New Polycaprolactone-Containing Self-Healing Coating Design for Enhance Corrosion Resistance of the Magnesium and Its Alloys
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Processing Procedures
2.1.1. Sample Preparation
2.1.2. PEO Coating Formation
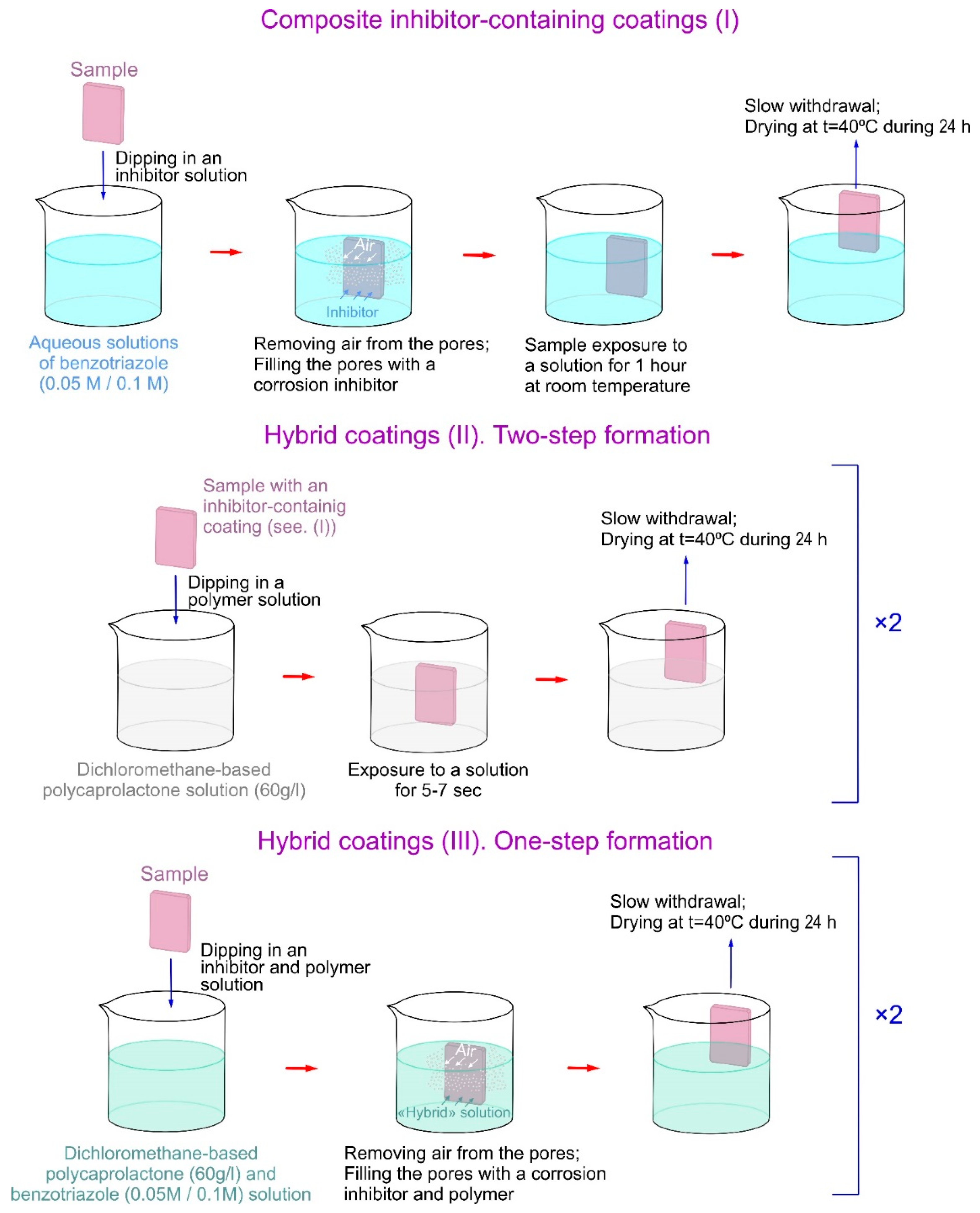
2.1.3. Inhibitor and Polymer Treatment
2.1.4. Cross-Section Preparation
2.2. Surface Characterization
2.2.1. SEM-EDX
2.2.2. XRD Analysis
2.2.3. XPS Analysis
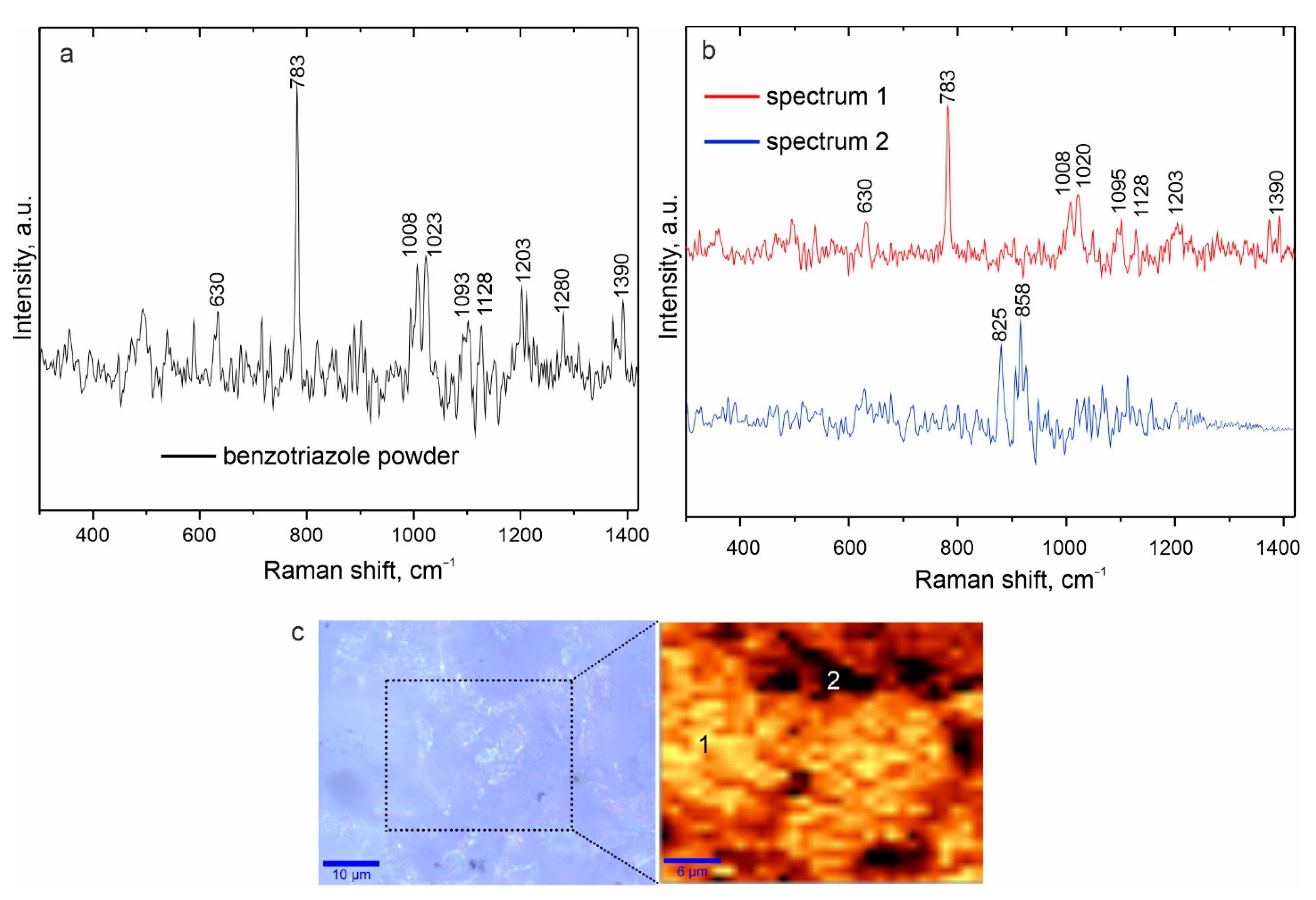
2.2.4. Raman Spectroscopy
2.3. Corrosion Studies
2.3.1. Electrochemical Measurements
2.3.2. Gravimetric and Volumetric Analysis
2.3.3. Inhibitor Release Test and Wettability Analysis
3. Results and Discussion
3.1. Coatings Obtained on MA8 Magnesium Alloy
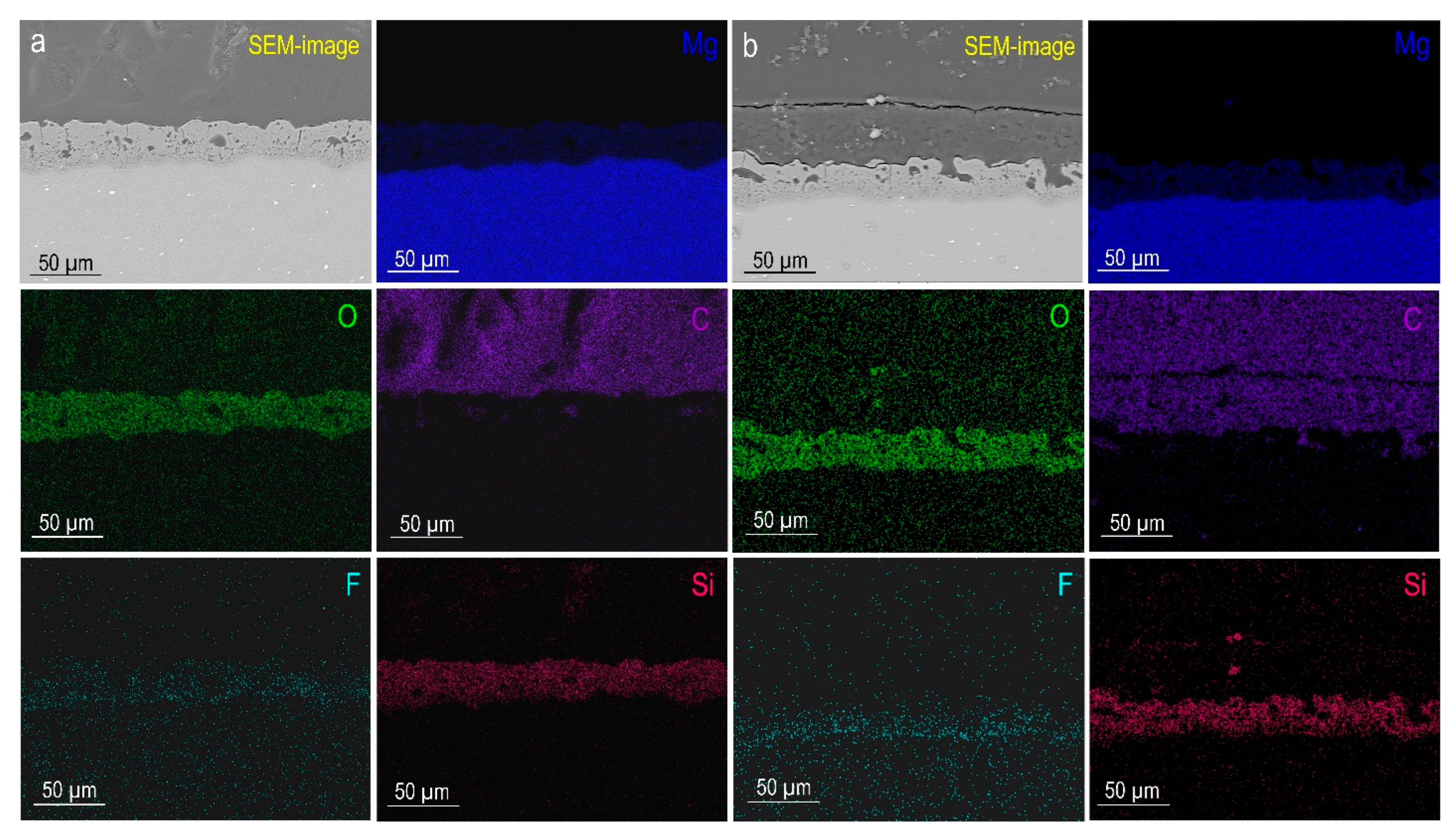
3.1.1. Structure and Composition
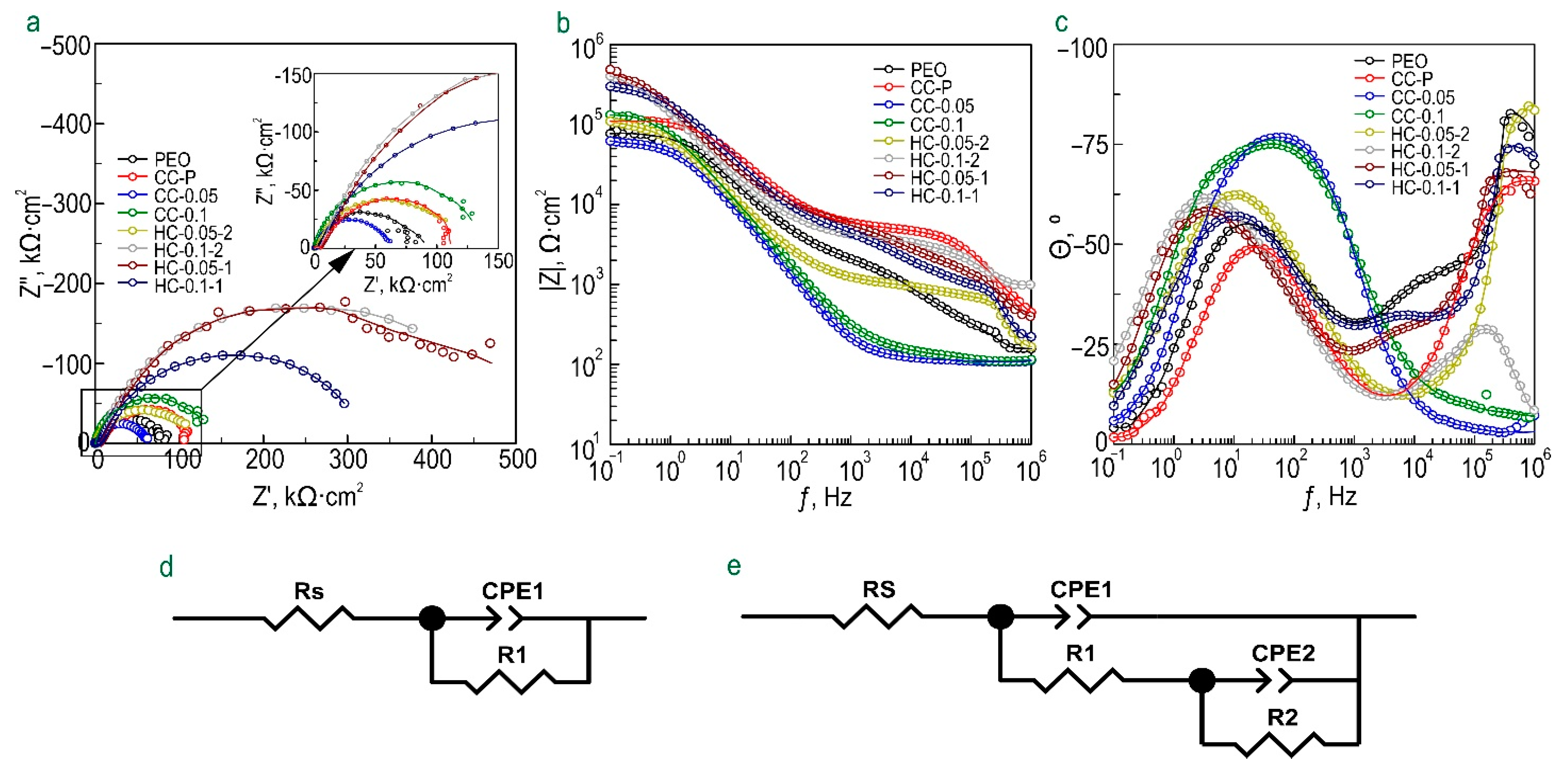
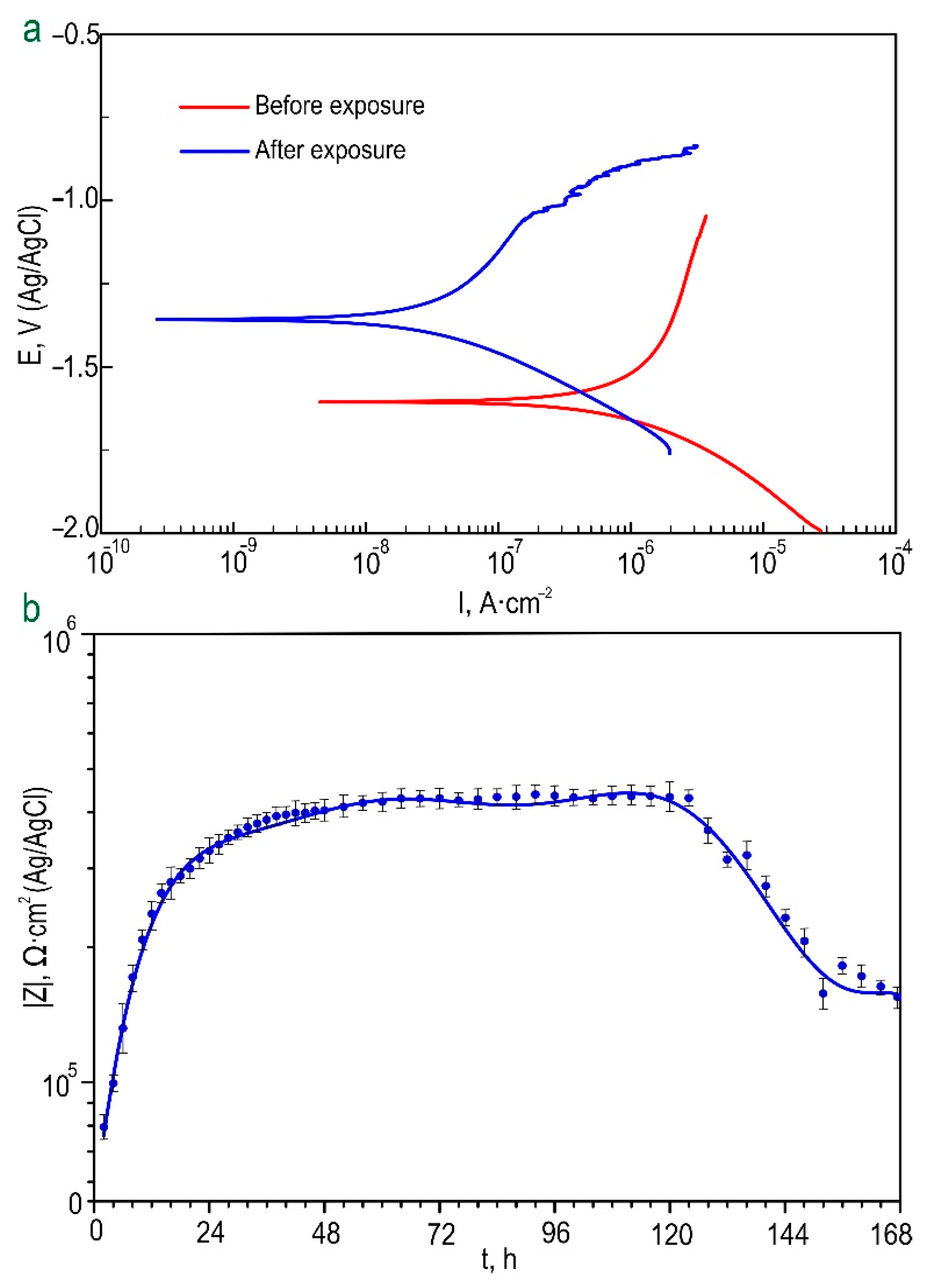
3.1.2. Electrochemical Properties
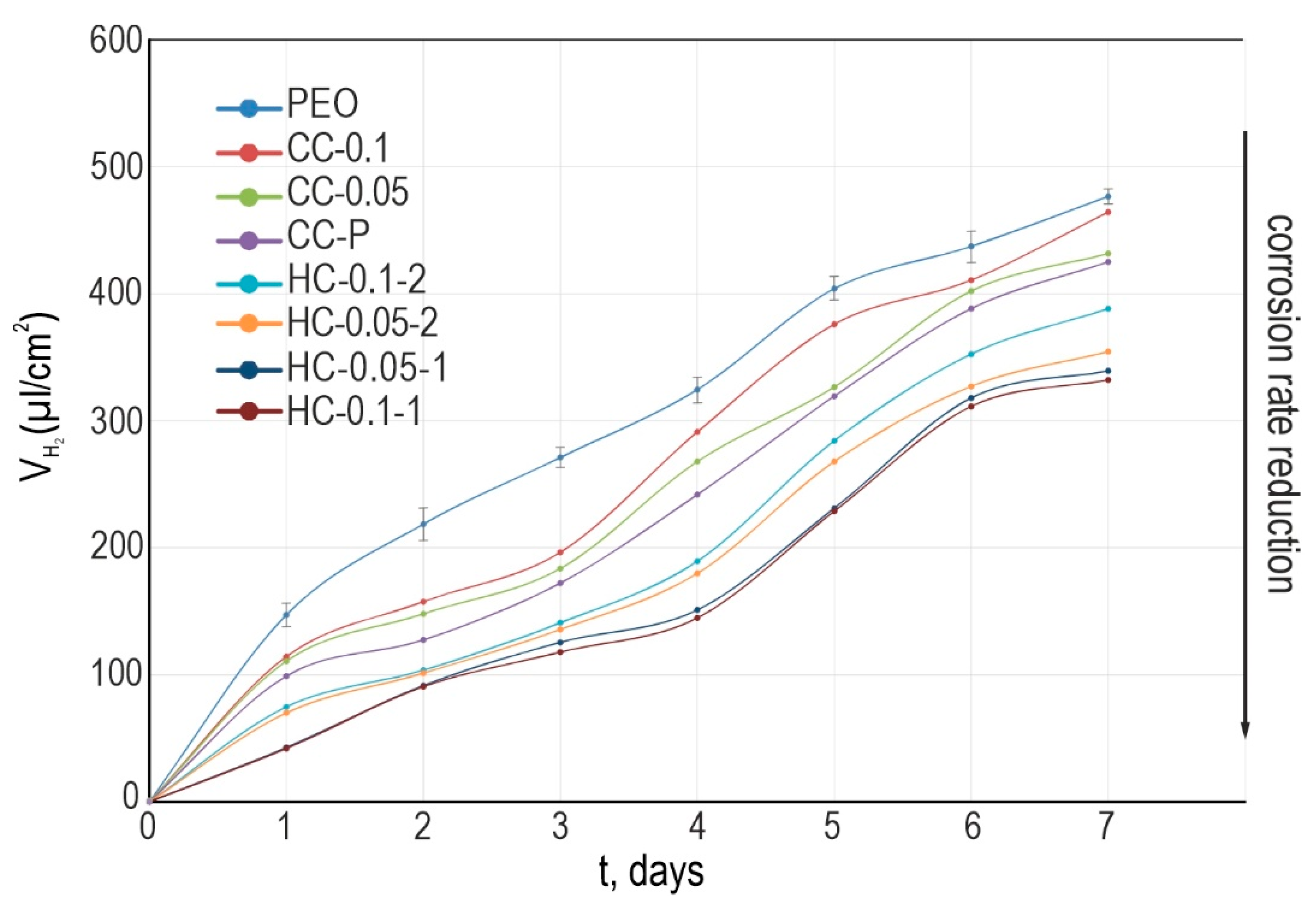
3.1.3. Hydrogen Release and Mass Loss Tests
3.1.4. Evaluation of Inhibitor Release into a Corrosive Medium and Surface Wettability Analysis
3.2. Coatings Obtained on AT-Mg
3.2.1. Microstructure Analysis
3.2.2. Electrochemical Properties
4. Conclusions
- Using plasma electrolytic oxidation, a biocompatible ceramic-like coating with a developed surface morphology was obtained. XRD analysis allowed determining periclase (MgO) and forsterite (Mg2SiO4) in the coating composition;
- The optimal method of surface treatment with benzotriazole (Btr) was selected in order to obtain the most efficient impregnation of PEO layer pores with corrosion inhibitors. The rate of Btr release from the hetero-oxide matrix was reduced by processing the obtained composite coatings with a bioresorbable polymeric material—polycaprolactone. Methods of hybrid coating formation using sequential impregnation of the base PEO layer with Btr at various concentrations and PCL (HC-0.05-2 and HC-0.1-2), as well as one-stage application of Btr and PCL from a solution based on dichloromethane (HC-0.05-1, HC-0.1-1), are presented;
- Using X-ray photoelectron spectroscopy and confocal Raman microspectroscopy, the composition of the formed surface composite inhibitor-containing layers was established. The presence of benzotriazole was confirmed, and its distribution over the coating surface was established at a microlevel;
- The level of corrosion protection of the MA8 magnesium alloy and AT-Mg was determined in 0.9 wt.% NaCl solution. A comparative analysis of the results of electrochemical tests made it possible to establish the best barrier properties for HC-0.1-1 and AT-Mg+HC coatings;
- Analysis of the electrochemical properties of samples with hybrid benzotriazole-containing layers showed stability of their corrosion behavior during 7 days of exposure to Hanks’ solution. Specimens with HC-0.1-1 are also characterized by the smallest volume of released hydrogen and the smallest weight loss among all the studied layers;
- Sealing the pores of the PEO coating with a polymeric material contributes to a significant reduction in inhibitor release into a corrosive medium. The obtained result allows the prolongation of the effect of active anticorrosion protection of the formed hybrid layers. The method of effective hybrid coating formation can provide the required level and duration of the corrosion protection period and controlled degradation of biomedical products from magnesium and its alloys.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; He, C.; Dianyu, E.; Yang, W.; Qi, F.; Xie, D.; Shen, L.; Peng, S.; Shuai, C. Mg Bone Implant: Features, Developments and Perspectives. Mater. Des. 2020, 185, 108259. [Google Scholar] [CrossRef]
- Kumar, S.; Katyal, P.; Chaudhary, R.N.; Singh, V. Assessment of Factors Influencing Bio-Corrosion of Magnesium Based Alloy Implants: A Review. Mater. Today Proc. 2022, 56, 2680–2689. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Lamaka, S.V.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Egorkin, V.S.; Imshinetskiy, I.M.; Zavidnaya, A.G.; Zheludkevich, M.L.; Gnedenkov, S.V. Electrochemical Behaviour of the MA8 Mg Alloy in Minimum Essential Medium. Corros. Sci. 2020, 168, 108552. [Google Scholar] [CrossRef]
- Rahman, M.; Li, Y.; Wen, C. HA Coating on Mg Alloys for Biomedical Applications: A Review. J. Magnes. Alloys 2020, 8, 929–943. [Google Scholar] [CrossRef]
- Amiri, H.; Mohammadi, I.; Afshar, A. Electrophoretic Deposition of Nano-Zirconia Coating on AZ91D Magnesium Alloy for Bio-Corrosion Control Purposes. Surf. Coatings Technol. 2017, 311, 182–190. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Filonina, V.S.; Plekhova, N.G.; Gnedenkov, S.V. Smart Composite Antibacterial Coatings with Active Corrosion Protection of Magnesium Alloys. J. Magnes. Alloys 2022, in press. [Google Scholar] [CrossRef]
- Stojadinović, S. Plasma Electrolytic Oxidation of Metals. J. Serbian Chem. Soc. 2013, 78, 713–716. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Protective Properties of Inhibitor-Containing Composite Coatings on a Mg Alloy. Corros. Sci. 2016, 102, 348–354. [Google Scholar] [CrossRef]
- Mohedano, M.; Luthringer, B.J.C.; Mingo, B.; Feyerabend, F.; Arrabal, R.; Sanchez-Egido, P.J.; Blawert, C.; Willumeit-Römer, R.; Zheludkevich, M.L.; Matykina, E. Bioactive Plasma Electrolytic Oxidation Coatings on Mg-Ca Alloy to Control Degradation Behaviour. Surf. Coatings Technol. 2017, 315, 454–467. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Features of the Magnesium Alloys Corrosion in the Chloride-Containing Media. Solid State Phenom. 2014, 213, 143–148. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Lamaka, S.V.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Egorkin, V.S.; Imshinetskiy, I.M.; Zheludkevich, M.L.; Gnedenkov, S.V. Control of the Mg Alloy Biodegradation via PEO and Polymer-Containing Coatings. Corros. Sci. 2021, 182, 109254. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Vyaliy, I.E.; Egorkin, V.S.; Gnedenkov, S.V. Corrosion of the Welded Aluminium Alloy in 0.5 M NaCl Solution. Part 2: Coating Protection. Materials 2018, 11, 2177. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Imshinetskiy, I.M.; Vyaliy, I.E.; Gnedenkov, S.V. Effect of Microstructure on the Corrosion Resistance of TIG Welded 1579 Alloy. Materials 2019, 12, 2615. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Inhibitor-Containing Composite Coatings on Mg Alloys: Corrosion Mechanism and Self-Healing Protection. Solid State Phenom. 2016, 245, 89–96. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Khrisanfova, O.A.; Sinebryukhov, S.L.; Puz, A.V.; Gnedenkov, A.S. Composite Protective Coatings on Nitinol Surface. Mater. Manuf. Process. 2008, 23, 879–883. [Google Scholar] [CrossRef]
- Zhang, F.; Ju, P.; Pan, M.; Zhang, D.; Huang, Y.; Li, G.; Li, X. Self-Healing Mechanisms in Smart Protective Coatings: A Review. Corros. Sci. 2018, 144, 74–88. [Google Scholar] [CrossRef]
- Guo, X.; An, M.; Yang, P.; Li, H.; Su, C. Effects of Benzotriazole on Anodized Film Formed on AZ31B Magnesium Alloy in Environmental-Friendly Electrolyte. J. Alloys Compd. 2009, 482, 487–497. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Vaghefinazari, B.; Mei, D.; Petrauskas, R.P.; Höche, D.; Zheludkevich, M.L. Comprehensive Screening of Mg Corrosion Inhibitors. Corros. Sci. 2017, 128, 224–240. [Google Scholar] [CrossRef]
- Wang, J.-L.; Ke, C.; Pohl, K.; Birbilis, N.; Chen, X.-B. The Unexpected Role of Benzotriazole in Mitigating Magnesium Alloy Corrosion: A Nucleating Agent for Crystalline Nanostructured Magnesium Hydroxide Film. J. Electrochem. Soc. 2015, 162, C403–C411. [Google Scholar] [CrossRef]
- Ostanina, T.N.; Rudoi, V.M.; Ovsyannikova, A.N.; Malkov, V.B. Magnesium Alloys Spontaneous Dissolution Features under External Anodic Polarization in Presence of Inhibitors. Russ. J. Electrochem. 2010, 46, 707–713. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of Magnesium-Based Biomaterials and Their Applications. J. Magnes. Alloys 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Sun, M.; Yerokhin, A.; Bychkova, M.Y.; Shtansky, D.V.; Levashov, E.A.; Matthews, A. Self-Healing Plasma Electrolytic Oxidation Coatings Doped with Benzotriazole Loaded Halloysite Nanotubes on AM50 Magnesium Alloy. Corros. Sci. 2016, 111, 753–769. [Google Scholar] [CrossRef]
- Lykins, W.R.; Bernards, D.A.; Schlesinger, E.B.; Wisniewski, K.; Desai, T.A. Tuning Polycaprolactone Degradation for Long Acting Implantables. Polymer 2022, 262, 125473. [Google Scholar] [CrossRef]
- Chunyan, Z.; Lan, C.; Jiajia, L.; Dongwei, S.; Jun, Z.; Huinan, L. In Vitro Evaluation of Degradation, Cytocompatibility and Antibacterial Property of Polycaprolactone/Hydroxyapatite Composite Coating on Bioresorbable Magnesium Alloy. J. Magnes. Alloys 2022, 10, 2252–2265. [Google Scholar] [CrossRef]
- Zomorodian, A.; Santos, C.; Carmezim, M.J.; Silva, T.M.E.; Fernandes, J.C.S.; Montemor, M.F. “In-Vitro” Corrosion Behaviour of the Magnesium Alloy with Al and Zn (AZ31) Protected with a Biodegradable Polycaprolactone Coating Loaded with Hydroxyapatite and Cephalexin. Electrochim. Acta 2015, 179, 431–440. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Wang, F.; Zhu, Y.; Gu, R.; Yang, F.; Xu, Y.; Xia, D.; Liu, Y. 3D-Printed Mg-1Ca/Polycaprolactone Composite Scaffolds with Promoted Bone Regeneration. J. Magnes. Alloys 2022, in press. [Google Scholar] [CrossRef]
- Nandhini, G.; Nivedha, B.; Pranesh, M.; Karthega, M. Study of Polycaprolactone/Curcumin Loaded Electrospun Nanofibers on AZ91 Magnesium Alloy. Mater. Today Proc. 2020, 33, 2170–2173. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Wang, J.; Hu, W. Corrosion Resistance and Near-Infrared Light Induced Self-Healing Behavior of Polycaprolactone Coating with MIL-53@TA on Magnesium Alloy. Appl. Surf. Sci. 2022, 585, 152729. [Google Scholar] [CrossRef]
- Xing, K.; Chen, Q.; Lin, J.; Hu, Z.; Li, Z.; Chen, J.; Xu, X.; Gu, C.; Tu, J. Polycaprolactone/ZnO Coating on WE43 Magnesium Alloy Combined with a MgO/MgCO3 Transition Layer for Promoting Anticorrosion and Interfacial Adhesion. Prog. Org. Coatings 2022, 171, 107029. [Google Scholar] [CrossRef]
- Ostrowski, N.; Lee, B.; Enick, N.; Carlson, B.; Kunjukunju, S.; Roy, A.; Kumta, P.N. Corrosion Protection and Improved Cytocompatibility of Biodegradable Polymeric Layer-by-Layer Coatings on AZ31 Magnesium Alloys. Acta Biomater. 2013, 9, 8704–8713. [Google Scholar] [CrossRef]
- Zomorodian, A.; Garcia, M.P.; Moura e Silva, T.; Fernandes, J.C.S.; Fernandes, M.H.; Montemor, M.F. Biofunctional Composite Coating Architectures Based on Polycaprolactone and Nanohydroxyapatite for Controlled Corrosion Activity and Enhanced Biocompatibility of Magnesium AZ31 Alloy. Mater. Sci. Eng. C 2015, 48, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, N.P.D.; Muthuvelu, K.S.; Arumugasamy, S.K. Recent Advancement in Biomedical Applications of Polycaprolactone and Polycaprolactone-Based Materials. In Encyclopedia of Materials: Plastics and Polymers; Elsevier: Amsterdam, The Netherlands, 2022; pp. 795–809. [Google Scholar]
- Abrisham, M.; Noroozi, M.; Panahi-Sarmad, M.; Arjmand, M.; Goodarzi, V.; Shakeri, Y.; Golbaten-Mofrad, H.; Dehghan, P.; Seyfi Sahzabi, A.; Sadri, M.; et al. The Role of Polycaprolactone-Triol (PCL-T) in Biomedical Applications: A State-of-the-Art Review. Eur. Polym. J. 2020, 131, 109701. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Egorkin, V.S.; Mashtalyar, D.V.; Vyaliy, I.E.; Nadaraia, K.V.; Imshinetskiy, I.M.; Nikitin, A.I.; Subbotin, E.P.; Gnedenkov, A.S. Magnesium Fabricated Using Additive Technology: Specificity of Corrosion and Protection. J. Alloys Compd. 2019, 808, 151629. [Google Scholar] [CrossRef]
- Maltseva, A.; Lamaka, S.V.; Yasakau, K.A.; Mei, D.; Kurchavov, D.; Zheludkevich, M.L.; Lefèvre, G.; Volovitch, P. In Situ Surface Film Evolution during Mg Aqueous Corrosion in Presence of Selected Carboxylates. Corros. Sci. 2020, 171, 108484. [Google Scholar] [CrossRef]
- Vaghefinazari, B.; Wang, C.; Mercier, D.; Mei, D.; Seyeux, A.; Marcus, P.; Blawert, C.; Lamaka, S.V.; Zheludkevich, M.L. Adverse Effect of 2,5PDC Corrosion Inhibitor on PEO Coated Magnesium. Corros. Sci. 2021, 192, 109830. [Google Scholar] [CrossRef]
- Zhang, Y.; Yaxu, W.; Jiang, Y.; Wang, L.; Zhang, J. Adsorbed Film and Synergistic Effect of Benzyltriphenylphosphonium Chloride and L-Histidine for Magnesium Alloys Corrosion in NaCl. J. Alloys Compd. 2020, 849, 156230. [Google Scholar] [CrossRef]
- Cao, F.; Shi, Z.; Song, G.L.; Liu, M.; Atrens, A. Corrosion Behaviour in Salt Spray and in 3.5% NaCl Solution Saturated with Mg(OH)2 of as-Cast and Solution Heat-Treated Binary Mg-X Alloys: X = Mn, Sn, Ca, Zn, Al, Zr, Si, Sr. Corros. Sci. 2013, 76, 60–97. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Filonina, V.S.; Egorkin, V.S.; Ustinov, A.Y.; Sergienko, V.I.; Gnedenkov, S.V. The Detailed Corrosion Performance of Bioresorbable Mg-0.8Ca Alloy in Physiological Solutions. J. Magnes. Alloys 2022, 10, 1326–1350. [Google Scholar] [CrossRef]
- Wagener, V.; Virtanen, S. Influence of Electrolyte Composition (Simulated Body Fluid vs. Dulbecco’s Modified Eagle’s Medium), Temperature, and Solution Flow on the Biocorrosion Behavior of Commercially Pure Mg. Corrosion 2017, 73, 1413–1422. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Khrisanfova, O.A.; Zavidnaya, A.G.; Sinebrukhov, S.L.; Gordienko, P.S.; Iwatsubo, S.; Matsui, A. Composition and Adhesion of Protective Coatings on Aluminum. Surf. Coatings Technol. 2001, 145, 146–151. [Google Scholar] [CrossRef]
- Lin, M.; Nemcova, A.; Voevodin, A.A.; Korenyi-Both, A.; Liskiewicz, T.W.; Laugel, N.; Matthews, A.; Yerokhin, A. Surface Characteristics Underpinning Fretting Wear Performance of Heavily Loaded Duplex Chameleon/PEO Coatings on Al. Tribol. Int. 2021, 154, 106723. [Google Scholar] [CrossRef]
- Mingo, B.; Arrabal, R.; Mohedano, M.; Llamazares, Y.; Matykina, E.; Yerokhin, A.; Pardo, A. Influence of Sealing Post-Treatments on the Corrosion Resistance of PEO Coated AZ91 Magnesium Alloy. Appl. Surf. Sci. 2018, 433, 653–667. [Google Scholar] [CrossRef]
- Yerokhin, A.; Parfenov, E.V.; Matthews, A. In Situ Impedance Spectroscopy of the Plasma Electrolytic Oxidation Process for Deposition of Ca- and P-Containing Coatings on Ti. Surf. Coatings Technol. 2016, 301, 54–62. [Google Scholar] [CrossRef]
- Wieduwilt, F.; Lenth, C.; Ctistis, G.; Plachetka, U.; Möller, M.; Wackerbarth, H. Evaluation of an On-Site Surface Enhanced Raman Scattering Sensor for Benzotriazole. Sci. Rep. 2020, 10, 8260. [Google Scholar] [CrossRef]
- Chan, H.Y.H.; Weaver, M.J. Vibrational Structural Analysis of Benzotriazole Adsorption and Phase Film Formation on Copper Using Surface-Enhanced Raman Spectroscopy. Langmuir 1999, 15, 3348–3355. [Google Scholar] [CrossRef]
- Thomas, S.; Venkateswaran, S.; Kapoor, S.; D’Cunha, R.; Mukherjee, T. Surface Enhanced Raman Scattering of Benzotriazole: A Molecular Orientational Study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 25–29. [Google Scholar] [CrossRef]
- Ur Rehman, Z.; Uzair, M.; Lim, H.T.; Koo, B.H. Structural and Electrochemical Properties of the Catalytic CeO2 Nanoparticles-Based PEO Ceramic Coatings on AZ91 Mg Alloy. J. Alloys Compd. 2017, 726, 284–294. [Google Scholar] [CrossRef]
- Chrissanthopoulos, A.; Bouropoulos, N.; Yannopoulos, S.N. Vibrational Spectroscopic and Computational Studies of Sol-Gel Derived CaO-MgO-SiO2 Binary and Ternary Bioactive Glasses. Vib. Spectrosc. 2008, 48, 118–125. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone Physiological Microenvironment and Healing Mechanism: Basis for Future Bone-Tissue Engineering Scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Mei, D.; Lamaka, S.V.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Vyaliy, I.E.; Zheludkevich, M.L.; Gnedenkov, S.V. Localized Currents and pH Distribution Studied during Corrosion of MA8 Mg Alloy in the Cell Culture Medium. Corros. Sci. 2020, 170, 108689. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Filonina, V.S.; Gnedenkov, S.V. Hydroxyapatite-containing PEO-coating design for biodegradable Mg-0.8Ca alloy: Formation and corrosion behaviour. J. Magnes. Alloy 2022, in press. [Google Scholar] [CrossRef]





| Sample’s Designation | Description |
|---|---|
| PEO | base PEO layer |
| CC-P | composite coating obtained by treating the PEO layer with 60 g/L polycaprolactone solution in dichloromethane |
| CC–0.05 | composite coating obtained by treating the PEO layer in a 0.05 M benzotriazole aqueous solution |
| CC–0.1 | composite coating obtained by treating the PEO layer in a 0.1 M benzotriazole aqueous solution |
| HC–0.05-2 | hybrid coating obtained in two steps: by treating the PEO layer in a 0.05 M benzotriazole aqueous solution, followed by applying the 60 g/L polycaprolactone in dichloromethane |
| HC–0.1-2 | hybrid coating obtained in two steps: by treating the PEO layer in a 0.1 M benzotriazole aqueous solution, followed by applying the 60 g/L polycaprolactone in dichloromethane |
| HC–0.05-1 | hybrid coating obtained in one step: by treating the PEO layer in the solution of 0.05 M benzotriazole and 60 g/L polycaprolactone in dichloromethane |
| HC–0.1-1 | hybrid coating obtained in one step: by treating the PEO layer in the solution of 0.1 M benzotriazole and 60 g/L polycaprolactone in dichloromethane |
| AT-Mg+PEO | PEO coating formed on magnesium obtained by additive technology |
| AT-Mg+CC | composite coating on AT-Mg obtained by impregnating the base PEO layer with 0.1 M benzotriazole aqueous solution |
| AT-Mg+HC | hybrid coating on AT-Mg obtained in two steps: by impregnating the base PEO layer with 0.1 M benzotriazole aqueous solution, followed by applying the 60 g/L polycaprolactone solution in dichloromethane |
| Element | Chemical State | Studied Surface | |
|---|---|---|---|
| Before Etching | After Etching (10 min) | ||
| Zn (2p) | Zn2+ | 1021.6 (0.2) | 1022.4 (0.4) |
| Na (1s) | Na+ | 1072.2 (0.6) | 1072.8 (0.7) |
| Ce (3d) | Ce4+ | 887.1 (0.2) | 887.0 (0.2) |
| F (1s) | MeF | 685.1 (1.8) | 686.8 (2.5) |
| Mn (2p) | Mn4+ | 641.4 (1.1) | 642.1 (1.7) |
| O (1s) | –NO –O–C– | – | 534.5 (11.1) |
| SiO2 O=C– | 533.1 (9.2) | 533.0 (12.6) | |
| MgO | 531.7 (8.2) | 531.5 (5.6) | |
| N (1s) | –N– l | 400.4 (10.0) | 400.6 (4.1) |
| =N- | 399.1 (4.7) | 399.2 (3.6) | |
| C (1s) | -C(O)O- | – | – |
| C–N –C-C(O)O- | 286.1 (14.7) | 286.4 (12.9) | |
| C–C, C–H | 285.0 (39.1) | 285.0 (20.5) | |
| Si (2p) | Si–O | – | 105.1 (8.2) |
| SiO2 | 103.8 (4.2) | 103.4 (4.1) | |
| Mg (2p) | Mg2+ | – | 51.0 (6.0) |
| Mg0 | 52.4 (8.7) | 49.8 (3.1) | |
| Coating Type | βa, mV/decade | –βc, mV/decade | IC, A·cm–2 | EC, V (Ag/AgCl) | RP, Ω·cm2 | |Z|f=0.1Hz, Ω·cm2 |
|---|---|---|---|---|---|---|
| CC-P before exposure | 220.70 | 218.18 | 2.40 × 10−6 | −1.50 | 1.99 × 104 | 105,880 |
| CC-P after 24 h of exposure | 242.30 | 96.79 | 1.43 × 10−8 | −1.49 | 2.10 × 106 | 177,130 |
| CC-0.05 before exposure | 263.46 | 145.81 | 5.56 × 10−7 | −1.49 | 7.35 × 104 | 61,301 |
| CC-0.05 after 24 h of exposure | 932.66 | 137.04 | 5.32 × 10−7 | −1.49 | 9.93 × 104 | 51,596 |
| CC-0.1 before exposure | 158.29 | 97.51 | 1.46 × 10−7 | −1.44 | 1.79 × 105 | 132,050 |
| CC-0.1 after 24 h of exposure | 557.88 | 161.15 | 1.30 × 10−7 | −1.38 | 4.18 × 105 | 54,112 |
| HC-0.05-2 before exposure | 565.54 | 202.45 | 3.58 × 10−7 | −1.45 | 1.81 × 105 | 109,830 |
| HC-0.05-2 after 24 h of exposure | 479.06 | 195.30 | 1.02 × 10−7 | −1.30 | 5.90 × 105 | 46,714 |
| HC-0.1-2 before exposure | 484.14 | 204.88 | 1.66 × 10−7 | −1.44 | 3.77 × 105 | 403,650 |
| HC-0.1-2 after 24 h of exposure | 599.30 | 199.76 | 4.97 × 10−8 | −1.27 | 1.29 × 106 | 45,042 |
| HC-0.05-1 before exposure | 384.80 | 181.46 | 1.00 × 10−7 | −1.43 | 5.34 × 105 | 485,760 |
| HC-0.05-1 after 24 h of exposure | 1026.80 | 218.04 | 5.27 × 10−8 | −1.34 | 1.48 × 106 | 515,040 |
| HC-0.1-1 before exposure | 483.89 | 274.71 | 3.43 × 10−8 | −1.22 | 2.22 × 106 | 300,280 |
| HC-0.1-1 after 24 h of exposure | 452.04 | 178.09 | 3.02 × 10−8 | −1.35 | 1.84 × 106 | 112,470 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gnedenkov, A.S.; Sinebryukhov, S.L.; Filonina, V.S.; Ustinov, A.Y.; Sukhoverkhov, S.V.; Gnedenkov, S.V. New Polycaprolactone-Containing Self-Healing Coating Design for Enhance Corrosion Resistance of the Magnesium and Its Alloys. Polymers 2023, 15, 202. https://doi.org/10.3390/polym15010202
Gnedenkov AS, Sinebryukhov SL, Filonina VS, Ustinov AY, Sukhoverkhov SV, Gnedenkov SV. New Polycaprolactone-Containing Self-Healing Coating Design for Enhance Corrosion Resistance of the Magnesium and Its Alloys. Polymers. 2023; 15(1):202. https://doi.org/10.3390/polym15010202
Chicago/Turabian StyleGnedenkov, Andrey S., Sergey L. Sinebryukhov, Valeriia S. Filonina, Alexander Yu. Ustinov, Sviatoslav V. Sukhoverkhov, and Sergey V. Gnedenkov. 2023. "New Polycaprolactone-Containing Self-Healing Coating Design for Enhance Corrosion Resistance of the Magnesium and Its Alloys" Polymers 15, no. 1: 202. https://doi.org/10.3390/polym15010202
APA StyleGnedenkov, A. S., Sinebryukhov, S. L., Filonina, V. S., Ustinov, A. Y., Sukhoverkhov, S. V., & Gnedenkov, S. V. (2023). New Polycaprolactone-Containing Self-Healing Coating Design for Enhance Corrosion Resistance of the Magnesium and Its Alloys. Polymers, 15(1), 202. https://doi.org/10.3390/polym15010202






