Graphene Oxide Molecularly Imprinted Polymers as Novel Adsorbents for Solid-Phase Microextraction for Selective Determination of Norfloxacin in the Marine Environment
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials and Chemicals
2.2. HPLC Analysis
2.3. Preparation of GO
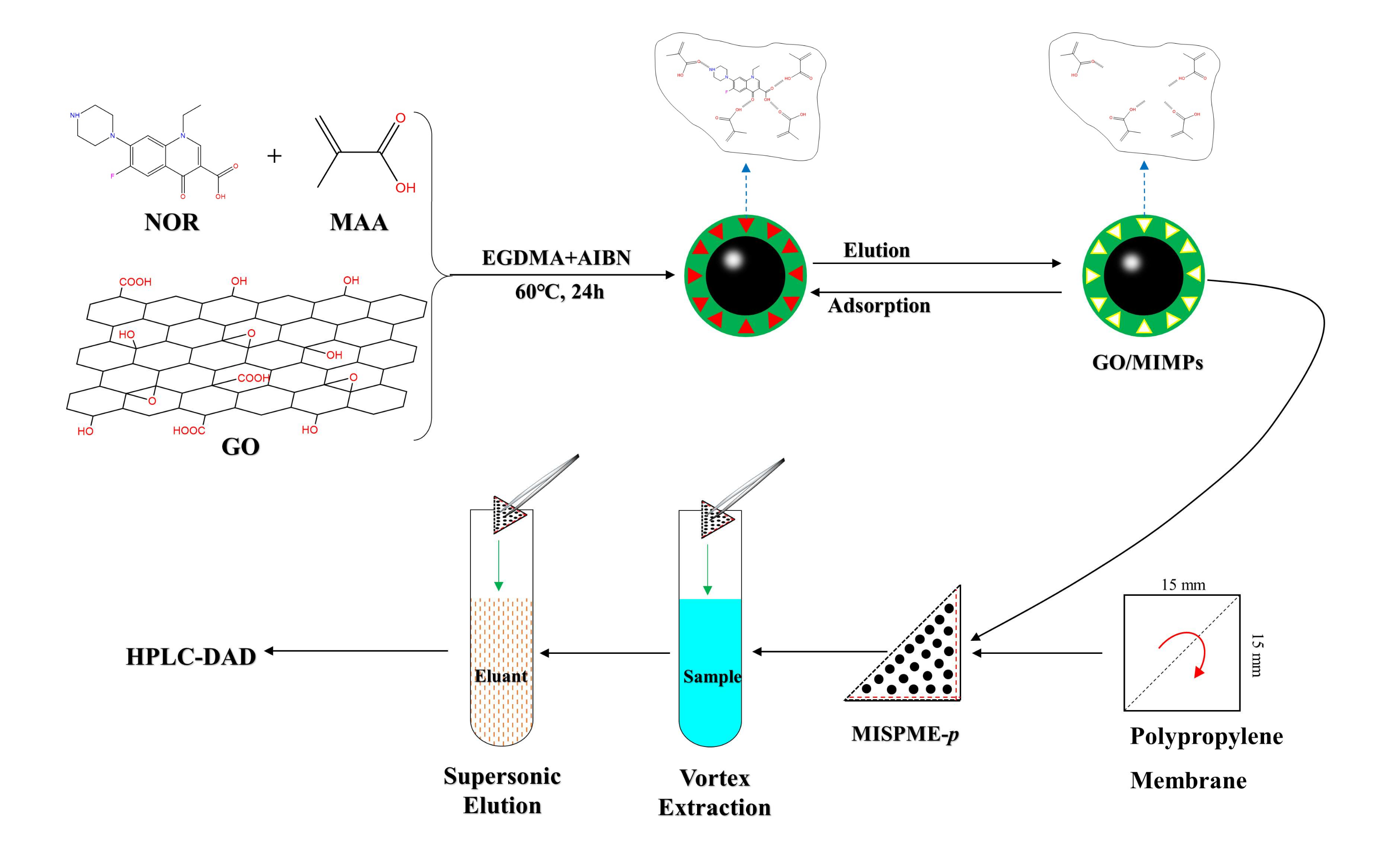
2.4. The Preparation of Polymers
2.5. Characterization of the Polymers
2.6. Adsorption Experiments
2.7. Preparation of MISPME Procedure
2.8. Samples Preparation
3. Results and Discussion
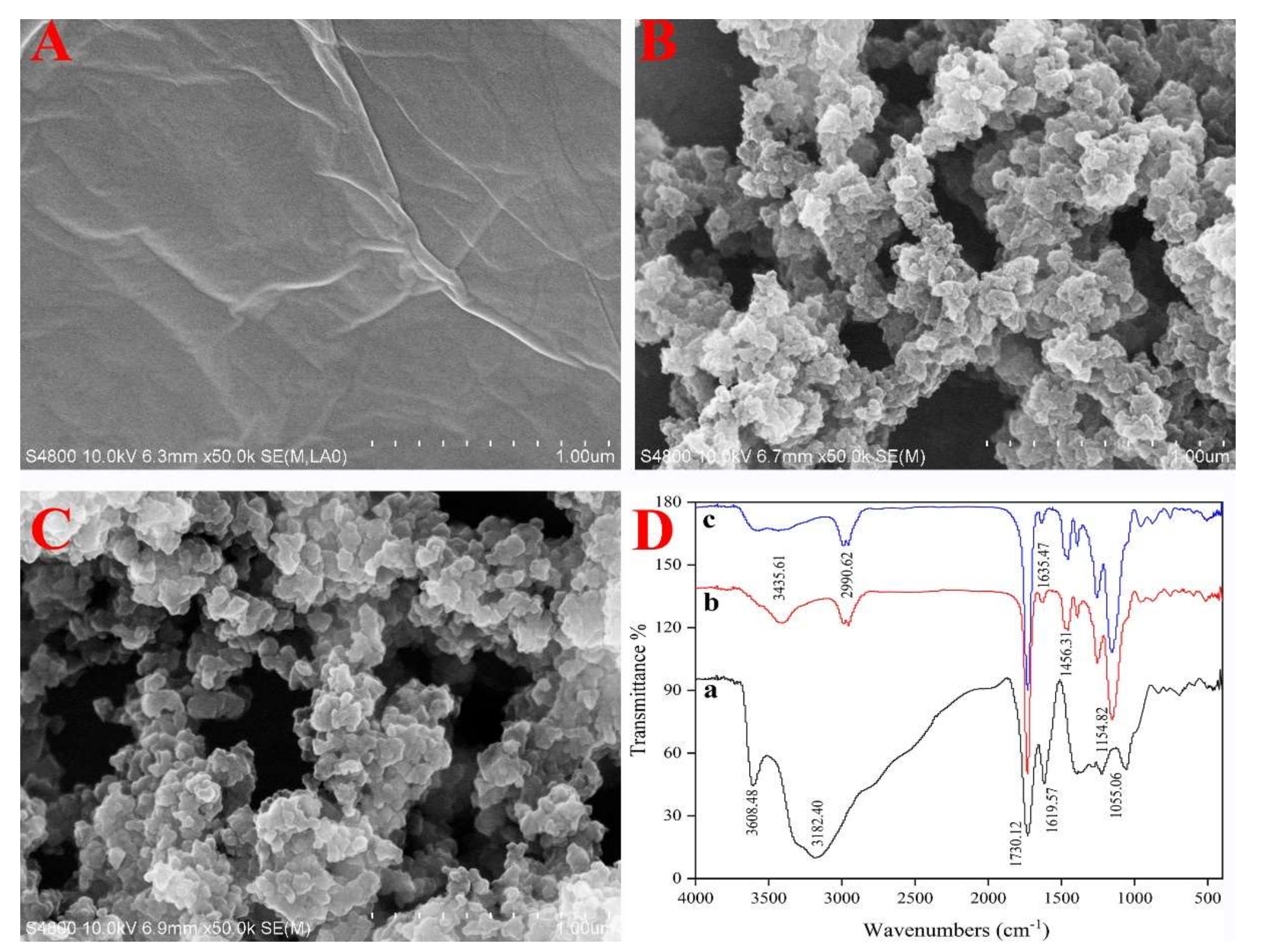
3.1. Characteristics of the Polymers
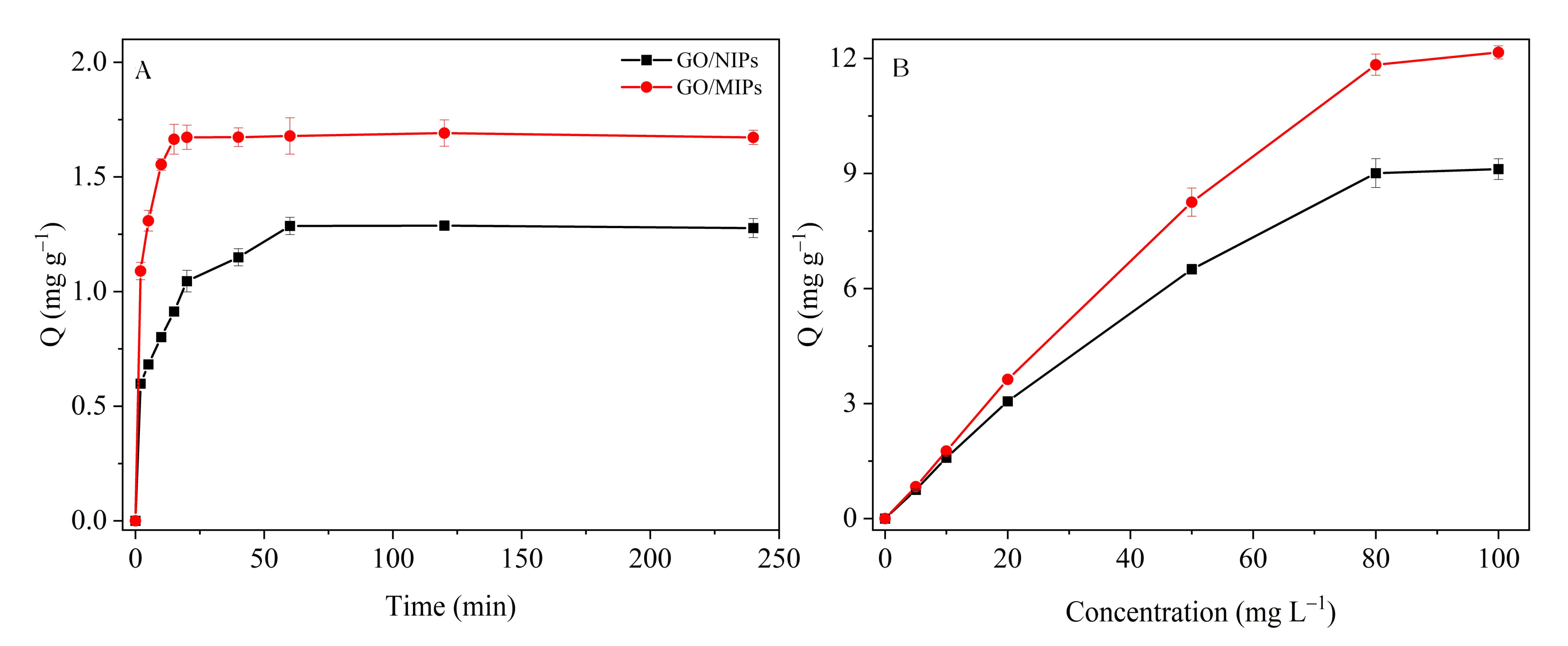
3.2. Adsorption Capacity of GO/MIPs
3.3. Optimization of the MISPME Procedure
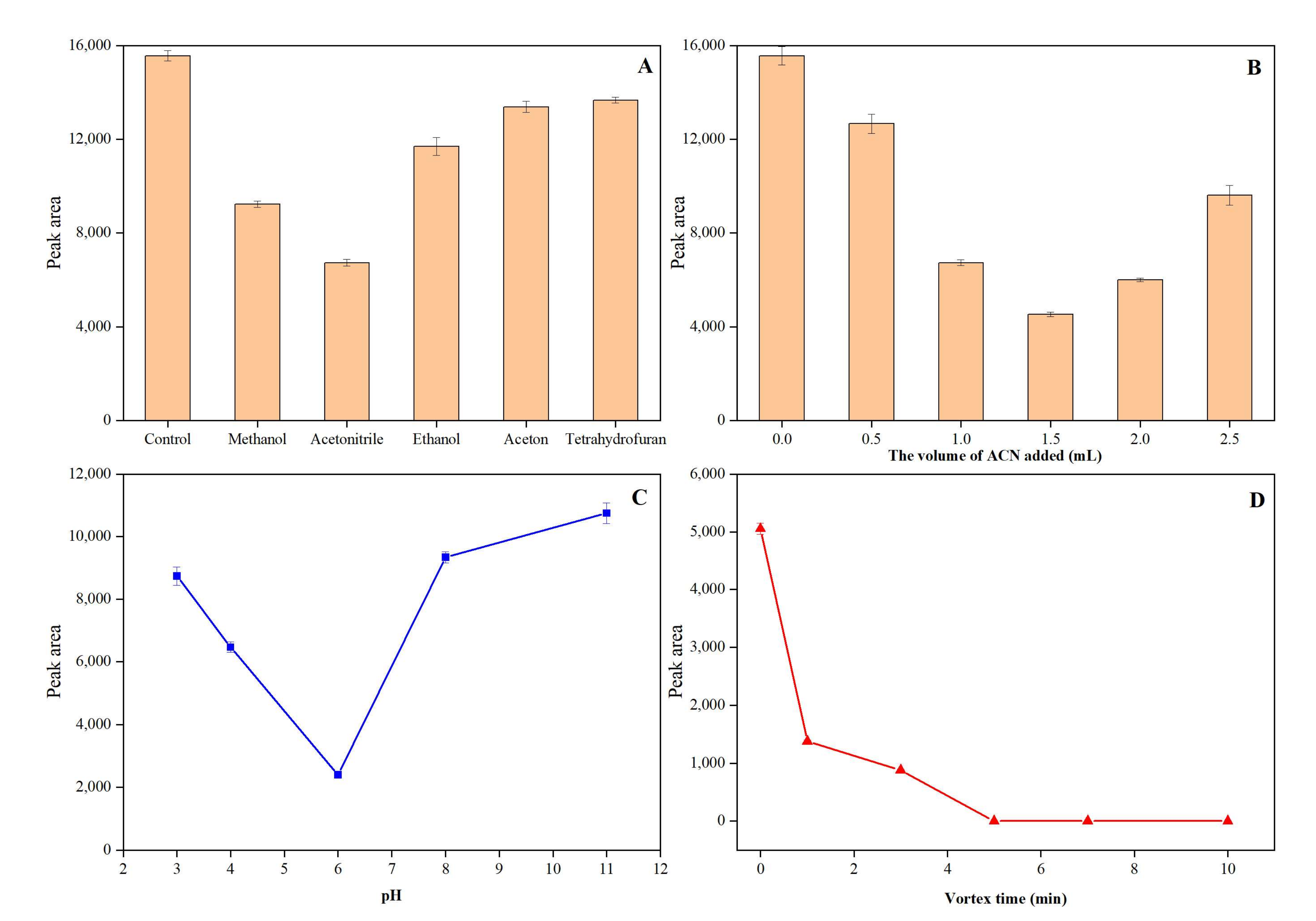
3.3.1. Type and Volume of Organic Cosolvent
3.3.2. Effect of pH and Extraction Time
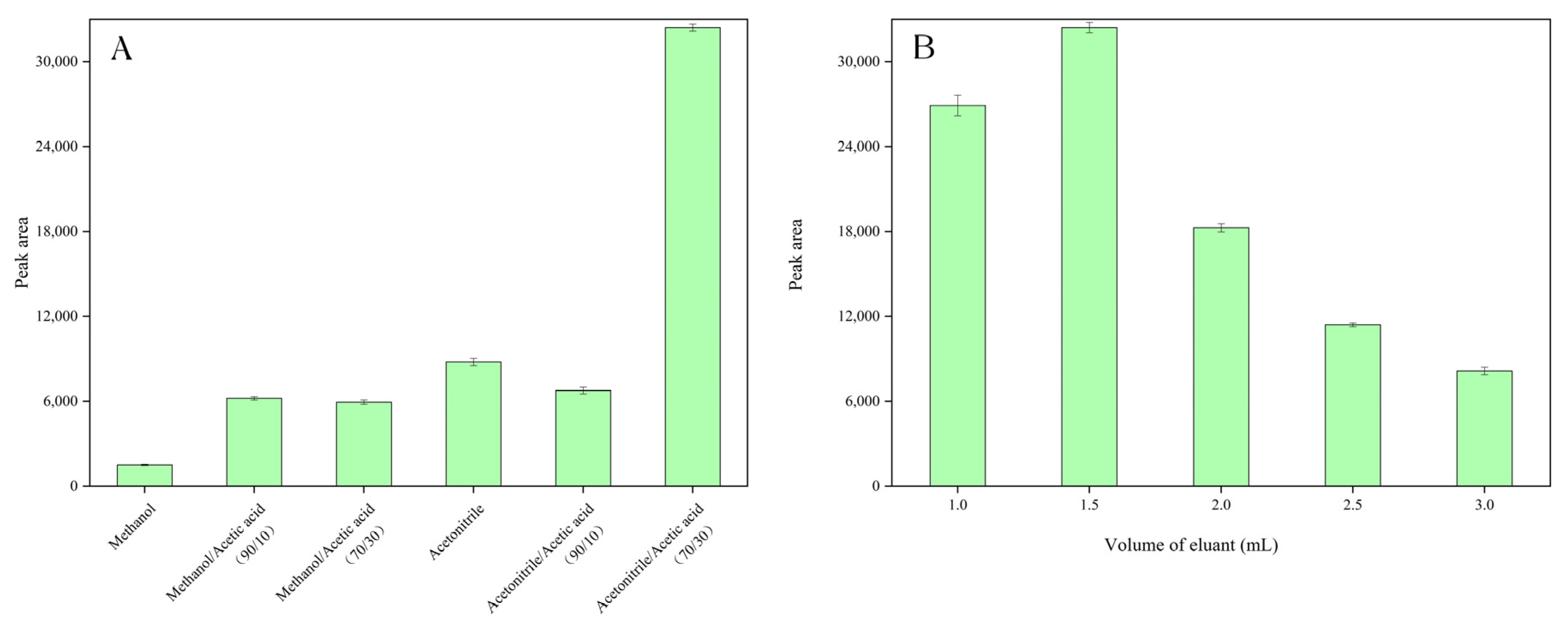
3.3.3. Effect of Eluent Type and Volume
3.4. Analytical Performance for NOR in Real Samples
3.5. Reusability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Urraca, J.L.; Castellari, M.; Barrios, C.A.; Moreno-Bondi, M.C. Multiresidue analysis of fluoroquinolone antimicrobials in chicken meat by molecularly imprinted solid-phase extraction and high-performance liquid chromatography. J. Chromatogr. A 2014, 1343, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y.; Zha, J.; Niu, M.C.; Hu, F.; Hui, X.H.; Tang, T.Y.; Fizir, M.; He, H. Bifunctional monomer molecularly imprinted sol-gel polymers based on the surface of magnetic halloysite nanotubes as an effective extraction approach for norfloxacin. Appl. Clay Sci. 2018, 162, 409–417. [Google Scholar] [CrossRef]
- Prieto, A.; Schrader, S.; Bauer, C.; Möder, M. Synthesis of a molecularly imprinted polymer and its application for microextraction by packed sorbent for the determination of fluoroquinolone related compounds in water. Anal. Chim. Acta 2011, 685, 146–152. [Google Scholar] [CrossRef]
- Prutthiwanasan, B.; Phechkrajang, C.; Suntornsuk, L. Fluorescent labelling of ciprofloxacin and norfloxacin and its application for residues analysis in surface water. Talanta 2016, 159, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Kahn, L.H. Antimicrobial resistance: A one health perspective. Trans. R. Soc. Trop. Med. Hyg. 2017, 6, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Babić, S.; Periša, M.; Škorić, I. Photolytic degradation of norfloxacin, enrofloxacin and ciprofloxacin in various aqueous media. Chemosphere 2013, 91, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Al-Mustafa, Z.H.; Al-Ghamdi, M.S. Use of norfloxacin in poultry production in the eastern province of Saudi Arabia and its possible impact on public health. Int. J. Environ. Health Res. 2000, 10, 291–299. [Google Scholar] [CrossRef]
- Hu, S.Y.; Wu, H.; Liang, X.J.; Xiao, C.L.; Zhao, Q.S.; Cao, Y.Q.; Han, X.R. A preliminary study on the eco-environmental geological issue of in-situ oil shale mining by a physical model. Chemosphere 2022, 287, 131987. [Google Scholar] [CrossRef]
- Gonzãlez-Pleiter, M.; Gonzalo, S.; Rodea-Palomares, I.; Leganés, F.; Rosal, R.; Boltes, K.; Marco, E.; Fernández-Piñas, F. Toxicity of five antibiotics and their mixtures towards photosynthetic aquatic organisms: Implications for environmental risk assessment. Water Res. 2013, 47, 2050–2064. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, K.; Huang, Q.; Yu, Y.; Peng, X. An indirect competitive enzyme-linked immunosorbent assay for determination of norfloxacin in waters using a specific polyclonal antibody. Anal. Chim. Acta 2011, 688, 84–89. [Google Scholar] [CrossRef]
- Peng, J.; Liu, L.; Kuang, H.; Cui, G.; Xu, C. Development of an icELISA and immunochromatographic strip for detection of norfloxacin and its analogs in milk. Food Agric. Immunol. 2016, 28, 288–298. [Google Scholar] [CrossRef] [Green Version]
- Kamal, A.H.; El-Malla, S.F. Mixed micellar liquid chromatographic method for simultaneous determination of norfloxacin and tinidazole in pharmaceutical tablets. Microchem. J. 2019, 150, 104151. [Google Scholar] [CrossRef]
- Stubbings, G.; Bigwood, T. The development and validation of a multiclass liquid chromatography tandem mass spectrometry (LC–MS/MS) procedure for the determination of veterinary drug residues in animal tissue using a QuEChERS (Quick, Easy, Cheap, Effective, Rugged and Safe) approach. Anal. Chim. Acta 2009, 637, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Chen, J.H.; Li, Z.Y.; Wang, S.; Shi, Q.; Cao, W.; Zheng, X.L.; Sun, C.J.; Wang, X.R.; Zheng, L. Determination of typical lipophilic marine toxins in marine sediments from three coastal bays of China using liquid chromatography–tandem mass spectrometry after accelerated solvent extraction. Mar. Pollut. Bull. 2015, 101, 954–960. [Google Scholar] [CrossRef]
- He, X.P.; Mei, X.Q.; Wang, J.T.; Lian, Z.R.; Tan, L.J.; Wu, W. Determination of diethylstilbestrol in seawater by molecularly imprinted solid-phase extraction coupled with high-performance liquid chromatography. Mar. Pollut. Bull. 2016, 102, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Andreu, V.; Blasco, C.; Picó, Y. Analytical strategies to determine quinolone residues in food and the environment. TrAC Trends Anal. Chem. 2007, 26, 534–556. [Google Scholar] [CrossRef]
- Lee, H.B.; Peart, T.E.; Svoboda, M.L. Determination of ofloxacin, norfloxacin, and ciprofloxacin in sewage by selective solid-phase extraction, liquid chromatography with fluorescence detection, and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2007, 1139, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Gaballah, M.S.; Li, X.; Zhang, Z.J.; Al-Anazi, A.; Sun, H.; Sobhi, M.; Philbert, M.; Ghorab, M.A.; Guo, J.B.; Dong, R.J. Determination of tetracycline, oxytetracycline, sulfadiazine, norfloxacin, and enrofloxacin in swine manure using a coupled method of on-line solid-phase extraction with the UHPLC-DAD. Antibiotics 2021, 10, 1397. [Google Scholar] [CrossRef] [PubMed]
- Mitani, K.; Kataoka, H. Determination of fluoroquinolones in environmental waters by in-tube solid-phase microextraction coupled with liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2006, 562, 16–22. [Google Scholar] [CrossRef]
- Pang, J.L.; Liao, Y.M.; Huang, X.J.; Ye, Z.W.; Yuan, D.X. Metal-organic framework-monolith composite-based in-tube solid phase microextraction on-line coupled to high-performance liquid chromatography-fluorescence detection for the highly sensitive monitoring of fluoroquinolones in water and food samples. Talanta 2019, 199, 499–506. [Google Scholar] [CrossRef]
- Yu, S.H.; Liu, Z.G.; Li, H.W.; Zhang, J.P.; Yuan, X.X.; Jia, X.Y.; Wu, Y.Q. Combination of a graphene SERS substrate and magnetic solid phase micro-extraction used for the rapid detection of trace illegal additives. Analyst 2018, 143, 883–890. [Google Scholar] [CrossRef]
- Barahona, F.; Albero, B.; Tadeo, J.L.; Martín-Esteban, A. Molecularly imprinted polymer-hollow fiber microextraction of hydrophilic fluoroquinolone antibiotics in environmental waters and urine samples. J. Chromatogr. A 2019, 1587, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Wang, C.C.; Yang, L.Y.; Zhang, W.; Lin, J.; Li, C. Determination of eight quinolones in milk using immunoaffinity microextraction in a packed syringe and liquid chromatography with fluorescence detection. J. Chromatogr. B 2017, 1064, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Moema, D.; Nindi, M.M.; Dube, S. Development of a dispersive liquid–liquid microextraction method for the determination of fluoroquinolones in chicken liver by high performance liquid chromatography. Anal. Chim. Acta 2012, 730, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Luliński, P.; Janczura, M.; Sobiech, M.; Giebułtowicz, J. Magnetic molecularly imprinted nano-conjugates for effective extraction of food components—a model study of tyramine determination in craft beers. Int. J. Mol. Sci. 2021, 22, 9560. [Google Scholar] [CrossRef]
- Dil, E.A.; Doustimotlagh, A.H.; Javadian, H.; Asfaram, A.; Ghaedi, M. Nano-sized Fe3O4@SiO2-molecular imprinted polymer as a sorbent for dispersive solid-phase microextraction of melatonin in the methanolic extract of Portulaca oleracea, biological, and water samples. Talanta 2021, 221, 121620. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wang, D.; Du, F.Y.; Zheng, W.Q.; Liu, Z.M.; Xu, Z.G.; Hu, X.Z.; Liu, H.C. Dummy-template molecularly imprinted micro-solid-phase extraction coupled with high-performance liquid chromatography for bisphenol A determination in environmental water samples. Microchem. J. 2019, 145, 337–344. [Google Scholar] [CrossRef]
- Lian, Z.R.; Liang, Z.L.; Wang, J.T. Determination of melamine in aquaculture feed samples based on molecularly imprinted solid-phase extraction. J. Sep. Sci. 2015, 38, 3655–3660. [Google Scholar] [CrossRef]
- Cantarella, M.; Carroccio, S.C.; Dattilo, S.; Avolio, R.; Castaldo, R.; Puglisi, C.; Privitera, V. Molecularly imprinted polymer for selective adsorption of diclofenac from contaminated water. Chem. Eng. J. 2019, 367, 180–188. [Google Scholar] [CrossRef]
- Zhao, M.; Hou, Z.H.; Lian, Z.R.; Qin, D.; Ge, C.Z. Direct extraction and detection of malachite green from marine sediments by magnetic nano-sized imprinted polymer coupled with spectrophotometric analysis. Mar. Pollut. Bull. 2020, 158, 111363. [Google Scholar] [CrossRef]
- Kasiri, E.; Haddadi, H.; Javadian, H.; Asfaram, A. Highly effective pre-concentration of thymol and carvacrol using nano-sized magnetic molecularly imprinted polymer based on experimental design optimization and their trace determination in summer savoury, Origanum majorana and Origanum vulgare extracts. J. Chromatogr. B 2021, 1182, 122941. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Raizada, P.; Thakur, V.K.; Saini, V.; Khan, A.A.P.; Singh, N.; Singh, P. An overview on polymeric carbon nitride assisted photocatalytic CO2 reduction: Strategically manoeuvring solar to fuel conversion efficiency. Chem. Eng. Sci. 2021, 230, 116219. [Google Scholar] [CrossRef]
- Hasija, V.; Nguyen, V.H.; Kumar, A.; Raizada, P.; Krishnan, V.; Khan, A.A.P.; Singh, P.; Lichtfouse, E.; Wang, C.Y.; Huong, P.T. Advanced activation of persulfate by polymeric g-C3N4 based photocatalysts for environmental remediation: A review. J. Hazard. Mater. 2021, 413, 125324. [Google Scholar] [CrossRef] [PubMed]
- Ning, F.; Qiu, T.; Wang, Q.; Peng, H.; Li, Y.; Wu, X.Q.; Zhang, Z.; Chen, L.X.; Xiong, H. Dummy-surface molecularly imprinted polymers on magnetic graphene oxide for rapid and selective quantification of acrylamide in heat-processed (including fried) foods. Food Chem. 2017, 221, 1797–1804. [Google Scholar] [CrossRef]
- Sedghi, R.; Heidari, B.; Yassari, M. Novel molecularly imprinted polymer based on β-cyclodextrin@ graphene oxide: Synthesis and application for selective diphenylamine determination. J. Colloid Interface Sci. 2017, 503, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Das, R.S.; Wankhade, A.V.; Kumar, A. Computationally designed ionic liquid based molecularly imprinted@ graphene oxide composite: Characterization and validation. J. Mol. Liq. 2021, 341, 116925. [Google Scholar] [CrossRef]
- Zheng, M.M.; Gong, R.; Zhao, X.; Feng, Y.Q. Selective sample pretreatment by molecularly imprinted polymer monolith for the analysis of fluoroquinolones from milk samples. J. Chromatogr. A 2010, 1217, 2075–2081. [Google Scholar] [CrossRef]
- Oliveira, H.L.D.; Teixeira, L.S.; Dinali, L.A.F.; Pires, B.C.; Simões, N.S.; Borges, K.B. Microextraction by packed sorbent using a new restricted molecularly imprinted polymer for the determination of estrogens from human urine samples. Microchem. J. 2019, 150, 104162. [Google Scholar] [CrossRef]
- Asfaram, A.; Arabi, M.; Ostovan, A.; Sadeghi, H.; Ghaedi, M. Simple and selective detection of quercetin in extracts of plants and food samples by dispersive-micro-solid phase extraction based on core–shell magnetic molecularly imprinted polymers. New J. Chem. 2018, 42, 16144. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Chen, J.L.; Wang, J.T.; Tan, L.J. Highly selective separation and detection of cyromazine from seawater using graphene oxide based molecularly imprinted solid-phase extraction. J. Sep. Sci. 2019, 42, 2100–2106. [Google Scholar] [CrossRef] [PubMed]
- Scorrano, S.; Mergola, L.; Bello, M.P.D.; Lazzoi, M.R.; Vasapollo, G.; Sole, R.D. Molecularly imprinted composite membranes for selective detection of 2-deoxyadenosine in urine samples. Int. J. Mol. Sci. 2015, 16, 13746–13759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.L.; Choi, H.J. Silica-graphene oxide hybrid composite particles and their electroresponsive characteristics. Langmuir 2012, 28, 7055–7062. [Google Scholar] [CrossRef]
- Li, J.M.; Zhou, Y.Q.; Sun, Z.A.; Cai, T.P.; Wang, X.X.; Zhao, S.W.; Liu, H.H.; Gong, B.L. Restricted access media-imprinted nanomaterials based on a metal–organic framework for highly selective extraction of fluoroquinolones in milk and river water. J. Chromatogr. A 2020, 1626, 461364. [Google Scholar] [CrossRef] [PubMed]
- Raevsky, O.A.; Schaper, K.J.; Seydel, J.K. H-Bond Contribution to Octanol-Water Partition Coefficients of Polar Compounds. Quant. Struct.-Act. Relatsh. 1995, 14, 433–436. [Google Scholar] [CrossRef]
- Tian, H.J.; Liu, T.; Mu, G.D.; Chen, F.M.; He, M.Y.; You, S.; Yang, M.L.; Li, Y.L.; Zhang, F. Rapid and sensitive determination of trace fluoroquinolone antibiotics in milk by molecularly imprinted polymer-coated stainless steel sheet electrospray ionization mass spectrometry. Talanta 2020, 219, 121282. [Google Scholar] [CrossRef]
- Lu, Z.L.; Deng, F.F.; He, R.; Tan, L.; Luo, X.Y.; Pan, X.H.; Yang, Z.C. A pass-through solid-phase extraction clean-up method for the determination of 11 quinolone antibiotics in chicken meat and egg samples using ultra-performance liquid chromatography tandem mass spectrometry. Microchem. J. 2019, 151, 104213. [Google Scholar] [CrossRef]
- Sun, X.L.; Wang, J.C.; Li, Y.; Yang, J.J.; Jin, J.; Shah, S.M.; Chen, J.P. Novel dummy molecularly imprinted polymers for matrix solid-phase dispersion extraction of eight fluoroquinolones from fish samples. J. Chromatogr. A 2014, 1359, 1–7. [Google Scholar] [CrossRef]
| Sample | Extraction Method | Detection | LOD | Recovery | Reference |
|---|---|---|---|---|---|
| Chicken | a MISPE | HPLC–FLD | 6.00 μg/kg | 94.0–101.0% | [1] |
| Water | b MIP-MEPS | LC–MS/MS | 3.80 μg/L | 82.0–104.0% | [3] |
| Fish | c DMI-MSPD | HPLC–FLD | 0.14 μg/kg | 95.0–99.2% | [48] |
| SeawaterFish | MISPME | HPLC–DAD | 0.15 μg/L0.10 μg/kg | 90.1–102.7% | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Tan, L.; Cui, Z.; Qu, K.; Wang, J. Graphene Oxide Molecularly Imprinted Polymers as Novel Adsorbents for Solid-Phase Microextraction for Selective Determination of Norfloxacin in the Marine Environment. Polymers 2022, 14, 1839. https://doi.org/10.3390/polym14091839
Chen J, Tan L, Cui Z, Qu K, Wang J. Graphene Oxide Molecularly Imprinted Polymers as Novel Adsorbents for Solid-Phase Microextraction for Selective Determination of Norfloxacin in the Marine Environment. Polymers. 2022; 14(9):1839. https://doi.org/10.3390/polym14091839
Chicago/Turabian StyleChen, Jianlei, Liju Tan, Zhengguo Cui, Keming Qu, and Jiangtao Wang. 2022. "Graphene Oxide Molecularly Imprinted Polymers as Novel Adsorbents for Solid-Phase Microextraction for Selective Determination of Norfloxacin in the Marine Environment" Polymers 14, no. 9: 1839. https://doi.org/10.3390/polym14091839
APA StyleChen, J., Tan, L., Cui, Z., Qu, K., & Wang, J. (2022). Graphene Oxide Molecularly Imprinted Polymers as Novel Adsorbents for Solid-Phase Microextraction for Selective Determination of Norfloxacin in the Marine Environment. Polymers, 14(9), 1839. https://doi.org/10.3390/polym14091839






