Relevance between Cassava Starch Liquefied by Phenol and Modification of Phenol-Formaldehyde Resin Wood Adhesive
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
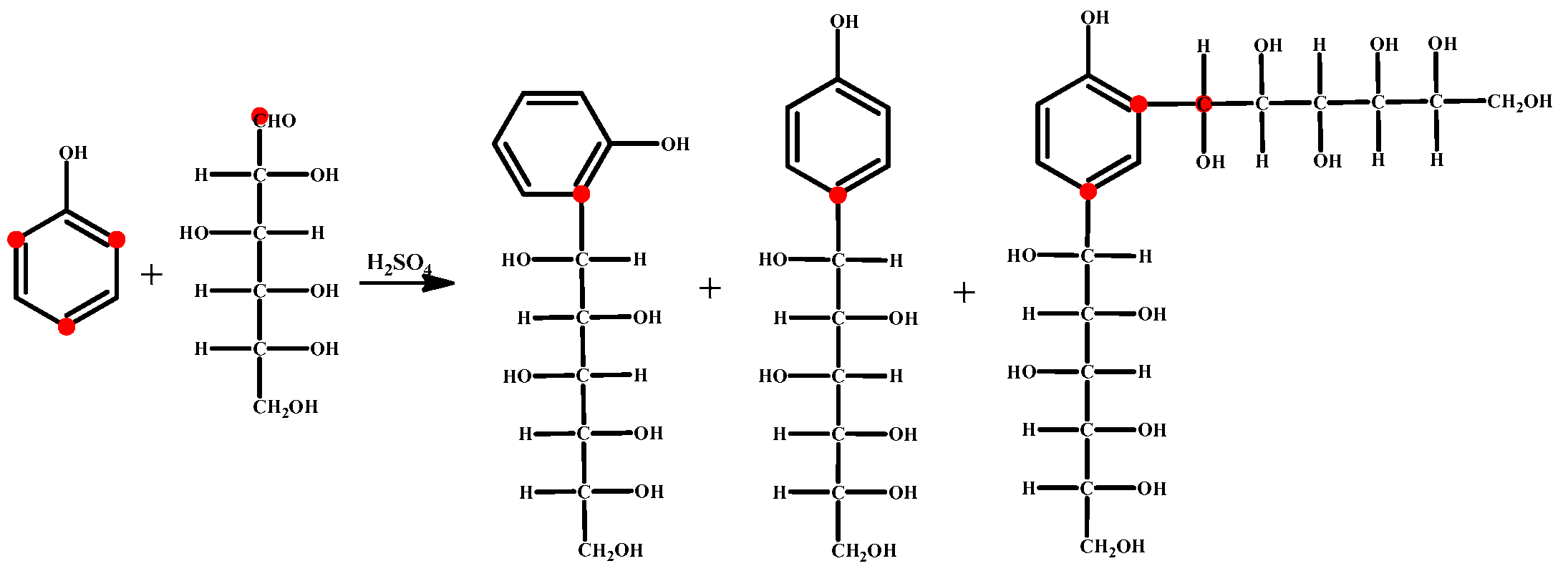
2.2.1. Liquefaction Process
2.2.2. Residue Rate
2.2.3. CPF Resin Synthesis
2.2.4. PF Resin Synthesis
2.2.5. Water Resistance Test
2.2.6. FT-IR Test
2.2.7. XRD Test
2.2.8. SEM Test
3. Results and Discussion
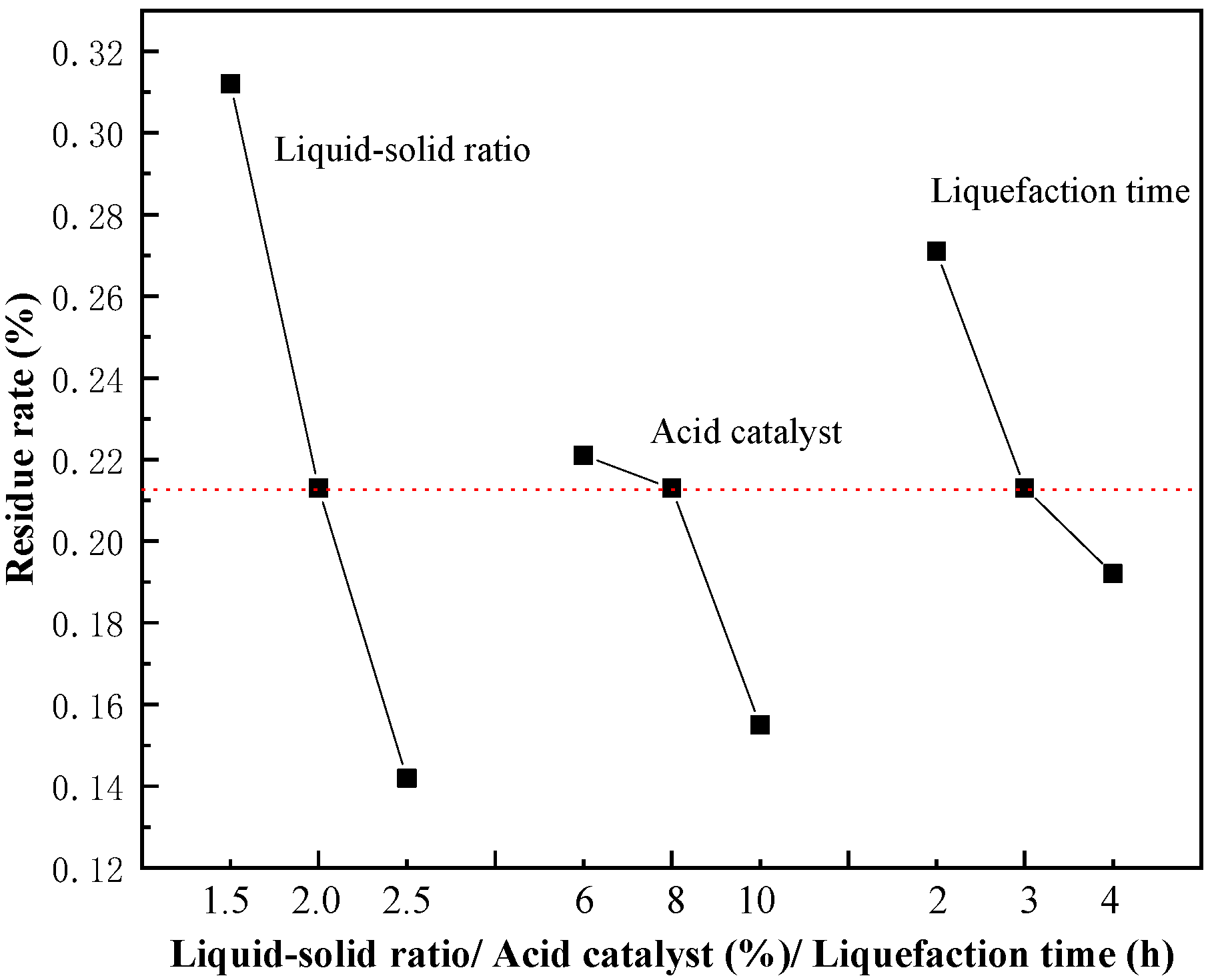
3.1. Effect of Liquefaction Parameters on Residue Rate
3.2. XRD Analysis
3.3. FT-IR Analysis
3.4. SEM Analysis
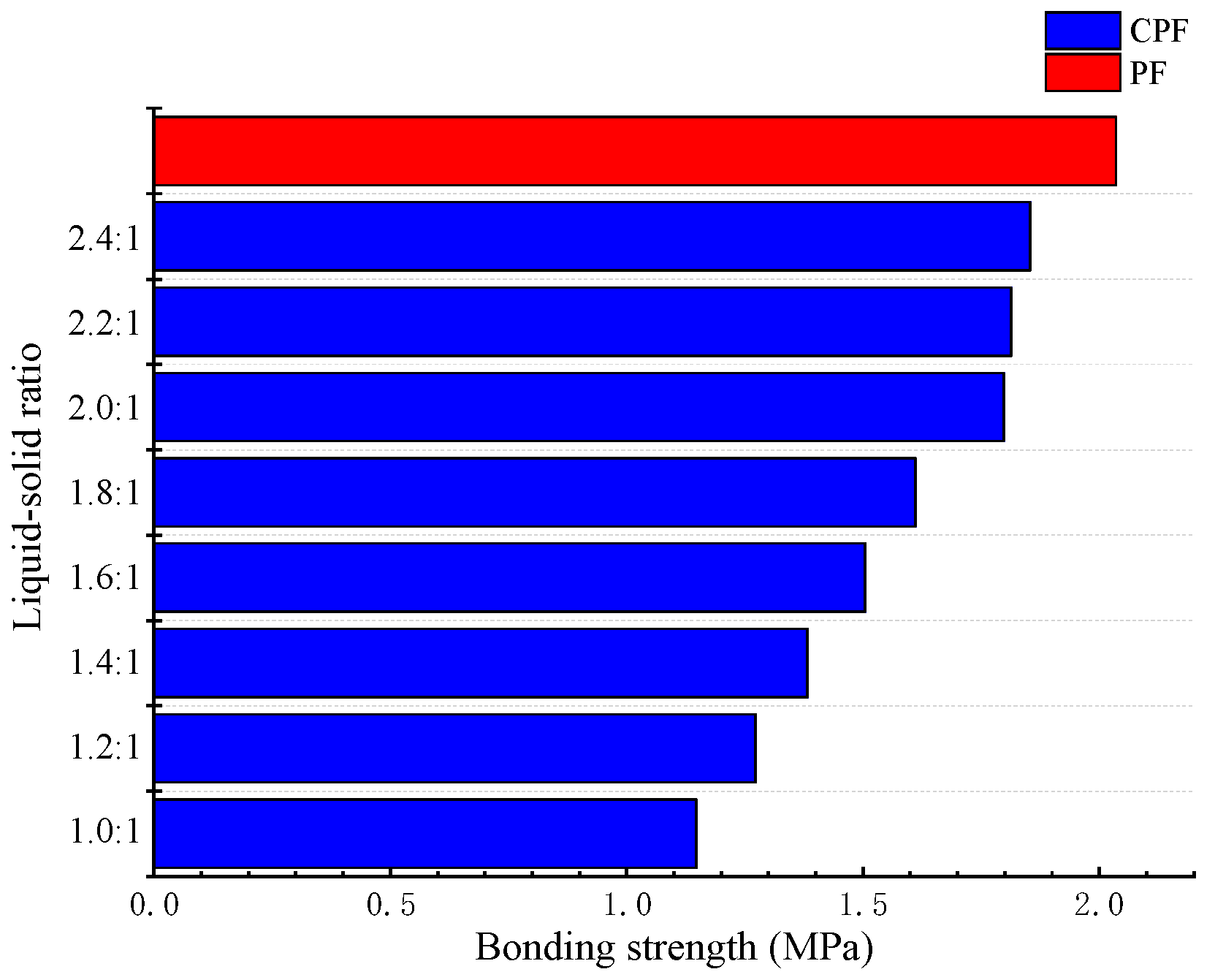
3.5. Water Resistance of CPF Resin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal Liquefaction of Biomass: Developments from Batch to Continuous Process. Bioresour. Technol. 2015, 178, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Li, B.; Yang, T.; Kai, X.; Wang, W.; Jie, Y.; Zhang, Y.; Chen, G. Sub-Supercritical Liquefaction of Rice Stalk for the Production of Bio-Oil: Effect of Solvents. Bioresour. Technol. 2015, 198, 94–100. [Google Scholar] [CrossRef]
- Gaurav, N.; Sivasankari, S.; Kiran, G.; Ninawe, A.; Selvin, J. Utilization of Bioresources for Sustainable Biofuels: A Review. Renew. Sustain. Energy Rev. 2017, 73, 205–214. [Google Scholar] [CrossRef]
- Manikandan, S.; Subbaiya, R.; Biruntha, M.; Krishnan, R.Y.; Muthusamy, G.; Karmegam, N. Recent Development Patterns, Utilization and Prospective of Biofuel Production: Emerging Nanotechnological Intervention for Environmental Sustainability—A Review. Fuel 2022, 314, 122757. [Google Scholar] [CrossRef]
- Antar, M. Biomass for a Sustainable Bioeconomy: An Overview of World Biomass Production and Utilization. Renew. Sustain. Energy Rev. 2021, 18, 110691. [Google Scholar] [CrossRef]
- Khoo, K.S.; Chia, W.Y.; Tang, D.Y.Y.; Show, P.L.; Chew, K.W.; Chen, W.-H. Nanomaterials Utilization in Biomass for Biofuel and Bioenergy Production. Energies 2020, 13, 892. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, K.; Kumagai, N.; Izumiya, K.; Takano, H.; Shinomiya, H.; Sasaki, Y.; Yoshida, T.; Kato, Z. The Use of Renewable Energy in the Form of Methane via Electrolytic Hydrogen Generation Using Carbon Dioxide as the Feedstock. Appl. Surf. Sci. 2016, 388, 608–615. [Google Scholar] [CrossRef]
- Jannatkhah, J.; Najafi, B.; Ghaebi, H. Energy-Exergy Analysis of Compression Ignition Engine Running with Biodiesel Fuel Extracted from Four Different Oil-Basis Materials. Int. J. Green Energy 2019, 16, 749–762. [Google Scholar] [CrossRef]
- Lu, S.; Li, J.; Xiao, B.; Guo, M. Analysis of Standard Accounting Method of Economic Compensation for Ecological Pollution in Watershed. Sci. Total Environ. 2020, 737, 138157. [Google Scholar] [CrossRef]
- Garcia, M.A.V.T.; Garcia, C.F.; Faraco, A.A.G. Pharmaceutical and Biomedical Applications of Native and Modified Starch: A Review. Starch-Stärke 2020, 72, 1900270. [Google Scholar] [CrossRef]
- Mahmood, K.; Kamilah, H.; Shang, P.L.; Sulaiman, S.; Ariffin, F.; Alias, A.K. A Review: Interaction of Starch/Non-Starch Hydrocolloid Blending and the Recent Food Applications. Food Biosci. 2017, 19, 110–120. [Google Scholar] [CrossRef]
- Zhu, F. Composition, Structure, Physicochemical Properties, and Modifications of Cassava Starch. Carbohydr. Polym. 2015, 122, 456–480. [Google Scholar] [CrossRef]
- Chen, L.; Din, Z.; Yang, D.; Hu, C.; Cai, J.; Xiong, H. Functional Nanoparticle Reinforced Starch-Based Adhesive Emulsion: Toward Robust Stability and High Bonding Performance. Carbohydr. Polym. 2021, 269, 118270. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, L.; Gu, J.; Tan, H.; Zhu, L. Preparation and Properties of a Starch-Based Wood Adhesive with High Bonding Strength and Water Resistance. Carbohydr. Polym. 2015, 115, 32–37. [Google Scholar] [CrossRef]
- Zheng, X.; Cheng, L.; Gu, Z.; Hong, Y.; Li, Z.; Li, C. Effects of Heat Pretreatment of Starch on Graft Copolymerization Reaction and Performance of Resulting Starch-Based Wood Adhesive. Int. J. Biol. Macromol. 2017, 96, 11–18. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, Z.; Hong, Y.; Cheng, L.; Li, Z. Bonding Strength and Water Resistance of Starch-Based Wood Adhesive Improved by Silica Nanoparticles. Carbohydr. Polym. 2011, 86, 72–76. [Google Scholar] [CrossRef]
- Ramdugwar, V.; Fernandes, H.; Gadekar, P. Study of Scavengers for Free Formaldehyde Reduction in Phenolic Resins Used in Polychloroprene Based Contact Adhesives. Int. J. Adhes. Adhes. 2022, 115, 103122. [Google Scholar] [CrossRef]
- Sarika, P.R.; Nancarrow, P.; Khansaheb, A.; Ibrahim, T. Bio-Based Alternatives to Phenol and Formaldehyde for the Production of Resins. Polymers 2020, 12, 2237. [Google Scholar] [CrossRef]
- Kim, S. The Reduction of Formaldehyde and VOCs Emission from Wood-Based Flooring by Green Adhesive Using Cashew Nut Shell Liquid (CNSL). J. Hazard. Mater. 2010, 182, 919–922. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Wu, Y.; He, Z.; Wan, H. “Greener” Adhesives Composed of Urea-Formaldehyde Resin and Cottonseed Meal for Wood-Based Composites. J. Clean. Prod. 2018, 187, 361–371. [Google Scholar] [CrossRef]
- Islam, M.N.; Rahman, F.; Das, A.K.; Hiziroglu, S. An Overview of Different Types and Potential of Bio-Based Adhesives Used for Wood Products. Int. J. Adhes. Adhes. 2022, 112, 102992. [Google Scholar] [CrossRef]
- Yang, W.; Jiao, L.; Wang, X.; Wu, W.; Lian, H.; Dai, H. Formaldehyde-Free Self-Polymerization of Lignin-Derived Monomers for Synthesis of Renewable Phenolic Resin. Int. J. Biol. Macromol. 2021, 166, 1312–1319. [Google Scholar] [CrossRef]
- Younesi-Kordkheili, H. Maleated Lignin Coreaction with Phenol-Formaldehyde Resins for Improved Wood Adhesives Performance. Int. J. Adhes. Adhes. 2022, 113, 103080. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, A.; Ge, T.; Liu, X.; Tang, X.; Li, Y. Research Progress on Modification of Phenolic Resin. Mater. Today Commun. 2021, 26, 101879. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, H.W.; Lee, S.M.; Jae, J.; Park, Y.-K. Overview of the Recent Advances in Lignocellulose Liquefaction for Producing Biofuels, Bio-Based Materials and Chemicals. Bioresour. Technol. 2019, 279, 373–384. [Google Scholar] [CrossRef]
- Čuk, N.; Kunaver, M.; Poljanšek, I.; Ugovšek, A.; Šernek, M.; Medved, S. Properties of Liquefied Wood Modified Melamine-Formaldehyde (MF) Resin Adhesive and Its Application for Bonding Particleboards. J. Adhes. Sci. Technol. 2015, 29, 1553–1562. [Google Scholar] [CrossRef]
- Yan, L.; Cui, Y.; Gou, G.; Wang, Q.; Jiang, M.; Zhang, S.; Hui, D.; Gou, J.; Zhou, Z. Liquefaction of Lignin in Hot-Compressed Water to Phenolic Feedstock for the Synthesis of Phenol-Formaldehyde Resins. Compos. Part B Eng. 2017, 112, 8–14. [Google Scholar] [CrossRef]
- Lee, S.-H.; Teramoto, Y.; Shiraishi, N. Acid-Catalyzed Liquefaction of Waste Paper in the Presence of Phenol and Its Application to Novolak-Type Phenolic Resin. J. Appl. Polym. Sci. 2002, 83, 1473–1481. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Carvalho, R.; Silva, E.R.; Bordado, J.C.; Cardoso, A.C.; do Rosário Costa, M.; Mateus, M.M. Natural Polymeric Water-Based Adhesive from Cork Liquefaction. Ind. Crops Prod. 2016, 84, 314–319. [Google Scholar] [CrossRef]
- Jiang, W. Liquefaction of Lignocellulosic Materials and Its Applications in Wood Adhesives—A Review. Ind. Crops Prod. 2018, 124, 325–342. [Google Scholar] [CrossRef]
- Li, L.; Geng, K.; Liu, D.; Song, H.; Li, H. Relationship between Starch Liquefaction Behavior and Properties of Polymer Coated Urea from Liquefied Starch. Prog. Org. Coat. 2020, 147, 105759. [Google Scholar] [CrossRef]
- Liu, Q.; Zhai, Z.; Guo, J.; Cheng, J.; Zhang, Y. Liquefaction of Starch Using Solid-Acid Catalysts Derived from Spent Coffee for the Production of Plasticized Poly (Vinyl Alcohol) Films. Carbohydr. Polym. 2021, 254, 117427. [Google Scholar] [CrossRef]
- Wu, L.; Hu, X.; Wang, S.; Mahmudul Hasan, M.D.; Jiang, S.; Li, T.; Li, C.-Z. Acid-Treatment of Bio-Oil in Methanol: The Distinct Catalytic Behaviours of a Mineral Acid Catalyst and a Solid Acid Catalyst. Fuel 2018, 212, 412–421. [Google Scholar] [CrossRef]
- Zeng, X.; Yu, T.; Wang, P.; Yuan, R.; Wen, Q.; Fan, Y.; Wang, C.; Shi, R. Preparation and Characterization of Polar Polymeric Adsorbents with High Surface Area for the Removal of Phenol from Water. J. Hazard. Mater. 2010, 177, 773–780. [Google Scholar] [CrossRef]
- Lin, L.; Yoshioka, M.; Yao, Y.; Shiraishi, N. Liquefaction of Wood in the Presence of Phenol Using Phosphoric Acid as a Catalyst and the Flow Properties of the Liquefied Wood. J. Appl. Polym. Sci. 1994, 52, 1629–1636. [Google Scholar] [CrossRef]
- Lin, L.; Nakagame, S.; Yao, Y.; Shiraishi, M.Y.; Shiraishi, N. Liquefaction Mechanism of β-O-4 Lignin Model Compound in the Presence of Phenol under Acid Catalysis. Part 2. Reaction Behaviour and Pathways. Holzforschung 2001, 55, 625–630. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, G.; Chen, J. Effects of Inorganic Acid Catalysts on Liquefaction of Wood in Phenol. Front. Forest. China 2006, 1, 214–218. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Q.; Apaer, P.; Kashiwagi, N.; Kurokawa, H.; Sugiyama, K.; Wang, X.; Guo, X. Liquefaction Processes and Characterization of Liquefied Products from Waste Woody Materials in Different Acidic Catalysts. In WIT Transactions on State of the Art in Science and Engineering; Syngellakis, S., Ed.; WIT Press: Southampton, UK, 2014; Volume 1, pp. 121–132. ISBN 978-1-78466-034-5. [Google Scholar]
- Kobayashi, M.; Asano, T.; Kajiyama, M.; Tomita, B. Analysis on Residue Formation during Wood Liquefaction with Polyhydric Alcohol. J. Wood Sci. 2004, 50, 407–414. [Google Scholar] [CrossRef]
- Yip, J.; Chen, M.; Szeto, Y.S.; Yan, S. Comparative Study of Liquefaction Process and Liquefied Products from Bamboo Using Different Organic Solvents. Bioresour. Technol. 2009, 100, 6674–6678. [Google Scholar] [CrossRef]
- Kunaver, M.; Jasiukaitytė, E.; Čuk, N. Ultrasonically Assisted Liquefaction of Lignocellulosic Materials. Bioresour. Technol. 2012, 103, 360–366. [Google Scholar] [CrossRef]
- Juhaida, M.F.; Paridah, M.T.; Mohd Hilmi, M.; Sarani, Z.; Jalaluddin, H.; Mohamad Zaki, A.R. Liquefaction of Kenaf (Hibiscus cannabinus L.) Core for Wood Laminating Adhesive. Bioresour. Technol. 2010, 101, 1355–1360. [Google Scholar] [CrossRef]
- Hu, S.; Li, Y. Two-Step Sequential Liquefaction of Lignocellulosic Biomass by Crude Glycerol for the Production of Polyols and Polyurethane Foams. Bioresour. Technol. 2014, 161, 410–415. [Google Scholar] [CrossRef]
- Ahmadzadeh, A.; Zakaria, S.; Rashid, R. Liquefaction of Oil Palm Empty Fruit Bunch (EFB) into Phenol and Characterization of Phenolated EFB Resin. Ind. Crops Prod. 2009, 30, 54–58. [Google Scholar] [CrossRef]
- da Silva, S.H.F.; Egüés, I.; Labidi, J. Liquefaction of Kraft Lignin Using Polyhydric Alcohols and Organic Acids as Catalysts for Sustainable Polyols Production. Ind. Crops Prod. 2019, 137, 687–693. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Meireles, M.A.A. Physicochemical Properties, Modifications and Applications of Starches from Different Botanical Sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zhou, G.; Gao, D.; Wei, Z.; Wang, N. Preparation and Performance Characterization of a Composite Dust Suppressant for Preventing Secondary Dust in Underground Mine Roadways. Chem. Eng. Res. Des. 2020, 156, 195–208. [Google Scholar] [CrossRef]
- de Aquino Cutrim Farias, F.; de Souza Moretti, M.M.; Costa, M.S.; BordignonJunior, S.E.; Cavalcante, K.B.; Boscolo, M.; Gomes, E.; Franco, C.M.L.; da Silva, R. Structural and Physicochemical Characteristics of Taioba Starch in Comparison with Cassava Starch and Its Potential for Ethanol Production. Ind. Crops Prod. 2020, 157, 112825. [Google Scholar] [CrossRef]
- Santha, N.; Sudha, K.G.; Vijayakumari, K.P.; Nayar, V.U.; Moorthy, S.N. Raman and Infrared Spectra of Starch Samples of Sweet Potato and Cassava. J. Chem. Sci. 1990, 102, 705–712. [Google Scholar] [CrossRef] [Green Version]
- Flores-Morales, A.; Jiménez-Estrada, M.; Mora-Escobedo, R. Determination of the Structural Changes by FT-IR, Raman, and CP/MAS 13C NMR Spectroscopy on Retrograded Starch of Maize Tortillas. Carbohydr. Polym. 2012, 87, 61–68. [Google Scholar] [CrossRef]
- Courvoisier, E.; Bicaba, Y.; Colin, X. Multi-Scale and Multi-Technique Analysis of the Thermal Degradation of Poly (Ether Ether Ketone). Polym. Degrad. Stab. 2018, 151, 65–79. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Palanichamy, M.; Chellamani, A.; Leo Bastin, J.A.; Madasamy, M.; Vallinayagam, P.; Madhavan, D. Liquid Phase Depolymerization of Neyveli Lignite over Phosphotungstic Acid. Asian J. Chem. 2013, 25, 7437–7440. [Google Scholar] [CrossRef]
- Tekin, N.; Pir, H.; Sagdinc, S. Study of the Solvent Effects on the Molecular Structure and CO Stretching Vibrations of Flurbiprofen. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 98, 122–131. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, N.; Li, J.; Ye, H.; Yu, Z.; Zhang, Y. CaCl2-Induced Interfacial Deposition for the Preparation of High-Strength and Flame-Retardant Plywood Using Geopolymer-Based Adhesive. Ceram. Int. 2021, 47, 33678–33686. [Google Scholar] [CrossRef]
- Darr, J.P.; Davis, S.Q.; Kohno, Y.; McKenna, K.; Morales, P. Morphological Effects on the Hygroscopic Properties of Sodium Chloride–Sodium Sulfate Aerosols. J. Aerosol Sci. 2014, 77, 158–167. [Google Scholar] [CrossRef]
- Ma, X.-S.; Wang, D.-H.; Cui, Y.-Z.; Tao, F.-R.; Wang, Y.-T.; Li, T.-D. A Novel Hydrophilic Conjugated Polymer Containing Hydroxyl Groups: Syntheses and Sensing Performance for NACs in Aqueous Solution. Sens. Actuators B Chem. 2017, 251, 851–857. [Google Scholar] [CrossRef]





| Biomass Material | Liquefacient | Liquid-Solid Ratio | Liquefaction Time/h | Liquefaction Temperature/°C | Catalyst | Residue Rate/% | Reference |
|---|---|---|---|---|---|---|---|
| Moso bamboo | Phenol | 10:1 | 14 | 180 | Hydrochloric acid | 1.00 | [40] |
| Ethylene glycol | 10:1 | 14 | 180 | Hydrochloric acid | 31.00 | [40] | |
| Ethylene carbonate | 10:1 | 14 | 180 | Hydrochloric acid | 20.00 | [40] | |
| Medium-densityfiberboard | Polyethylene glycol mixed glycerol | 5:1 | 1.5 | 180 | Concentrated sulfuric acid | 6.23 | [41] |
| Veneered particleboard | 5:1 | 2 | 180 | Concentrated sulfuric acid | 5.04 | [41] | |
| Particleboard | 5:1 | 1.5 | 180 | Concentrated sulfuric acid | 6.24 | [41] | |
| Oriented strand board | 5:1 | 1.5 | 180 | Concentrated sulfuric acid | 3.00 | [41] | |
| Plywood | 5:1 | 1.5 | 180 | Concentrated sulfuric acid | 1.09 | [41] | |
| Wheat straw | 5:1 | 1.5 | 180 | Concentrated sulfuric acid | 5.58 | [41] | |
| Spruce sawdust | 5:1 | 1.5 | 180 | Concentrated sulfuric acid | 2.60 | [41] | |
| Kenaf core | 18:1 | 1.5 | 160 | Concentrated sulfuric acid | 2.94 | [42] | |
| Corn stover | Crude glycerol | 10:1 | 2.5 | 150 | Concentrated sulfuric acid | 11.90 | [43] |
| 10:1 | 3.0 | 240 | Sodium hydroxide | 7.90 | [43] | ||
| Oil palm empty fruit bunch | Phenol | 4:1 | 1.5 | 130 | Concentrated sulfuric acid | 5.40 | [44] |
| Kraft lignin | Polyethylene glycol mixed glycerol | 6.7:1 | 1.0 | 160 | Acetic acid | 12.51 | [45] |
| 6.7:1 | 1.0 | 160 | Lactic acid | 11.93 | [45] | ||
| 6.7:1 | 1.0 | 160 | Citric acid | 14.32 | [45] |
| Factor | Parameter | 2θ | d/nm | f | D/nm | c/% |
|---|---|---|---|---|---|---|
| Cassava starch | - | 37.78 | 0.2376 | 0.253 | 0.5845 | 37.7 |
| Catalyst usage | 6% | 37.68 | 0.2390 | 0.274 | 0.5513 | 6.93 |
| 8% | 37.68 | 0.2388 | 0.278 | 0.5318 | 6.86 | |
| 10% | 37.66 | 0.2386 | 0.266 | 0.5518 | 6.56 | |
| Liquefaction time | 2 h | 37.65 | 0.2393 | 0.266 | 0.5359 | 7.02 |
| 3 h | 37.68 | 0.2388 | 0.278 | 0.5318 | 6.86 | |
| 4 h | 37.63 | 0.2391 | 0.273 | 0.5342 | 6.93 | |
| Liquid–solid ratio | 1.5:1 | 37.70 | 0.2385 | 0.282 | 0.5213 | 7.21 |
| 2.0:1 | 37.68 | 0.2388 | 0.278 | 0.5318 | 6.86 | |
| 2.5:1 | 37.66 | 0.2385 | 0.279 | 0.5225 | 5.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Fang, J.; Xie, E.; Gan, W. Relevance between Cassava Starch Liquefied by Phenol and Modification of Phenol-Formaldehyde Resin Wood Adhesive. Polymers 2022, 14, 1914. https://doi.org/10.3390/polym14091914
Liu J, Fang J, Xie E, Gan W. Relevance between Cassava Starch Liquefied by Phenol and Modification of Phenol-Formaldehyde Resin Wood Adhesive. Polymers. 2022; 14(9):1914. https://doi.org/10.3390/polym14091914
Chicago/Turabian StyleLiu, Jinming, Jianlin Fang, Enjun Xie, and Weixing Gan. 2022. "Relevance between Cassava Starch Liquefied by Phenol and Modification of Phenol-Formaldehyde Resin Wood Adhesive" Polymers 14, no. 9: 1914. https://doi.org/10.3390/polym14091914
APA StyleLiu, J., Fang, J., Xie, E., & Gan, W. (2022). Relevance between Cassava Starch Liquefied by Phenol and Modification of Phenol-Formaldehyde Resin Wood Adhesive. Polymers, 14(9), 1914. https://doi.org/10.3390/polym14091914





