Immobilization of Strontium Aluminate into Recycled Polycarbonate Plastics towards an Afterglow and Photochromic Smart Window
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Polycarbonate Bars
2.3. Characterization Methods
3. Results and Discussion
3.1. Preparation of Polycarbonate Window
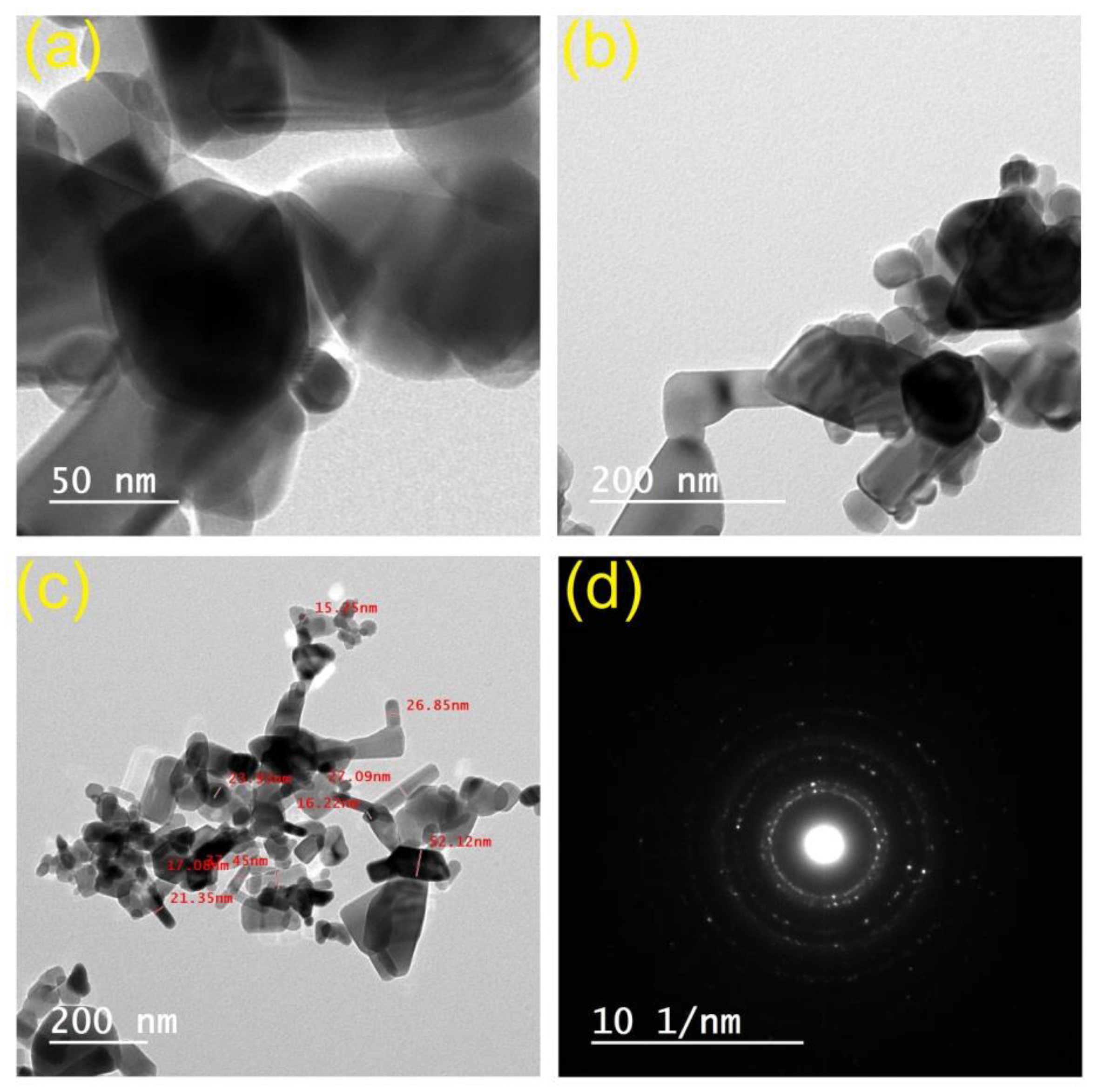
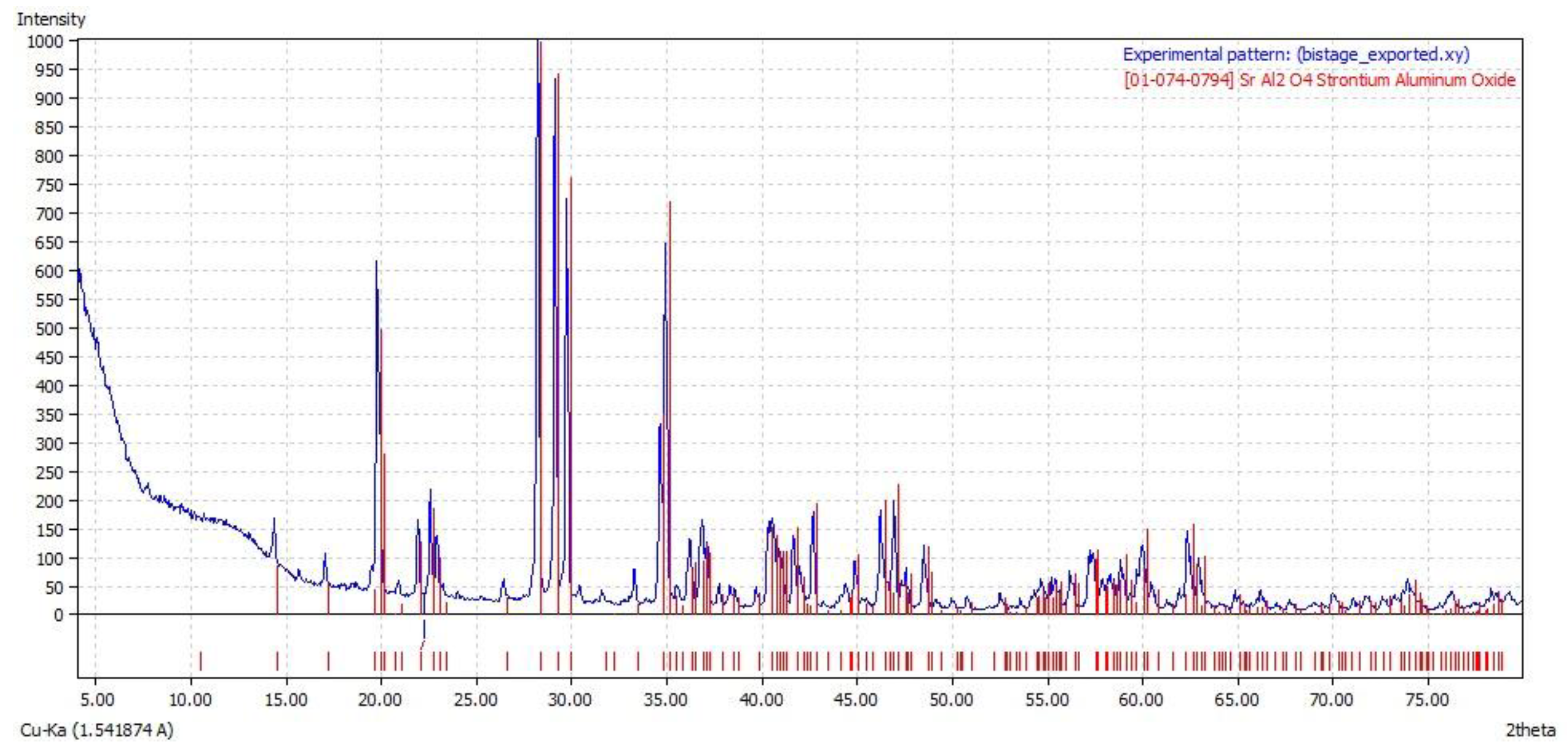
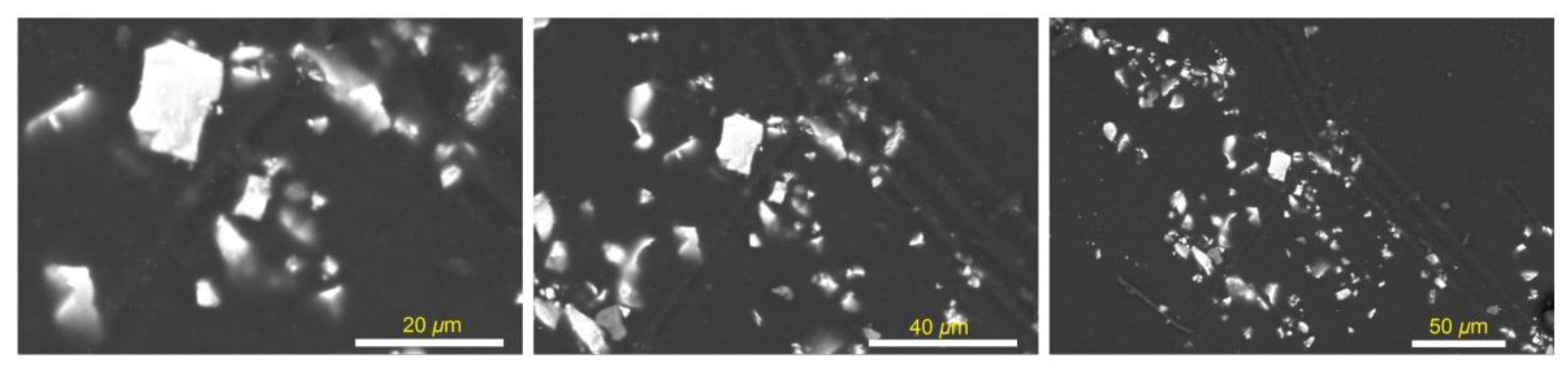
3.2. Morphological Features
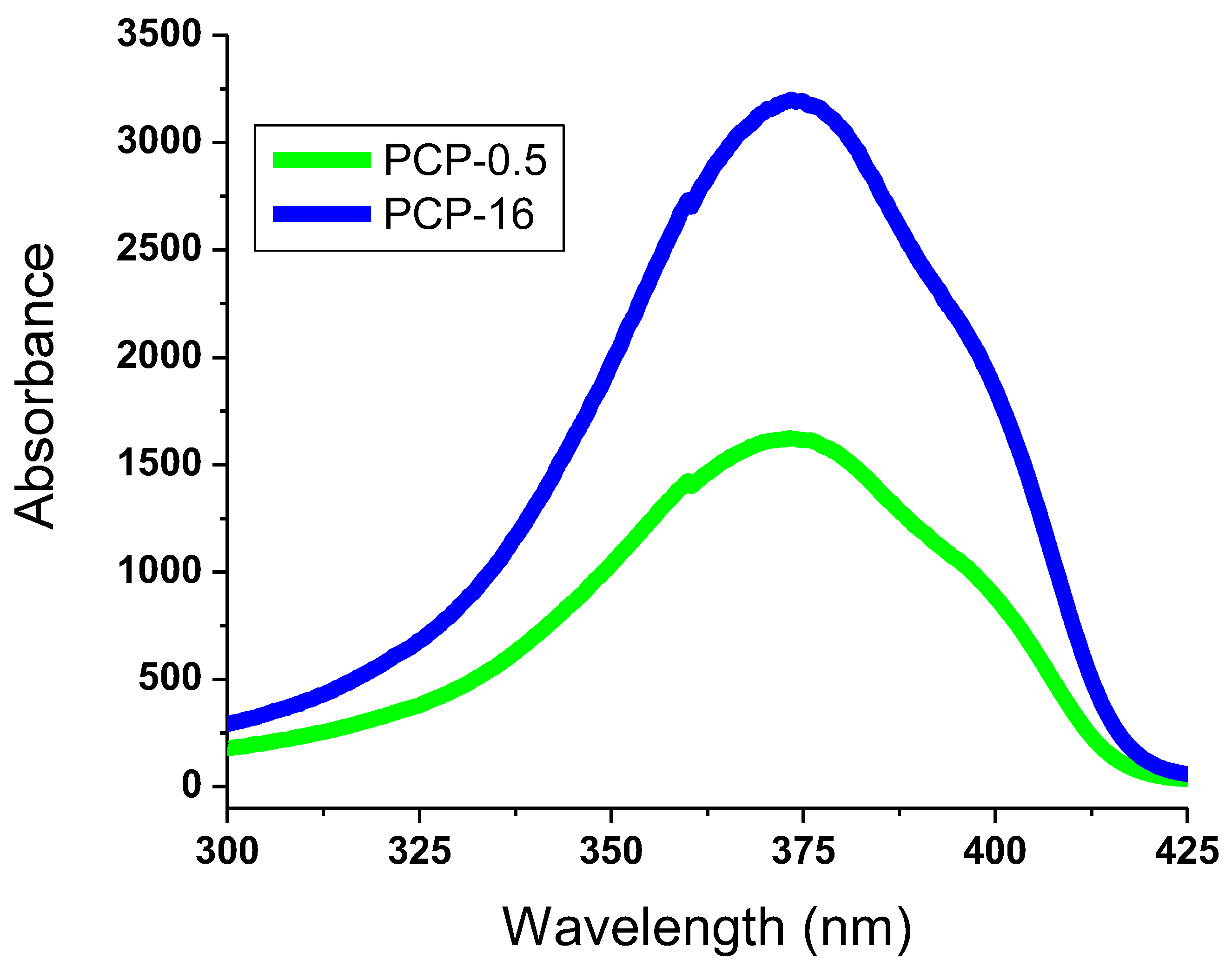
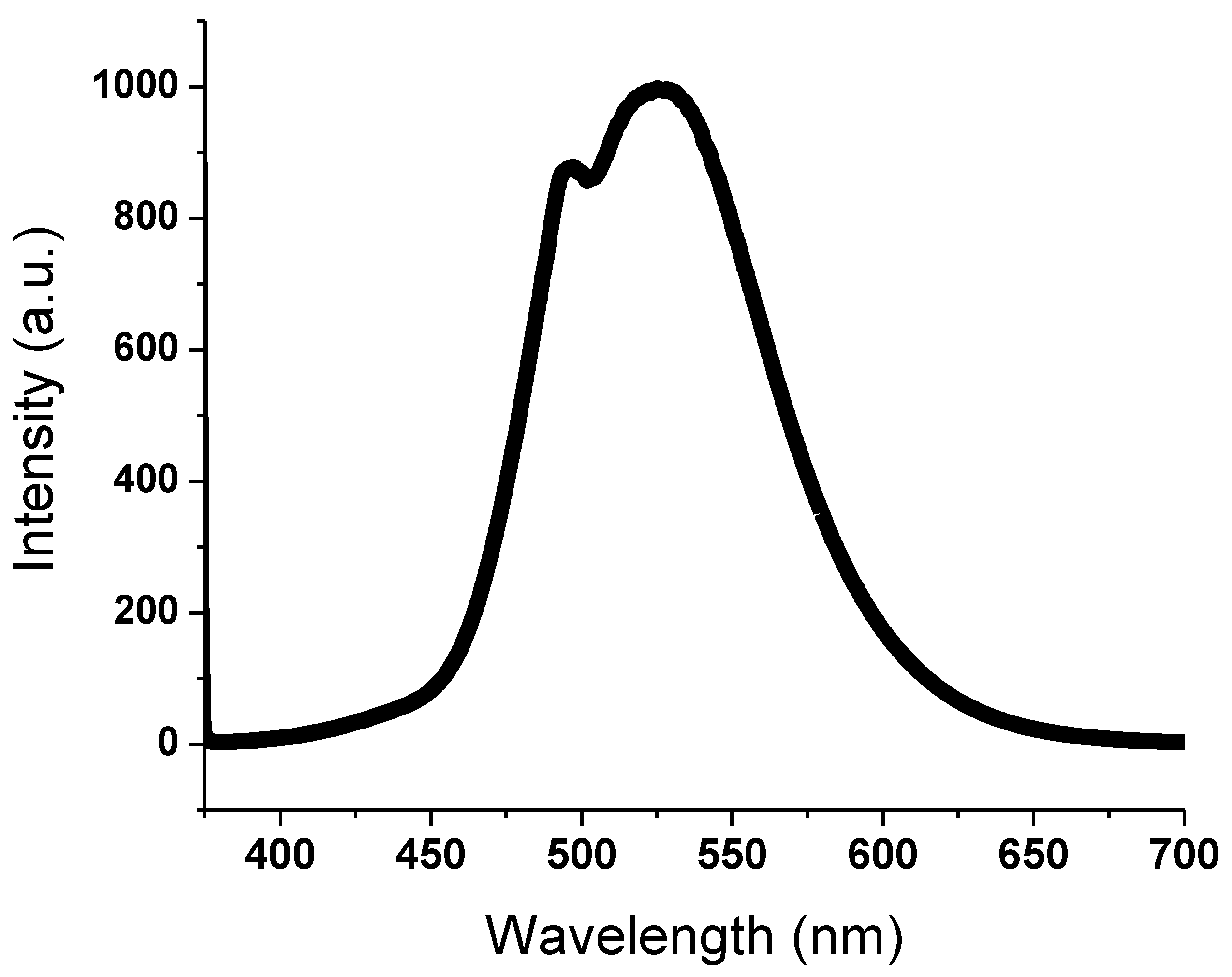
3.3. Photoluminescence Spectra
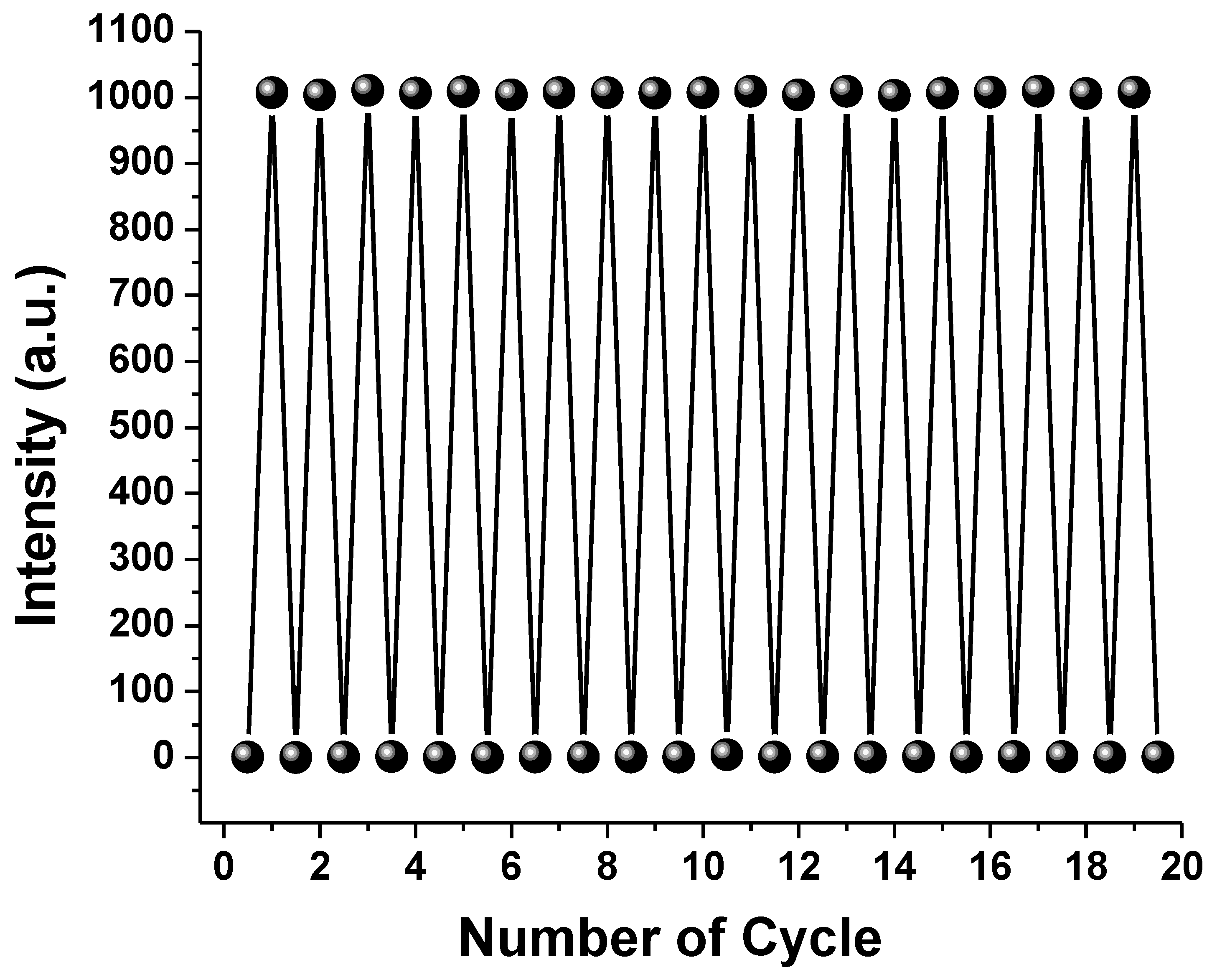
3.4. Photostability Study
3.5. UV-Protection and Hydrophobicity Screening
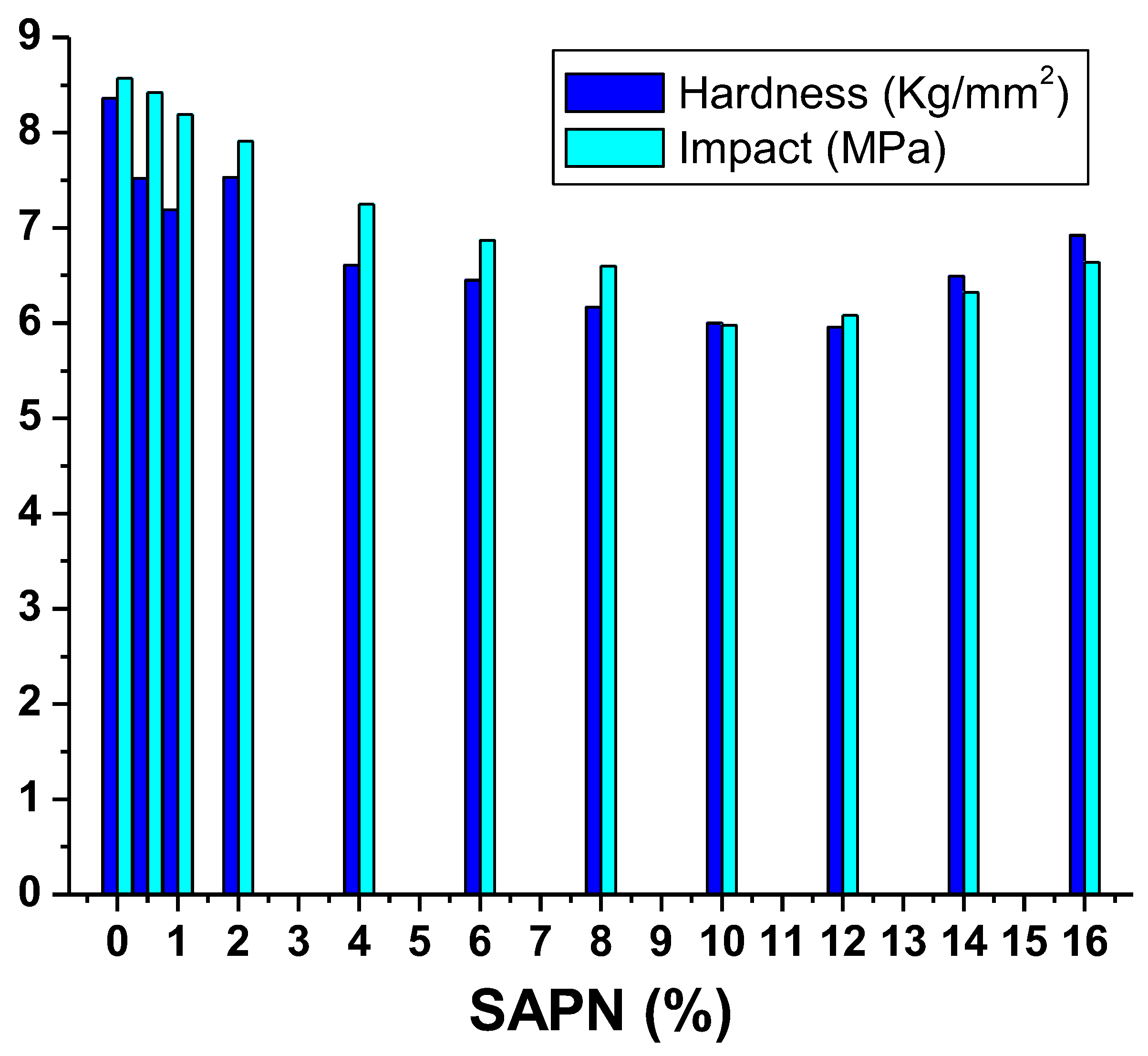
3.6. Hardness Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Liu, Y.; Zhan, X.; Luo, D.; Sun, X. Photochromic Transparent Wood for Photo-Switchable Smart Window Applications. J. Mater. Chem. C 2019, 7, 8649–8654. [Google Scholar] [CrossRef]
- Keaney, J.F., Jr.; Larson, M.G.; Vasan, R.S.; Wilson, P.W.F.; Lipinska, I.; Corey, D.; Massaro, J.M.; Sutherland, P.; Vita, J.A.; Benjamin, E.J. Obesity and Systemic Oxidative Stress: Clinical Correlates of Oxidative Stress in the Framingham Study. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, J.; Cui, H.; Ye, T.; Hao, F.; Xiong, W.; Liu, P.; Luo, H. Preparation of Organic–Inorganic Hybrid Methylene Blue Polymerized Organosilane/Sepiolite Pigments with Superhydrophobic and Self-Cleaning Properties. Text. Res. J. 2019, 89, 4220–4229. [Google Scholar] [CrossRef]
- Bagri, A.; Mattevi, C.; Acik, M.; Chabal, Y.J.; Chhowalla, M.; Shenoy, V.B. Structural Evolution during the Reduction of Chemically Derived Graphene Oxide. Nat. Chem. 2010, 2, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Kavaliauskaite, R.; Klimaviciute, R.; Zemaitaitis, A. Factors Influencing Production of Cationic Starches. Carbohydr. Polym. 2008, 73, 665–675. [Google Scholar] [CrossRef]
- Wakayama, Y.; Hayakawa, R.; Higashiguchi, K.; Matsuda, K. Photochromism for Optically Functionalized Organic Field-Effect Transistors: A Comprehensive Review. J. Mater. Chem. C 2020, 8, 10956–10974. [Google Scholar] [CrossRef]
- Guo, S.; Zhou, S.; Chen, J.; Guo, P.; Ding, R.; Sun, H.; Feng, H.; Qian, Z. Photochromism and Fluorescence Switch of Furan-Containing Tetraarylethene Luminogens with Aggregation-Induced Emission for Photocontrolled Interface-Involved Applications. ACS Appl. Mater. Interfaces 2020, 12, 42410–42419. [Google Scholar] [CrossRef]
- Kayani, A.B.A.; Kuriakose, S.; Monshipouri, M.; Khalid, F.A.; Walia, S.; Sriram, S.; Bhaskaran, M. UV Photochromism in Transition Metal Oxides and Hybrid Materials. Small 2021, 17, 2100621. [Google Scholar] [CrossRef]
- El-Nour, K.M.M.A.; Eftaiha, A.; Al-Warthan, A.; Ammar, R.A.A. Synthesis and Applications of Silver Nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef]
- Al-Qahtani, S.; Aljuhani, E.; Felaly, R.; Alkhamis, K.; Alkabli, J.; Munshi, A.; El-Metwaly, N. Development of Photoluminescent Translucent Wood toward Photochromic Smart Window Applications. Ind. Eng. Chem. Res. 2021, 60, 8340–8350. [Google Scholar] [CrossRef]
- Bite, I.; Krieke, G.; Zolotarjovs, A.; Laganovska, K.; Liepina, V.; Smits, K.; Auzins, K.; Grigorjeva, L.; Millers, D.; Skuja, L. Novel Method of Phosphorescent Strontium Aluminate Coating Preparation on Aluminum. Mater. Des. 2018, 160, 794–802. [Google Scholar] [CrossRef]
- Elsayed, M.T.; Hassan, A.A.; Abdelaal, S.A.; Taher, M.M.; khalaf Ahmed, M.; Shoueir, K.R. Morphological, Antibacterial, and Cell Attachment of Cellulose Acetate Nanofibers Containing Modified Hydroxyapatite for Wound Healing Utilizations. J. Mater. Res. Technol. 2020, 9, 13927–13936. [Google Scholar] [CrossRef]
- Sneha, K.; Sathishkumar, M.; Kim, S.; Yun, Y.-S. Counter Ions and Temperature Incorporated Tailoring of Biogenic Gold Nanoparticles. Process Biochem. 2010, 45, 1450–1458. [Google Scholar] [CrossRef]
- Li, J.; Tang, S.; Ge, M.; Zhu, Y.; Yu, Y. Preparation and Photochromic Properties of Phosphomolybdic Acid/Rare Earth Strontium Aluminate Luminous Fiber. Mater. Res. Express 2020, 7, 85501. [Google Scholar] [CrossRef]
- Pourjavaher, S.; Almasi, H.; Meshkini, S.; Pirsa, S.; Parandi, E. Development of a Colorimetric PH Indicator Based on Bacterial Cellulose Nanofibers and Red Cabbage (Brassica Oleraceae) Extract. Carbohydr. Polym. 2017, 156, 193–201. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Alzahrani, H.K.; Azher, O.A.; Owidah, Z.O.; Abualnaja, M.; Habeebullah, T.M.; El-Metwaly, N.M. Immobilization of Anthocyanin-Based Red-Cabbage Extract onto Cellulose Fibers toward Environmentally Friendly Biochromic Diagnostic Biosensor for Recognition of Urea. J. Environ. Chem. Eng. 2021, 9, 105493. [Google Scholar] [CrossRef]
- Erickson, J.G. Reactions of Long-Chain Amines. II. Reactions with Urea1. J. Am. Chem. Soc. 1954, 76, 3977–3978. [Google Scholar] [CrossRef]
- Poulose, A.M.; Anis, A.; Shaikh, H.; Alhamidi, A.; Siva Kumar, N.; Elnour, A.Y.; Al-Zahrani, S.M. Strontium Aluminate-Based Long Afterglow PP Composites: Phosphorescence, Thermal, and Mechanical Characteristics. Polymers 2021, 13, 1373. [Google Scholar] [CrossRef]
- Kumar, K.G.; Bhargav, P.B.; Aravinth, K.; Arumugam, R.; Ramasamy, P. Dysprosium Activated Strontium Aluminate Phosphor: A Potential Candidate for WLED Applications. J. Lumin. 2020, 223, 117126. [Google Scholar] [CrossRef]
- Kim, D. Recent Developments in Lanthanide-Doped Alkaline Earth Aluminate Phosphors with Enhanced and Long-Persistent Luminescence. Nanomaterials 2021, 11, 723. [Google Scholar] [CrossRef]
- Singh, T.A.; Das, J.; Sil, P.C. Zinc Oxide Nanoparticles: A Comprehensive Review on Its Synthesis, Anticancer and Drug Delivery Applications as Well as Health Risks. Adv. Colloid Interface Sci. 2020, 286, 102317. [Google Scholar] [CrossRef] [PubMed]
- Abumelha, H.M. Simple Production of Photoluminescent Polyester Coating Using Lanthanide-doped Pigment. Luminescence 2021, 36, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Abou-Melha, K. Preparation of Photoluminescent Nanocomposite Ink toward Dual-Mode Secure Anti-Counterfeiting Stamps. Arab. J. Chem. 2022, 15, 103604. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Alzahrani, S.O.; Snari, R.M.; Al-Ahmed, Z.A.; Alkhamis, K.; Alhasani, M.; El-Metwaly, N.M. Preparation of photoluminescent and photochromic smart glass window using sol-gel technique and lanthanides-activated aluminate phosphor. Ceram. Int. 2022, 48, 17489–17498. [Google Scholar] [CrossRef]
- Fu, X.; Cai, J.; Zhang, X.; Li, W.-D.; Ge, H.; Hu, Y. Top-down Fabrication of Shape-Controlled, Monodisperse Nanoparticles for Biomedical Applications. Adv. Drug Deliv. Rev. 2018, 132, 169–187. [Google Scholar] [CrossRef]
- Li, G.; Cao, F.; Zhang, K.; Hou, L.; Gao, R.; Zhang, W.; Wang, Y. Design of Anti-UV Radiation Textiles with Self-Assembled Metal–Organic Framework Coating. Adv. Mater. Interfaces 2020, 7, 1901525. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Li, Z.; McClements, D.J. Comparison of Emulsifying Properties of Food-Grade Polysaccharides in Oil-in-Water Emulsions: Gum Arabic, Beet Pectin, and Corn Fiber Gum. Food Hydrocoll. 2017, 66, 144–153. [Google Scholar] [CrossRef]
- Zheng, H.; Du, Y.; Yu, J.; Huang, R.; Zhang, L. Preparation and Characterization of Chitosan/Poly (Vinyl Alcohol) Blend Fibers. J. Appl. Polym. Sci. 2001, 80, 2558–2565. [Google Scholar] [CrossRef]
- Terzano, R.; Alfeld, M.; Janssens, K.; Vekemans, B.; Schoonjans, T.; Vincze, L.; Tomasi, N.; Pinton, R.; Cesco, S. Spatially resolved (semi) quantitative determination of iron (Fe) in plants by means of synchrotron micro X-ray fluorescence. Anal. Bioanal. Chem. 2013, 405, 3341–3350. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A Review on Electrospinning for Membrane Fabrication: Challenges and Applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]


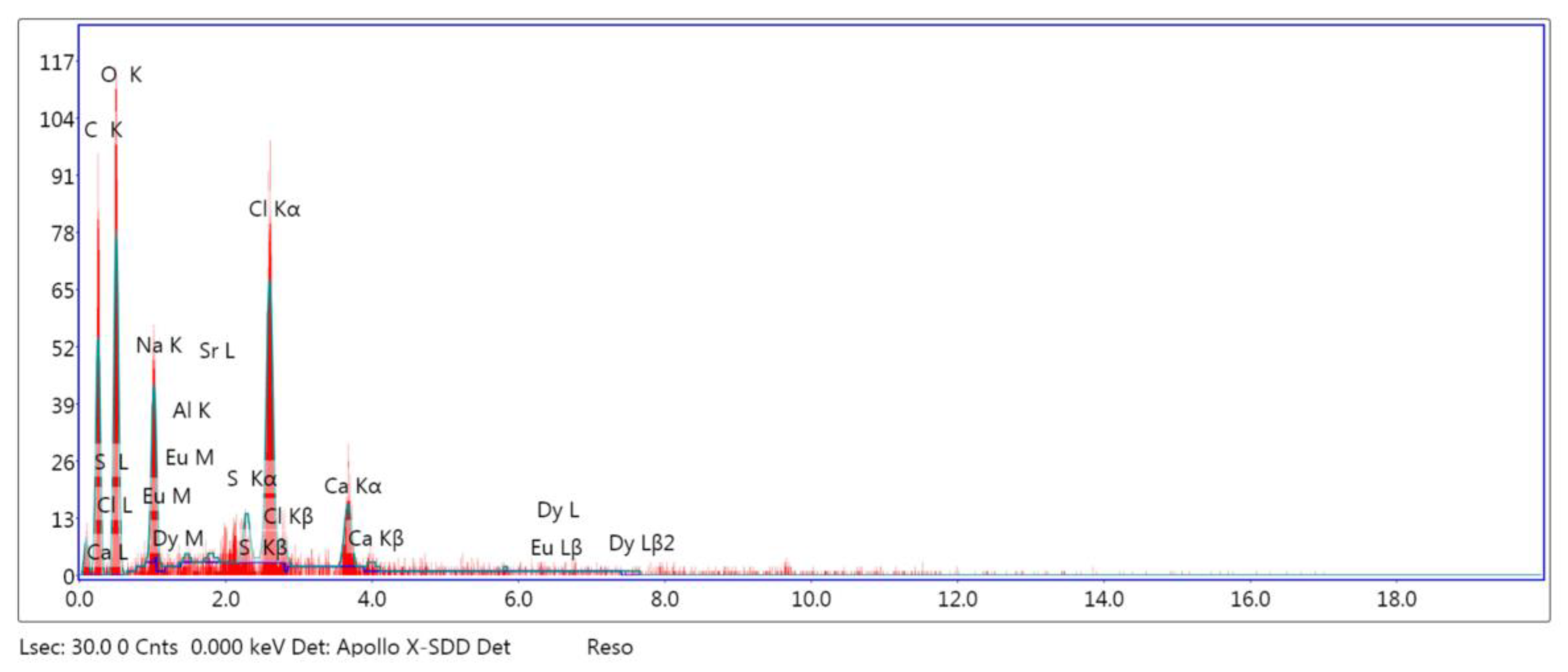
| SAPN@PCP | C | O | Al | Sr | Eu | Dy | |
|---|---|---|---|---|---|---|---|
| PCP-0 | P1 | 65.82 | 34.18 | 0 | 0 | 0 | 0 |
| P2 | 66.24 | 33.76 | 0 | 0 | 0 | 0 | |
| P3 | 66.19 | 33.81 | 0 | 0 | 0 | 0 | |
| PCP-0.5 | P1 | 64.00 | 33.74 | 1.07 | 0.69 | 0.34 | 0.14 |
| P2 | 64.05 | 33.80 | 1.12 | 0.66 | 0.26 | 0.11 | |
| P3 | 64.10 | 33.80 | 1.09 | 0.65 | 0.22 | 0.14 | |
| PCP-4 | P1 | 61.11 | 33.59 | 2.57 | 1.67 | 0.72 | 0.34 |
| P2 | 60.81 | 33.81 | 2.64 | 1.72 | 0.59 | 0.43 | |
| P3 | 60.75 | 33.76 | 2.76 | 1.80 | 0.61 | 0.32 | |
| PCP-8 | P1 | 58.23 | 33.51 | 3.78 | 3.00 | 0.94 | 0.50 |
| P2 | 58.33 | 33.67 | 3.75 | 2.68 | 0.92 | 0.65 | |
| P3 | 58.74 | 33.38 | 3.74 | 2.50 | 0.95 | 0.69 | |
| PCP-12 | P1 | 56.20 | 32.13 | 5.50 | 4.34 | 1.06 | 0.82 |
| P2 | 56.11 | 32.03 | 5.83 | 4.10 | 1.17 | 0.76 | |
| P3 | 56.43 | 32.32 | 5.30 | 3.99 | 1.34 | 0.73 | |
| PCP-16 | P1 | 53.10 | 31.93 | 7.26 | 5.44 | 1.47 | 0.93 |
| P2 | 53.20 | 31.62 | 7.62 | 5.21 | 1.56 | 0.89 | |
| P3 | 53.38 | 31.93 | 7.29 | 5.05 | 1.61 | 0.84 | |
| Element | Elemental Contents (wt%) | ||||
|---|---|---|---|---|---|
| PCP-0.5 | PCP-4 | PCP-8 | PCP-12 | PCP-16 | |
| Na | 2.60 | 2.48 | 1.63 | 1.38 | 0.84 |
| K | 2.55 | 2.26 | 1.85 | 0.71 | 0.77 |
| Sr | 25.72 | 27.88 | 30.33 | 32.17 | 33.06 |
| Ca | 3.06 | 2.48 | 1.82 | 1.08 | 0.80 |
| Cl | 3.39 | 2.37 | 1.41 | 0.96 | 0.75 |
| Al | 53.98 | 56.29 | 58.17 | 60.31 | 60.71 |
| Si | 8.70 | 6.14 | 4.79 | 2.39 | 2.07 |
| SAPN@PCP | UPF | Contact Angle (°) |
|---|---|---|
| PCP-0 | 75 | 134.0 |
| PCP-0.5 | 90 | 137.6 |
| PCP-1 | 117 | 138.1 |
| PCP-2 | 136 | 140.1 |
| PCP-4 | 180 | 142.4 |
| PCP-6 | 200 | 145.7 |
| PCP-8 | 246 | 147.5 |
| PCP-10 | 299 | 148.3 |
| PCP-12 | 322 | 149.1 |
| PCP-14 | 331 | 150.2 |
| PCP-16 | 336 | 150.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Hefnawy, M.E.; Ismail, A.I.; Alhayyani, S.; Al-Goul, S.T.; Zayed, M.M.; Abou Taleb, M. Immobilization of Strontium Aluminate into Recycled Polycarbonate Plastics towards an Afterglow and Photochromic Smart Window. Polymers 2023, 15, 119. https://doi.org/10.3390/polym15010119
El-Hefnawy ME, Ismail AI, Alhayyani S, Al-Goul ST, Zayed MM, Abou Taleb M. Immobilization of Strontium Aluminate into Recycled Polycarbonate Plastics towards an Afterglow and Photochromic Smart Window. Polymers. 2023; 15(1):119. https://doi.org/10.3390/polym15010119
Chicago/Turabian StyleEl-Hefnawy, Mohamed E., Ali I. Ismail, Sultan Alhayyani, Soha T. Al-Goul, Mohamed M. Zayed, and Manal Abou Taleb. 2023. "Immobilization of Strontium Aluminate into Recycled Polycarbonate Plastics towards an Afterglow and Photochromic Smart Window" Polymers 15, no. 1: 119. https://doi.org/10.3390/polym15010119
APA StyleEl-Hefnawy, M. E., Ismail, A. I., Alhayyani, S., Al-Goul, S. T., Zayed, M. M., & Abou Taleb, M. (2023). Immobilization of Strontium Aluminate into Recycled Polycarbonate Plastics towards an Afterglow and Photochromic Smart Window. Polymers, 15(1), 119. https://doi.org/10.3390/polym15010119






