Ethylene-Vinyl Acetate Copolymers as Potential Thermoplastic Modifiers of Photopolymer Compositions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Methods Characterization
2.3.1. Refractometry
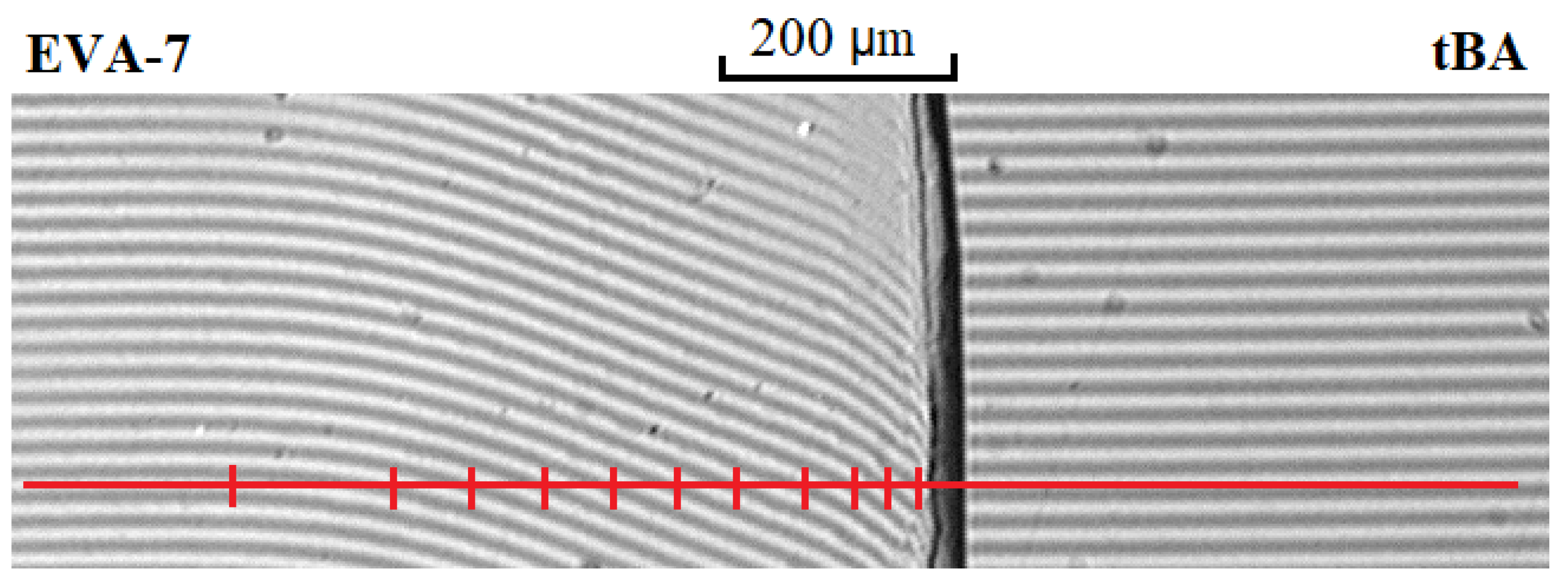
2.3.2. Optical Micro-Interferometry
3. Results and Discussion
3.1. Refractometry Results
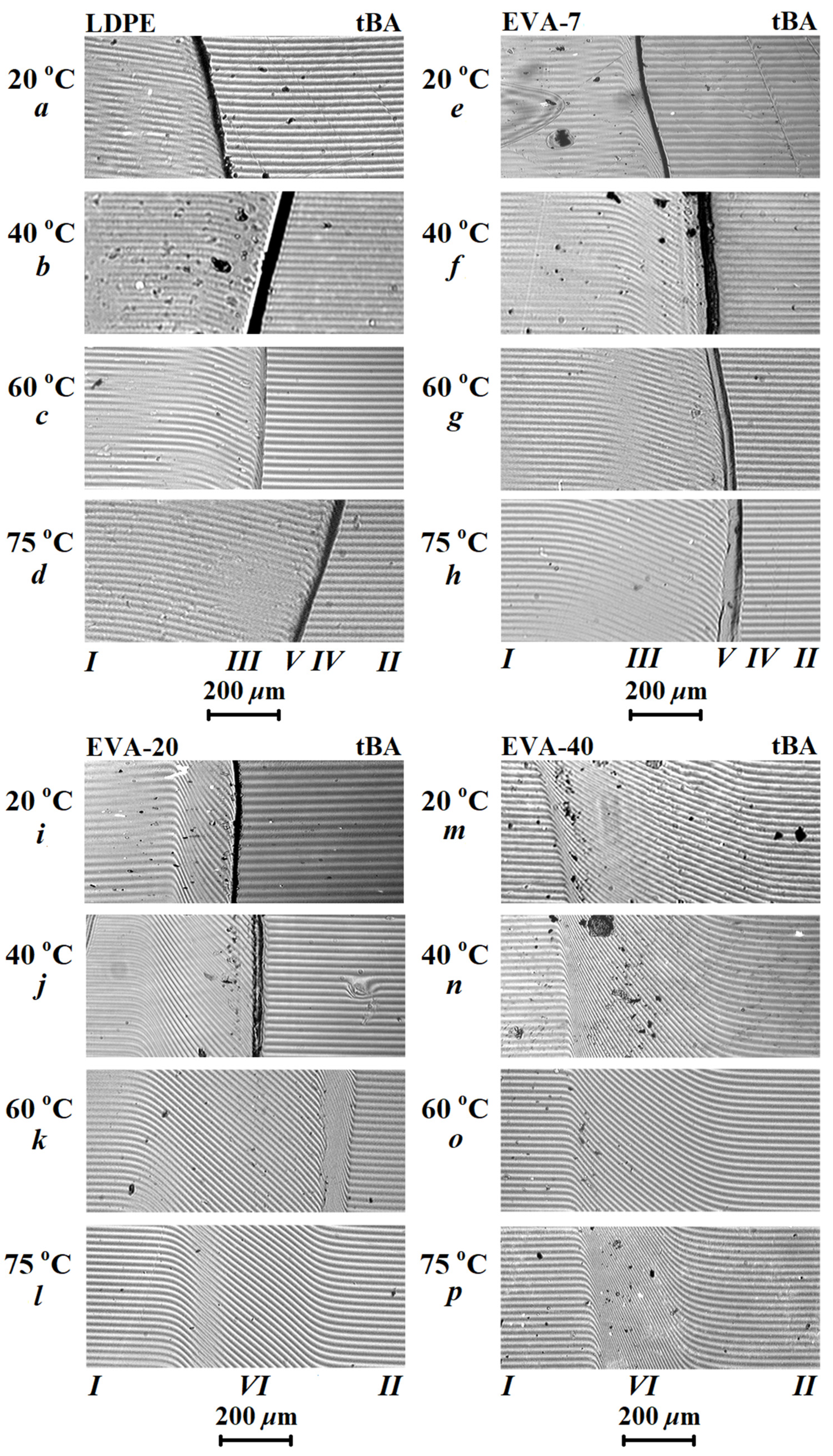
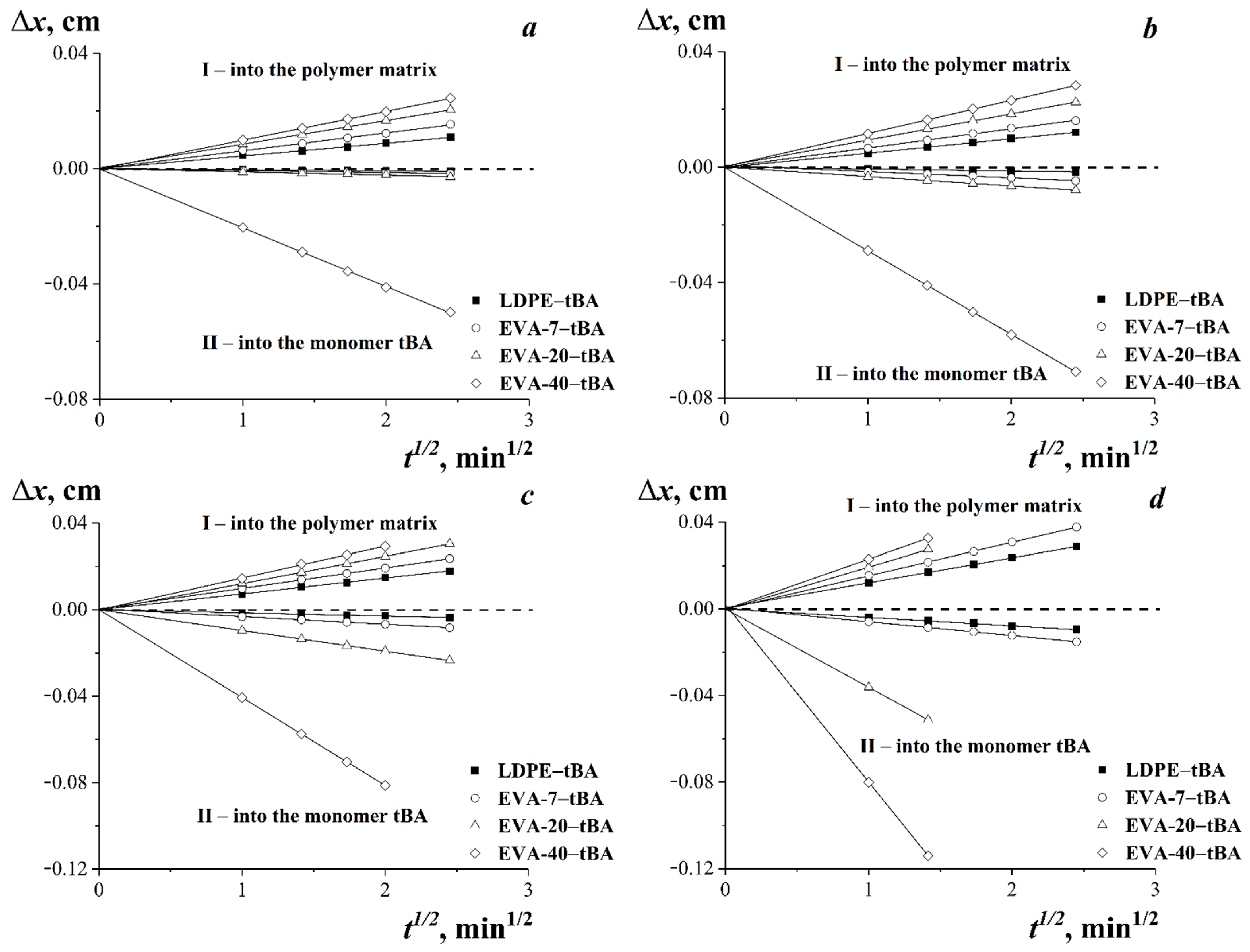
3.2. Diffusion Zones
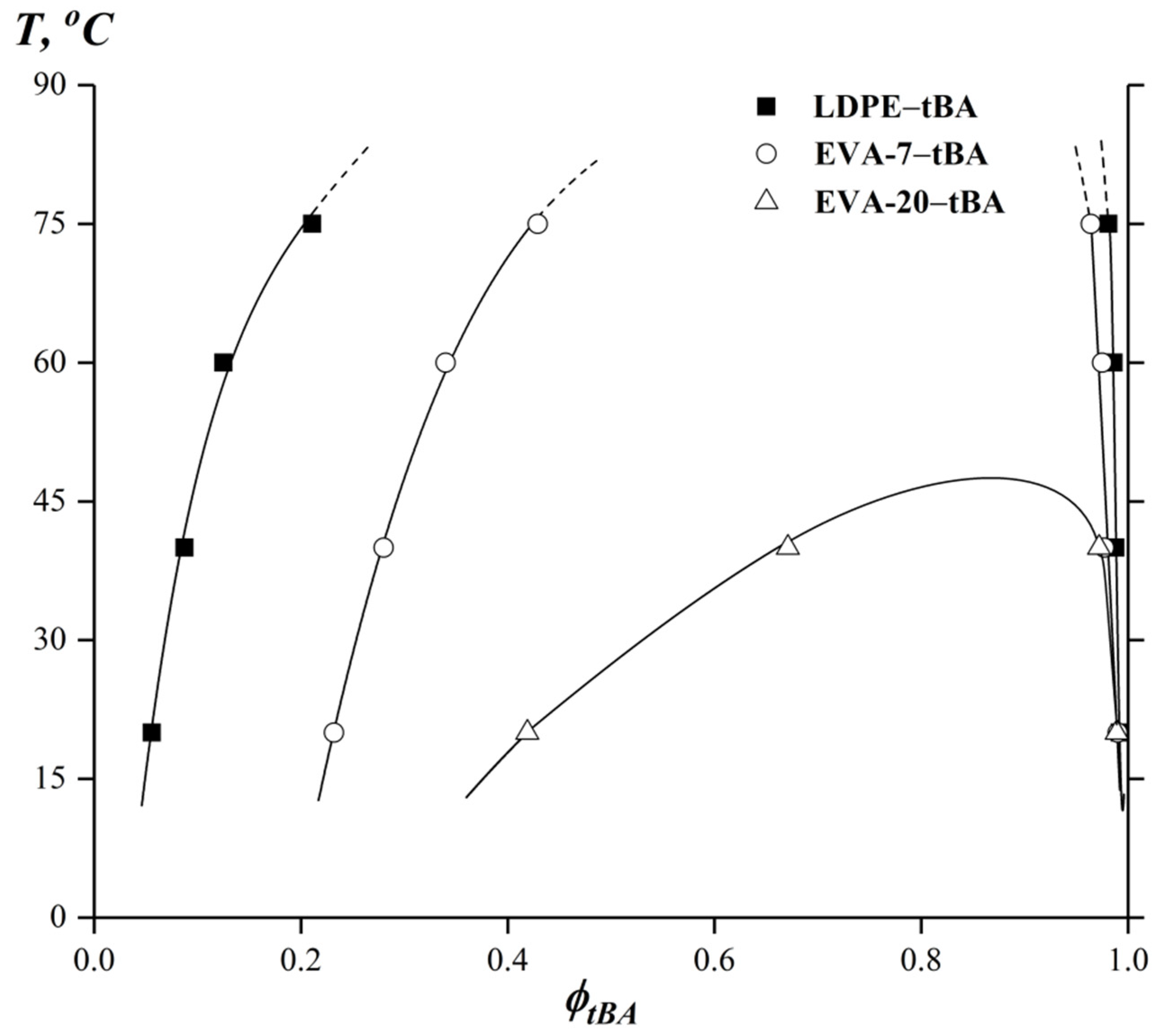
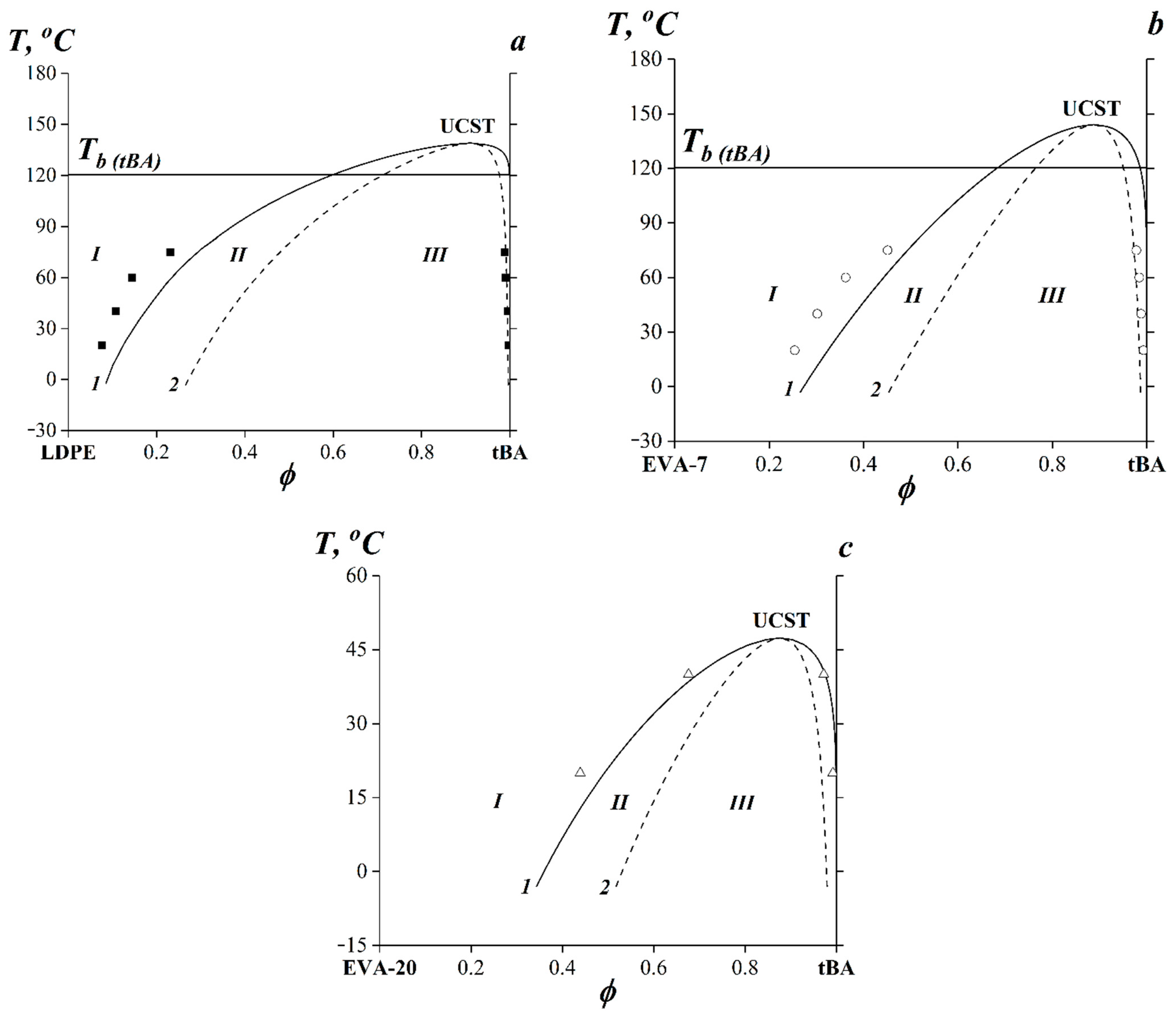
3.3. Phase State Diagrams in the tBA-Based Compositions
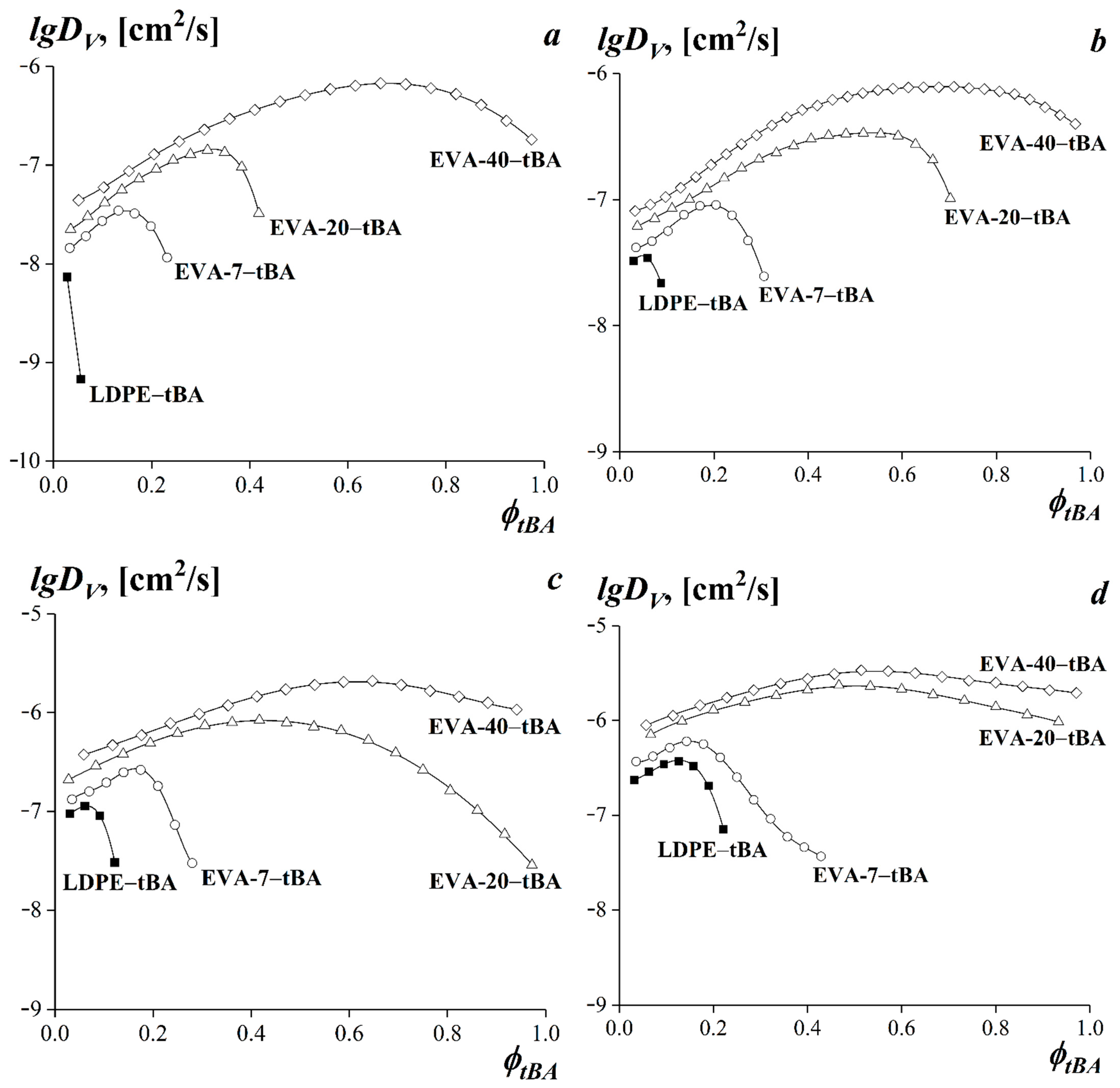
3.4. Diffusion in tBA-(LDPE or EVA) Systems
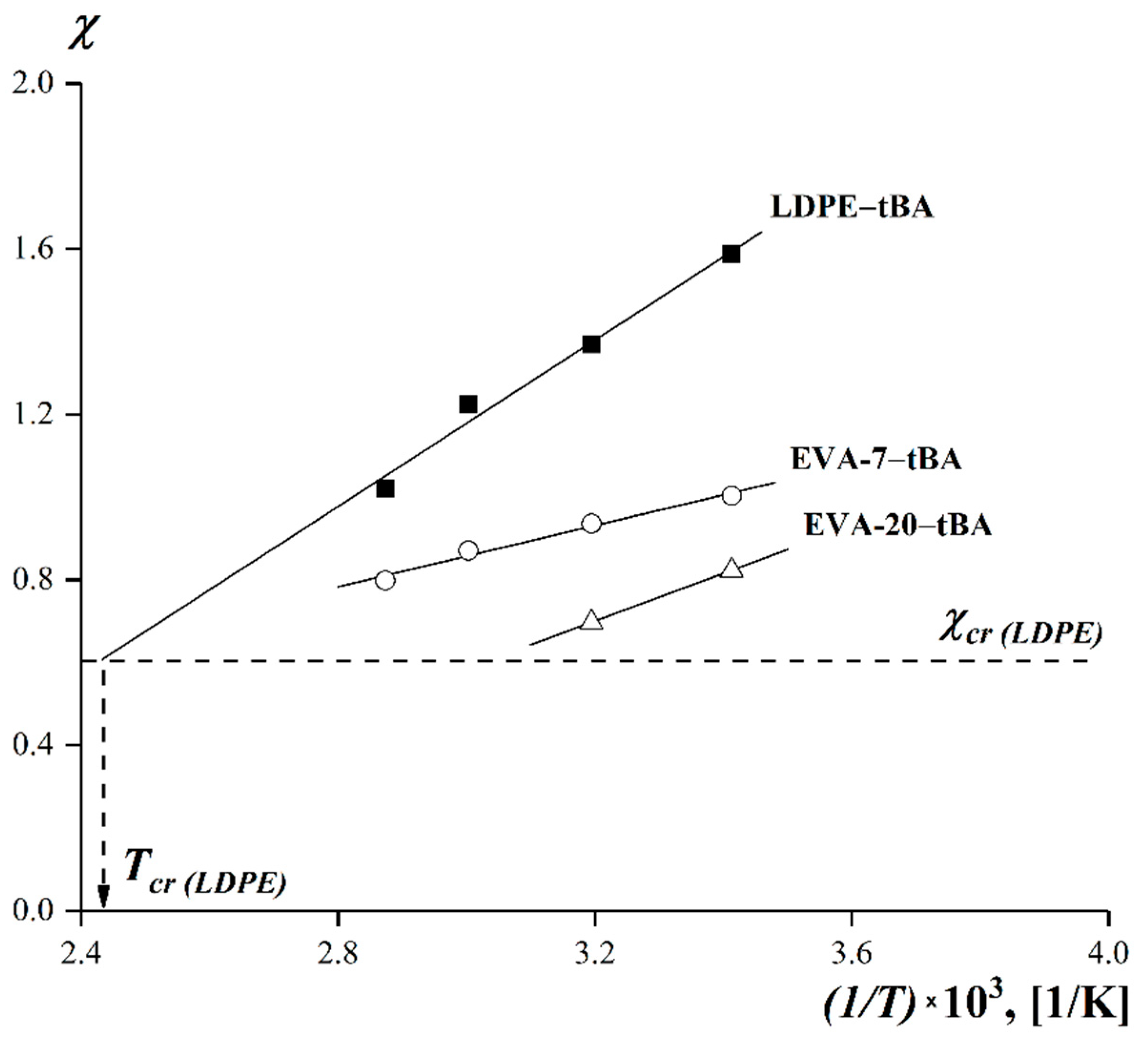
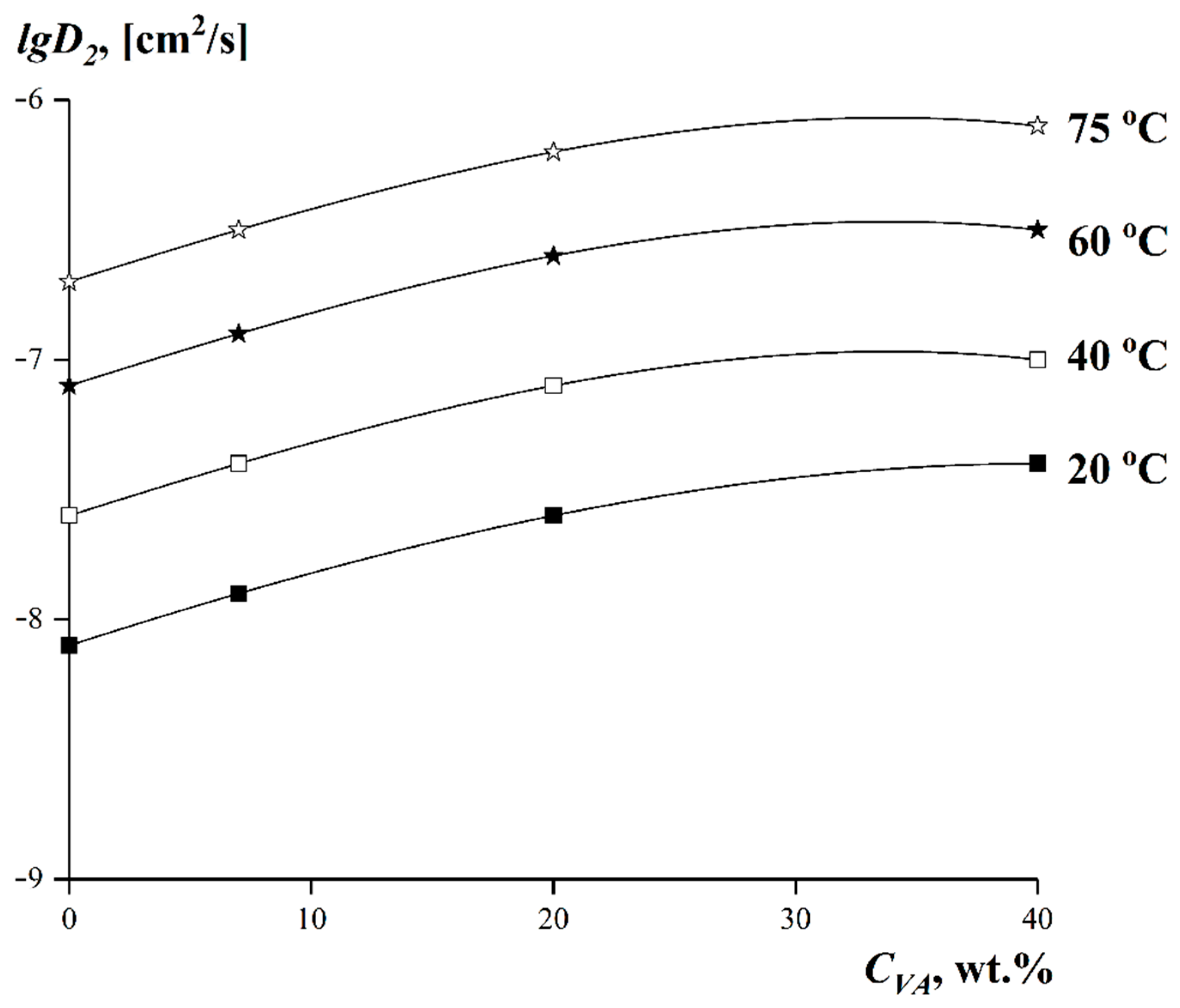
3.5. Dependences of the Limiting Values of Interdiffusion Coefficients on the Experimental Temperature and on the VA Content in the Copolymer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Regassa, Y.; Lemu, H.G.; Sirabizuh, B. Trends of using polymer composite materials in additive manufacturing. IOP Conf. Ser. Mater. Sci. Eng. 2019, 659, 12–21. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.W.; Stucker, B. Photopolymerization Processes. In Additive Manufacturing Technologies; Springer: Boston, MA, USA, 2010; pp. 78–119. ISBN 978-1-4419-1119-3. [Google Scholar]
- Lin, D.; Jin, S.; Zhang, F.; Wang, C.; Wang, Y.; Zhou, C.; Cheng, G.J. 3D stereolithography printing of graphene oxide reinforced complex architectures. Nanotechnology 2015, 26, 434003. [Google Scholar] [CrossRef] [PubMed]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. Patent U.S. Patent 4575330A, 3 November 1986. [Google Scholar]
- Manapat, J.Z.; Chen, Q.; Ye, P.; Advincula, R.C. 3D printing of polymer nanocomposites via stereolithography. Macromol. Mater. Eng. 2017, 302, 1600553. [Google Scholar] [CrossRef]
- Perera, S.D.; Durand-Silva, A.; Remy, A.K.; Diwakara, S.D.; Smaldone, R.A. 3D Printing of Aramid Nanofiber Composites by Stereolithography. ACS Appl. Nano Mater. 2021, 5, 13705–13710. [Google Scholar] [CrossRef]
- Attaran, M. The rise of 3-D printing: The advantages of additive manufacturing over traditional manufacturing. Bus. Horiz. 2017, 60, 677–688. [Google Scholar] [CrossRef]
- Maines, E.M.; Porwal, M.K.; Ellison, C.J.; Reineke, T.M. Sustainable advances in SLA/DLP 3D printing materials and processes. Green Chem. 2021, 23, 6863–6897. [Google Scholar] [CrossRef]
- Kroll, E.; Artzi, D. Enhancing aerospace engineering students’ learning with 3D printing wind-tunnel models. Rapid Prototyp. J. 2011, 17, 393–402. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D printing of polymer matrix composites: A review and prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Purtov, J.; Rogin, P.; Verch, A.; Johansen, V.E.; Hensel, R. Nanopillar diffraction gratings by two-photon lithography. Nanomaterials 2019, 9, 1495. [Google Scholar] [CrossRef]
- Shao, J.; Huang, Y.; Fan, Q. Visible light initiating systems for photopolymerization: Status, development and challenges. Polym. Chem. 2014, 5, 4195–4210. [Google Scholar] [CrossRef]
- Crivello, J.V.; Reichmanis, E. Photopolymer materials and processes for advanced technologies. Chem. Mater. 2014, 26, 533–548. [Google Scholar] [CrossRef]
- Lemu, H.G. On Opportunities and Limitations of Additive Manufacturing Technology for Industry 4.0 Era. In Advanced Manufacturing and Automation VIII. IWAMA.; Wang, K., Wang, Y., Strandhagen, J., Yu, T., Eds.; Springer: Singapore, 2018; Volume 484, pp. 106–113. ISBN 978-981-13-2374-4. [Google Scholar]
- Kamat, A.M.; Pei, Y.; Kottapalli, A.G.P. Bioinspired cilia sensors with graphene sensing elements fabricated using 3D printing and casting. Nanomaterials 2019, 9, 954. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Chua, Z.Y.; Moon, S.K.; Song, J.; Bi, G.; Zheng, H.; Lee, B.; Koo, J. Laser-induced graphene on additive manufacturing parts. Nanomaterials 2019, 9, 90. [Google Scholar] [CrossRef]
- Liu, J.; Gaynor, A.T.; Chen, S.; Kang, Z.; Suresh, K.; Takezawa, A.; Li, L.; Kato, J.; Tang, J.; Wang, C.C.L.; et al. Current and future trends in topology optimization for additive manufacturing. Struct. Multidiscip. Optim. 2018, 57, 2457–2483. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Berry, D.R.; Cortés-Guzmán, K.P.; Durand-Silva, A.; Perera, S.D.; Remy, A.K.; Yan, Q.; Smaldone, R.A. Supramolecular tools for polymer additive manufacturing. MRS Commun. 2021, 11, 146–156. [Google Scholar] [CrossRef]
- ASTM F2792-12a; Standard Terminology for Additive Manufacturing Technologies. ASTM International: West Conshohocken, PA, USA, 2012; p. 11.
- Mubarak, S.; Dhamodharan, D.; Kale, M.B.; Divakaran, N.; Senthil, T.; Sathiyanathan, P.; Wu, L.; Wang, J. A novel approach to enhance mechanical and thermal properties of SLA 3D printed structure by incorporation of metal–metal oxide nanoparticles. Nanomaterials 2020, 10, 217. [Google Scholar] [CrossRef]
- Mu, Q.; Wang, L.; Dunn, C.K.; Kuang, X.; Duan, F.; Zhang, Z.; Qi, H.J.; Wang, T. Digital light processing 3D printing of conductive complex structures. Addit. Manuf. 2017, 18, 74–83. [Google Scholar] [CrossRef]
- Skliutas, E.; Kašėtaitė, S.; Grigalevičiūtė, G.; Jonušauskas, L.; Rekštytė, S.; Ostrauskaitė, J.; Malinauskas, M. Bioresists from renewable resources as sustainable photoresins for 3D laser microlithography: Material synthesis, cross-linking rate and characterization of the structures. Adv. Fabr. Technol. Micro/Nano Opt. Photonics X SPIE 2017, 10115, 226–236. [Google Scholar] [CrossRef]
- Yu, C.; Schimelman, J.; Wang, P.; Miller, K.L.; Ma, X.; You, S.; Guan, J.; Sun, B.; Zhu, W.; Chen, S. Photopolymerizable biomaterials and light-based 3D printing strategies for biomedical applications. Chem. Rev. 2020, 120, 10695–10743. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Jin, J. Photopolymerization in 3D printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. Additive Manufacturing Technologies; Springer: Cham, Switzerland, 2021; Volume 17, ISBN 978-3-030-56126-0. [Google Scholar]
- Monti, J.; Blasco, E. Hierarchical ordering in light-triggered additive manufacturing. Polym. Chem. 2020, 11, 7316–7329. [Google Scholar] [CrossRef]
- Appuhamillage, G.A.; Chartrain, N.; Meenakshisundaram, V.; Feller, K.D.; Williams, C.B.; Long, T.E. 110th anniversary: Vat photopolymerization-based additive manufacturing: Current trends and future directions in materials design. Ind. Eng. Chem. Res. 2019, 58, 15109–15118. [Google Scholar] [CrossRef]
- Durand-Silva, A.; Cortés-Guzmán, K.P.; Johnson, R.M.; Perera, S.D.; Diwakara, S.D.; Smaldone, R.A. Balancing Self-Healing and Shape Stability in Dynamic Covalent Photoresins for Stereolithography 3D Printing. ACS Macro Lett. 2021, 10, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.H.A.; Zhakeyev, A.; Leung, D.Y.C.; Xuan, J. GO-modified flexible polymer nanocomposites fabricated via 3D stereolithography. Front. Chem. Sci. Eng. 2019, 13, 736–743. [Google Scholar] [CrossRef]
- Yee, D.W.; Greer, J.R. Three-dimensional chemical reactors: In situ materials synthesis to advance vat photopolymerization. Polym. Int. 2021, 70, 964–976. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef]
- Choi, J.S.; Kang, H.W.; Lee, I.H.; Ko, T.J.; Cho, D.W. Development of micro-stereolithography technology using a UV lamp and optical fiber. Int. J. Adv. Manuf. Technol. 2009, 41, 281–286. [Google Scholar] [CrossRef]
- Vaezi, M.; Seitz, H.; Yang, S. A review on 3D micro-additive manufacturing technologies. Int. J. Adv. Manuf. Technol. 2013, 67, 1721–1754. [Google Scholar] [CrossRef]
- Gorsche, C.; Seidler, K.; Knaack, P.; Dorfinger, P.; Koch, T.; Stampfl, J.; Moszner, N.; Liska, R. Rapid formation of regulated methacrylate networks yielding tough materials for lithography-based 3D printing. Polym. Chem. 2016, 7, 2009–2014. [Google Scholar] [CrossRef]
- Fuh, J.Y.H.; Lu, L.; Tan, C.C.; Shen, Z.X.; Chew, S. Processing and characterising photo-sensitive polymer in the rapid prototyping process. J. Mater. Process. Technol. 1999, 89, 211–217. [Google Scholar] [CrossRef]
- Korhonen, H.; Sinh, L.H.; Luong, N.D.; Lehtinen, P.; Verho, T.; Partanen, J.; Seppälä, J. Fabrication of graphene-based 3D structures by stereolithography. Phys. Status Solidi (A) 2016, 213, 982–985. [Google Scholar] [CrossRef]
- Jose, J.P.; Malhotra, S.K.; Thomas, S.; Kuruvilla, J.; Goda, K.; Sreekala, M.S. Advances in polymer composites: Macro- and microcomposites—State of the art, new challenges, and opportunities. In Polymer Composites; Thomas, S., Kuruvilla, J., Malhotra, S.K., Goda, K., Sreekala, M.S., Eds.; Wiley-VCH Verlag & Co. KGaA: Weinheim, Germany, 2012; Volume 1, pp. 3–16. ISBN 978-3-527-32624-2. [Google Scholar]
- Kurimoto, M.; Yamashita, Y.; Ozaki, H.; Kato, T.; Funabashi, T.; Suzuoki, Y. 3D printing of conical insulating spacer using alumina/UV-cured-resin composite. In Proceedings of the 2015 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Ann Arbor, MI, USA, 18–21 October 2015; pp. 463–466. [Google Scholar] [CrossRef]
- Martin, J.J.; Fiore, B.E.; Erb, R.M. Designing bioinspired composite reinforcement architectures via 3D magnetic printing. Nat. Commun. 2015, 6, 8641. [Google Scholar] [CrossRef]
- Kalsoom, U.; Peristyy, A.; Nesterenko, P.N.; Paull, B. A 3D printable diamond polymer composite: A novel material for fabrication of low cost thermally conducting devices. RSC Adv. 2016, 6, 38140–38147. [Google Scholar] [CrossRef]
- Sandoval, J.H.; Wicker, R.B. Functionalizing stereolithography resins: Effects of dispersed multi-walled carbon nanotubes on physical properties. Rapid Prototyp. J. 2006, 12, 292–303. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Yang, X.; Zhang, T.; Zhu, K.; Si, W.; Liu, Z.; Sun, H. Additive manufacturing of carbon nanotube-photopolymer composite radar absorbing materials. Polym. Compos. 2018, 39, E671–E676. [Google Scholar] [CrossRef]
- Yugang, D.; Yuan, Z.; Yiping, T.; Dichen, L. Nano-TiO2-modified photosensitive resin for RP. Rapid Prototyp. J. 2011, 17, 247–252. [Google Scholar] [CrossRef]
- Chen, S.G.; Yang, J.; Jia, Y.G.; Lu, B.; Ren, L. TiO2 and PEEK reinforced 3D printing PMMA composite resin for dental denture base applications. Nanomaterials 2019, 9, 1049. [Google Scholar] [CrossRef]
- Schwartz, J.J. Additive manufacturing: Frameworks for chemical understanding and advancement in vat photopolymerization. MRS Bull. 2022, 47, 628–641. [Google Scholar] [CrossRef]
- Sameni, F.; Ozkan, B.; Karmel, S.; Engstrøm, D.S.; Sabet, E. Large Scale Vat-Photopolymerization of Investment Casting Master Patterns: The Total Solution. Polymers 2022, 14, 4593. [Google Scholar] [CrossRef] [PubMed]
- Shapagin, A.V.; Budylin, N.Y.; Chalykh, A.E.; Solodilov, V.I.; Korokhin, R.A.; Poteryaev, A.A. Phase Equilibrium, Morphology, and Physico-Mechanics in Epoxy–Thermoplastic Mixtures with Upper and Lower Critical Solution Temperatures. Polymers 2020, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Brantseva, T.V.; Solodilov, V.I.; Antonov, S.V.; Gorbunova, I.Y.; Korohin, R.A.; Shapagin, A.V.; Smirnova, N.M. Epoxy modification with poly (vinyl acetate) and poly (vinyl butyral). J. Appl. Polym. Sci. 2016, 133, 44081. [Google Scholar] [CrossRef]
- Tretyakov, I.V.; Vyatkina, M.A.; Cherevinskiy, A.P.; Solodilov, V.I.; Shapagin, A.V.; Korokhin, R.A.; Budylin, N.Y.; Kireinov, A.V.; Gorbatkina, Y.A. Effect of Polyethersulfone on the Properties of Epoxy Resin and Wound Unidirectional Glass Fiber Reinforced Plastics Based on It. Bull. Russ. Acad. Sci. Phys. 2021, 85, 876–880. [Google Scholar] [CrossRef]
- Korokhin, R.A.; Shapagin, A.V.; Solodilov, V.I.; Zvereva, U.G.; Solomatin, D.V.; Gorbatkina, Y.A. Epoxy polymers modified with polyetherimide. Part I: Rheological and thermomechanical characteristics. Polym. Bull. 2021, 78, 1573–1584. [Google Scholar] [CrossRef]
- Shapagin, A.V.; Budylin, N.Y.; Chalykh, A.E. Regulation of a phase structure at the interface in epoxy–polysulfone systems. Russ. Chem. Bull. 2018, 67, 2172–2177. [Google Scholar] [CrossRef]
- Ioffe, B.V. Refractometric Methods of Chemistry; Chemistry Publisher: Leningrad, Russia, 1983; 352p. [Google Scholar]
- Chalykh, A.E.; Zagaitov, A.I.; Gromov, V.V.; Korotchenko, D.P. Optical Diffusiometer “ODA-2”; IFKh RAN: Moscow, Russia, 1996; 34p. [Google Scholar]
- Malkin, A.Y.; Ascadsky, A.A.; Kovriga, V.V.; Chalykh, A.E. Experimental Methods of Polymer Physics; Mir Publisher: Moscow, Russia, 1983; 520p. [Google Scholar]
- Petrova, T.V.; Tretyakov, I.V.; Kireynov, A.V.; Shapagin, A.V.; Budylin, N.Y.; Alexeeva, O.V.; Beshtoev, B.Z.; Solodilov, V.I.; Yurkov, G.Y.; Berlin, A.A. Structure and Properties of Epoxy Polysulfone Systems Modified with an Active Diluent. Polymers 2022, 14, 5320. [Google Scholar] [CrossRef]
- Chalykh, A.E. Diffusion in Polymer Systems; Chemistry Publisher: Moscow, Russia, 1987; 312p. [Google Scholar]
- Nikulova, U.V.; Chalykh, A.E. Phase Equilibrium and Interdiffusion in Blends of Polystyrene with Polyacrylates. Polymers 2021, 13, 2283. [Google Scholar] [CrossRef]
- Chalykh, A.E.; Nikulova, U.V.; Gerasimov, V.K. Simulation of Binodal and Spinodal Curves of Phase State Diagrams for Binary Polymer Systems. Polymers 2022, 14, 2524. [Google Scholar] [CrossRef]
- Ramesh, N.; Davis, P.K.; Zielinski, J.M.; Danner, R.P.; Duda, J.L. Application of free-volume theory to self diffusion of solvents in polymers below the glass transition temperature: A review. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1629–1644. [Google Scholar] [CrossRef]
- Nikulova, U.V. PhaDiag Software for Analysis and Prediction of Phase State Diagrams. Patent RU 2022661504, 2022. 22p. [Google Scholar]
- Bekman, I.N. Diffusion Mathematics; OntoPrint Publisher: Moscow, Russia, 2016; 400p. [Google Scholar]
- Nikulova, U.V.; Chalykh, A.E. Phase Equilibrium and Interdiffusion in Poly (Vinyl Methyl Ether)-Water System. Polymers 2020, 12, 2445. [Google Scholar] [CrossRef] [PubMed]


| Object | Producer | CVA, [wt. %] | Tg, [°C] | Tm, [°C] | ρ, [g/cm3] | nD20 | Mw, [Da] |
|---|---|---|---|---|---|---|---|
| tBA | Acros Organics BVBA, Geel, Belgium | — | — | −69.0 2 | 0.883 2 | 1.4119 3 | 128 |
| LDPE | Naftan, New Polotsk, Belarus | 0 | −20.1 1 | 113.3 1 | 0.916 2 | 1.5193 3 | 2900 4 |
| EVA-7 | Savilen, Kazan, Russia | 5.0–7.0 2 | −34.3 1 | 95.4 1 | 0.925 2 | 1.5026 3 | 2800 4 |
| EVA-20 | Total Fina Elf S.A., Kulbua, France | 19.0–21.0 2 | −34.6 1 | 53.3 1 | 0.940 2 | 1.4979 3 | 2500 4 |
| EVA-40 | DuPont de Nemours, Wilmington, DE, USA | 40.0 2 | −30.6 1 | 49.3 1 | 0.966 2 | 1.4775 3 | 2000 4 |
| System | χcr | Tcr, [°C] | φcr |
|---|---|---|---|
| LDPE–tBA | 0.6035 | 138 | 0.9102 |
| EVA-7–tBA | 0.6345 | 143 | 0.8877 |
| EVA-20–tBA | 0.6514 | 47 | 0.8761 |
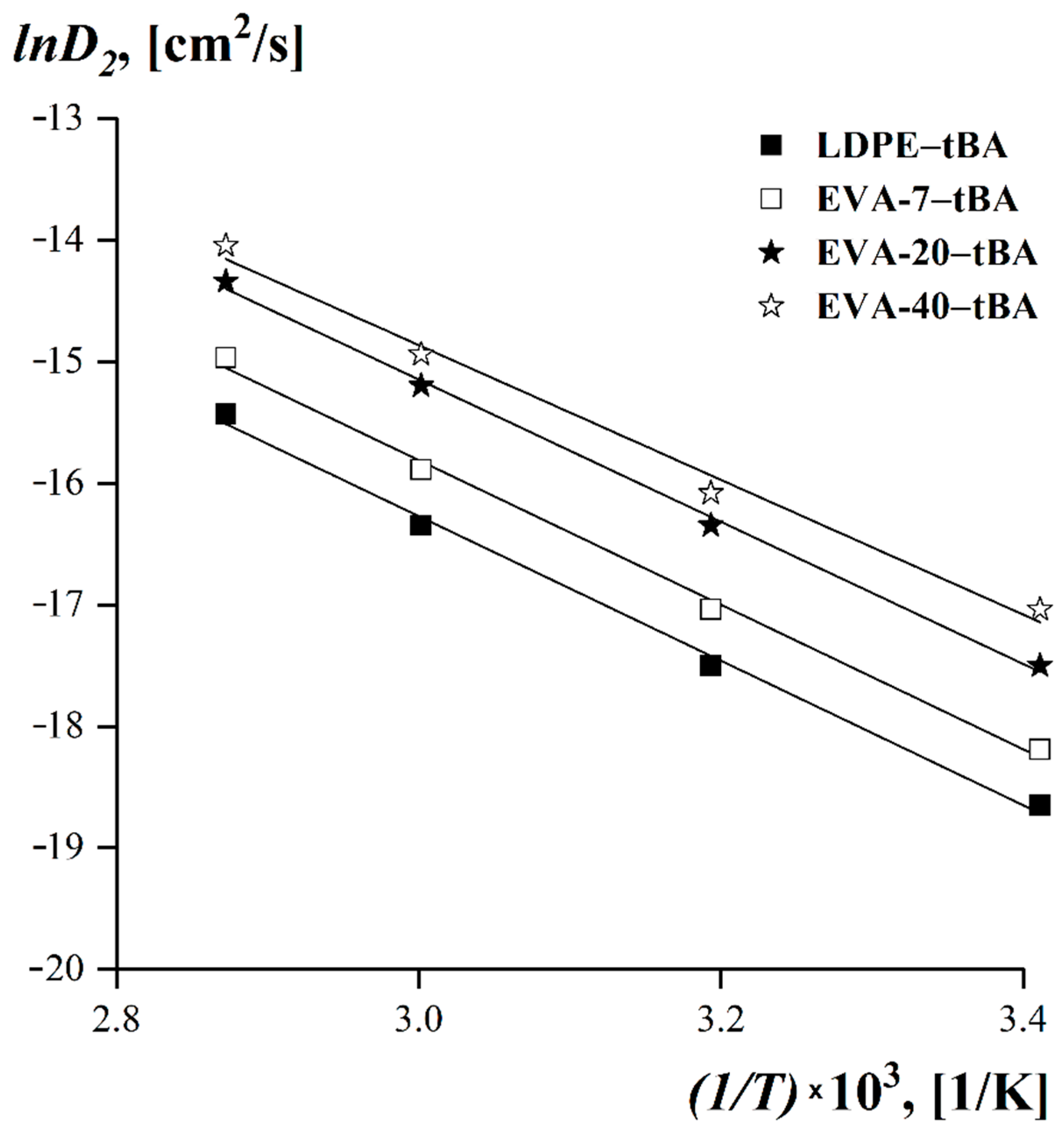
| System | Texp, [°C] | lgD2, [cm2/s] | System | Texp, [°C] | lgD2, [cm2/s] |
|---|---|---|---|---|---|
| LDPE–tBA | 20 | −8.1 | EVA−20–tBA | 20 | −7.6 |
| 40 | −7.6 | 40 | −7.1 | ||
| 60 | −7.1 | 60 | −6.6 | ||
| 75 | −6.7 | 75 | −6.2 | ||
| EVA−7–tBA | 20 | −7.9 | EVA−40–tBA | 20 | −7.4 |
| 40 | −7.4 | 40 | −7.1 | ||
| 60 | −6.9 | 60 | −6.5 | ||
| 75 | −6.5 | 75 | −6.1 |
| System | Ea, [kJ/mol] |
|---|---|
| LDPE–tBA | 49.37 |
| EVA-7–tBA | 49.36 |
| EVA-20–tBA | 48.28 |
| EVA-40–tBA | 46.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazhanov, D.A.; Poteryaev, A.A.; Shapagin, A.V.; Shcherbina, A.A. Ethylene-Vinyl Acetate Copolymers as Potential Thermoplastic Modifiers of Photopolymer Compositions. Polymers 2023, 15, 131. https://doi.org/10.3390/polym15010131
Bazhanov DA, Poteryaev AA, Shapagin AV, Shcherbina AA. Ethylene-Vinyl Acetate Copolymers as Potential Thermoplastic Modifiers of Photopolymer Compositions. Polymers. 2023; 15(1):131. https://doi.org/10.3390/polym15010131
Chicago/Turabian StyleBazhanov, Dmitriy A., Arkadiy A. Poteryaev, Alexey V. Shapagin, and Anna A. Shcherbina. 2023. "Ethylene-Vinyl Acetate Copolymers as Potential Thermoplastic Modifiers of Photopolymer Compositions" Polymers 15, no. 1: 131. https://doi.org/10.3390/polym15010131
APA StyleBazhanov, D. A., Poteryaev, A. A., Shapagin, A. V., & Shcherbina, A. A. (2023). Ethylene-Vinyl Acetate Copolymers as Potential Thermoplastic Modifiers of Photopolymer Compositions. Polymers, 15(1), 131. https://doi.org/10.3390/polym15010131








