Study on Efficient Degradation of Waste PU Foam
Abstract
1. Introduction
2. Experimental Section
2.1. Raw Materials and Reagents
2.2. Instruments and Equipment
3. Experimental Methods
3.1. Degradation Experiment of Waste Polyurethane Foam
3.2. Preparation of Regenerated Polyurethane Foam
4. Testing and Characterization
4.1. Viscosity Test
4.2. Hydroxyl Value Test
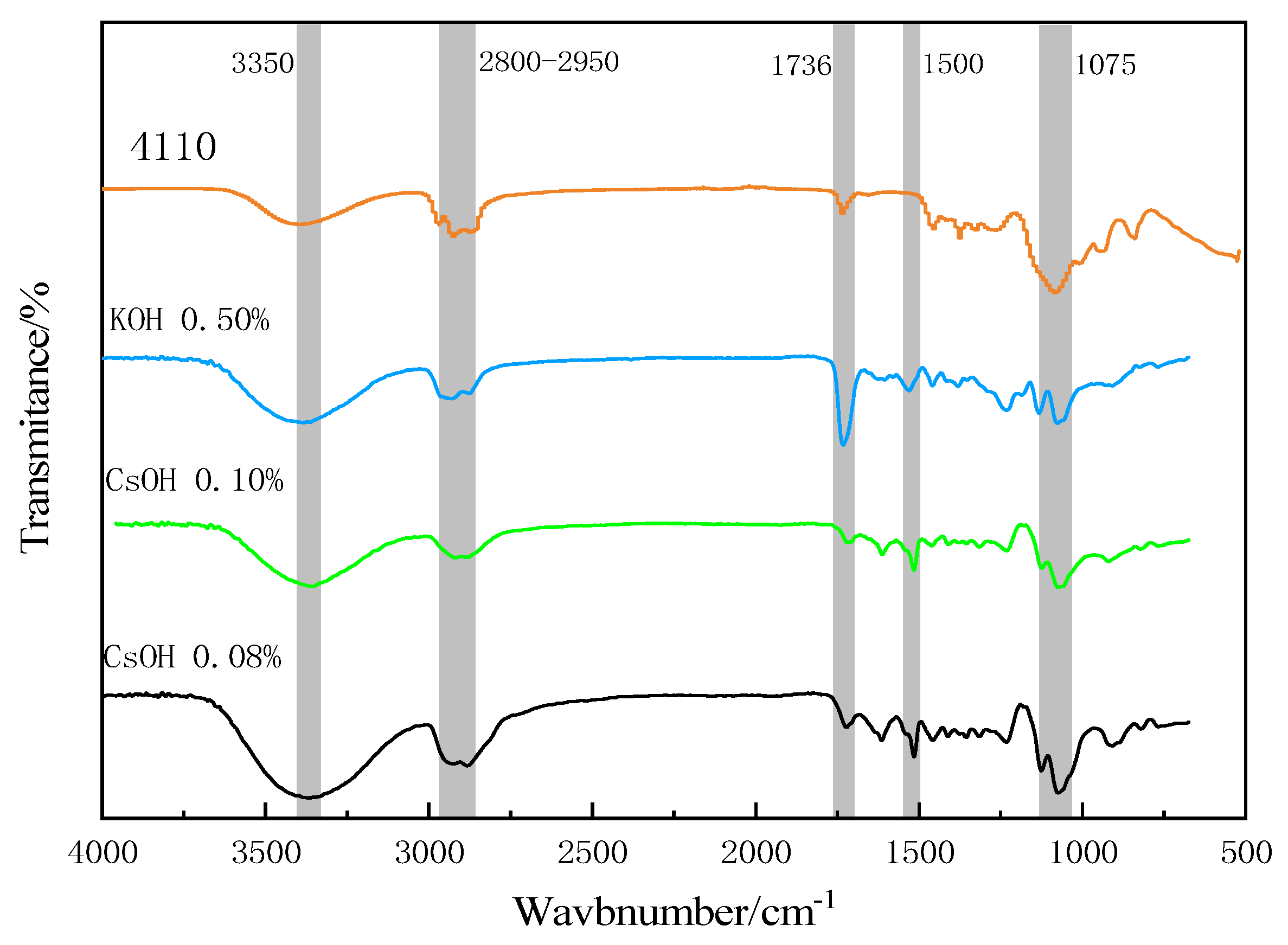
4.3. Fourier Transform Infrared Spectrum Analysis
4.4. Gel Permeation Chromatography Analysis
4.5. Apparent Density Test
4.6. Compression Strength Test
4.7. Scanning Electron Microscope
5. Results and Discussion
5.1. Discussion on the Degradation Mechanism of Waste Polyurethane
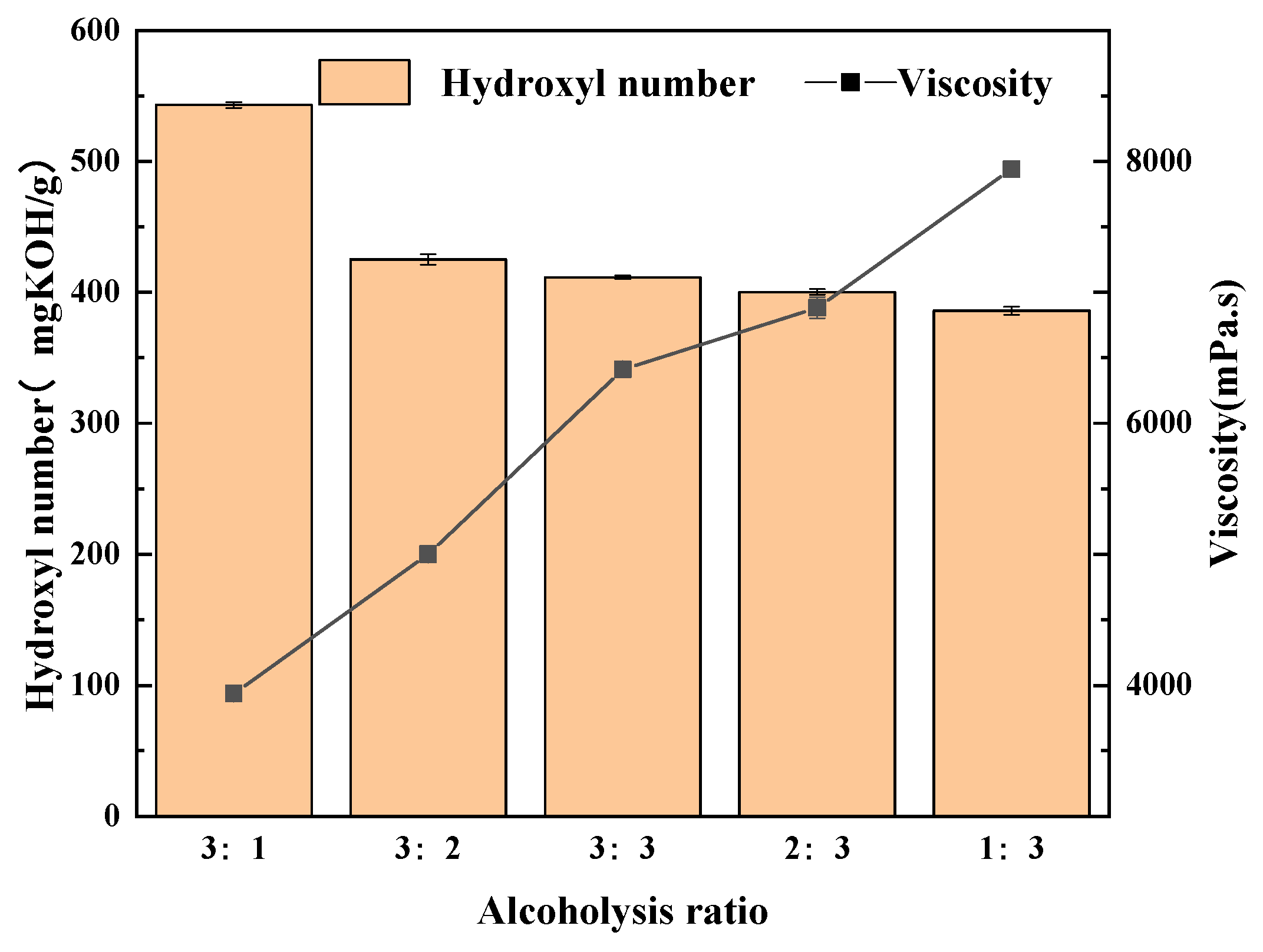
5.2. Selecting the Proportions of Alcoholysis Agents
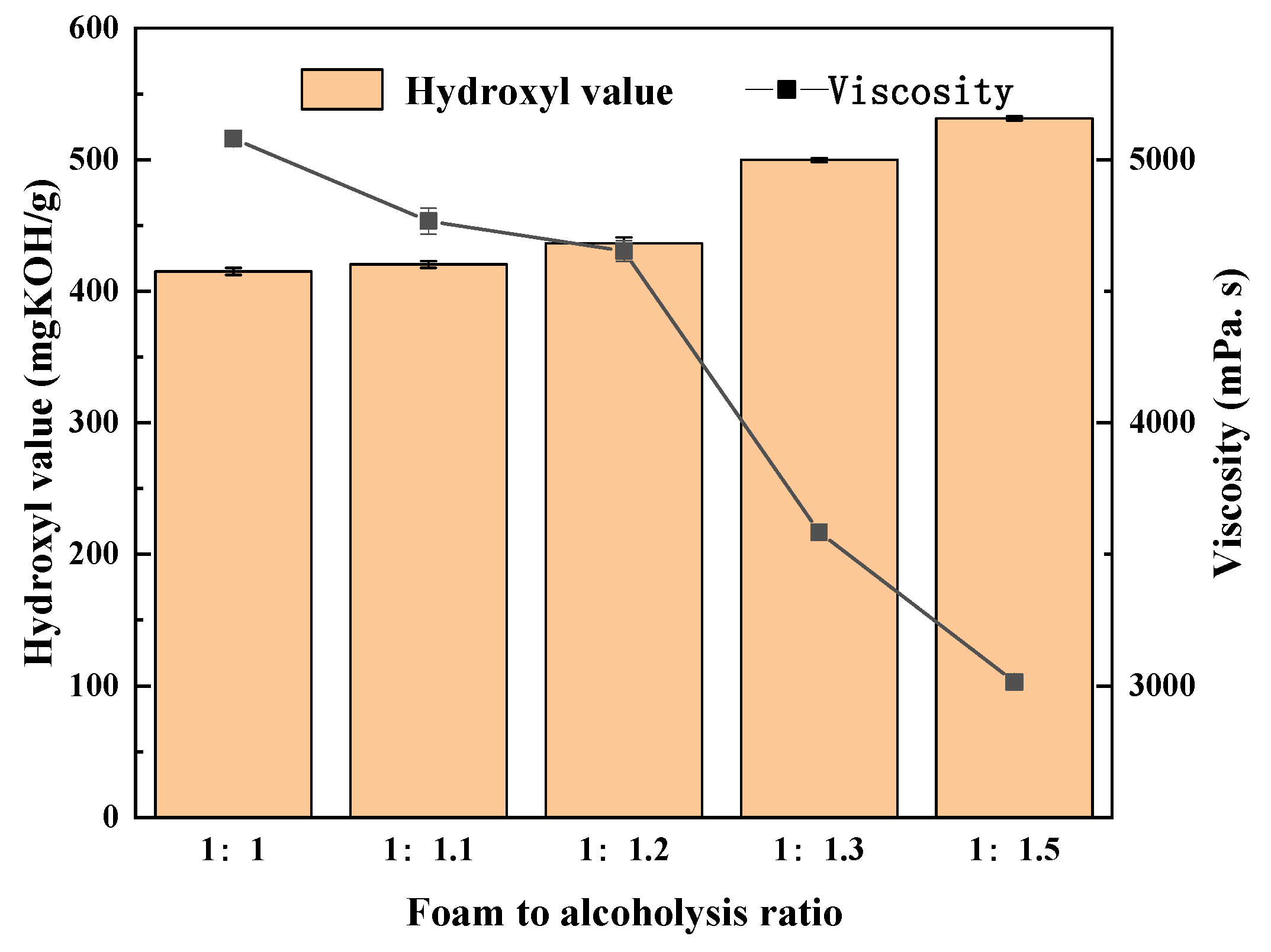
5.3. Choice of Foam and Alcoholysis Agent Ratio
5.4. Research on the Amount of Cesium Hydroxide Catalyst
5.5. Infrared Spectrum Analysis of Recovered Polyols
5.6. Gel Permeation Chromatography Analysis
5.7. Effect of Foaming Catalyst and Additive Content on Regenerated Polyurethane Foam
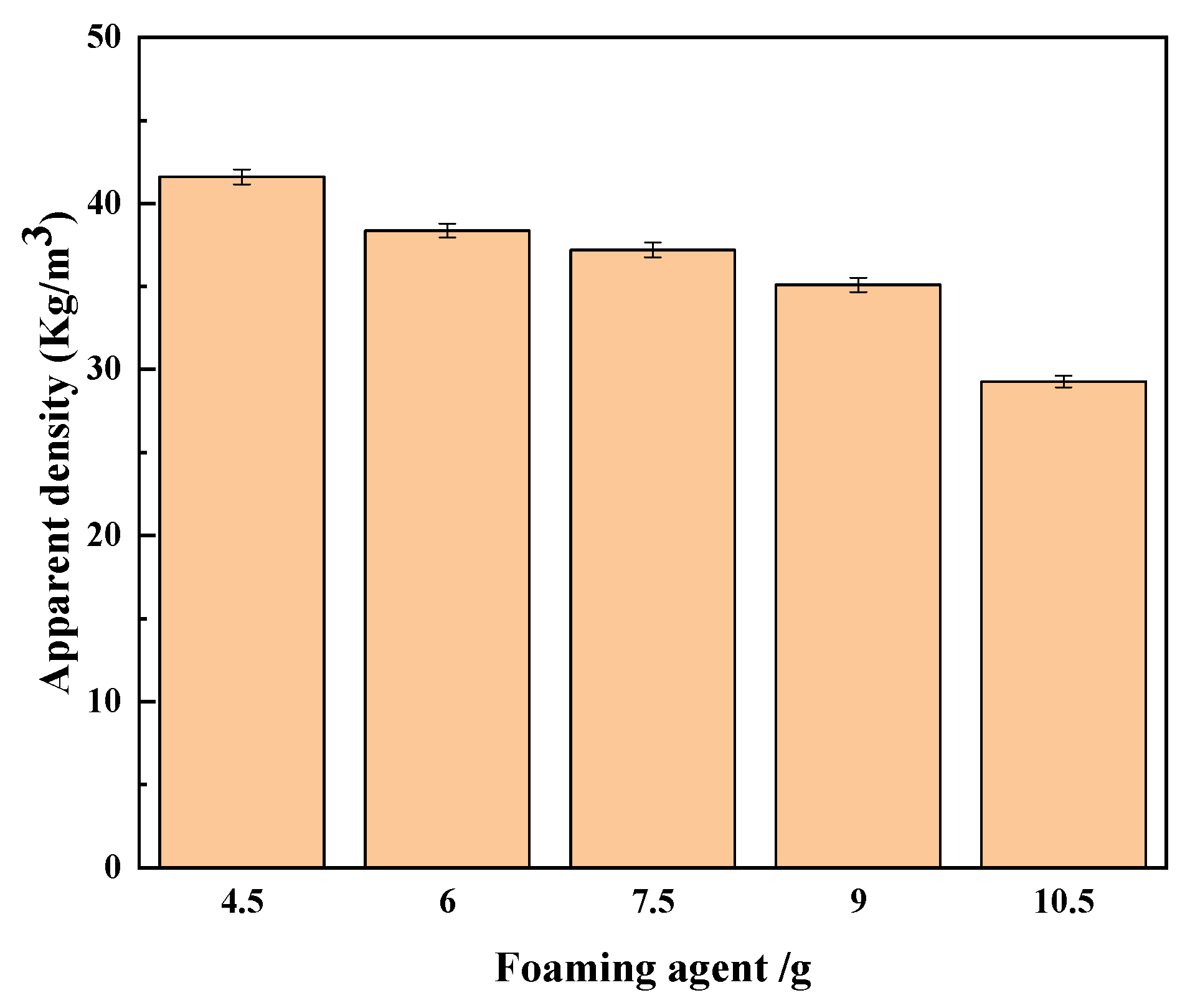
5.7.1. Effect of Foaming Agent Addition Amount on Regenerated Polyurethane Foam
- (1)
- Foaming time: The time was recorded by adding polyisocyanate (PAPI) and stirring until the surface of the regenerated polyurethane foam hardened and no longer adhered. Each group of experiments was repeated five times from which the average value was calculated. The experimental results are shown in Table 5.
- (2)
- Apparent density of regenerated polyurethane foam: After standing the prepared reclaimed polyurethane foam sample in a cool place for 72 h, the reclaimed polyurethane sample was cut into 50 mm × 50 mm × 50 mm sample blocks, and its apparent density was tested according to the GB/T6343-2009 standard. The sample blocks were weighed using an electronic balance, and the apparent density of the sample was then calculated using the formula ρ = m/V. The experiment was repeated five times for each group of samples from which the average value was calculated. The measurement results are shown in Figure 5.
- (3)
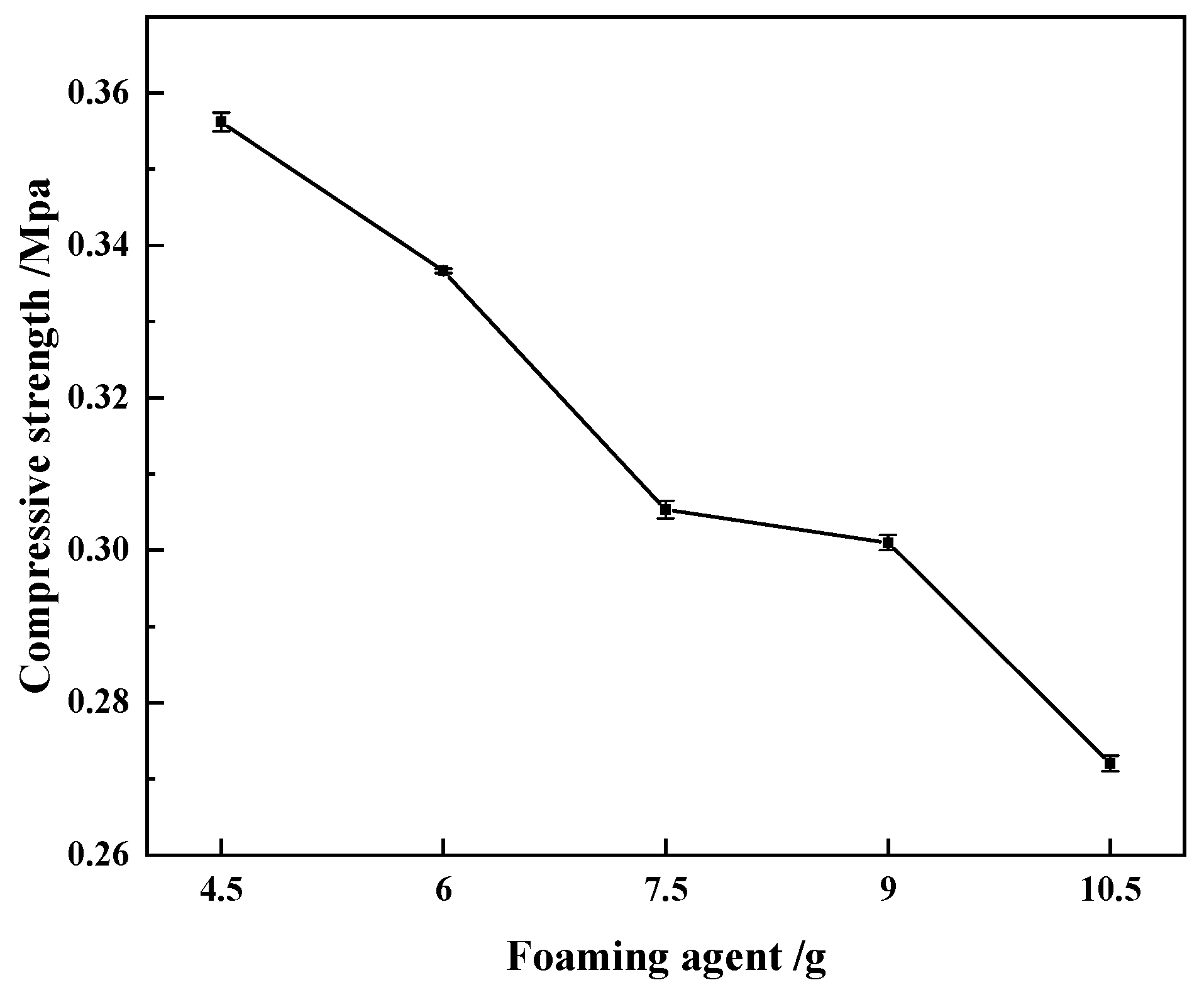
- Compressive strength of regenerated polyurethane foam: The compressive strength of the regenerated polyurethane foam was tested according to GB/T 8813-2008. Samples with specifications of 50 mm × 50 mm × 50 mm were tested using a universal testing machine, and the displacement velocity of the testing machine was adjusted to 20 mm/min. Five measurements were made for each group of samples from which the average value was calculated. The test results are shown in Figure 6.
- (4)
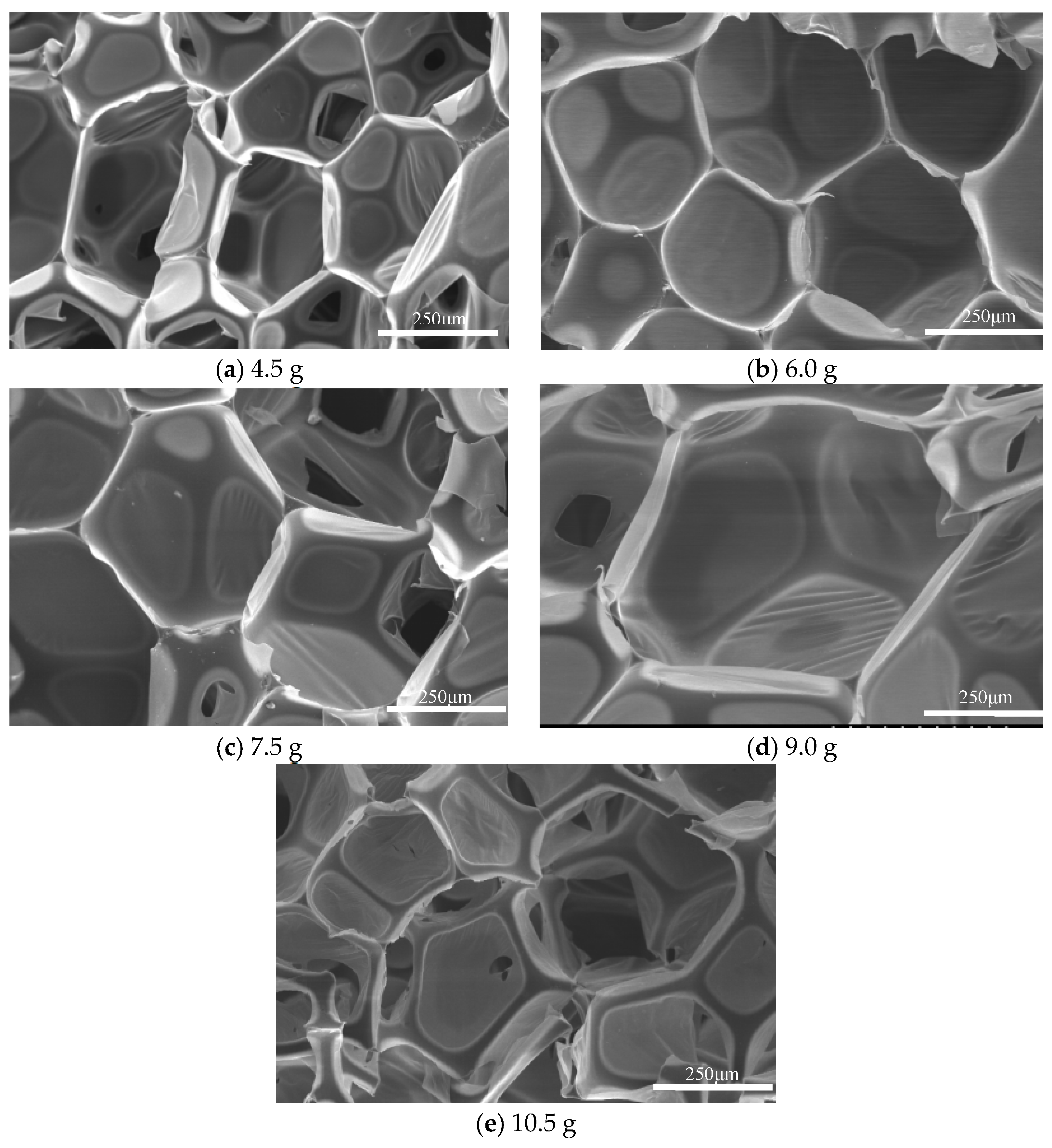
- Scanning electron microscopy and analysis of regenerated polyurethane foam: The samples of regenerated polyurethane foams prepared with different amounts of foaming agent were sliced. Scanning electron microscopy was used to observe them, and clear areas were selected for shooting. The bubble holes in each sample are shown in Figure 7.
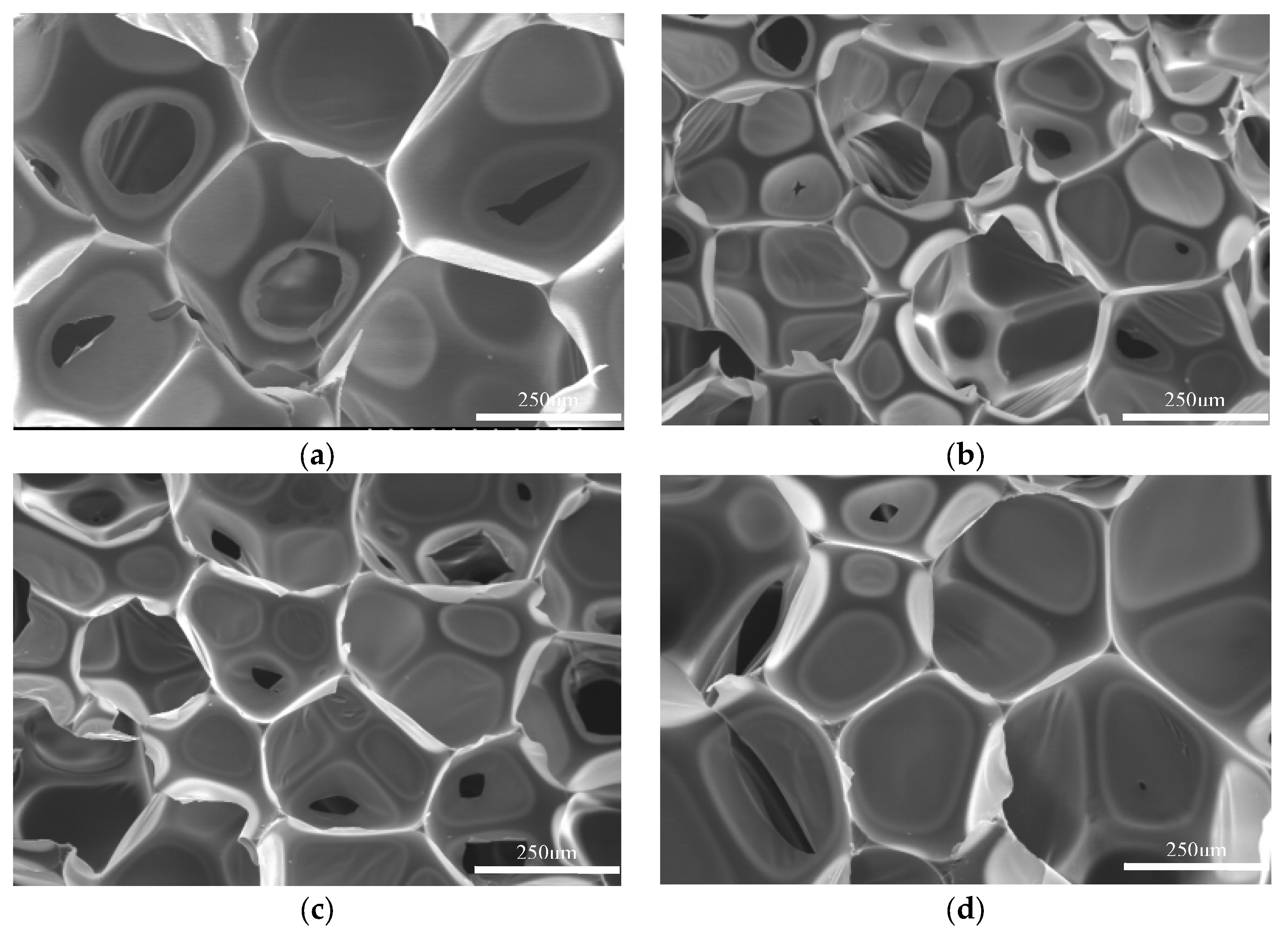
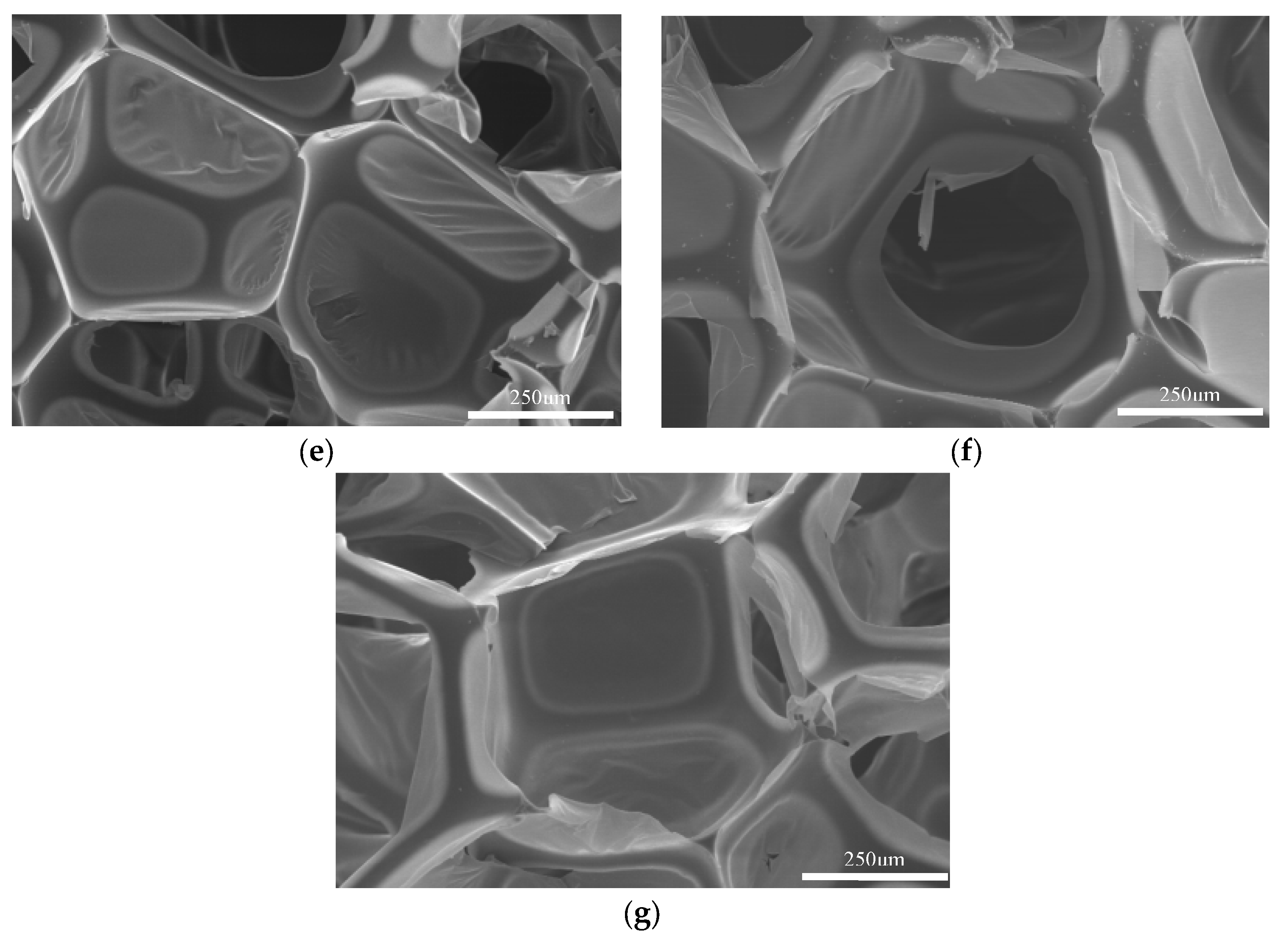
5.7.2. Effect of Catalyst Addition Amount on Regenerated Polyurethane Foam
6. Conclusions
- (1)
- The optimal conditions of alcoholysis were obtained when the mass ratio of glycerol to butanediol was 3:2, the mass ratio of the used polyurethane foam to alcoholysis agents was 1:1.2, the amount of cesium hydroxide catalyst was 0.08%, the reaction temperature was 170 °C, and the reaction time was 2.5 h. Under these conditions, waste polyurethane was successfully degraded.
- (2)
- When the amount of regenerated polyol was 9 g and the amount of polyether polyol 4110 was 21 g, adding 9.0 g of foaming agent, 0.30 g of triethanolamine catalyst, and 0.15 g of dibutyltin dilaurate produced regenerated polyurethane foam with a density of 34.1 kg/m3 and compression strength of 0.30 mpa. The regenerated polyurethane foam meets national standards, the sample bubble holes were complete, the skeleton was strong, and the best reaction conditions were achieved.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [PubMed]
- Piszczyk, A.; Kosmela, P.; Strankowski, M. Elastic polyurethane foams containing graphene nanoplatelets. Adv. Polym. Technol. 2017, 37, 1625–1634. [Google Scholar] [CrossRef]
- Namgoong, S.; Jung, J.E.; Yoon, Y.D.; Han, S.K.; Lee, Y.N.; Son, J.W. Highly hydrophilic polyurethane foam dressing versus early hydrophilic polyurethane foam dressing on skin graft donor site healing in patients with diabetes: An exploratory clinical trial. Adv. Ski. Wound Care. 2020, 33, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Beneš, H.; Vlčková, V.; Paruzel, A.; Trhlíková, O.; Chalupa, J.; Kanizsová, L.; Skleničková, K.; Halecký, M. Multifunctional and fully aliphatic biodegradable polyurethane foam as porous biomass carrier for biofiltration. Polym. Degrad. Stab. 2020, 176, 109156. [Google Scholar] [CrossRef]
- Magnin, A.; Pollet, E.; Phalip, V.; Avérous, L. Evaluation of biological degradation of polyurethanes. Biotechnol. Adv. 2019, 39, 107457. [Google Scholar] [CrossRef]
- Hamidov, M.; Çakmakçi, E.; Kahraman, M.V. Autocatalytic reactive flame retardants for rigid polyurethane foams. Mater. Chem. Phys. 2021, 267, 124636. [Google Scholar] [CrossRef]
- Gheydari, M.; Dorraji, M.S.S.; Fazli, M.; Rasoulifard, M.H.; Almaie, S.; Daneshvar, H.; Ashjari, H.R. Preparation of open-cell polyurethane nanocomposite foam with Ag3PO4 and GO: Antibacterial and adsorption characteristics. J. Polym. Res. 2021, 28, 69. [Google Scholar] [CrossRef]
- Li, H.; Lin, S.; Feng, X.; Pan, Q. Preparation of superhydrophobic and superoleophilic polyurethane foam for oil spill cleanup. J. Macromol. Sci. Part A 2021, 58, 758–768. [Google Scholar] [CrossRef]
- De Nino, A.; Olivito, F.; Algieri, V.; Costanzo, P.; Jiritano, A.; Tallarida, M.A.; Maiuolo, L. Efficient and fast removal of oils from water surfaces via highly oleophilic polyurethane composites. Toxics 2021, 9, 186. [Google Scholar] [CrossRef]
- Andersons, J.; Kirpluks, M.; Cabulis, P.; Kalnins, K. Bio-based rigid high-density polyurethane foams as a structural thermal break material. Constr. Build. Mater. 2020, 260, 120471. [Google Scholar] [CrossRef]
- Shao, H.; Zhang, Q.; Liu, H.; Guo, W.; Jiang, Y.; Chen, L.; He, L.; Qi, J.; Xiao, H.; Chen, Y.; et al. Renewable natural resources reinforced polyurethane foam for use of lightweight thermal insulation. Mater. Res. Express 2020, 7, 055302. [Google Scholar] [CrossRef]
- Uram, K.; Prociak, A.; Vevere, L.; Pomilovskis, R.; Cabulis, U.; Kirpluks, M. Natural Oil-Based Rigid Polyurethane Foam Thermal Insulation Applicable at Cryogenic Temperatures. Polymers 2021, 13, 4276. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.B. Revisiting flexible polyurethane foam flammability in furniture and bedding in the United States. Fire Mater. 2021, 45, 68–80. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, J.; Sun, P.; Zhang, L.; Qian, X.; Jiang, S.; Shi, C. Green, tough and highly efficient flame-retardant rigid polyurethane foam enabled by double network hydrogel coatings. Soft Matter 2021, 17, 10555–10565. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Liu, P.; Wang, H.; Dai, W.; Wang, J.; Wang, Z.; Shiu, B.C.; Lou, C.W.; Lin, J.H. Preparation and characteristics of flexible polyurethane foam filled with expanded vermiculite powder and concave-convex structural panel. J. Mater. Res. Technol. 2021, 12, 1288–1302. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, X.; Li, Q.; Ji, Z. Cushion properties of polyurethane foam-in-place materials. Emerg. Mater. Res. 2017, 6, 60–68. [Google Scholar] [CrossRef]
- Silva, C.S.; Lima, A.; Rodrigues, S.J.F.; Gonçalves, L.F.; Sampaio, Á.M.; Oliveira, L.; Fernandes, A.; Pontes, A.J. Development of functionalised foam for electrostatic discharge applications. Plast. Rubber Compos. 2020, 49, 470–478. [Google Scholar] [CrossRef]
- Moon, J.; Sinha, T.K.; Kwak, S.B.; Ha, J.U.; Oh, J.S. Study on Seating Comfort of Polyurethane Multilayer Seat Cushions. Int. J. Automot. Technol. 2020, 21, 1089–1095. [Google Scholar] [CrossRef]
- Yang, W.; Dong, Q.; Liu, S.; Xie, H.; Liu, L.; Li, J. Recycling and Disposal Methods for Polyurethane Foam Wastes. Procedia Environ. Sci. 2012, 16, 167–175. [Google Scholar] [CrossRef]
- Zia, K.M.; Bhatti, H.N.; Bhatti, I.A. Methods for polyurethane and polyurethane composites, recycling and recovery: A review. React. Funct. Polym. 2007, 67, 675–692. [Google Scholar] [CrossRef]
- Vanbergen, T.; Verlent, I.; De Geeter, J.; Haelterman, B.; Claes, L.; De Vos, D. Recycling of Flexible Polyurethane Foam by Split-Phase Alcoholysis: Identification of Additives and Alcoholyzing Agents to Reach Higher Efficiencies. Chemsuschem 2020, 13, 3835–3843. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Hatano, B.; Kadokawa, J.-I.; Tagaya, H. Effect of diaminotoluene on the decomposition of polyurethane foam waste in superheated water. Polym. Degrad. Stab. 2002, 76, 179–184. [Google Scholar] [CrossRef]
- Troev, K.; Grancharov, G.; Tsevi, R. Chemical degradation of polyurethanes 3. Degradation of microporous polyurethane elastomer by diethyl phosphonate and tris (1-methyl-2-chloroethyl) phosphate. Polym. Degrad. Stab. 2000, 70, 43–48. [Google Scholar] [CrossRef]
- Kemona, A.; Piotrowska, M. Polyurethane Recycling and Disposal: Methods and Prospects. Polymers 2020, 12, 1752. [Google Scholar] [CrossRef]
- Kiss, G.; Rusu, G.; Peter, F.; Tănase, I.; Bandur, G. Recovery of Flexible Polyurethane Foam Waste for Efficient Reuse in Industrial Formulations. Polymers 2020, 12, 1533. [Google Scholar] [CrossRef]
- Kiss, G.; Rusu, G.; Bandur, G.; Hulka, I.; Romecki, D.; Péter, F. Advances in Low-Density Flexible Polyurethane Foams by Optimized Incorporation of High Amount of Recycled Polyol. Polymers 2021, 13, 1736. [Google Scholar] [CrossRef]
- Yao, Z.; Yu, S.; Su, W.; Wu, W.; Tang, J.; Qi, W. Comparative study on the pyrolysis kinetics of polyurethane foam from waste refrigerators. Waste Manag. Res. J. Sustain. Circ. Econ. 2019, 38, 271–278. [Google Scholar] [CrossRef]
- Shin, S.R.; Kim, H.N.; Liang, J.Y.; Lee, S.H.; Lee, D.S. Sustainable rigid polyurethane foams based on recycled polyols from chemical recycling of waste polyurethane foams. J. Appl. Polym. Sci. 2019, 136, 47916. [Google Scholar] [CrossRef]
- Borowicz, M.; Paciorek-Sadowska, J.; Lubczak, J.; Czupryński, B. Biodegradable, Flame-Retardant, and Bio-Based Rigid Polyurethane/Polyisocyanurate Foams for Thermal Insulation Application. Polymers 2019, 11, 1816. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, X.; Chen, W.; Zhang, H.; Han, D. Modification of rigid polyurethane foams with the addition of nano-SiO2 or lignocellulosic biomass. Polymers 2020, 12, 107. [Google Scholar] [CrossRef]
- Kucharek, M.; MacRae, W.; Yang, L. Investigation of the effects of silica aerogel particles on thermal and mechanical properties of epoxy composites. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106108. [Google Scholar] [CrossRef]
- Molero, C.; Lucas, D.; Rodríguez, A. Polym. and Degrad; Chongqing University: Chongqing, China, 2016; Volume 91, pp. 1132–1152. [Google Scholar]
- Zhang, X.L. Industrial Application of Environmentally Friendly Titanium-Based Polyester Catalyst; Tianjin University: Tianjin, China, 2017; Volume 51, pp. 1087–1095. [Google Scholar]
- Hakim, A.A.; Nassar, M.; Emam, A.; Sultan, M. Preparation and characterization of rigid polyurethane foam prepared from sugar-cane bagasse polyol. Mater. Chem. Phys. 2011, 129, 301–307. [Google Scholar] [CrossRef]
- Fournier, D.; Du Prez, F. “Click” chemistry as a promising tool for side-chain functionalization of polyurethanes. Macromolecules 2008, 41, 4622–4630. [Google Scholar] [CrossRef]

| Reagent Name | Purity | Factory of Production |
|---|---|---|
| Waste polyurethane rigid foam | Industrial waste | Shanghai Hecheng Polymer Material Co., Ltd. (Shanghai, China) |
| Glycerine | AR | Tianjin Chemical Reagent Factory 1 (Tianjim, China) |
| Butane-1,4-diol (BDO) | AR | Tianjin Kaitong Chemical Reagent Co., Ltd. (Tianjin, China) |
| CsOH | AR | Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) |
| KOH | AR | Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) |
| Silicone oil stabilizer | CP | Guangzhou Feirui Chemical Co., Ltd. (Guangzhou, China) |
| TEA | AR | Shanghai Demao Chemical Co., Ltd. (Shanghai, China) |
| Dibutyltin dilaurate | AR | Shanghai Jieer Technology Co., Ltd. (Shanghai, China) |
| Foaming agent | CP | Shenzhen Huachang Chemical Co., Ltd. (Shenzhen, China) |
| Polyether 4110 | CP | Shandong Lianhaoyao New Material Co., Ltd. (Shandong, China) |
| Polyaryl polymethylene isocyanate (PAPI) | CP | Wuhan Fude Chemical Co., Ltd. (Wuhan, China) |
| Name of Instrument | Model | Factory of Production |
|---|---|---|
| Electronic analytical balance | JA3003C | Sartorius Scientific Instruments Co., Ltd. (Beijing China) |
| Cantilever constant-speed power electric mixer | TJ-1200W | Changzhou Huaao Instrument Manufacturing Co., Ltd. (Changzhou China) |
| Spherical reactor (1 L) | ZNHW-200 | Shanghai Leighton Industrial Co., Ltd. (Shanghai, China) |
| Digital blast drying oven | WX881 | Wujiang Weixin Electric Heating Equipment Co., Ltd. (Wujiang, China) |
| Digital viscometer | NDJ-5 | Shanghai Pingxuan Scientific Instrument Co., Ltd. (Shanghai, China) |
| Disposable plastic cup | 350 mL | Top Daily Chemicals Co., Ltd. (Yangzhou, China) |
| Constant-temperature heating sleeve | FDSG-420 | Wuxi Huachang Chemical Co., Ltd. (Wuxi, China) |
| Additive Amount (%) | Hydroxyl Number/(mgKOH/g) | Viscosity (mPa·s) |
|---|---|---|
| 0.04 (CsOH) | 429 | 4498 |
| 0.06 (CsOH) | 438 | 4254 |
| 0.08 (CsOH) | 445 | 3834 |
| 0.10 (CsOH) | 441 | 4432 |
| 0.12 (CsOH) | 436 | 5139 |
| 0.50 (KOH) | 437 | 4678 |
| Additive Amount/(%) | Mn | PDI |
|---|---|---|
| 0.50 (KOH) | 3827 | 1.466 |
| 0.04 (CsOH) | 3794 | 1.401 |
| 0.06 (CsOH) | 3526 | 1.324 |
| 0.08 (CsOH) | 2804 | 1.267 |
| 0.10 (CsOH) | 2683 | 1.269 |
| 0.12 (CsOH) | 2511 | 1.274 |
| Polyether 4110 | 1105 | 1.168 |
| Number | Foaming Agent/(g) | Cream Time/(s) | Tack-Free Time/(s) |
|---|---|---|---|
| 1 | 4.50 | 3 | 58 |
| 2 | 6.00 | 2 | 53 |
| 3 | 7.50 | 3 | 50 |
| 4 | 9.00 | 3 | 48 |
| 5 | 10.50 | 2 | 39 |
| Sample | TEA/(g) | DBTDL/(g) | Morphology |
|---|---|---|---|
| a | 0.15 | 0.15 | Slightly smaller, cavity collapsed |
| b | 0.15 | 0.30 | Smaller, cavity collapse |
| c | 0.15 | 0.45 | Smaller, cavity collapse |
| d | 0.30 | 0.45 | Slightly smaller, cavity collapsed |
| e | 0.30 | 0.15 | Moderate, no hole collapse |
| f | 0.45 | 0.30 | Large, crispy skin |
| g | 0.45 | 0.15 | Large, crispy skin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, X.; Wang, X.; Guo, X.; Liu, S.; Lou, C.; Liu, Y. Study on Efficient Degradation of Waste PU Foam. Polymers 2023, 15, 2359. https://doi.org/10.3390/polym15102359
Gu X, Wang X, Guo X, Liu S, Lou C, Liu Y. Study on Efficient Degradation of Waste PU Foam. Polymers. 2023; 15(10):2359. https://doi.org/10.3390/polym15102359
Chicago/Turabian StyleGu, Xiaohua, Xiaoyao Wang, Xinyu Guo, Siwen Liu, Chunhua Lou, and Yan Liu. 2023. "Study on Efficient Degradation of Waste PU Foam" Polymers 15, no. 10: 2359. https://doi.org/10.3390/polym15102359
APA StyleGu, X., Wang, X., Guo, X., Liu, S., Lou, C., & Liu, Y. (2023). Study on Efficient Degradation of Waste PU Foam. Polymers, 15(10), 2359. https://doi.org/10.3390/polym15102359







