Jeziorny Method Should Be Avoided in Avrami Analysis of Nonisothermal Crystallization
Abstract
1. Introduction
2. Avrami Model
3. Jeziorny Method
4. Nonisothermal Form of Avrami Model
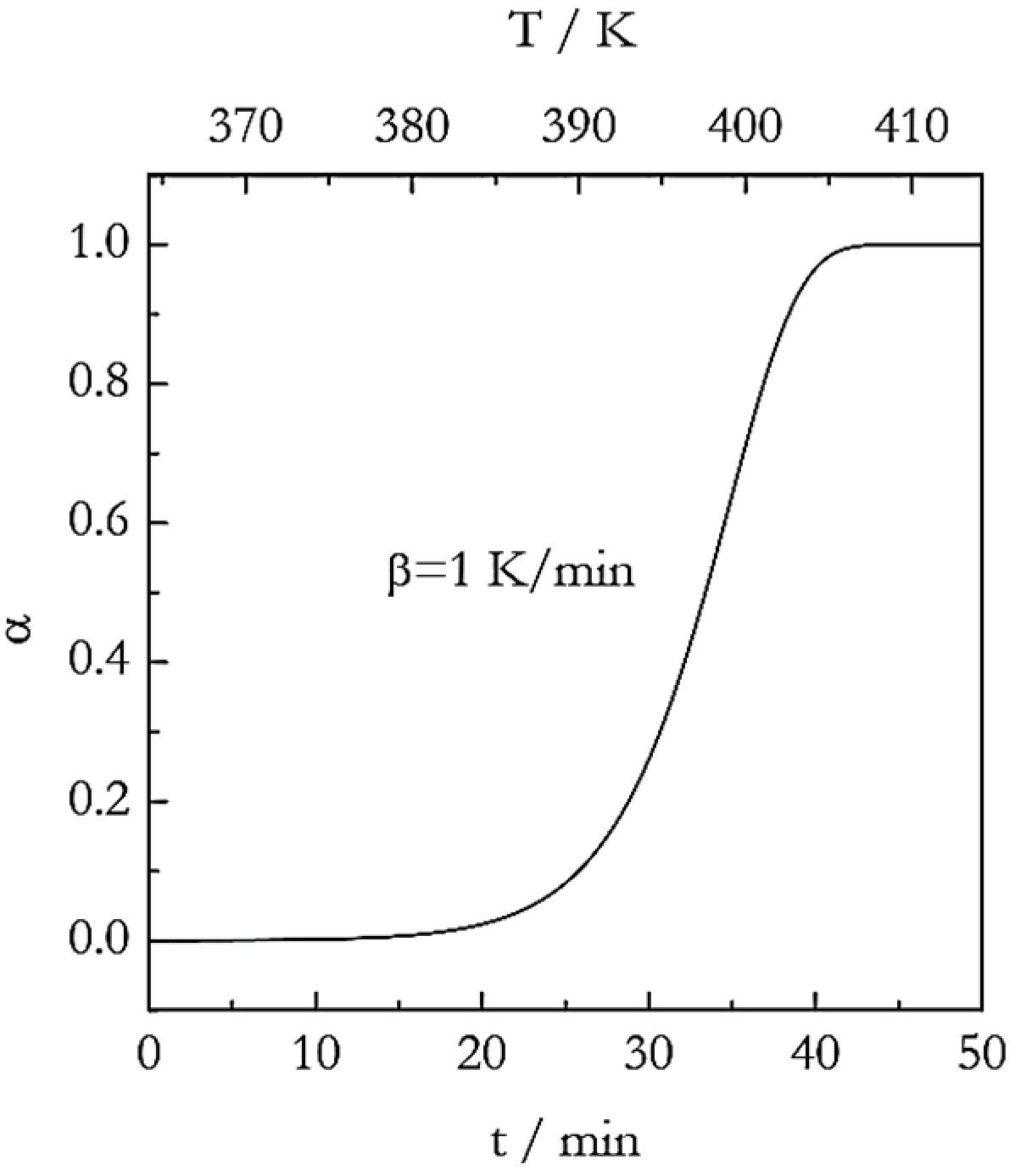
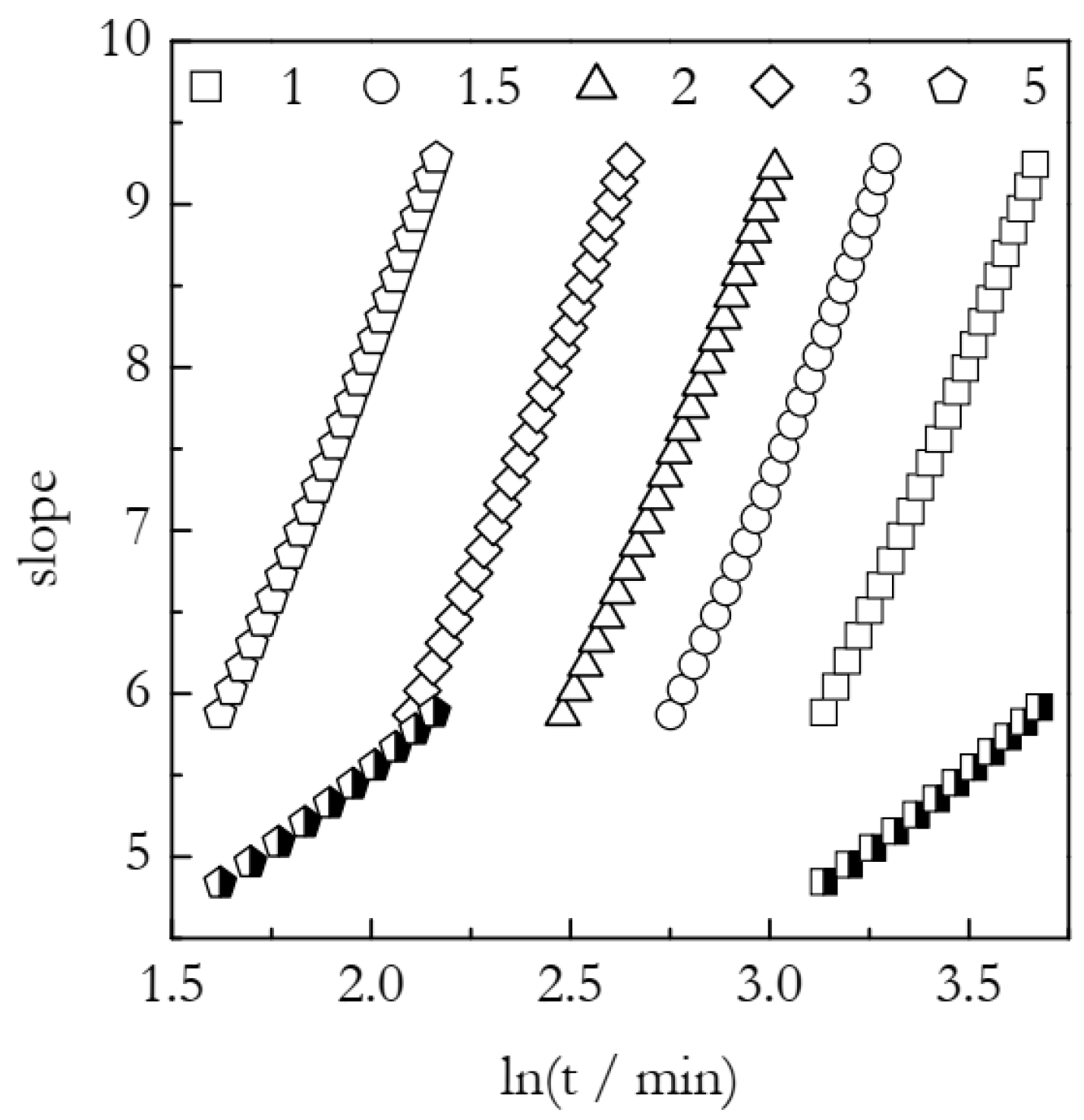
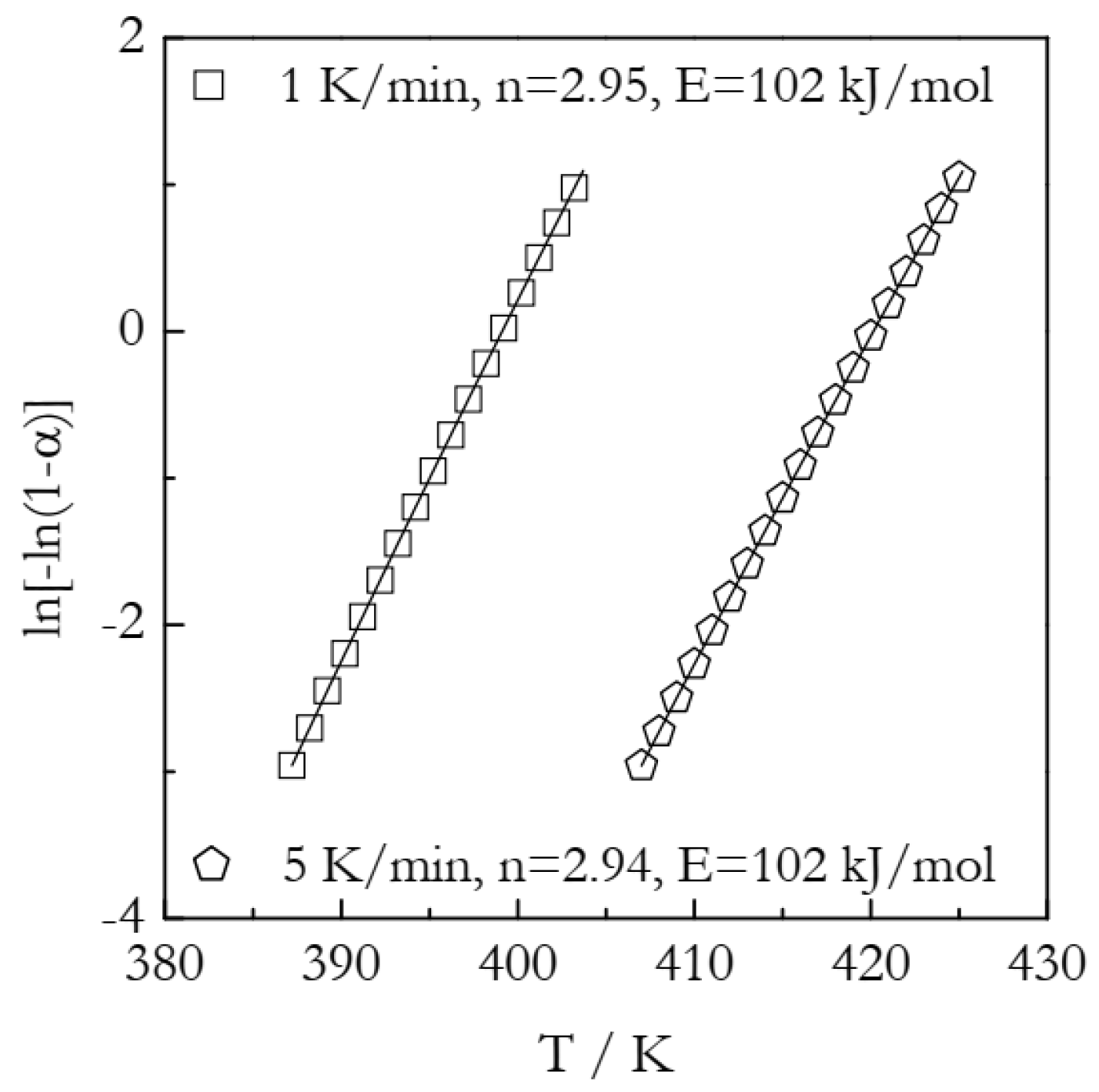
5. Simulations
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schultz, J.M. Polymer Crystallization; ACS & Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Mandelkern, L. Crystallization of Polymers; Cambridge University Press: Cambridge, UK, 2004; Volume 2. [Google Scholar]
- Ozawa, T. Kinetics of nonisothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Available online: Scopus.com (accessed on 4 December 2022).
- Jeziorny, A. Parameters characterizing the kinetics of the non-isothermal crystallization of poly(ethylene terephthalate) determined by d.s.c. Polymer 1978, 19, 1142–1144. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, C.; Dong, Z. Comparison of the Ozawa and modified Avrami models of polymer crystallization under nonisothermal conditions using a computer simulation method. Thermochim. Acta 2007, 466, 22–28. [Google Scholar] [CrossRef]
- Kourtidou, D.; Chrissafis, K. Nonisothermal Crystallization Kinetics: Studying the Validity of Different Johnson–Mehl–Avrami–Erofeev–Kolmogorov (JMAEK) Based Equations. Thermochim. Acta 2021, 704, 179030. [Google Scholar]
- Qu, D.; Gao, H.; Wang, Q.; Bai, Y.; Li, N. Non-isothermal crystallization kinetics of bio-based poly(butylene-co-isosorbide succinate) (PBIS). J. Therm. Anal. Calorim. 2020, 139, 1931–1939. [Google Scholar] [CrossRef]
- Nouira, S.; Hassine, T.; Fitoussi, J.; Shirinbayan, M.; Gamaoun, F.; Tcharkhtchi, A. Non-isothermal crystallization kinetics and its effect on the mechanical properties of homopolymer isotactic polypropylene. J. Polym. Res. 2022, 29, 26. [Google Scholar] [CrossRef]
- Lou, S.; Zhang, H.; Liu, F.; Yin, W.; Ren, G.; Chen, Z.; Su, C. Effects of post-treatment on crystallization behavior of glass fiber-reinforced polyamide 66 composite with red phosphorus flame retardant. J. Therm. Anal. Calorim. 2022, 147, 7229–7242. [Google Scholar] [CrossRef]
- Nakamura, K.; Watanabe, T.; Katayama, K.; Amano, T. Some Aspects of Nonisothermal Crystallization of Polymers. I. Relationship Between Crystallization Temperature, Crystallinity, and Cooling Conditions. J. Appl. Polym. Sci. 1972, 16, 1077–1091. [Google Scholar] [CrossRef]
- Piorkowska, E.; Galeski, A.; Haudin, J.-M. Critical assessment of overall crystallization kinetics theories and predictions. Prog. Polym. Sci. 2006, 31, 549–575. [Google Scholar] [CrossRef]
- Vyazovkin, S. Nonisothermal crystallization of polymers: Getting more out of kinetic analysis of differential scanning calorimetry data. Polym. Cryst. 2018, 1, e10003. [Google Scholar] [CrossRef]
- Vyazovkin, S. Activation energies and temperature dependencies of the rates of crystallization and melting of polymers. Polymers 2020, 12, 1070. [Google Scholar] [CrossRef]
- Cebe, P.; Hong, S.D. Crystallization behaviour of poly(etherether-ketone). Polymer 1986, 27, 1183–1192. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Bai, Y.; Ding, L. Non-isothermal melt-crystallization kinetics of poly (ethyleneterephthalate-co-sodium-5-sulfo-iso-phthalate). Thermochim. Acta 2016, 645, 43–49. [Google Scholar] [CrossRef]
- Jhu, Y.S.; Yang, T.C.; Hung, K.C.; Xu, J.W.; Wu, T.L.; Wu, J.H. Nonisothermal Crystallization Kinetics of Acetylated Bamboo Fiber-Reinforced Polypropylene Composites. Polymers 2019, 11, 1078. [Google Scholar] [CrossRef]
- Miyagawa, Y.; Adachi, S. Analysis of Nonisothermal Crystallization of Rapeseed Oil by Deconvolution of Differential Scanning Calorimetry Curve. J. Oleo Sci. 2019, 68, 1215–1222. [Google Scholar] [CrossRef]
- Chen, W.M.; Yang, M.C.; Hong, S.G.; Hsieh, Y.S. Effect of soft segment content of Pebax® Rnew on the properties of Nylon-6/SMA/PEBA blends. J. Polym. Res. 2019, 26, 25. [Google Scholar] [CrossRef]
- Gaonkar, A.A.; Murudkar, V.V.; Deshpande, V.D. Comparison of crystallization kinetics of polyethylene terephthalate (PET) and reorganized PET. Thermochim. Acta 2020, 683, 178472. [Google Scholar] [CrossRef]
- Li, X.; Zou, M.; Lei, L.; Xi, L. Non-Isothermal Crystallization Kinetics of Poly(ethylene glycol) and Poly(ethylene glycol)-B-Poly(ε-caprolactone) by Flash DSC Analysis. Polymers 2021, 13, 3713. [Google Scholar] [CrossRef]
- de Bruijn, T.J.W.; de Jong, W.A.; van den Berg, P.J. Kinetic parameters in Avrami-Erofeev type reactions from isothermal and non-isothermal experiments. Thermochim. Acta 1981, 45, 315–325. [Google Scholar] [CrossRef]
- Yinnon, H.; Uhlmann, D.R. Applications of thermoanalytical techniques to the study of crystallization kinetics in glass-forming liquids, Part I: Theory. J. Non-Cryst. Solids 1983, 54, 253–275. [Google Scholar] [CrossRef]
- Fatemi, N.; Whitehead, R.; Price, D.; Dollimore, D. Some comments on the use of Avrami-Erofeev expressions and solid state decomposition rate constants. Thermochim. Acta 1986, 104, 93–100. [Google Scholar] [CrossRef]
- Khanna, Y.P.; Taylor, T.J. Comments and Recommendations on the Use of the Avrami Equation for Physico-Chemical Kinetics. Polym. Eng. Sci. 1988, 28, 1042–1045. [Google Scholar] [CrossRef]
- Brown, M.E.; Galwey, A.K. Arrhenius Parameters for Solid-State Reactions from Isothermal Rate-Time Curves. Anal. Chem. 1989, 61, 1136–1139. [Google Scholar] [CrossRef]
- Henderson, D.W. Experimental analysis of nonisotermal transformations involving nucleation and growth. J. Therm. Anal. 1979, 15, 325–331. [Google Scholar] [CrossRef]
- Henderson, D.W. Thermal analysis of non-isothermal crystallization kinetic in glass forming liquids. J. Non-Cryst. Solids 1979, 30, 301–315. [Google Scholar] [CrossRef]
- Farjas, J.; Roura, P. Modification of the Kolmogorov–Johnson–Mehl–Avrami rate equation for non-isothermal experiments and its analytical solution. Acta Mater. 2006, 54, 5573–5579. [Google Scholar] [CrossRef]
- Lauritzen, J.I.; Hoffman, J.D. Extension of theory of growth of chainfolded polymer crystals to large undercoolings. J. Appl. Phys. 1973, 44, 4340–4352. [Google Scholar] [CrossRef]
- Starink, M.J. Activation energy determination for linear heating experiments: Deviations due to neglecting the low temperature end of the temperature integral. J. Mater. Sci. 2007, 42, 483–489. [Google Scholar] [CrossRef]
- Flynn, J.H. The ‘Temperature Integral’—Its use and abuse. Thermochim. Acta 1997, 300, 83–92. [Google Scholar] [CrossRef]
- Starink, M.J. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Dranca, I. Isoconversional Analysis of Combined Melt and Glass Crystallization Data. Macromol. Chem. Phys. 2006, 207, 20–25. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Bikiaris, D.N.; Achilias, D.S. Effect of molecular weight on the cold-crystallization, of biodegradable poly(ethylene succinate). Thermochim. Acta 2007, 457, 41–54. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Galukhin, A. Problems with Applying the Ozawa–Avrami Crystallization Model to Non-Isothermal Crosslinking Polymerization. Polymers 2022, 14, 693. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vyazovkin, S. Jeziorny Method Should Be Avoided in Avrami Analysis of Nonisothermal Crystallization. Polymers 2023, 15, 197. https://doi.org/10.3390/polym15010197
Vyazovkin S. Jeziorny Method Should Be Avoided in Avrami Analysis of Nonisothermal Crystallization. Polymers. 2023; 15(1):197. https://doi.org/10.3390/polym15010197
Chicago/Turabian StyleVyazovkin, Sergey. 2023. "Jeziorny Method Should Be Avoided in Avrami Analysis of Nonisothermal Crystallization" Polymers 15, no. 1: 197. https://doi.org/10.3390/polym15010197
APA StyleVyazovkin, S. (2023). Jeziorny Method Should Be Avoided in Avrami Analysis of Nonisothermal Crystallization. Polymers, 15(1), 197. https://doi.org/10.3390/polym15010197






