Matrix-Assisted Laser Desorption and Electrospray Ionization Tandem Mass Spectrometry of Microbial and Synthetic Biodegradable Polymers
Abstract
1. Introduction
2. MALDI Tandem Mass Spectrometry Studies
3. ESI Tandem Mass Spectrometry Studies
3.1. Fragmentation Analysis and Dissociation Tools
3.2. Structural Characterization
3.3. Degradation Investigations
3.4. Bioresorbable Polymers and DDS Studies by LC-ESI Tandem MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalita, N.K.; Hakkarainen, M. Integrating biodegradable polyesters in a circular economy. Curr. Opin. Green Sustain. Chem. 2023, 40, 100751. [Google Scholar] [CrossRef]
- Von Vacano, B.; Mangold, H.; Vandermeulen, G.W.M.; Battagliarin, G.; Hofmann, M.; Bean, J.; Künkel, A. Sustainable Design of Structural and Functional Polymers for a Circular Economy. Angew. Chem. Int. Ed. 2023, 62, e202210823. [Google Scholar] [CrossRef] [PubMed]
- Swetha, T.A.; Bora, A.; Mohanrasu, K.; Balaji, P.; Raja, R.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A comprehensive review on polylactic acid (PLA)—Synthesis, processing and application in food packaging. Int. J. Biol. Macromol. 2023, 234, 123715. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, Y.; Liu, M.; Meng, L.; Li, C. A review of research and application of polylactic acid composites. J. Appl. Polym. Sci. 2023, 140, e53477. [Google Scholar] [CrossRef]
- Behera, S.; Priyadarshanee, M.; Vandana; Das, S. Polyhydroxyalkanoates, the bioplastics of microbial origin: Properties, biochemical synthesis, and their applications. Chemosphere 2022, 294, 133723. [Google Scholar] [CrossRef]
- Fernandez-Bunster, G.; Pavez, P. Novel Production Methods of Polyhydroxyalkanoates and Their Innovative Uses in Biomedicine and Industry. Molecules 2022, 27, 8351. [Google Scholar] [CrossRef]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Yao, W.; Ge, J.; Hu, Q.; Ma, J.; Yuan, D.; Fu, X.; Qi, Y.; Volmer, D.A. An advanced LC–MS/MS protocol for simultaneous detection of pharmaceuticals and personal care products in the environment. Rapid Commun. Mass Spectrom. 2023, 37, e9397. [Google Scholar] [CrossRef]
- Keating, A.R.; Wesdemiotis, C. Rapid and simple determination of average molecular weight and composition of synthetic polymers via electrospray ionization-mass spectrometry and a Bayesian universal charge deconvolution. Rapid Commun. Mass Spectrom. 2023, 37, e9478. [Google Scholar] [CrossRef]
- Elzahhar, P.; Belal, A.S.F.; Elamrawy, F.; Helal, N.A.; Nounou, M.I. Bioconjugation in Drug Delivery: Practical Perspectives and Future Perceptions. Pharm. Nanotechnol. 2019, 2000, 125–182. [Google Scholar] [CrossRef]
- Wrona, M.; Nerin, C. Risk Assessment of Plastic Packaging for Food Applications. In Food Contact Materials: Mass Spectrometry Techniques; RSC: London, UK, 2019; pp. 163–191. [Google Scholar] [CrossRef]
- Altuntaş, E.; Schubert, U.S. “Polymeromics”: Mass spectrometry based strategies in polymer science toward complete sequencing approaches: A review. Anal. Chim. Acta 2014, 808, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Crecelius, A.C.; Vitz, J.; Schubert, U.S. Mass spectrometric imaging of synthetic polymers. Anal. Chim. Acta 2013, 808, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Rizzarelli, P.; Carroccio, S. Modern mass spectrometry in the characterization and degradation of biodegradable polymers. Anal. Chim. Acta 2014, 808, 18–43. [Google Scholar] [CrossRef] [PubMed]
- Kowalczuk, M.; Adamus, G. Mass spectrometry for the elucidation of the subtle molecular structure of biodegradable polymers and their degradation products. Mass Spectrom. Rev. 2015, 35, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Montaudo, G.; Samperi, F.; Montaudo, M.S. Characterization of synthetic polymers by MALDI-MS. Prog. Polym. Sci. 2006, 31, 277–357. [Google Scholar] [CrossRef]
- Yoo, H.-J.; Kim, D.-H.; Shin, D.; Oh, Y.; Lee, S.; Lee, J.Y.; Choi, Y.-J.; Lee, S.H.; Lee, K.-S.; Kim, Y.; et al. Recent developments in pre-treatment and analytical techniques for synthetic polymers by MALDI-TOF mass spectrometry. Anal. Methods 2020, 12, 5767–5800. [Google Scholar] [CrossRef]
- De Bruycker, K.; Welle, A.; Hirth, S.; Blanksby, S.J.; Barner-Kowollik, C. Mass spectrometry as a tool to advance polymer science. Nat. Rev. Chem. 2020, 4, 257–268. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Rapisarda, M.; Valenti, G. Mass spectrometry in bioresorbable polymer development, degradation and drug-release tracking. Rapid Commun. Mass Spectrom. 2020, 34, e8697. [Google Scholar] [CrossRef]
- Montaudo, G.; Lattimer, R. Mass Spectrometry of Polymers; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar] [CrossRef]
- Li, L. Maldi Mass Spectrometry for Synthetic Polymer Analysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Tsuge, S.; Ohtani, H.; Watanabe, C. Pyrolysis-GC/MS data book of synthetic polymers. In Pyrograms, Thermograms and MS of Pyrolyzates; Elsevier: Oxford, UK, 2011; ISBN 978-0-444-53892-5. [Google Scholar]
- Barner-Kowollik, C.; Gruendling, T.; Falkenhagen, J.; Weidner, S. Mass Spectrometry in Polymer Chemistry; Wiley-VCH: Weinheim, Germany, 2011; ISBN 978-3-527-32924-3. [Google Scholar]
- Hakkarainnen, M. Mass Spectrometry of Polymers—New Techniques. In Advances in Polymer Science; Springer: Heidelberg, Germany, 2012; ISBN 978-3-642-28041-2. [Google Scholar]
- Li, L. Overview of MS and MALDI MS for Polymer Analysis. In Maldi Mass Spectrometry for Synthetic Polymer Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; Volume 175, ISBN 978-0-471-77579-9. [Google Scholar]
- Dimzon, I.K.D.; Knepper, T.P. MALDI–TOF MS for Characterization of Synthetic Polymers in Aqueous Environment. In TOF-MS within Food and Environmental Analysis; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Dworzanski, J.; Meuzelaar, H. Pyrolysis Mass Spectrometry, Methods. In Encyclopedia of Spectroscopy and Spectrometry; Academic Press, Elsevier: Oxford, UK, 2017. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Carroccio, S. Recent trends in the structural characterization and degradation of biodegradable polymers by modern mass spectrometry. In Biodegradable Polymers; Nova Science Publisher: Ithaca, NY, USA, 2016. [Google Scholar]
- Rizzarelli, P.; Carroccio, S. Role of Mass Spectrometry in the Elucidation of Thermal Degradation Mechanisms in Polymeric Materials. In Reaction Mechanisms in Thermal Analysis of Materials; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Harvey, D.J. Mass Spectrometry: (1) Matrix-Assisted Laser Desorption/Ionization (MALDI). In Encyclopedia of Analytical Science; Academic Press: Oxford, UK, 2019. [Google Scholar] [CrossRef]
- Lee, J.-W.; Gardella, J.A. Simultaneous Time-of-Flight Secondary Ion MS Quantitative Analysis of Drug Surface Concentration and Polymer Degradation Kinetics in Biodegradable Poly(l-lactic acid) Blends. Anal. Chem. 2003, 75, 2950–2958. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Zampino, D.; Ferreri, L.; Impallomeni, G. Direct Electrospray Ionization Mass Spectrometry Quantitative Analysis of Sebacic and Terephthalic Acids in Biodegradable Polymers. Anal. Chem. 2011, 83, 654–660. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Cirica, M.; Pastorelli, G.; Puglisi, C.; Valenti, G. Aliphatic poly(ester amide)s from sebacic acid and aminoalcohols of different chain length: Synthesis, characterization and soil burial degradation. Polym. Degrad. Stab. 2015, 121, 90–99. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Arrigo, R.; Baiamonte, M.; Rizzarelli, P.; Curcuruto, G. Concentration-dependent anti-/pro-oxidant activity of natural phenolic compounds in bio-polyesters. Polym. Degrad. Stab. 2017, 142, 21–28. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Rapisarda, M.; Perna, S.; Mirabella, E.F.; La Carta, S.; Puglisi, C.; Valenti, G. Determination of polyethylene in biodegradable polymer blends and in compostable carrier bags by Py-GC/MS and TGA. J. Anal. Appl. Pyrolysis 2016, 117, 72–81. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Piredda, G.; La Carta, S.; Mirabella, E.F.; Valenti, G.; Bernet, R.; Impallomeni, G. Characterization and laser-induced degradation of a medical grade polylactide. Polym. Degrad. Stab. 2019, 169, 108991. [Google Scholar] [CrossRef]
- Impallomeni, G.; Mineo, P.G.; Vento, F.; Ballistreri, A. Novel pranoprofen-poly(ε-caprolactone) conjugates: Microwave-assisted synthesis and structural characterization. Polym. Int. 2020, 70, 604–611. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Rapisarda, M. Tandem Mass Spectrometry in the Analysis of Biodegradable Polymers. In Mass Spectrometry: Theory and Applications; Nichols, W.O., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2021; pp. 127–181. ISBN 978-1-53619-790-7. [Google Scholar]
- Crecelius, A.C.; Baumgaertel, A.; Schubert, U.S. Tandem mass spectrometry of synthetic polymers. J. Mass Spectrom. 2009, 44, 1277–1286. [Google Scholar] [CrossRef]
- Wesdemiotis, C.; Solak, N.; Polce, M.J.; Dabney, D.E.; Chaicharoen, K.; Katzenmeyer, B.C. Fragmentation pathways of polymer ions. Mass Spectrom. Rev. 2011, 30, 523–559. [Google Scholar] [CrossRef]
- Crotty, S.; Gerişlioğlu, S.; Endres, K.J.; Wesdemiotis, C.; Schubert, U.S. Polymer architectures via mass spectrometry and hyphenated techniques: A review. Anal. Chim. Acta 2016, 932, 1–21. [Google Scholar] [CrossRef]
- Wesdemiotis, C. Multidimensional Mass Spectrometry of Synthetic Polymers and Advanced Materials. Angew. Chem. Int. Ed. 2017, 56, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Hart-Smith, G. A review of electron-capture and electron-transfer dissociation tandem mass spectrometry in polymer chemistry. Anal. Chim. Acta 2014, 808, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Jahn, B.O.; Hager, M.D.; Crecelius, A.; Gottschaldt, M.; Schubert, U.S. Systematic MALDI-TOF CID Investigation on Different Substituted mPEG 2000. Macromol. Chem. Phys. 2010, 211, 677–684. [Google Scholar] [CrossRef]
- Scionti, V.; Wesdemiotis, C. Tandem Mass Spectrometry Analysis of Polymer Structures and Architectures. In Mass Spectrometry in Polymer Chemistry; Barner-Kowollik, C., Gruendling, T., Falkenhagen, J., Weidner, S., Eds.; John Wiley & Sons, Ltd.: Weinheim, Germany, 2012; pp. 57–84. [Google Scholar] [CrossRef]
- Yol, A.M.; Dabney, D.E.; Wang, S.-F.; Laurent, B.A.; Foster, M.D.; Quirk, R.P.; Grayson, S.M.; Wesdemiotis, C. Differentiation of Linear and Cyclic Polymer Architectures by MALDI Tandem Mass Spectrometry (MALDI-MS2). J. Am. Soc. Mass Spectrom. 2013, 24, 74–82. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Puglisi, C.; Montaudo, G. Sequence determination in aliphatic poly(ester amide)s by matrix-assisted laser desorption/ionization time-of-flight and time-of-flight/time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 2407–2418. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Puglisi, C.; Montaudo, G. Matrix-assisted laser desorption/ionization time-of-flight/time-of-flight tandem mass spectra of poly(butylene adipate). Rapid Commun. Mass Spectrom. 2006, 20, 1683–1694. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Puglisi, C. Structural characterization of synthetic poly(ester amide) from sebacic acid and 4-amino-1-butanol by matrix-assisted laser desorption ionization time-of-flight/time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 739–754. [Google Scholar] [CrossRef]
- De Winter, J.; Lemaur, V.; Marsal, P.; Coulembier, O.; Cornil, J.; Dubois, P.; Gerbaux, P. Mechanistic study of the collision-induced dissociation of sodium-cationized polylactide oligomers: A joint experimental and theoretical investigation. J. Am. Soc. Mass Spectrom. 2010, 21, 1159–1168. [Google Scholar] [CrossRef]
- Scionti, V.; Wesdemiotis, C. Electron transfer dissociation versus collisionally activated dissociation of cationized biodegradable polyesters. J. Mass Spectrom. 2012, 47, 1442–1449. [Google Scholar] [CrossRef]
- Rizzarelli, P. Matrix-assisted laser desorption ionization time-of-flight/time-of-flight tandem mass spectra of biodegradable polybutylenesuccinate. Rapid Commun. Mass Spectrom. 2013, 27, 2213–2225. [Google Scholar] [CrossRef]
- Prian, K.; Aloui, I.; Legros, V.; Buchmann, W. Study of the gas-phase decomposition of multiply lithiated polycaprolactone, polytetrahydrofurane and their copolymer by two different activation methods: Collision-induced dissociation and electron transfer dissociation. Anal. Chim. Acta 2019, 1048, 85–95. [Google Scholar] [CrossRef]
- Rizzarelli, P.; La Carta, S.; Mirabella, E.F.; Rapisarda, M.; Impallomeni, G. Sequencing Biodegradable and Potentially Biobased Polyesteramide of Sebacic Acid and 3-Amino-1-propanol by MALDI TOF-TOF Tandem Mass Spectrometry. Polymers 2022, 14, 1500. [Google Scholar] [CrossRef]
- Duale, K.; Latos, P.; Chrobok, A.; Domiński, A.; Maksymiak, M.M.; Adamus, G.; Kowalczuk, M. Towards Advances in Molecular Understanding of Boric Acid Biocatalyzed Ring-Opening (Co)Polymerization of δ-Valerolactone in the Presence of Ethylene Glycol as an Initiator. Molecules 2021, 26, 4859. [Google Scholar] [CrossRef] [PubMed]
- Koster, S.; Duursma, M.C.; Guo, X.; van Benthem, R.A.T.M.; de Koster, C.G.; Boon, J.J.; Heeren, R.M.A. Isomer separation of hyperbranched polyesteramides with gas-phase H/D exchange and a novel MSn approach: DoDIP. J. Mass Spectrom. 2002, 37, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Terrier, P.; Buchmann, W.; Desmazières, B.; Tortajada, J. Block Lengths and Block Sequence of Linear Triblock and Glycerol Derivative Diblock Copolyethers by Electrospray Ionization−Collision-Induced Dissociation Mass Spectrometry. Anal. Chem. 2006, 78, 1801–1806. [Google Scholar] [CrossRef]
- Adamus, G. Structural analysis of poly[(R,S)-3-hydroxybutyrate-co-L-lactide] copolyesters by electrospray ionization ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 2477–2490. [Google Scholar] [CrossRef]
- Weidner, S.M.; Falkenhagen, J.; Maltsev, S.; Sauerland, V.; Rinken, M. A novel software tool for copolymer characterization by coupling of liquid chromatography with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 2750–2758. [Google Scholar] [CrossRef]
- Osaka, I.; Yoshimoto, A.; Watanabe, M.; Takama, M.; Murakami, M.; Kawasaki, H.; Arakawa, R. Quantitative determination of cyclic polylactic acid oligomers in serum by direct injection liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2008, 870, 247–250. [Google Scholar] [CrossRef]
- Adamus, G. Molecular Level Structure of (R,S)-3-Hydroxybutyrate/(R,S)-3-Hydroxy-4-ethoxybutyrate Copolyesters with Dissimilar Architecture. Macromolecules 2009, 42, 4547–4557. [Google Scholar] [CrossRef]
- Weidner, S.M.; Falkenhagen, J.; Knop, K.; Thünemann, A. Structure and end-group analysis of complex hexanediol-neopentylglycol-adipic acid copolyesters by matrix-assisted laser desorption/ionization collision-induced dissociation tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 2768–2774. [Google Scholar] [CrossRef]
- Kwiecień, I.; Adamus, G.; Kowalczuk, M. Electrospray ionisation mass spectrometry molecular-level structural characterisation of novel phenoxycarboxylic acid-oligo(3-hydroxybutyrate) conjugates with potential agricultural applications. Rapid Commun. Mass Spectrom. 2012, 26, 2673–2682. [Google Scholar] [CrossRef]
- De Winter, J.; Coulembier, O.; Dubois, P.; Gerbaux, P. Collision-induced dissociation of polymer ions: Charge driven decomposition for sodium-cationized polylactides and isomeric end-group distinction. Int. J. Mass Spectrom. 2011, 308, 11–17. [Google Scholar] [CrossRef]
- Maksymiak, M.; Debowska, R.; Jelonek, K.; Kowalczuk, M.; Adamus, G. Structural characterization of biocompatible lipoic acid-oligo-(3-hydroxybutyrate) conjugates by electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Khare, V.; Kour, S.; Alam, N.; Dubey, R.D.; Saneja, A.; Koul, M.; Gupta, A.P.; Singh, D.; Singh, S.K.; Saxena, A.K.; et al. Synthesis, characterization and mechanistic-insight into the anti-proliferative potential of PLGA-gemcitabine conjugate. Int. J. Pharm. 2014, 470, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Maksymiak, M.; Kowalczuk, M.; Adamus, G. Electrospray tandem mass spectrometry for the structural characterization of p-coumaric acid–oligo(3-hydroxybutyrate) conjugates. Int. J. Mass Spectrom. 2014, 359, 6–11. [Google Scholar] [CrossRef]
- Josse, T.; De Winter, J.; Dubois, P.; Coulembier, O.; Gerbaux, P.; Memboeuf, A. A tandem mass spectrometry-based method to assess the architectural purity of synthetic polymers: A case of a cyclic polylactide obtained by click chemistry. Polym. Chem. 2014, 6, 64–69. [Google Scholar] [CrossRef]
- Shirangi, M.; Hennink, W.E.; Somsen, G.W.; Van Nostrum, C.F. Identification and Assessment of Octreotide Acylation in Polyester Microspheres by LC–MS/MS. Pharm. Res. 2015, 32, 3044–3054. [Google Scholar] [CrossRef]
- Kwiecień, I.; Radecka, I.; Kowalczuk, M.; Adamus, G. Transesterification of PHA to Oligomers Covalently Bonded with (Bio)Active Compounds Containing Either Carboxyl or Hydroxyl Functionalities. PLoS ONE 2015, 10, e0120149. [Google Scholar] [CrossRef]
- Kwiecień, I.; Radecka, I.; Kwiecień, M.; Adamus, G. Synthesis and Structural Characterization of Bioactive PHA and γ-PGA Oligomers for Potential Applications as a Delivery System. Materials 2016, 9, 307. [Google Scholar] [CrossRef]
- Maksymiak, M.; Bałakier, T.; Jurczak, J.; Kowalczuk, M.; Adamus, G. Bioactive (co)oligoesters with antioxidant properties—Synthesis and structural characterization at the molecular level. RSC Adv. 2016, 6, 57751–57761. [Google Scholar] [CrossRef]
- Kang, M.J.; Seong, Y.; Kim, M.Y.; Pyun, J.C. Analysis of polymer characteristics using matrix-assisted laser desorp-tion/ionization time-of-flight mass spectrometry. Appl. Chem. Eng. 2017, 28, 263–271. [Google Scholar] [CrossRef]
- Johnston, B.; Jiang, G.; Hill, D.; Adamus, G.; Kwiecień, I.; Zięba, M.; Sikorska, W.; Green, M.; Kowalczuk, M.; Radecka, I. The Molecular Level Characterization of Biodegradable Polymers Originated from Polyethylene Using Non-Oxygenated Polyethylene Wax as a Carbon Source for Polyhydroxyalkanoate Production. Bioengineering 2017, 4, 73. [Google Scholar] [CrossRef]
- Adamus, G.; Kurcok, P.; Radecka, I.; Kowalczuk, M. Bioactive oligomers from natural polyhydroxyalkanoates and their synthetic analogues. Polimery 2017, 62, 317–322. [Google Scholar] [CrossRef]
- Alalwiat, A.; Tang, W.; Gerişlioğlu, S.; Becker, M.L.; Wesdemiotis, C. Mass Spectrometry and Ion Mobility Characterization of Bioactive Peptide–Synthetic Polymer Conjugates. Anal. Chem. 2016, 89, 1170–1177. [Google Scholar] [CrossRef]
- Sallam, S.; Luo, Y.; Becker, M.L.; Wesdemiotis, C. Multidimensional mass spectrometry characterization of isomeric biodegradable polyesters. Eur. J. Mass Spectrom. 2017, 23, 402–410. [Google Scholar] [CrossRef]
- Sallam, S.; Dolog, I.; Paik, B.A.; Jia, X.; Kiick, K.L.; Wesdemiotis, C. Sequence and Conformational Analysis of Peptide–Polymer Bioconjugates by Multidimensional Mass Spectrometry. Biomacromolecules 2018, 19, 1498–1507. [Google Scholar] [CrossRef]
- Omer, E.; Cariou, R.; Remaud, G.; Guitton, Y.; Germon, H.; Hill, P.; Dervilly-Pinel, G.; Le Bizec, B. Elucidation of non-intentionally added substances migrating from polyester-polyurethane lacquers using automated LC-HRMS data processing. Anal. Bioanal. Chem. 2018, 410, 5391–5403. [Google Scholar] [CrossRef]
- Peptu, C.; Danchenko, M.; Škultéty, L.; Mosnáček, J. Structural Architectural Features of Cyclodextrin Oligoesters Revealed by Fragmentation Mass Spectrometry Analysis. Molecules 2018, 23, 2259. [Google Scholar] [CrossRef]
- Johnston, B.; Radecka, I.; Hill, D.; Chiellini, E.; Ilieva, V.I.; Sikorska, W.; Musioł, M.; Zięba, M.; Marek, A.A.; Keddie, D.; et al. The Microbial Production of Polyhydroxyalkanoates from Waste Polystyrene Fragments Attained Using Oxidative Degradation. Polymers 2018, 10, 957. [Google Scholar] [CrossRef]
- Duale, K.; Zięba, M.; Chaber, P.; Di Fouque, D.J.; Memboeuf, A.; Peptu, C.; Radecka, I.; Kowalczuk, M.; Adamus, G. Molecular Level Structure of Biodegradable Poly(Delta-Valerolactone) Obtained in the Presence of Boric Acid. Molecules 2018, 23, 2034. [Google Scholar] [CrossRef]
- Ashby, R.D.; Solaiman, D.K.; Nuñez, A.; Strahan, G.D.; Johnston, D.B. Burkholderia sacchari DSM 17165: A source of compositionally-tunable block-copolymeric short-chain poly(hydroxyalkanoates) from xylose and levulinic acid. Bioresour. Technol. 2018, 253, 333–342. [Google Scholar] [CrossRef]
- Fouquet, T.N.J.; Pizzala, H.; Rollet, M.; Crozet, D.; Giusti, P.; Charles, L. Mass Spectrometry-Based Analytical Strategy for Comprehensive Molecular Characterization of Biodegradable Poly(lactic-co-glycolic Acid) Copolymers. J. Am. Soc. Mass Spectrom. 2020, 31, 1554–1562. [Google Scholar] [CrossRef]
- Alalwiat, A.; Grieshaber, S.E.; Paik, B.A.; Kiick, K.L.; Jia, X.; Wesdemiotis, C. Top-down mass spectrometry of hybrid materials with hydrophobic peptide and hydrophilic or hydrophobic polymer blocks. Analyst 2015, 140, 7550–7564. [Google Scholar] [CrossRef] [PubMed]
- Mikami, K.; Lonnecker, A.T.; Gustafson, T.P.; Zinnel, N.F.; Pai, P.-J.; Russell, D.H.; Wooley, K.L. Polycarbonates Derived from Glucose via an Organocatalytic Approach. J. Am. Chem. Soc. 2013, 135, 6826–6829. [Google Scholar] [CrossRef] [PubMed]
- Blaj, D.-A.; Balan-Porcarasu, M.; Petre, B.A.; Harabagiu, V.; Peptu, C. MALDI mass spectrometry monitoring of cyclodextrin-oligolactide derivatives synthesis. Polymer 2021, 233, 124186. [Google Scholar] [CrossRef]
- Peptu, C.; Blaj, D.-A.; Balan-Porcarasu, M.; Rydz, J. Cyclodextrin-Oligocaprolactone Derivatives—Synthesis and Advanced Structural Characterization by MALDI Mass Spectrometry. Polymers 2022, 14, 1436. [Google Scholar] [CrossRef]
- Brochhausen, C.; Zehbe, R.; Watzer, B.; Halstenberg, S.; Gabler, F.; Schubert, H.; Kirkpatrick, C.J. Immobilization and controlled release of prostaglandin E2from poly-L-lactide-co-glycolide microspheres. J. Biomed. Mater. Res. Part A 2008, 91, 454–462. [Google Scholar] [CrossRef]
- Owen, S.C.; Li, H.; Sanders, W.G.; Cheung, A.K.; Terry, C.M. Correlation of tissue drug concentrations with in vivo magnetic resonance images of polymer drug depot around arteriovenous graft. J. Control. Release 2010, 146, 23–30. [Google Scholar] [CrossRef]
- Chen, S.; Pederson, D.; Oak, M.; Singh, J. In Vivo Absorption of Steroidal Hormones from Smart Polymer Based Delivery Systems. J. Pharm. Sci. 2010, 99, 3381–3388. [Google Scholar] [CrossRef]
- Ashiru, D.A.; Karu, K.; Zloh, M.; Patel, R.; Basit, A.W. Relative quantification of polyethylene glycol 400 excreted in the urine of male and female volunteers by direct injection electrospray-selected ion monitoring mass spectrometry. Int. J. Pharm. 2011, 414, 35–41. [Google Scholar] [CrossRef]
- Bhaskar, V.V.; Middha, A.; Tiwari, S.; Shivakumar, S. Liquid chromatography/tandem mass spectrometry method for quantitative estimation of polyethylene glycol 400 and its applications. J. Chromatogr. B 2013, 926, 68–76. [Google Scholar] [CrossRef]
- Warrack, B.M.; Redding, B.P.; Chen, G.; Bolgar, M.S. Determination of the molecular weight of poly(ethylene glycol) in biological samples by reversed-phase LC–MS with in-source fragmentation. Anal. Bioanal. Chem. 2013, 405, 4283–4287. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, X.; Song, F.; Liu, Z.; Xie, Z.; Jing, X. Studies on the biological character of a new pH-sensitive doxorubicin prodrug with tumor targeting using a LC-MS/MS method. Anal. Methods 2014, 6, 3159–3166. [Google Scholar] [CrossRef]
- Tappa, K.; Jammalamadaka, U.; Ballard, D.H.; Bruno, T.; Israel, M.R.; Vemula, H.; Meacham, J.; Mills, D.K.; Woodard, P.K.; Weisman, J.A. Medication eluting devices for the field of OBGYN (MEDOBGYN): 3D printed biodegradable hormone eluting constructs, a proof of concept study. PLoS ONE 2017, 12, e0182929. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, J.; Li, B.; Yang, X.; Zeng, R.; Liu, Y.; Li, T.; Ho, R.J.; Shao, J. PLGA-PEG-PLGA triblock copolymeric micelles as oral drug delivery system: In vitro drug release and in vivo pharmacokinetics assessment. J. Colloid Interface Sci. 2017, 490, 542–552. [Google Scholar] [CrossRef]
- Lv, L.; Liu, C.; Li, Z.; Song, F.; Li, G.; Huang, X. Pharmacokinetics of Quercetin-Loaded Methoxy Poly(ethylene glycol)-b-poly(L-lactic acid) Micelle after Oral Administration in Rats. BioMed Res. Int. 2017, 2017, 1750895. [Google Scholar] [CrossRef]
- Zhou, X.; Meng, X.; Cheng, L.; Su, C.; Sun, Y.; Sun, L.; Tang, Z.; Fawcett, J.P.; Yang, Y.; Gu, J. Development and Application of an MSALL-Based Approach for the Quantitative Analysis of Linear Polyethylene Glycols in Rat Plasma by Liquid Chromatography Triple-Quadrupole/Time-of-Flight Mass Spectrometry. Anal. Chem. 2017, 89, 5193–5200. [Google Scholar] [CrossRef]
- Perteghella, S.; Sottani, C.; Coccè, V.; Negri, S.; Cavicchini, L.; Alessandri, G.; Cottica, D.; Torre, M.L.; Grignani, E.; Pessina, A. Paclitaxel-Loaded Silk Fibroin Nanoparticles: Method Validation by UHPLC-MS/MS to Assess an Exogenous Approach to Load Cytotoxic Drugs. Pharmaceutics 2019, 11, 285. [Google Scholar] [CrossRef]
- Samy, K.E.; Cao, Y.; Kim, J.; da Silva, N.R.K.; Phone, A.; Bloomer, M.M.; Bhisitkul, R.B.; Desai, T.A. Co-Delivery of Timolol and Brimonidine with a Polymer Thin-Film Intraocular Device. J. Ocul. Pharmacol. Ther. 2019, 35, 124–131. [Google Scholar] [CrossRef]
- Creighton, R.L.; Suydam, I.T.; Ebner, M.E.; Afunugo, W.E.; Bever, A.M.; Cao, S.; Jiang, Y.; Woodrow, K.A. Sustained Intracellular Raltegravir Depots Generated with Prodrugs Designed for Nanoparticle Delivery. ACS Biomater. Sci. Eng. 2019, 5, 4013–4022. [Google Scholar] [CrossRef]
- Alshetaili, A.S.; Ansari, M.J.; Anwer, K.; Ganaie, M.A.; Iqbal, M.; Alshahrani, S.M.; Alalaiwe, A.S.; Alsulays, B.B.; Alshehri, S.; Sultan, A.S. Enhanced Oral Bioavailability of Ibrutinib Encapsulated Poly(Lactic-co-Glycolic Acid) Nanoparticles: Pharmacokinetic Evaluation in Rats. Curr. Pharm. Anal. 2019, 15, 661–668. [Google Scholar] [CrossRef]
- Nedorubov, A.A.; Pavlov, A.N.; Pyatigorskaya, N.V.; Brkich, G.E.; Shabalina, M.M. Pharmacokinetics of Nanosomal Form of Levodopa in Intranasal Administration. Maced. J. Med. Sci. 2019, 7, 3509–3513. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, D.; Zhang, X.; Wang, D.; Dongye, M.; Chen, W.; Lin, D.; Zhu, F.; Chen, W.; Lin, H. Development and effects of tacrolimus-loaded nanoparticles on the inhibition of corneal allograft rejection. Drug Deliv. 2019, 26, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Jiang, H.; Yin, L.; Liu, Y.; Xu, M. Development of an UPLC-MS/MS method coupled with in-source CID for quantitative analysis of PEG-PLA copolymer and its application to a pharmacokinetic study in rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1125, 121716. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Alam, A.; Ahmad, F.; Amir, M.; Pottoo, F.; Sarafroz, M.; Jafar, M.; Umar, K. Daunorubicin oral bioavailability enhancement by surface coated natural biodegradable macromolecule chitosan based polymeric nanoparticles. Int. J. Biol. Macromol. 2019, 128, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, D.; Sun, D.; Gu, J. Current status of in vivo bioanalysis of nano drug delivery systems. J. Pharm. Anal. 2020, 10, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Ren, T.; Zhao, S.; Shi, M.; Gu, J. Comparative pharmacokinetic study of PEGylated gemcitabine and gemcitabine in rats by LC-MS/MS coupled with pre-column derivatization and MSALL technique. Talanta 2019, 206, 120184. [Google Scholar] [CrossRef]
- Lv, L.; Li, D.; Wang, H.; Li, C.; Qian, X.; Dong, H.-T.; Sun, J.; Zhao, L. Quantitation of lanosterol in the vitreous humor of rabbits after ocular administration of lanosterol/thermogel formulation by ultra high performance liquid chromatography–tandem mass spectrometry with the electrospray ionization mode. J. Chromatogr. A 2017, 1519, 83–90. [Google Scholar] [CrossRef]
- Woo, G.; Mittelman, M.; Santerre, J. Synthesis and characterization of a novel biodegradable antimicrobial polymer. Biomaterials 2000, 21, 1235–1246. [Google Scholar] [CrossRef]
- Elliott, S.L.; Fromstein, J.D.; Woodhous, K.A. Identification of biodegradation products formed by L-phenylalanine based segmented polyurethaneureas. J. Biomater. Sci. Polym. Ed. 2002, 13, 691–711. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Impallomeni, G.; Montaudo, G. Evidence for Selective Hydrolysis of Aliphatic Copolyesters Induced by Lipase Catalysis. Biomacromolecules 2004, 5, 433–444. [Google Scholar] [CrossRef]
- Osaka, I.; Watanabe, M.; Takama, M.; Murakami, M.; Arakawa, R. Characterization of linear and cyclic polylactic acids and their solvolysis products by electrospray ionization mass spectrometry. J. Mass Spectrom. 2006, 41, 1369–1377. [Google Scholar] [CrossRef]
- Hakkarainen, M.; Adamus, G.; Höglund, A.; Kowalczuk, M.; Albertsson, A.-C. ESI-MS Reveals the Influence of Hydrophilicity and Architecture on the Water-Soluble Degradation Product Patterns of Biodegradable Homo- and Copolyesters of 1,5-dioxepan-2-one and ε-Caprolactone. Macromolecules 2008, 41, 3547–3554. [Google Scholar] [CrossRef]
- Höglund, A.; Hakkarainen, M.; Kowalczuk, M.; Adamus, G.; Albertsson, A.-C. Fingerprinting the degradation product patterns of different polyester-ether networks by electrospray ionization mass spectrometry. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 4617–4629. [Google Scholar] [CrossRef]
- Pulkkinen, M.; Palmgrén, J.J.; Auriola, S.; Malin, M.; Seppälä, J.; Järvinen, K. High-performance liquid chromatography/electrospray ionization tandem mass spectrometry for characterization of enzymatic degradation of 2,2′-bis(2-oxazoline)-linked poly-ε-caprolactone. Rapid Commun. Mass Spectrom. 2007, 22, 121–129. [Google Scholar] [CrossRef]
- Song, J.; Šišková, A.; Simons, M.G.; Kowalski, W.J.; Kowalczuk, M.M.; van den Brink, O.F. LC-Multistage Mass Spectrometry for the Characterization of Poly(Butylene Adipate-co-Butylene Terephthalate) Copolyester. J. Am. Soc. Mass Spectrom. 2011, 22, 641–648. [Google Scholar] [CrossRef]
- Wang, H.-J.; Zhang, Y.; Kato, S.; Nakagawa, K.; Kimura, F.; Miyazawa, T.; Wang, J.-Y. HPLC-MS/MS: A potential method to track the in vivo degradation of zein-based biomaterial. J. Biomed. Mater. Res. Part A 2017, 106, 606–613. [Google Scholar] [CrossRef]
- Musioł, M.; Jurczyk, S.; Sobota, M.; Klim, M.; Sikorska, W.; Zięba, M.; Janeczek, H.; Rydz, J.; Kurcok, P.; Johnston, B.; et al. (Bio)Degradable Polymeric Materials for Sustainable Future—Part 3: Degradation Studies of the PHA/Wood Flour-Based Composites and Preliminary Tests of Antimicrobial Activity. Materials 2020, 13, 2200. [Google Scholar] [CrossRef]
- Kwiecień, I.; Bałakier, T.; Jurczak, J.; Kowalczuk, M.; Adamus, G. Molecular architecture of novel potentially bioactive (co)oligoesters containing pesticide moieties established by electrospray ionization multistage mass spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 533–544. [Google Scholar] [CrossRef]
- Johnston, B.; Radecka, I.; Chiellini, E.; Barsi, D.; Ilieva, V.I.; Sikorska, W.; Musioł, M.; Zięba, M.; Chaber, P.; Marek, A.A.; et al. Mass Spectrometry Reveals Molecular Structure of Polyhydroxyalkanoates Attained by Bioconversion of Oxidized Polypropylene Waste Fragments. Polymers 2019, 11, 1580. [Google Scholar] [CrossRef]
- Radecka, I.; Irorere, V.; Jiang, G.; Hill, D.; Williams, C.; Adamus, G.; Kwiecień, M.; Marek, A.A.; Zawadiak, J.; Johnston, B.; et al. Oxidized Polyethylene Wax as a Potential Carbon Source for PHA Production. Materials 2016, 9, 367. [Google Scholar] [CrossRef]
- Ekere, A.I.; Johnston, B.; Zięba, M.; Chaber, P.; Adamus, G.; Tchuenbou-Magaia, F.; Barsi, D.; Amaro, L.P.; Chiellini, E.; Radecka, I.; et al. Environmental cleaning mission. Chim. Oggi-Chem. Today 2019, 37, 36–39. [Google Scholar]
- Ekere, I.; Johnston, B.; Tchuenbou-Magaia, F.; Townrow, D.; Wojciechowski, S.; Marek, A.; Zawadiak, J.; Duale, K.; Zieba, M.; Sikorska, W.; et al. Bioconversion Process of Polyethylene from Waste Tetra Pak Packaging to Polyhydroxyalkanoates. Polymers 2022, 14, 2840. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.-J.; Zhang, X.-H.; Zhang, Y.-Y.; Du, B.-Y.; Fan, Z.-Q.; Qi, G.-R. Functional poly(carbonate-co-ether) synthesis from glycidyl methacrylate/CO2 copolymerization catalyzed by Zn–Co(iii) double metal cyanide complex catalyst. RSC Adv. 2014, 4, 3188–3194. [Google Scholar] [CrossRef]
- Arnould, M.; Vargas, R.; Buehner, R.W.; Wesdemiotis, C. Tandem Mass Spectrometry Characteristics of Polyester Anions and Cations Formed by Electrospray Ionization. Eur. J. Mass Spectrom. 2005, 11, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Rizzarelli, P.; La Carta, S.; Rapisarda, M.; Valenti, G. Chapter 13-Analytical methods in resorbable polymer development and degradation tracking. In Materials for Biomedical Engineering: Absorbable Polymers; Grumezescu, V., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 351–408. [Google Scholar] [CrossRef]
- Byrd, H.C.M.; McEwen, C.N. The Limitations of MALDI-TOF Mass Spectrometry in the Analysis of Wide Polydisperse Polymers. Anal. Chem. 2000, 72, 4568–4576. [Google Scholar] [CrossRef]
- Nielen, M.W. Maldi time-of-flight mass spectrometry of synthetic polymers. Mass Spectrom. Rev. 1999, 18, 309–344. [Google Scholar] [CrossRef]
- Medzihradszky, K.F.; Campbell, J.M.; Baldwin, M.A.; Falick, A.M.; Juhasz, P.; Vestal, M.L.; Burlingame, A.L. The Characteristics of Peptide Collision-Induced Dissociation Using a High-Performance MALDI-TOF/TOF Tandem Mass Spectrometer. Anal. Chem. 2000, 72, 552–558. [Google Scholar] [CrossRef]
- Weidner, S.M.; Kricheldorf, H.R. Matrix-assisted laser desorption/ionization behavior of neat linear and cyclic poly(L-lactide)s and their blends. Rapid Commun. Mass Spectrom. 2020, 34, e8673. [Google Scholar] [CrossRef]
- Naito, Y.; Kotani, M.; Ohmura, T. A novel laser desorption/ionization method using through hole porous alumina membranes. Rapid Commun. Mass Spectrom. 2018, 32, 1851–1858. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of UV Degradation and Stabilization, 2nd ed.; ChemTec Publishing: Toronto, ON, Canada, 2015. [Google Scholar] [CrossRef]
- Perale, G.; Hilborn, J. Bioresorbable Polymers for Biomedical Applications. In From Fundamentals to Translational Medicine; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Natarajan, J.V.; Nugraha, C.; Ng, X.W.; Venkatraman, S. Sustained-release from nanocarriers: A review. J. Control. Release 2014, 193, 122–138. [Google Scholar] [CrossRef]

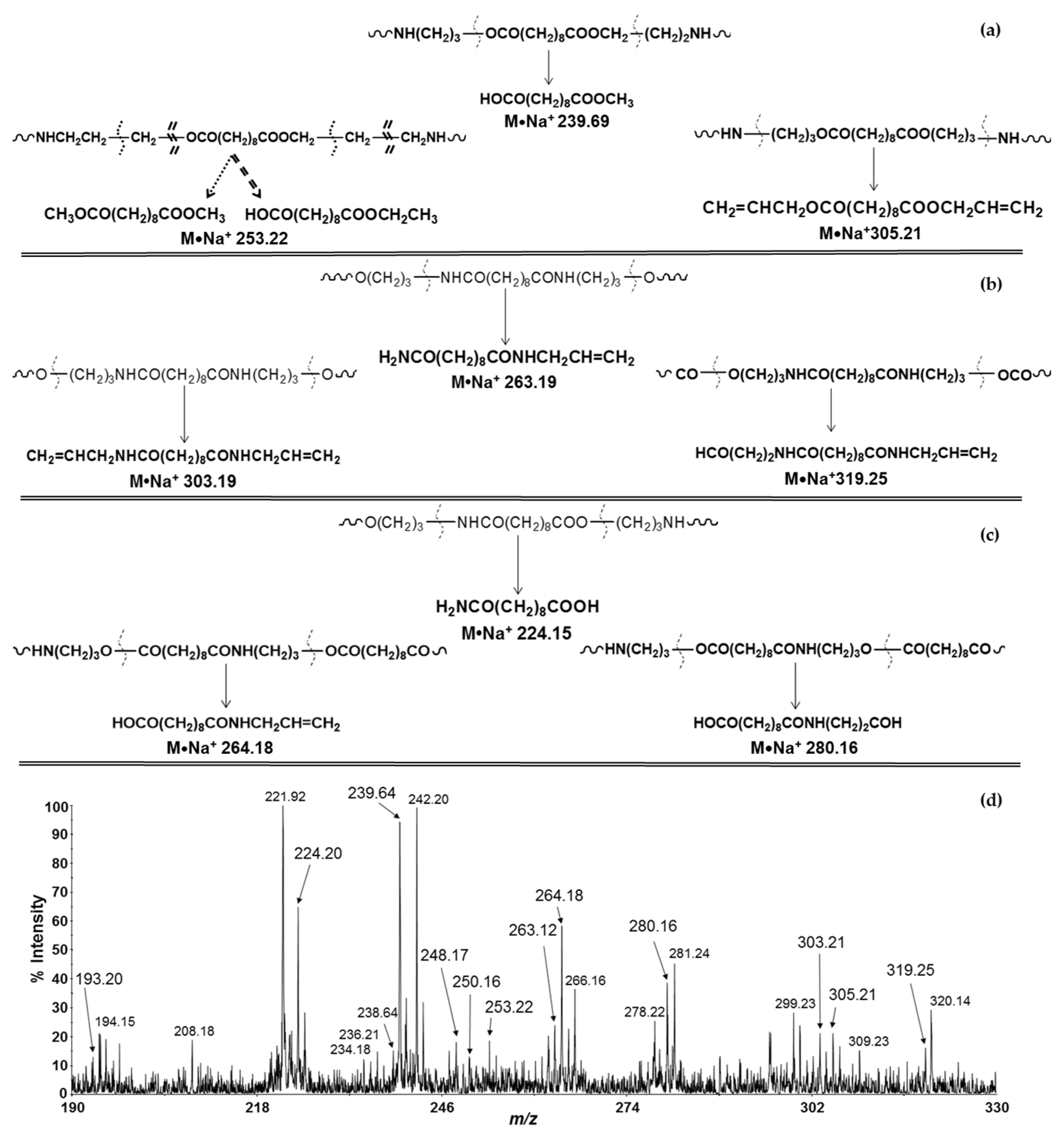
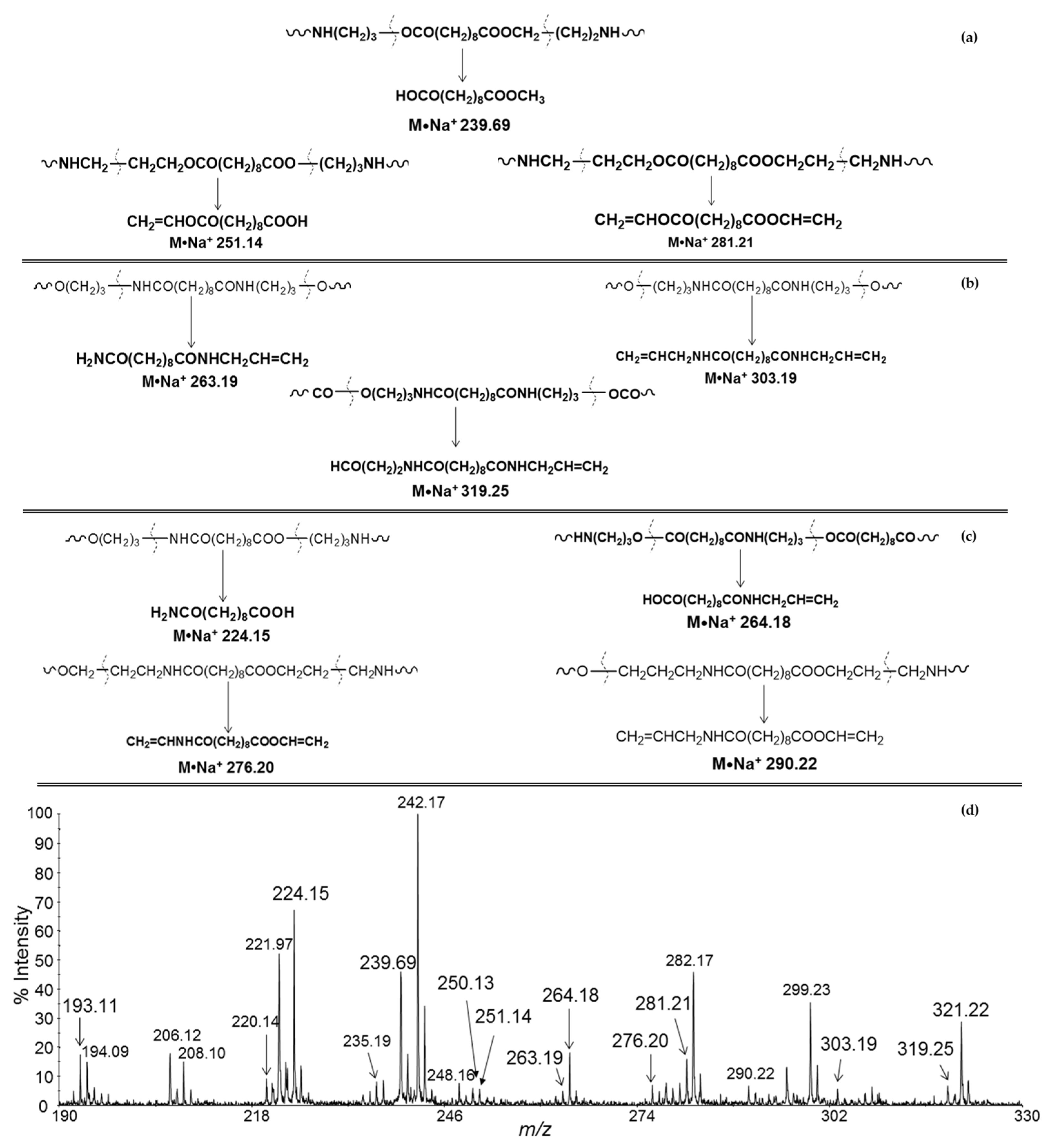
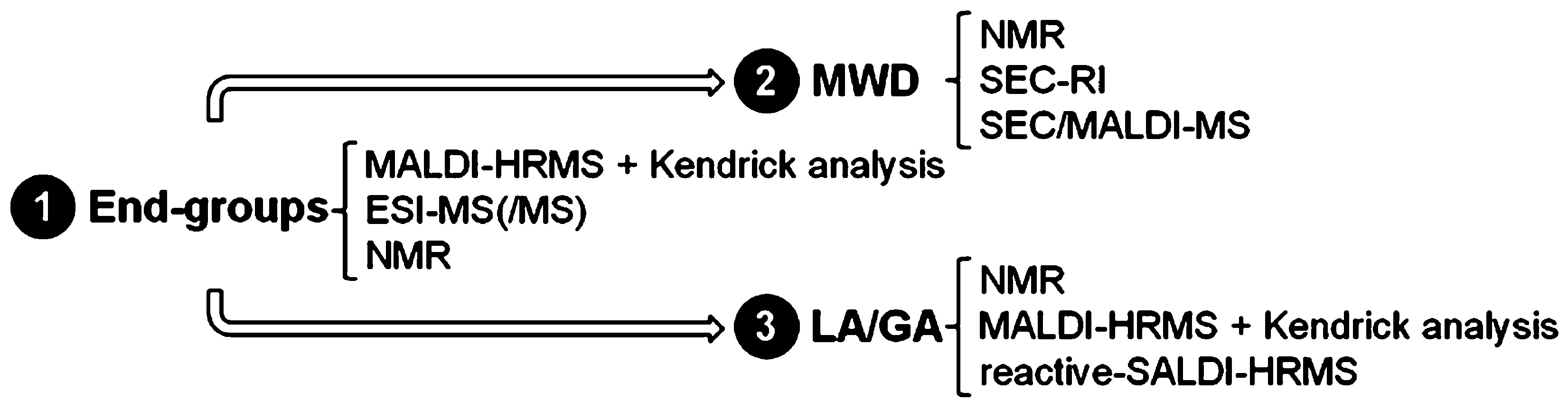
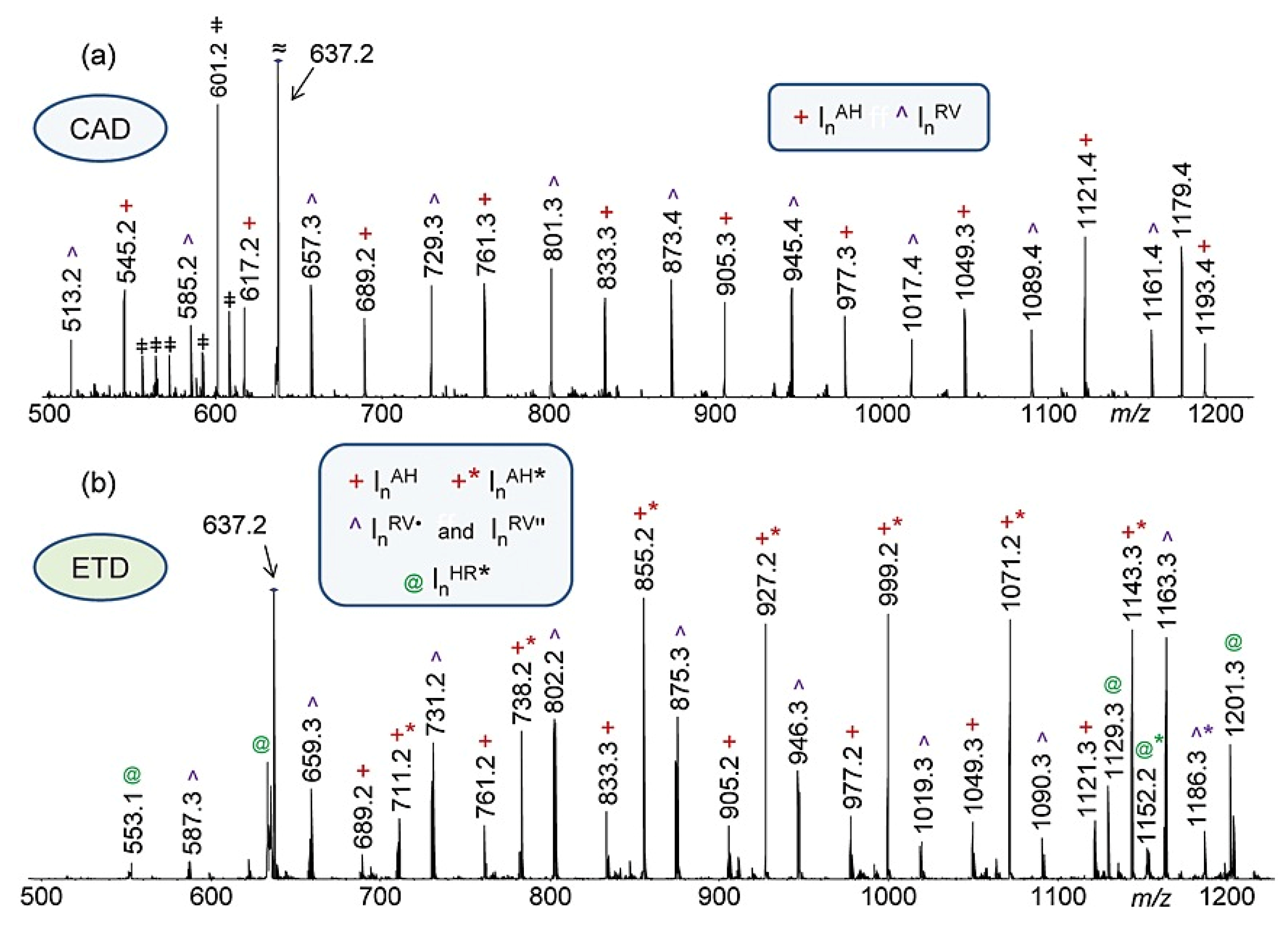
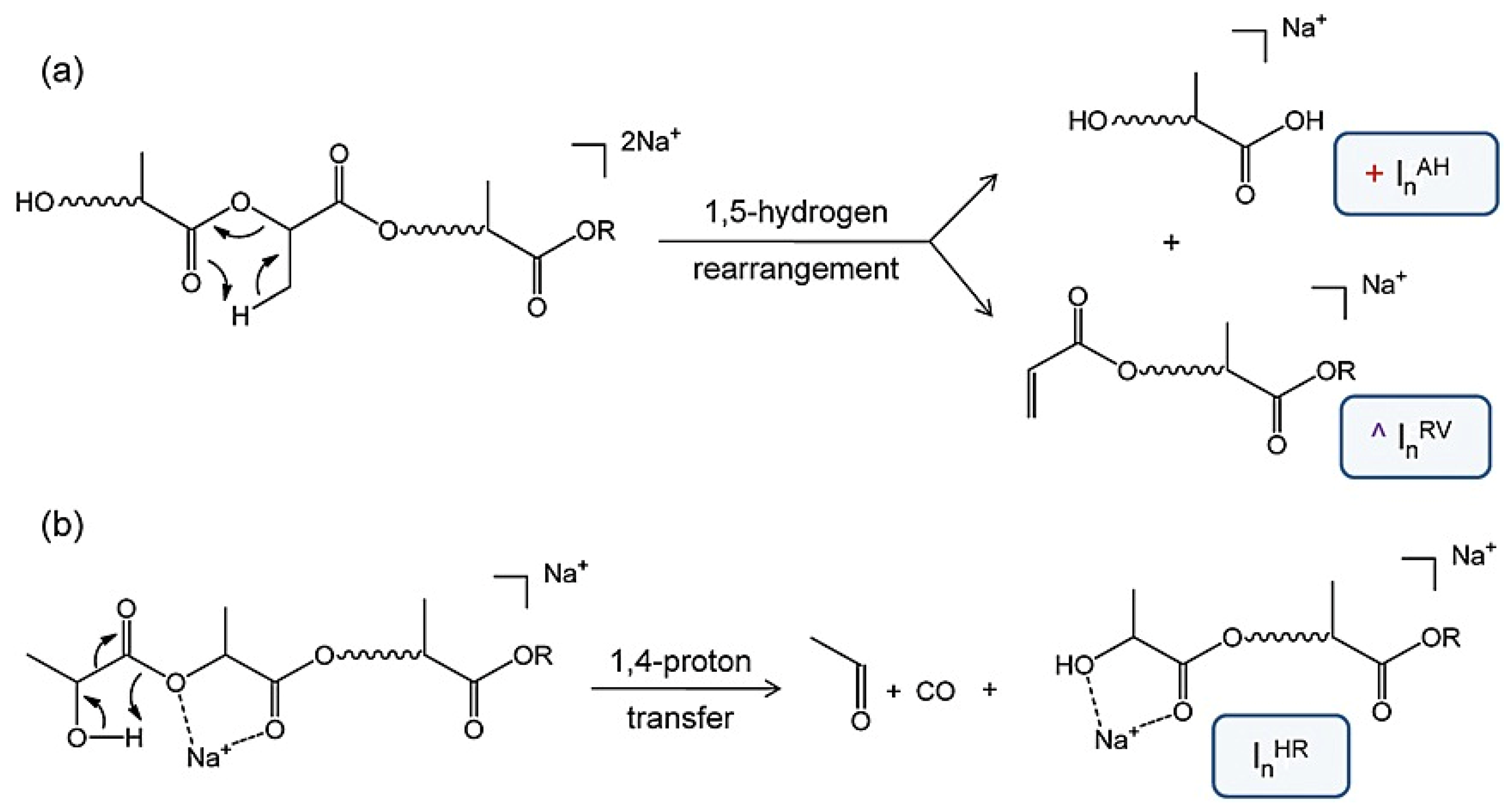
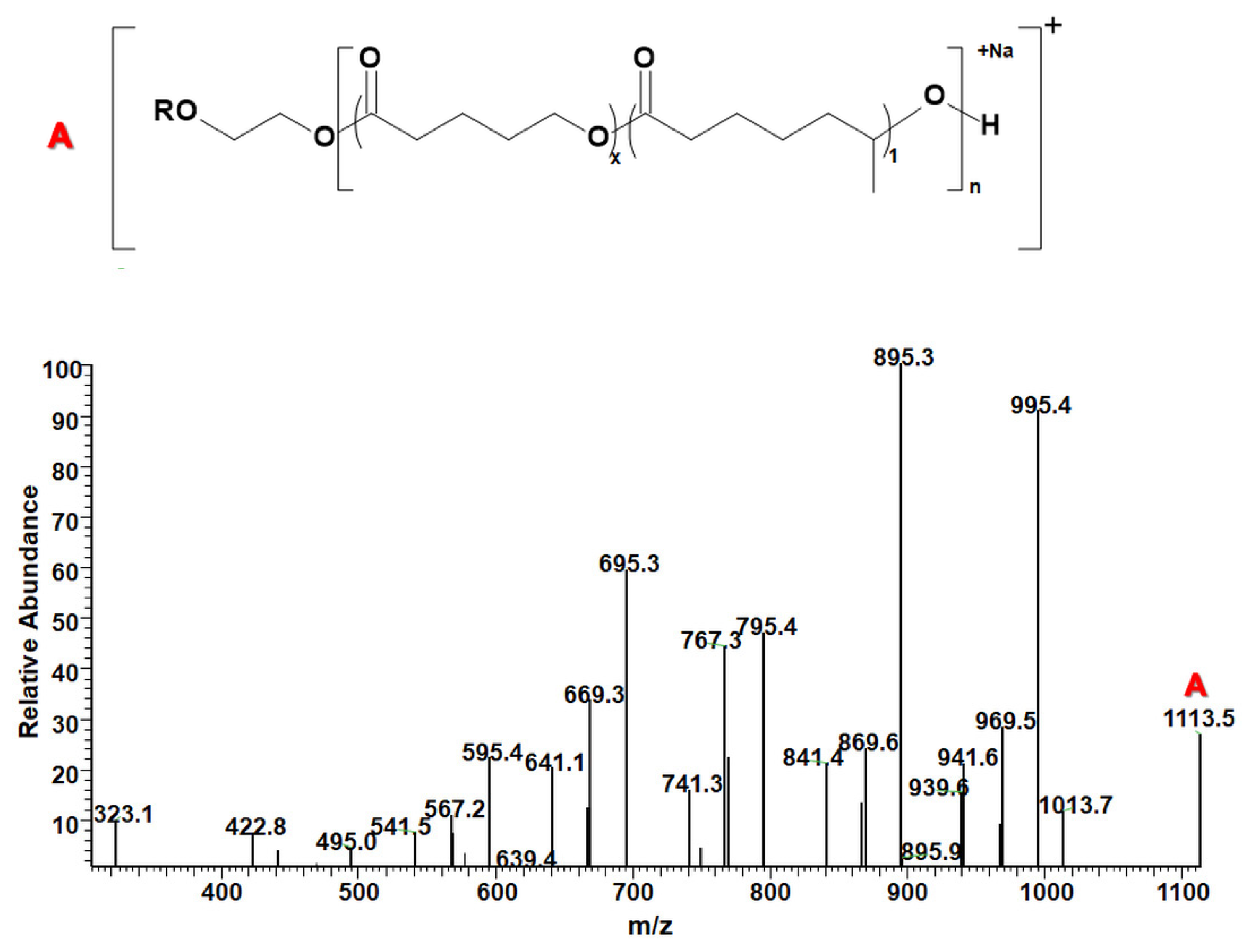
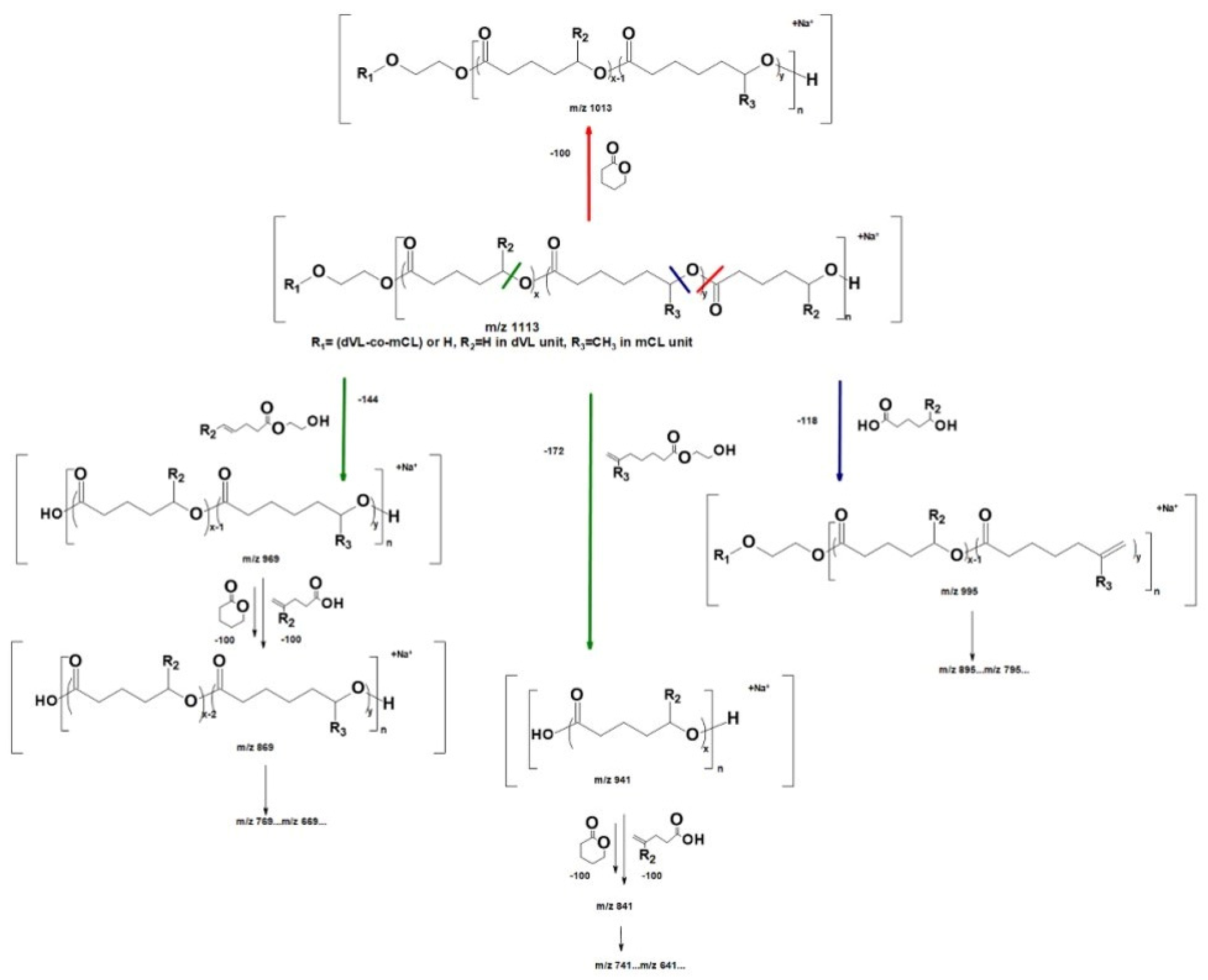
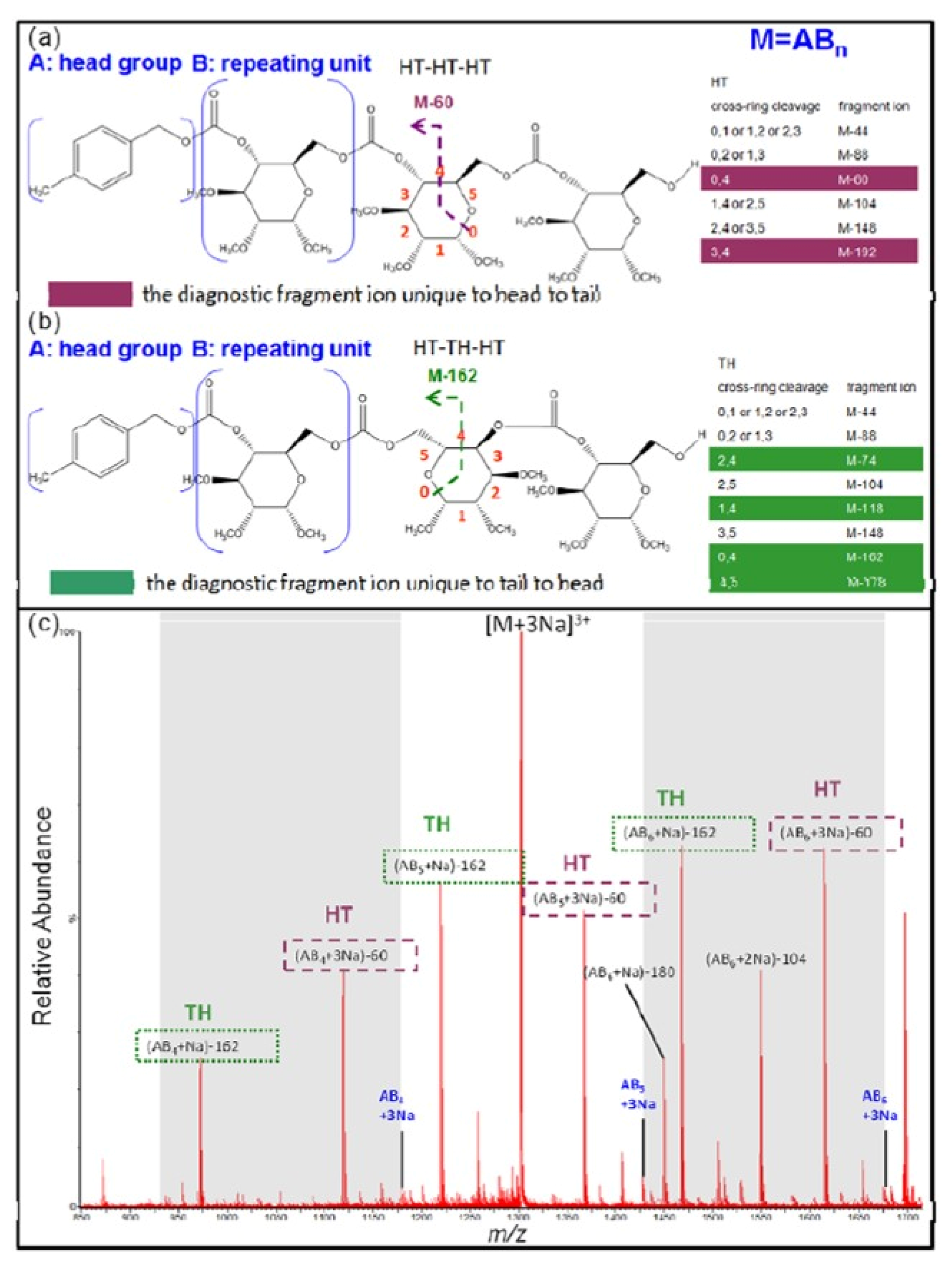

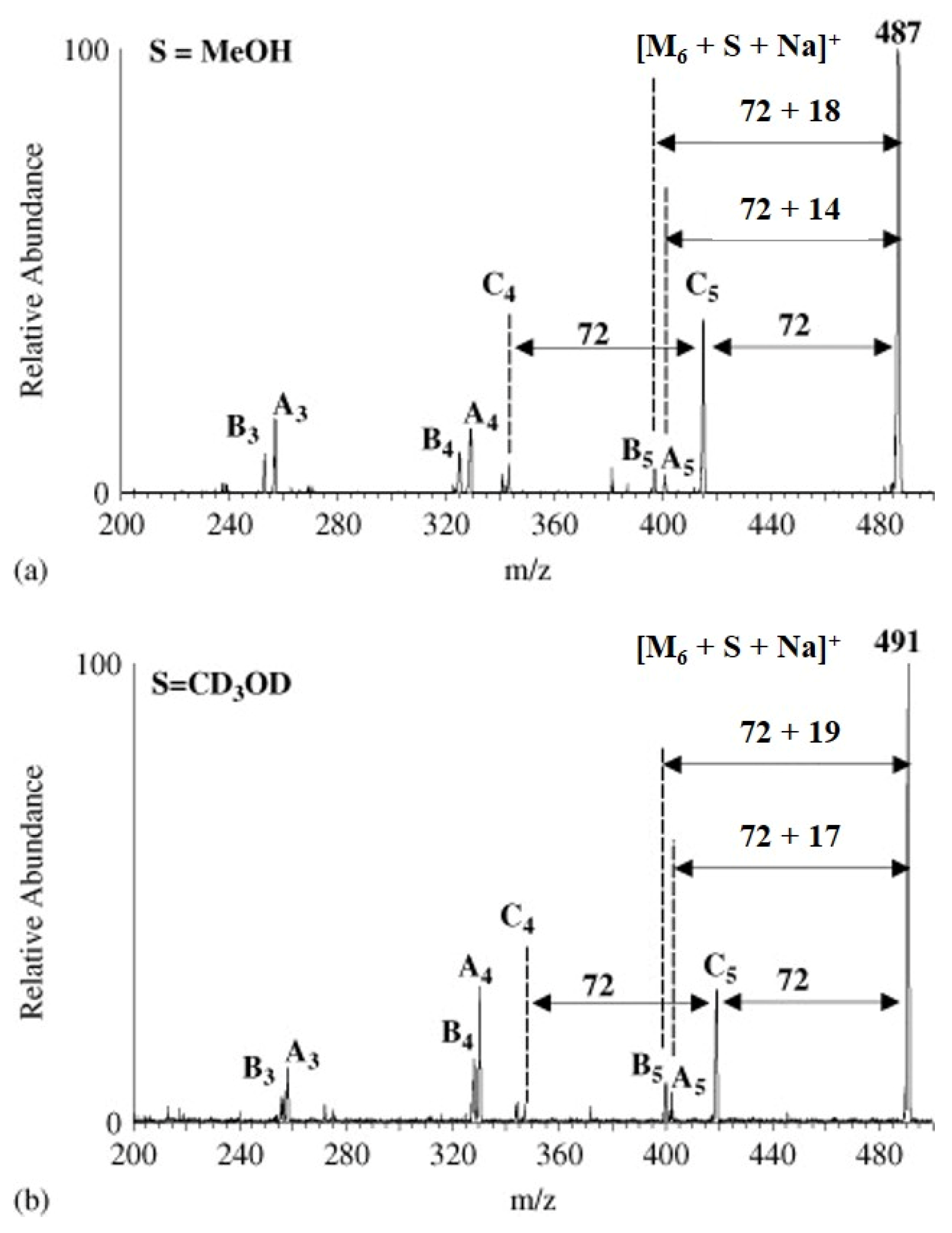
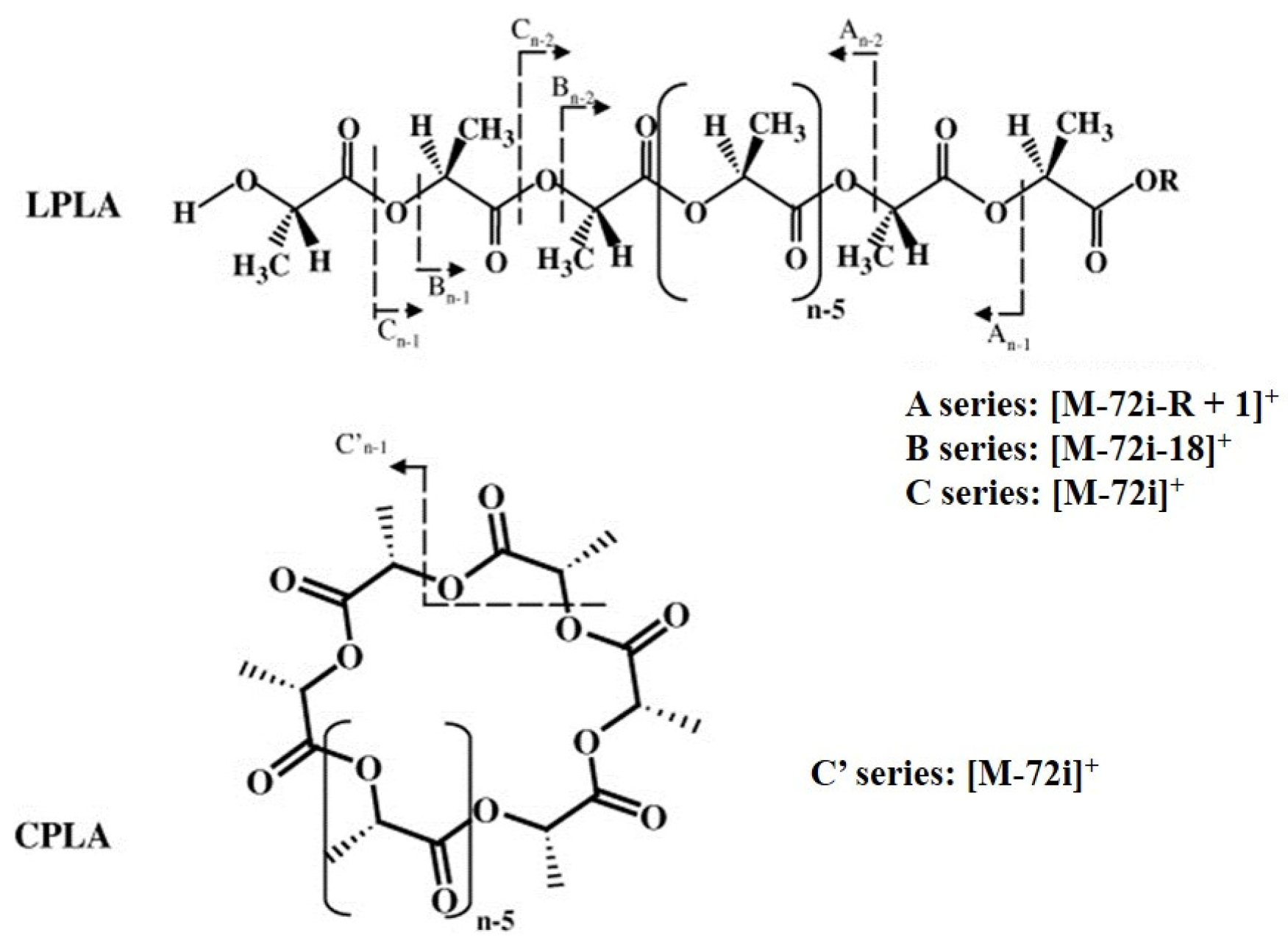
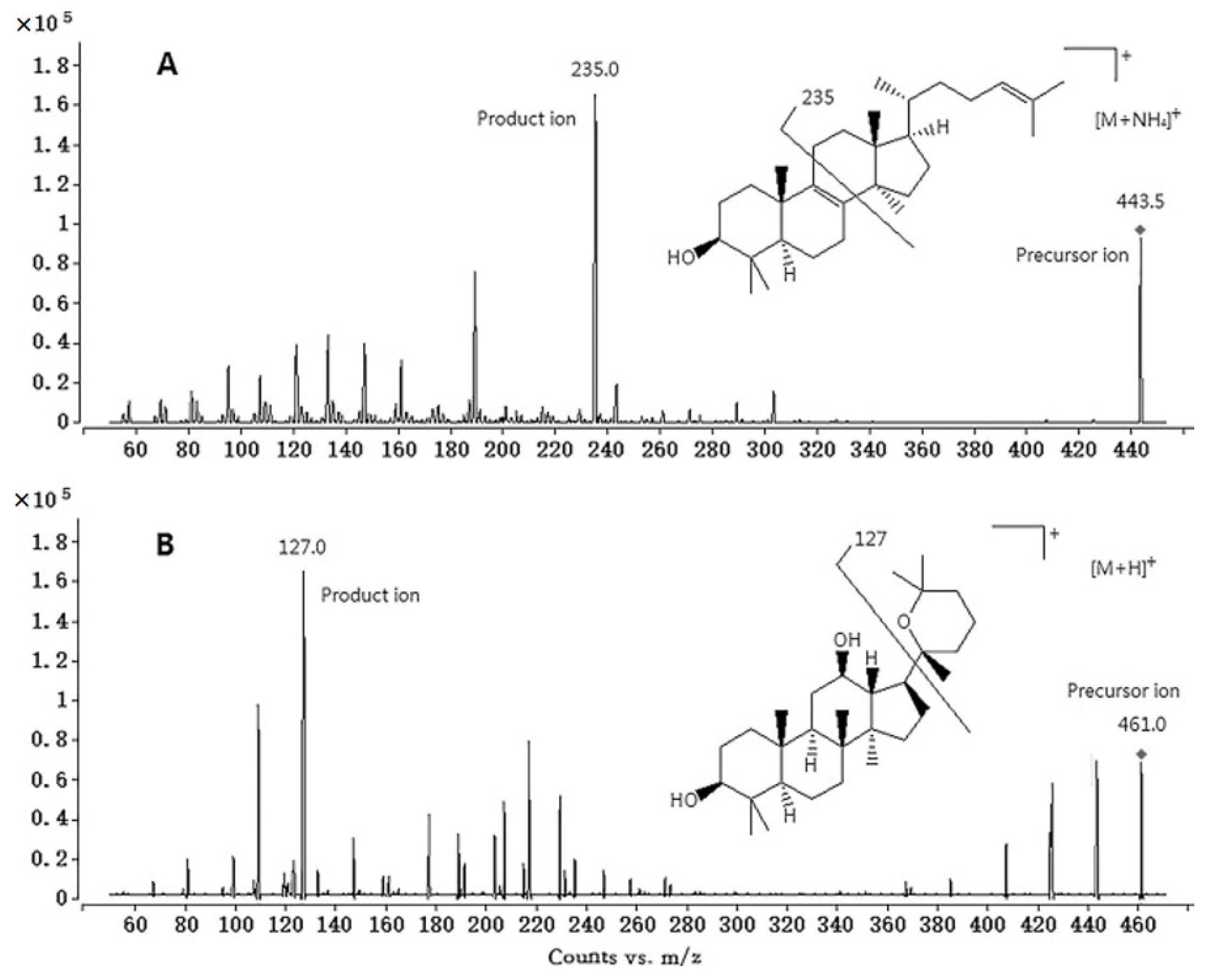
| Polymers | MS/MS Techniques | Other Methods | Information Acquired by MS/MS | Ref. |
|---|---|---|---|---|
| mPEG | MALDI–CID | SEC; 1H NMR | Nature of end groups and architectural modifications | [44] |
| PEAm | MALDI–CID | PSD-MALDI-TOF | Fragmentation mechanisms and ester/amide sequences | [47] |
| PBA | MALDI–CID | / | Fragmentation mechanisms | [48] |
| PEAm | MALDI–CID | / | Fragmentation mechanisms and ester/amide sequences | [49] |
| PLA | ESI–CID | / | Mechanistic study | [50] |
| Poly(ethylene adipate) and PBA, PLA and copolymers | ESI–CAD/ETD | / | CAD/ETD comparison | [51] |
| PBSu | MALDI–CID | / | Fragmentation mechanisms | [52] |
| PCL, PTHF and copolymer | ESI–CAD/ETD | / | CAD/ETD comparison | [53] |
| PEAm | MALDI–CID | / | Fragmentation mechanisms and ester/amide sequences | [54] |
| PdVL and P(dVL-co-mCL) | ESI–CID | 1H NMR | Fragmentation mechanisms, end groups and molecular structures | [55] |
| Hyperbranched PEAm | ESI-FT-ICR–CAD | / | Fragmentation mechanisms, isomeric structures and composition | [56] |
| PEO, PPO, linear triblock and glycerol derivative diblock copolyethers | ESI–CID | / | Fragmentation mechanisms, block lengths and sequences | [57] |
| P[(R,S)-3HB-co-LA] | ESI–CID | GPC; 1H NMR | Fragmentation mechanisms, molecular structures, end groups and sequences | [58] |
| PEO-co-PPO | MALDI–CID | LAC–MALDI offline; software tool | Differentiation between isobaric structures | [59] |
| CPLA oligomers | LC-ESI–CID | / | Quantitative determination in serum | [60] |
| Poly[(R,S)3-hydroxy-4-ethoxybutyrate-co-(R,S)-3-hydroxybutyrate)], Poly[(R,S)-3-hydroxybutyrate)-block-(R,S)-3-hydroxy-4-ethoxybutyrate)] | ESI–CID | SEC; FT-IR; 1H NMR | Molecular structures and sequence distribution | [61] |
| Hexanediol-neopentylglycol-adipic acid copolyesters | MALDI–CID | / | Fragmentation mechanism, differentiation between cyclic and linear oligomers | [62] |
| Phenoxycarboxylic acid–oligo(3-hydroxybutyrate) conjugates | ESI–CID | GPC; FT-IR; 1H NMR | Confirmation of structure assignment and functionalization | [63] |
| PLA with different isomeric end groups | ESI–CID | / | Isomeric end-group distinction | [64] |
| Lipoic acid–oligo-(3-HB) conjugates | ESI–CID | GPC; HPLC-ESI/MS; 1H NMR; FTIR | Structural characterization | [65] |
| p-coumaric acid–oligo(3-HB) conjugates | ESI–CID | GPC; FT-IR; 1H NMR | Confirmation of structure assignment and functionalization | [67] |
| CPLA | ESI–CID | SEC; MALDI-MS; 1H NMR | Detection of architectural impurity | [68] |
| Pesticide PHAs conjugates | ESI–CID | GPC; 1H NMR | Confirmation of structure assignment and functionalization | [70] |
| Tyrosol-P(3HB-co-4HB) and γ-PGA conjugates | ESI–CID | GPC; 1H NMR | Structural characterization and successful polymerization | [71] |
| (p-AA-CH2-HP)n, p-AA-CH2-HP)m/HBn | ESI–CID | SEC; FT-IR; 1H NMR | Fragmentation mechanism, molecular structures, end groups and copolymer composition | [72] |
| Microbial PHAs | ESI–CID | GPC; FT-IR; 1H NMR | Structure identification | [74] |
| Peptides-PEG conjugates | MALDI–CID; ESI–CAD/ETD, ESI-IM-MS CAD | CD | Elucidation of alanine-rich polypeptides sequence and conformation | [76,78] |
| PPM, PPF | MALDI–CID; ESI–CAD/ETD, ESI-IM-MS CAD | SEC, Molecular modeling | Composition, end groups and chain sequence differentiate the isomeric PPM and PPF | [77] |
| CDLA | MALDI–LID, ESI–CID | GPC; 1H NMR | Structural architecture, influence of the cationization agent on fragmentation profiles, average length of the PLA chains attached to the CD | [80] |
| Microbial PHAs | ESI–CID | GPC; FT-IR; 1H NMR, TGA | Structure identification | [81] |
| PdVL | ESI–CID | GPC; MALDI-MS; 1H NMR | Molecular structure, end groups | [82] |
| P3HB-b-3HV | MALDI–CID | GPC; ESI-MS; 1H and 13C NMR, solution-state NMR; DSC | Fragmentation mechanisms, presence of some randomness | [83] |
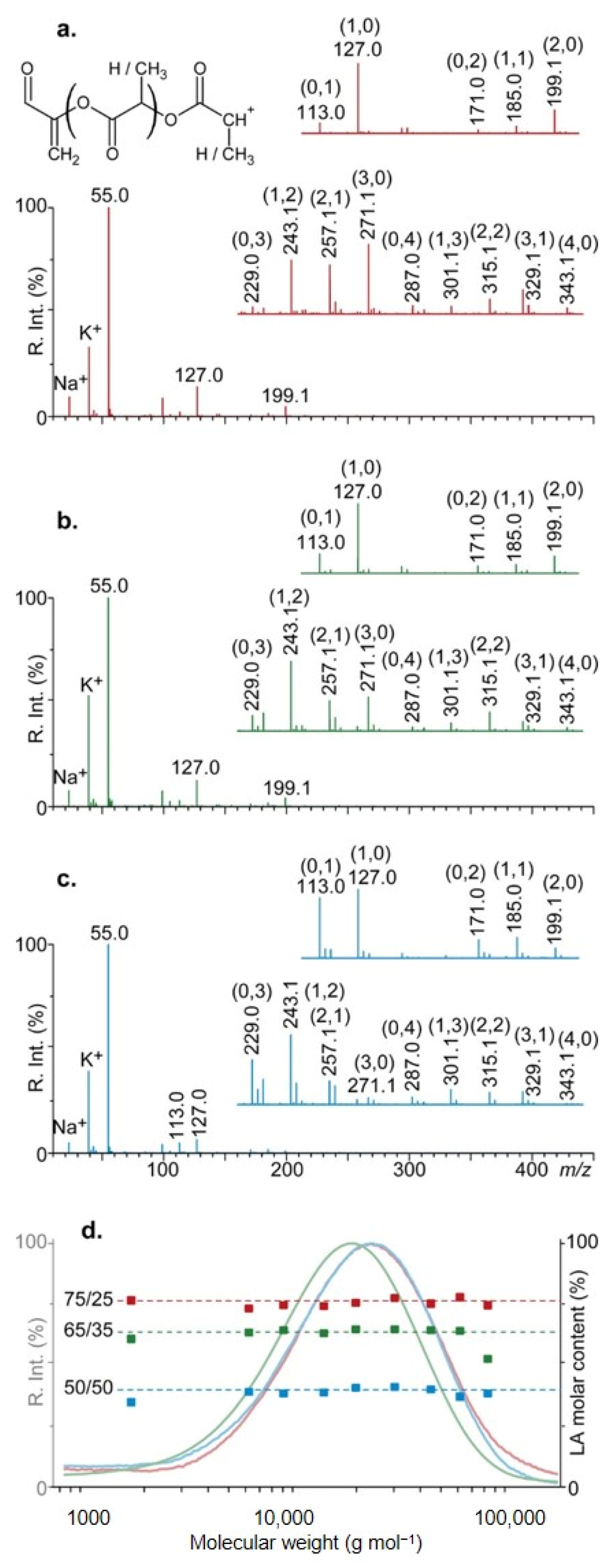
| PLGA | ESI–CID | SEC; NMR; SEC/MALDI-MS offline; reactive-SALDI; Kendrick analysis | Structure and end groups | [84] |
| Peptides-polymers conjugates | MALDI–CID; ESI–CAD/ETD, ESI-IM-MS CAD | Molecular modeling | Architectural microstructure | [85] |
| PC | ESI–ETD | 1H and 13C NMR; MALDI-MS; DSC; TGA | Distribution of head-to-head, head-to-tail, and tail-to-tail regiochemistries | [86] |
| CDLA | MALDI–LID | GPC; 1H NMR | Structural architecture, interference of the solvents | [87] |
| CDCL | MALDI–LID | 1D and 2D NMR | Structure confirmation | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzarelli, P.; Rapisarda, M. Matrix-Assisted Laser Desorption and Electrospray Ionization Tandem Mass Spectrometry of Microbial and Synthetic Biodegradable Polymers. Polymers 2023, 15, 2356. https://doi.org/10.3390/polym15102356
Rizzarelli P, Rapisarda M. Matrix-Assisted Laser Desorption and Electrospray Ionization Tandem Mass Spectrometry of Microbial and Synthetic Biodegradable Polymers. Polymers. 2023; 15(10):2356. https://doi.org/10.3390/polym15102356
Chicago/Turabian StyleRizzarelli, Paola, and Marco Rapisarda. 2023. "Matrix-Assisted Laser Desorption and Electrospray Ionization Tandem Mass Spectrometry of Microbial and Synthetic Biodegradable Polymers" Polymers 15, no. 10: 2356. https://doi.org/10.3390/polym15102356
APA StyleRizzarelli, P., & Rapisarda, M. (2023). Matrix-Assisted Laser Desorption and Electrospray Ionization Tandem Mass Spectrometry of Microbial and Synthetic Biodegradable Polymers. Polymers, 15(10), 2356. https://doi.org/10.3390/polym15102356






