Analysis, and Fluid Model Derivation. Polymers 2022, 14, 4023. [Google Scholar] [CrossRef]
- Omeiza, A.A.; Samsuri, A.B. Viscoelastic surfactants application in hydraulic fracturing, it’s set back and mitigation—An overview. ARPN J. Eng. Appl. Sci. 2006, 9, 25–29. [Google Scholar]
- Dantas, T.N.C.; Santanna, V.C.; Neto, A.A.D.; Moura, M.C.P.A. Hydraulic Gel Fracturing. J. Dispers. Sci. Technol. 2005, 26, 1–4. [Google Scholar] [CrossRef]
- Gupta, D.V.S. Unconventional Fracturing Fluids: What, Where and Why, EPA’s Report. 2010. Available online: https://www.epa.gov/hfstudy/unconventional-fracturing-fluids-what-where-and-why (accessed on 6 February 2023).
- Akano, T.T.; James, C.C. An assessment of ensemble learning approaches and single-based machine learning algorithms for the characterization of undersaturated oil viscosity. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 1–18. [Google Scholar] [CrossRef]
- Fogang, L.T.; Kamal, M.S.; Sultan, A.S. Viscosity-Reducing Agents (Breakers) for Viscoelastic Surfactant Gels for Well Stimulation. Energy Fuels 2020, 34, 15686–15700. [Google Scholar] [CrossRef]
- Dutta, R.; Lee, C.H.; Odumabo, S.; Ye, P.; Walker, S.C.; Karpyn, Z.T.; Ayala, L.F. Quantification of Fracturing Fluid Migration due to Spontaneous Imbibition in Fractured Tight Formations, SPE 154939. In Proceedings of the Americas Unconventional Resources Conference, Pittsburgh, PA, USA, 5–6 June 2012. [Google Scholar] [CrossRef]
- Ayoub, J.; Hutchins, R.; van der Bas, F.; Intl, S.E.; Cobianco, S.; Emiliani, C. SPE 98746 New Findings in Fracture Cleanup Change Common Industry Perceptions. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, CA, USA, 24–26 February 2006. [Google Scholar] [CrossRef]











| Group 1 Fluids (Low Surfactant Concentration Fluids) | Group 2 Fluids (High Surfactant Concentration Fluids) | ||
|---|---|---|---|
| Type 1 Fluids | Type 2 Nanofluids | Type 3 Fluids | Type 4 Nanofluids |
| Fluid 1 (0.2 M NaNO3) | Fluid 1 (0.2 M NaNO3) | Fluid 1 (0.2 M NaNO3) | Fluid 1 (0.2 M NaNO3) |
| Fluid 2 (0.4 M NaNO3) | Fluid 2 (0.4 M NaNO3) | Fluid 2 (0.4 M NaNO3) | Fluid 2 (0.4 M NaNO3) |
| Fluid 3 (0.6 M NaNO3) | Fluid 3 (0.6 M NaNO3) | Fluid 3 (0.6 M NaNO3) | Fluid 3 (0.6 M NaNO3) |
| Fluid 4 (0.8 M NaNO3) | Fluid 4 (0.8 M NaNO3) | Fluid 4 (0.8 M NaNO3) | Fluid 4 (0.8 M NaNO3) |
| Fluid 5 (1.0 M NaNO3) | Fluid 5 (1.0 M NaNO3) | Fluid 5 (1.0 M NaNO3) | |
| Fluid 6 (1.5 M NaNO3) | Fluid 6 (1.5 M NaNO3) | Fluid 6 (1.2 M NaNO3) | |
| Fluid 7 (2.0 M NaNO3) | Fluid 7 (2.0 M NaNO3) | Fluid 7 (1.4 M NaNO3) | |
| Fluid | SD | Mean | SEM |
|---|---|---|---|
| Optimum SBVE nanofluid of type 4 | 1892.556 | 689.6557 | 77.26326 |
| Optimum SBVE fluid of type 3 | 2384.984 | 818.0965 | 97.44782 |
| Guar Gum gel | 1258.263 | 688 | 51.36839 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, M.C.; Ayoub, M.A.; Idress, M.B.; Sircar, A. Development of a Novel Surfactant-Based Viscoelastic Fluid System as an Alternative Nonpolymeric Fracturing Fluid and Comparative Analysis with Traditional Guar Gum Gel Fluid. Polymers 2023, 15, 2444. https://doi.org/10.3390/polym15112444
Patel MC, Ayoub MA, Idress MB, Sircar A. Development of a Novel Surfactant-Based Viscoelastic Fluid System as an Alternative Nonpolymeric Fracturing Fluid and Comparative Analysis with Traditional Guar Gum Gel Fluid. Polymers. 2023; 15(11):2444. https://doi.org/10.3390/polym15112444
Chicago/Turabian StylePatel, Mahesh Chandra, Mohammed Abdalla Ayoub, Mazlin Bt Idress, and Anirbid Sircar. 2023. "Development of a Novel Surfactant-Based Viscoelastic Fluid System as an Alternative Nonpolymeric Fracturing Fluid and Comparative Analysis with Traditional Guar Gum Gel Fluid" Polymers 15, no. 11: 2444. https://doi.org/10.3390/polym15112444
APA StylePatel, M. C., Ayoub, M. A., Idress, M. B., & Sircar, A. (2023). Development of a Novel Surfactant-Based Viscoelastic Fluid System as an Alternative Nonpolymeric Fracturing Fluid and Comparative Analysis with Traditional Guar Gum Gel Fluid. Polymers, 15(11), 2444. https://doi.org/10.3390/polym15112444







