Biocompatibility and Mechanical Properties of Carboxymethyl Chitosan Hydrogels
Abstract
1. Introduction
2. Materials and Methods
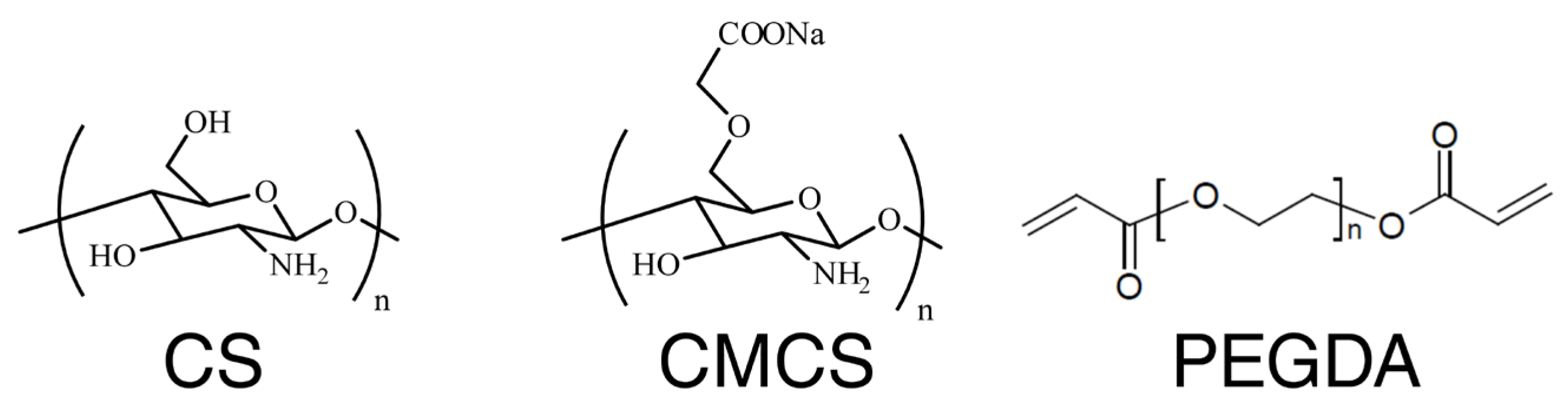
2.1. Chemicals/Materials
2.2. Hydrogel Manufacturing
2.3. Compression Test
2.4. Live/Dead Test
2.4.1. Preparation of Eluates
2.4.2. Cell Culture
2.4.3. Cell Viability
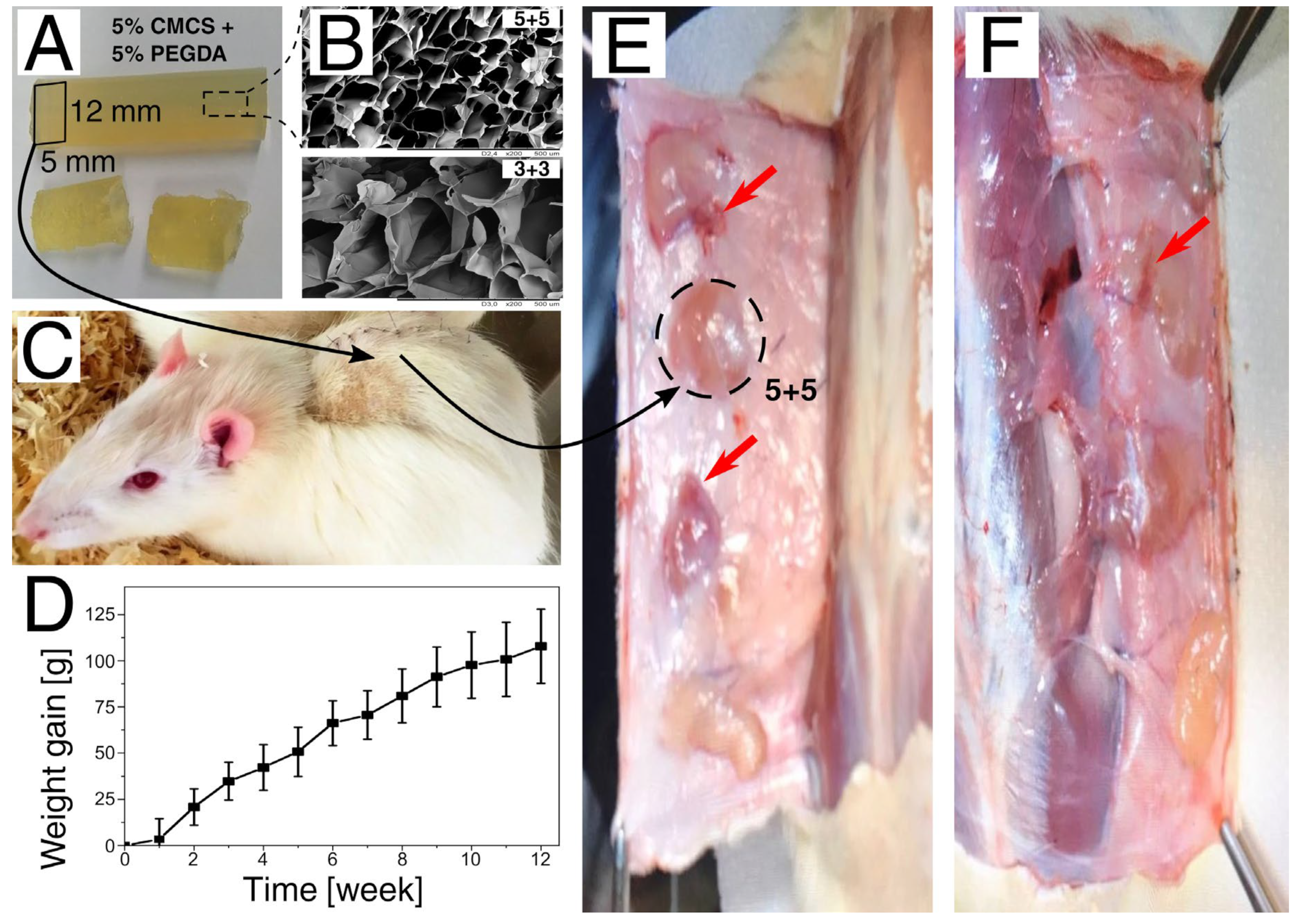
2.5. Animal Studies
2.5.1. Animals
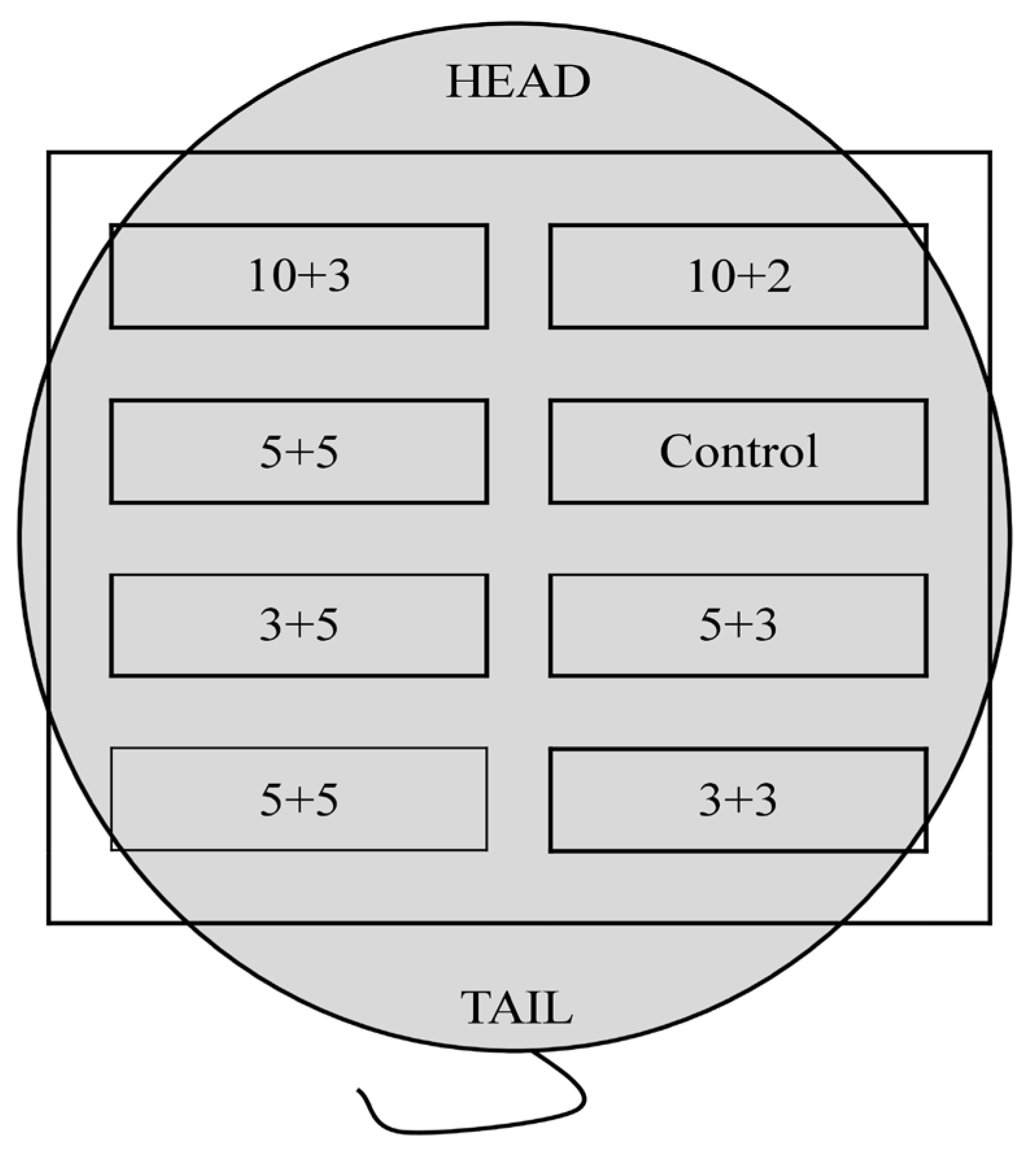
2.5.2. Biocompatibility Test
- CMCS 3% and PEGDA 3%;
- CMCS 3% and PEGDA 5%;
- CMCS 5% and PEGDA 3%;
- CMCS 5%and PEGDA 5%;
- CMCS 10% and PEGDA 2%;
- CMCS 10% and PEGDA 3%.
2.6. Statistical Analysis
3. Results and Discussion
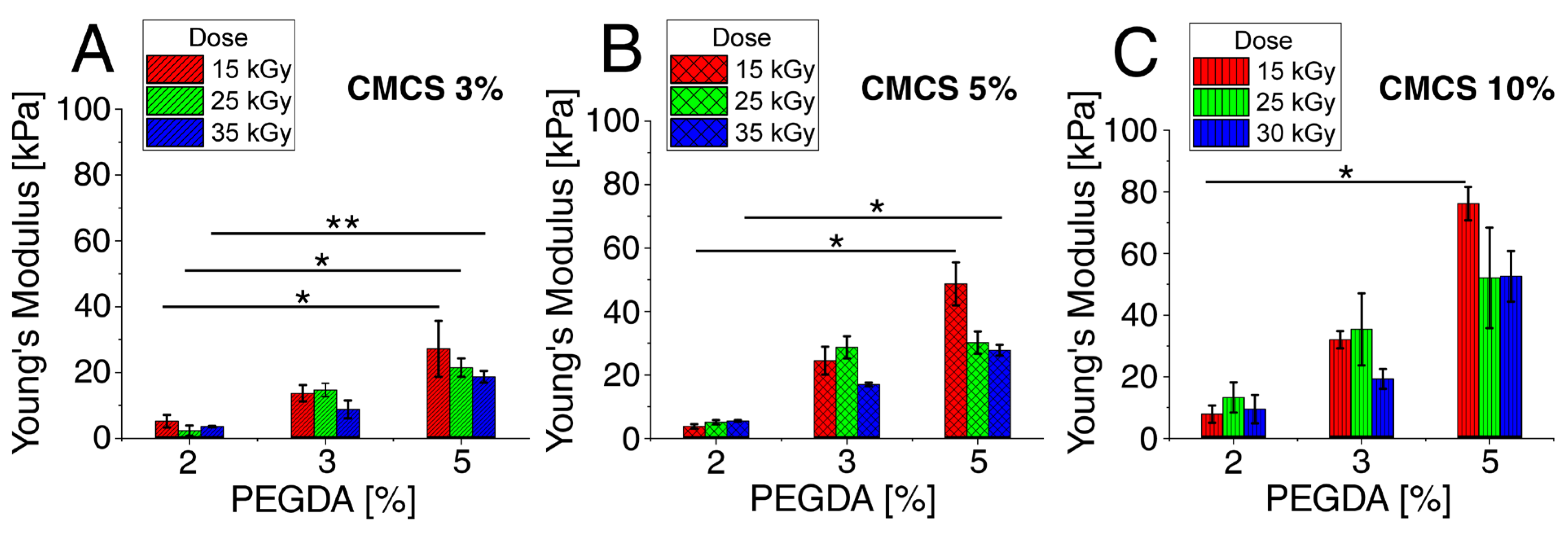
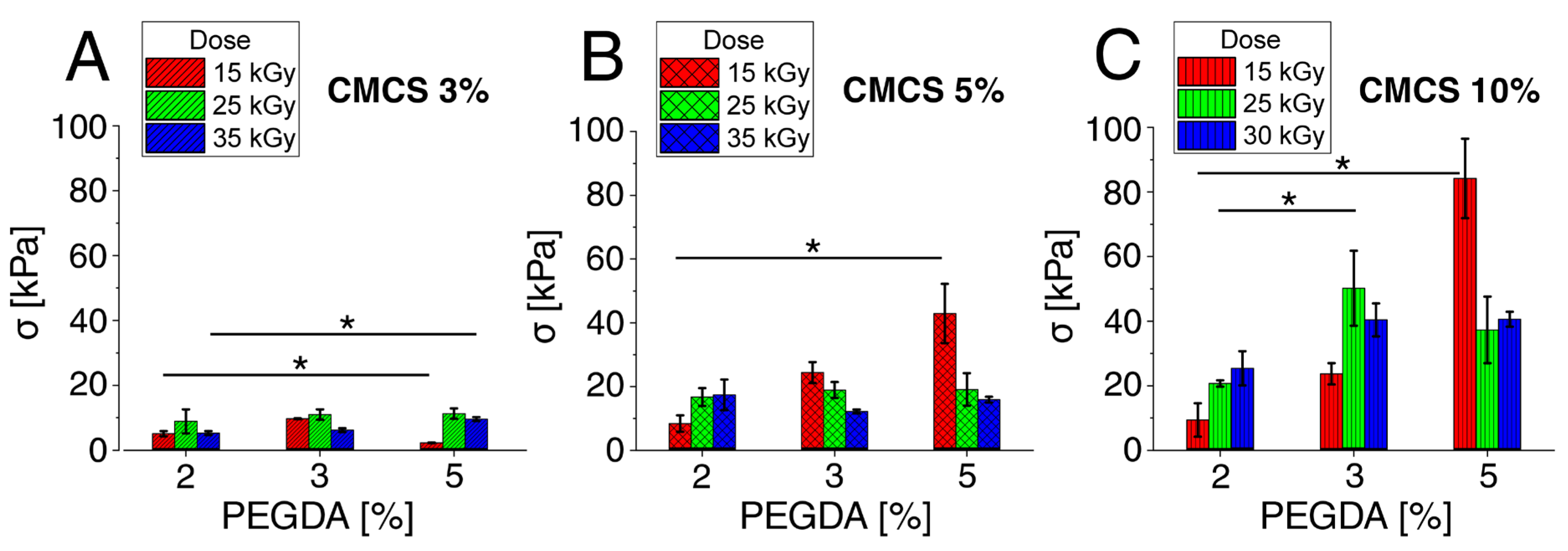
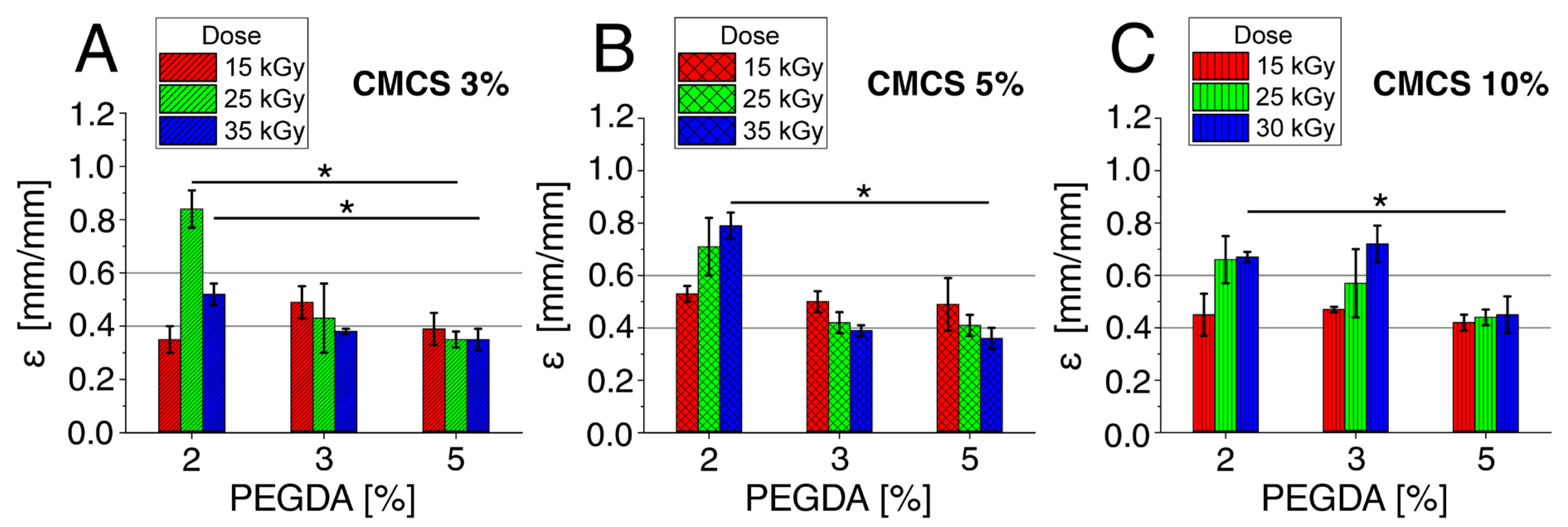
3.1. Mechanical Properties Tests
3.1.1. Young’s Modulus—Flexibility of Hydrogels
3.1.2. Compressive Strength–Maximum Pressure
3.1.3. Deformation at the Max Compression Stress
3.2. Live/Dead Test
3.3. Assessment of In Vivo Biocompatibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Andrade, J. Hydrogels in medicine and pharmacy. J. Control. Release 1989, 10. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.; Javed, F.; Ahmad, Z.; Md Akil, H. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.L.; Yu, A.C.; Agmon, G.; Appel, E.A. Supramolecular polymeric biomaterials. Biomater. Sci. 2017, 6, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Liu, L.; He, G.; Zhang, T.; Yang, M.; Cai, J.; Fan, L.; Tao, S. Preparation and properties of carboxymethyl chitosan/oxidized hydroxyethyl cellulose hydrogel. Int. J. Biol. Macromol. 2020, 162, 1692–1698. [Google Scholar] [CrossRef]
- Rosiak, J.M. Hydrogel Dressings. In Radiation Effects on Polymers; ACS Symposium Series: Washington, DC, USA, 1991; pp. 271–299. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, H.; Lei, X.; Lau, J.N.Y.; Yuan, M.; Wang, X.; Zhang, F.; Zhou, F.; Qi, S.; Shu, B.; et al. A Systematic Review and Meta-Analysis of Clinical Effectiveness and Safety of Hydrogel Dressings in the Management of Skin Wounds. Front. Bioeng. Biotechnol. 2019, 7, 342. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Ulański, P. Synthesis of hydrogels by irradiation of polymers in aqueous solution. Radiat. Phys. Chem. 1999, 55, 139–151. [Google Scholar] [CrossRef]
- Wasikiewicz, J.M.; Mitomo, H.; Nagasawa, N.; Yagi, T.; Tamada, M.; Yoshii, F. Radiation crosslinking of biodegradable carboxymethylchitin and carboxymethylchitosan. J. Appl. Polym. Sci. 2006, 102, 758–767. [Google Scholar] [CrossRef]
- Yoshii, F.; Zhao, L.; Wach, R.A.; Nagasawa, N.; Mitomo, H.; Kume, T. Hydrogels of polysaccharide derivatives crosslinked with irradiation at paste-like condition. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2003, 208, 320–324. [Google Scholar] [CrossRef]
- Szafulera, K.; Wach, R.A.; Olejnik, A.K.; Rosiak, J.M.; Ulański, P. Radiation synthesis of biocompatible hydrogels of dextran methacrylate. Radiat. Phys. Chem. 2018, 142, 115–120. [Google Scholar] [CrossRef]
- Al-Assaf, S.; Gulrez, K.; Czechowska-Biskup, R.; Wach, R.; Rosiak, J.; Ulanski, P. Radiation Modification of Polysaccharides. The Radiation Chemistry of Polysaccharides; Al-Assaf, S., Coqueret, X., Dahlan, K.M., Sen, M., Ulanski, P., Eds.; International Atomic Energy Agency: Vienna, Austria, 2016; pp. 77–116. [Google Scholar]
- Charlesby, A. The degradation of cellulose by ionizing radiation. J. Polym. Sci. 1955, 15, 263–270. [Google Scholar] [CrossRef]
- Ershov, B.G. Radiation-chemical degradation of cellulose and other polysaccharides. Russ. Chem. Rev. 1998, 67, 315–334. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Carboxymethylated chitins and chitosans. Carbohydr. Polym. 1988, 8, 1–21. [Google Scholar] [CrossRef]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control. Release 2010, 145, 182–195. [Google Scholar] [CrossRef]
- Rodrigues, S.; Dionisio, M.; Lopez, C.R.; Grenha, A. Biocompatibility of chitosan carriers with application in drug delivery. J. Funct. Biomater. 2012, 3, 615–641. [Google Scholar] [CrossRef]
- Von Palubitzki, L.; Wang, Y.; Hoffmann, S.; Vidal, Y.S.S.; Zobiak, B.; Failla, A.V.; Schmage, P.; John, A.; Osorio-Madrazo, A.; Bauer, A.T.; et al. Differences of the tumour cell glycocalyx affect binding of capsaicin-loaded chitosan nanocapsules. Sci. Rep. 2020, 10, 22443. [Google Scholar] [CrossRef]
- Doench, I.; Torres-Ramos, M.E.W.; Montembault, A.; Nunes de Oliveira, P.; Halimi, C.; Viguier, E.; Heux, L.; Siadous, R.; Thire, R.; Osorio-Madrazo, A. Injectable and Gellable Chitosan Formulations Filled with Cellulose Nanofibers for Intervertebral Disc Tissue Engineering. Polymers 2018, 10, 1202. [Google Scholar] [CrossRef]
- Gorzelanny, C.; Poppelmann, B.; Pappelbaum, K.; Moerschbacher, B.M.; Schneider, S.W. Human macrophage activation triggered by chitotriosidase-mediated chitin and chitosan degradation. Biomaterials 2010, 31, 8556–8563. [Google Scholar] [CrossRef]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef]
- Rao, S.B.; Sharma, C.P. Use of chitosan as a biomaterial: Studies on its safety and hemostatic potential. J. Biomed. Mater. Res. 1997, 34, 21–28. [Google Scholar] [CrossRef]
- Amine, S.; Montembault, A.; Fumagalli, M.; Osorio-Madrazo, A.; David, L. Controlled Polyelectrolyte Association of Chitosan and Carboxylated Nano-Fibrillated Cellulose by Desalting. Polymers 2021, 13, 2023. [Google Scholar] [CrossRef] [PubMed]
- Desorme, M.; Montembault, A.; Lucas, J.M.; Rochas, C.; Bouet, T.; David, L. Spinning of hydroalcoholic chitosan solutions. Carbohydr. Polym. 2013, 98, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Madrazo, A.; David, L.; Trombotto, S.; Lucas, J.M.; Peniche-Covas, C.; Domard, A. Kinetics study of the solid-state acid hydrolysis of chitosan: Evolution of the crystallinity and macromolecular structure. Biomacromolecules 2010, 11, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Madrazo, A.; David, L.; Peniche-Covas, C.; Rochas, C.; Putaux, J.L.; Trombotto, S.; Alcouffe, P.; Domard, A. Fine microstructure of processed chitosan nanofibril networks preserving directional packing and high molecular weight. Carbohydr. Polym. 2015, 131, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Domenge, O.; Ragot, H.; Deloux, R.; Crepet, A.; Revet, G.; Boitard, S.E.; Simon, A.; Mougenot, N.; David, L.; Delair, T.; et al. Efficacy of epicardial implantation of acellular chitosan hydrogels in ischemic and nonischemic heart failure: Impact of the acetylation degree of chitosan. Acta Biomater. 2021, 119, 125–139. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef]
- Pita-López, M.L.; Fletes-Vargas, G.; Espinosa-Andrews, H.; Rodríguez-Rodríguez, R. Physically cross-linked chitosan-based hydrogels for tissue engineering applications: A state-of-the-art review. Eur. Polym. J. 2021, 145, 110176. [Google Scholar] [CrossRef]
- Chen, L.; Du, Y.; Tian, Z.; Sun, L. Effect of the degree of deacetylation and the substitution of carboxymethyl chitosan on its aggregation behavior. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 296–305. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Della Sala, F.; Biondi, M.; Guarnieri, D.; Borzacchiello, A.; Ambrosio, L.; Mayol, L. Mechanical behavior of bioactive poly(ethylene glycol) diacrylate matrices for biomedical application. J. Mech. Behav. Biomed. Mater. 2020, 110, 103885. [Google Scholar] [CrossRef]
- Wei, Q.; Ma, L.; Zhang, W.; Ma, G.; Hu, Z. EGCG-crosslinked carboxymethyl chitosan-based hydrogels with inherent desired functions for full-thickness skin wound healing. J. Mater. Chem. B 2022, 10, 3927–3935. [Google Scholar] [CrossRef]
- Fan, L.; Yi, J.; Tong, J.; Zhou, X.; Ge, H.; Zou, S.; Wen, H.; Nie, M. Preparation and characterization of oxidized konjac glucomannan/carboxymethyl chitosan/graphene oxide hydrogel. Int. J. Biol. Macromol. 2016, 91, 358–367. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Zhang, Z. Calcium-carboxymethyl chitosan hydrogel beads for protein drug delivery system. J. Appl. Polym. Sci. 2007, 103, 3164–3168. [Google Scholar] [CrossRef]
- Zhao, L.; Mitomo, H.; Nagasawa, N.; Yoshii, F.; Kume, T. Radiation synthesis and characteristic of the hydrogels based on carboxymethylated chitin derivatives. Carbohydr. Polym. 2003, 51, 169–175. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.; Wach, R.A.; Stojek, P.; Kamińska, M.; Rosiak, J.M.; Ulański, P. Synthesis of Chitosan and Carboxymethyl Chitosan Hydrogels by Electron Beam Irradiation. Prog. Chem. Appl. Chitin Its Deriv. 2016, 21, 27–45. [Google Scholar] [CrossRef]
- Kłosiński, K.K.; Pasieka, Z.; Arkuszewski, P.T.; Girek, M.K.; Szymański, P.B.; Wach, R.A.; Czechowska-Biskup, R. Synthesis and Potential Cytotoxicity Evaluation of Carboxymethyl Chitosan Hydrogels. Prog. Chem. Appl. Chitin Its Deriv. 2017, XXII, 82–96. [Google Scholar] [CrossRef][Green Version]
- Singh, D.; Singh, D.; Choi, S.M.; Zo, S.M.; Painuli, R.M.; Kwon, S.W.; Han, S.S. Effect of Extracts ofTerminalia chebulaon Proliferation of Keratinocytes and Fibroblasts Cells: An Alternative Approach for Wound Healing. Evid.-Based Complement. Altern. Med. 2014, 2014, 701656. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.M.; Abdel-Mohsen, A.M.; Hrdina, R.; Burgert, L.; Fohlerova, Z.; Pavliňák, D.; Sayed, O.N.; Jancar, J. Wound dressing based on chitosan/hyaluronan/nonwoven fabrics: Preparation, characterization and medical applications. Int. J. Biol. Macromol. 2016, 89, 725–736. [Google Scholar] [CrossRef]
- He, M.; Potuck, A.; Zhang, Y.; Chu, C.-C. Arginine-based polyester amide/polysaccharide hydrogels and their biological response. Acta Biomater. 2014, 10, 2482–2494. [Google Scholar] [CrossRef]
- Hassan, W.; Dong, Y.; Wang, W. Encapsulation and 3D culture of human adipose-derived stem cells in an in-situ crosslinked hybrid hydrogel composed of PEG-based hyperbranched copolymer and hyaluronic acid. Stem Cell Res. Ther. 2013, 4, 32. [Google Scholar] [CrossRef]
- Sotocinal, S.G.; Sorge, R.E.; Zaloum, A.; Tuttle, A.H.; Martin, L.J.; Wieskopf, J.S.; Mapplebeck, J.C.; Wei, P.; Zhan, S.; Zhang, S.; et al. The Rat Grimace Scale: A partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain 2011, 7, 55. [Google Scholar] [CrossRef]
- Wu, X.; Li, H. Incorporation of Bioglass Improved the Mechanical Stability and Bioactivity of Alginate/Carboxymethyl Chitosan Hydrogel Wound Dressing. ACS Appl. Bio Mater. 2021, 4, 1677–1692. [Google Scholar] [CrossRef]
- Bollen, L.S. New trends in biological evaluation of medical devices. Med. Device Technol. 2005, 16, 10–15. [Google Scholar]
- Schuh, J.C. Medical device regulations and testing for toxicologic pathologists. Toxicol. Pathol. 2008, 36, 63–69. [Google Scholar] [CrossRef]
- McAllister, P.; Jeswiet, J. Medical device regulation for manufacturers. Proc. Inst. Mech. Eng. H 2003, 217, 459–467. [Google Scholar] [CrossRef]
- Schuh, J.C.L.; Funk, K.A. Compilation of International Standards and Regulatory Guidance Documents for Evaluation of Biomaterials, Medical Devices, and 3-D Printed and Regenerative Medicine Products. Toxicol. Pathol. 2019, 47, 344–357. [Google Scholar] [CrossRef]
- Reeve, L.; Baldrick, P. Biocompatibility assessments for medical devices—Evolving regulatory considerations. Expert Rev. Med. Devices 2017, 14, 161–167. [Google Scholar] [CrossRef]
- Swift, W.; Bair, J.A.; Chen, W.; Li, M.; Lie, S.; Li, D.; Yang, M.; Shatos, M.A.; Hodges, R.R.; Kolko, M.; et al. Povidone iodine treatment is deleterious to human ocular surface conjunctival cells in culture. BMJ Open Ophthalmol. 2020, 5, e000545. [Google Scholar] [CrossRef]
- Huber, D.; Grzelak, A.; Baumann, M.; Borth, N.; Schleining, G.; Nyanhongo, G.S.; Guebitz, G.M. Anti-inflammatory and anti-oxidant properties of laccase-synthesized phenolic-O-carboxymethyl chitosan hydrogels. New Biotechnol. 2018, 40, 236–244. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, X.; Hu, W.; Han, X.; Fan, L.; Tao, S. Preparation and characterization of carboxymethyl chitosan/collagen peptide/oxidized konjac composite hydrogel. Int. J. Biol. Macromol. 2020, 149, 31–40. [Google Scholar] [CrossRef]
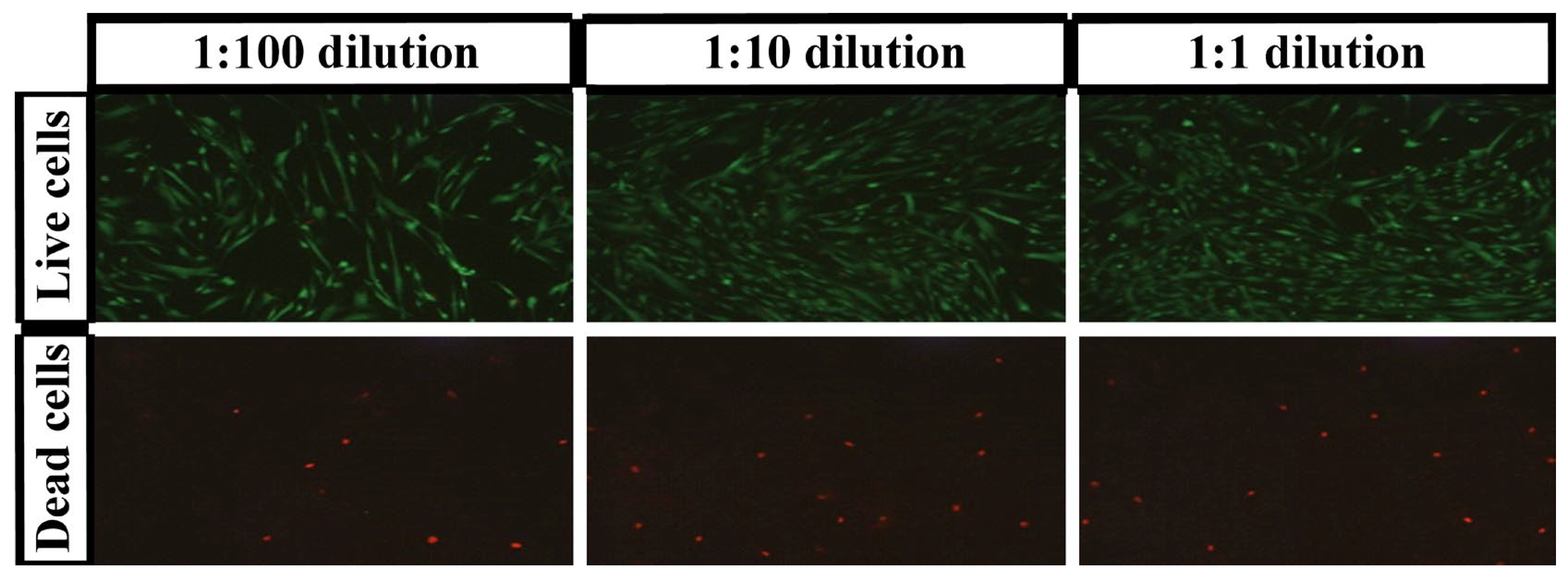
| % CMCS | % PEGDA | % Live Cells | SD (%) | % Dead Cells | SD (%) |
|---|---|---|---|---|---|
| 1:1 ratio | |||||
| 10% | 5 | 95.8 | 7.2 | 8.3 | 1.3 |
| 3 | 91.6 | 9.5 | 8.0 | 1.4 | |
| 2 | 88.1 | 7.2 | 5.8 | 1.4 | |
| 5% | 5 | 100.0 | 10.8 | 9.5 | 1.5 |
| 3 | 94.7 | 7.4 | 7.8 | 1.8 | |
| 2 | 90.5 | 10.7 | 7.1 | 1.8 | |
| 3% | 5 | 98.2 | 9.8 | 9.2 | 1.6 |
| 3 | 99.7 | 5.3 | 7.8 | 0.6 | |
| 2 | 90.0 | 5.0 | 5.1 | 1.1 | |
| 1:10 ratio | |||||
| 10% | 5 | 92.8 | 6.1 | 6.7 | 1.5 |
| 3 | 97.6 | 8.8 | 6.8 | 1.7 | |
| 2 | 99.9 | 8.6 | 6.6 | 1.8 | |
| 5% | 5 | 100.7 | 11.8 | 9.5 | 1.2 |
| 3 | 95.6 | 9.7 | 7.5 | 2.0 | |
| 2 | 92.7 | 9.5 | 7.7 | 1.2 | |
| 3% | 5 | 100.8 | 7.0 | 7.4 | 1.0 |
| 3 | 110.8 | 7.0 | 9.4 | 1.1 | |
| 2 | 93.8 | 3.4 | 5.6 | 1.7 | |
| 1:100 ratio | |||||
| 10% | 5 | 92.7 | 5.6 | 6.4 | 1.5 |
| 3 | 96.6 | 8.4 | 7.0 | 1.2 | |
| 2 | 96.9 | 7.2 | 7.2 | 1.1 | |
| 5% | 5 | 103.3 | 10.9 | 9.3 | 1.7 |
| 3 | 84.6 | 10.6 | 6.5 | 1.3 | |
| 2 | 90.3 | 11.2 | 10.0 | 1.3 | |
| 3% | 5 | 100.0 | 5.3 | 8.9 | 1.0 |
| 3 | 110.4 | 9.8 | 9.0 | 1.9 | |
| 2 | 99.3 | 7.9 | 6.3 | 1.8 | |
| Negative control | 100.0 | 4.65 | 0.0 | 0.15 | |
| Positive control | 0.0 | 1.21 | 100.0 | 3.26 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kłosiński, K.K.; Wach, R.A.; Girek-Bąk, M.K.; Rokita, B.; Kołat, D.; Kałuzińska-Kołat, Ż.; Kłosińska, B.; Duda, Ł.; Pasieka, Z.W. Biocompatibility and Mechanical Properties of Carboxymethyl Chitosan Hydrogels. Polymers 2023, 15, 144. https://doi.org/10.3390/polym15010144
Kłosiński KK, Wach RA, Girek-Bąk MK, Rokita B, Kołat D, Kałuzińska-Kołat Ż, Kłosińska B, Duda Ł, Pasieka ZW. Biocompatibility and Mechanical Properties of Carboxymethyl Chitosan Hydrogels. Polymers. 2023; 15(1):144. https://doi.org/10.3390/polym15010144
Chicago/Turabian StyleKłosiński, Karol K., Radosław A. Wach, Małgorzata K. Girek-Bąk, Bożena Rokita, Damian Kołat, Żaneta Kałuzińska-Kołat, Barbara Kłosińska, Łukasz Duda, and Zbigniew W. Pasieka. 2023. "Biocompatibility and Mechanical Properties of Carboxymethyl Chitosan Hydrogels" Polymers 15, no. 1: 144. https://doi.org/10.3390/polym15010144
APA StyleKłosiński, K. K., Wach, R. A., Girek-Bąk, M. K., Rokita, B., Kołat, D., Kałuzińska-Kołat, Ż., Kłosińska, B., Duda, Ł., & Pasieka, Z. W. (2023). Biocompatibility and Mechanical Properties of Carboxymethyl Chitosan Hydrogels. Polymers, 15(1), 144. https://doi.org/10.3390/polym15010144










