Sustained and Microenvironment-Accelerated Release of Minocycline from Alginate Injectable Hydrogel for Bacteria-Infected Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation of SA@MC Injectable Hydrogels
2.2. Characterization of SA@MC Injectable Hydrogels
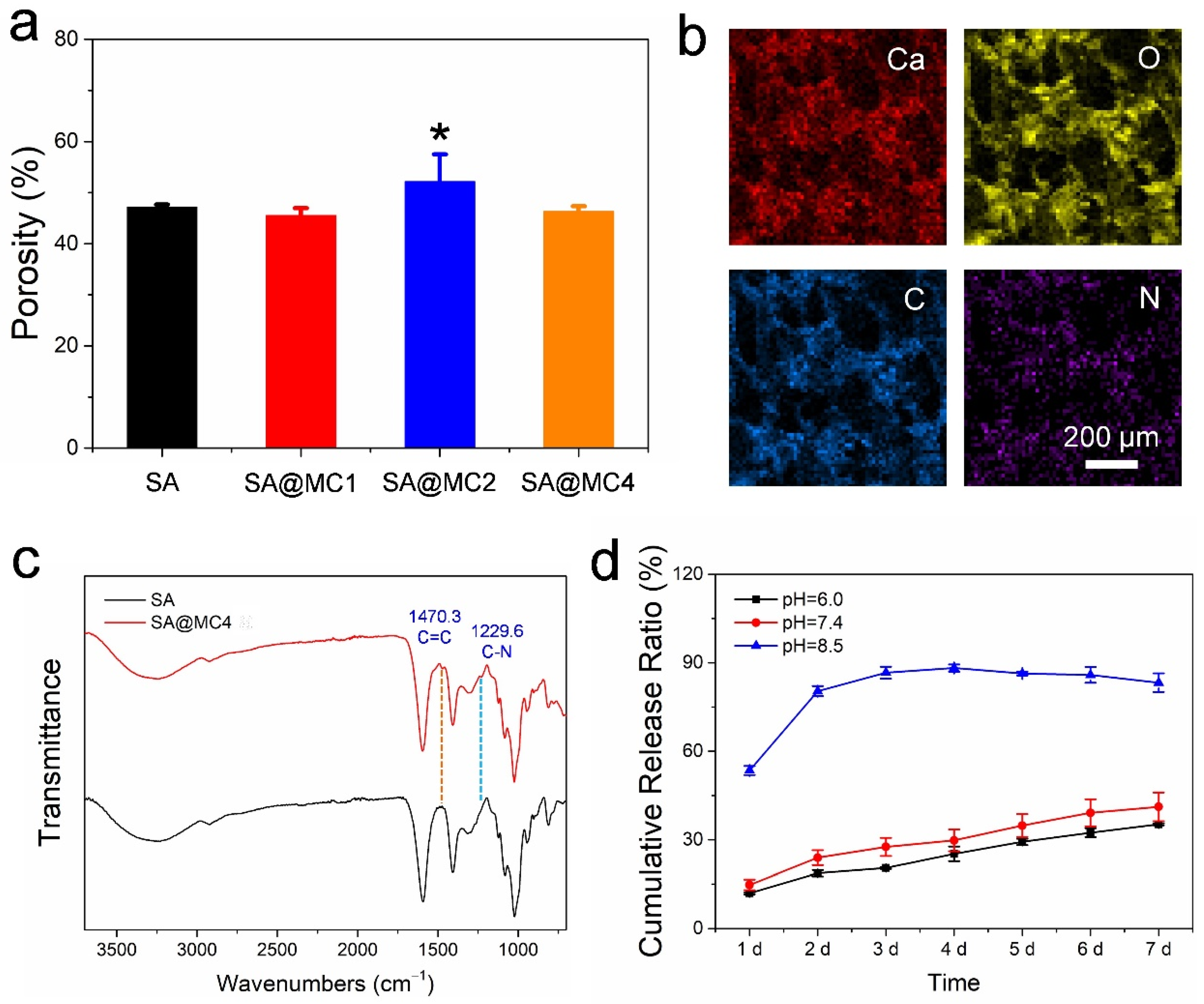
2.3. Drug Release Kinetics
2.4. Cytocompatibility Evaluation of SA@MC Injectable Hydrogels
2.5. Antibacterial Activity Evaluation
2.6. In Vivo Wound Healing in a Full-Thickness Skin Defect Model
2.7. Statistical Analyses
3. Results and Discussion
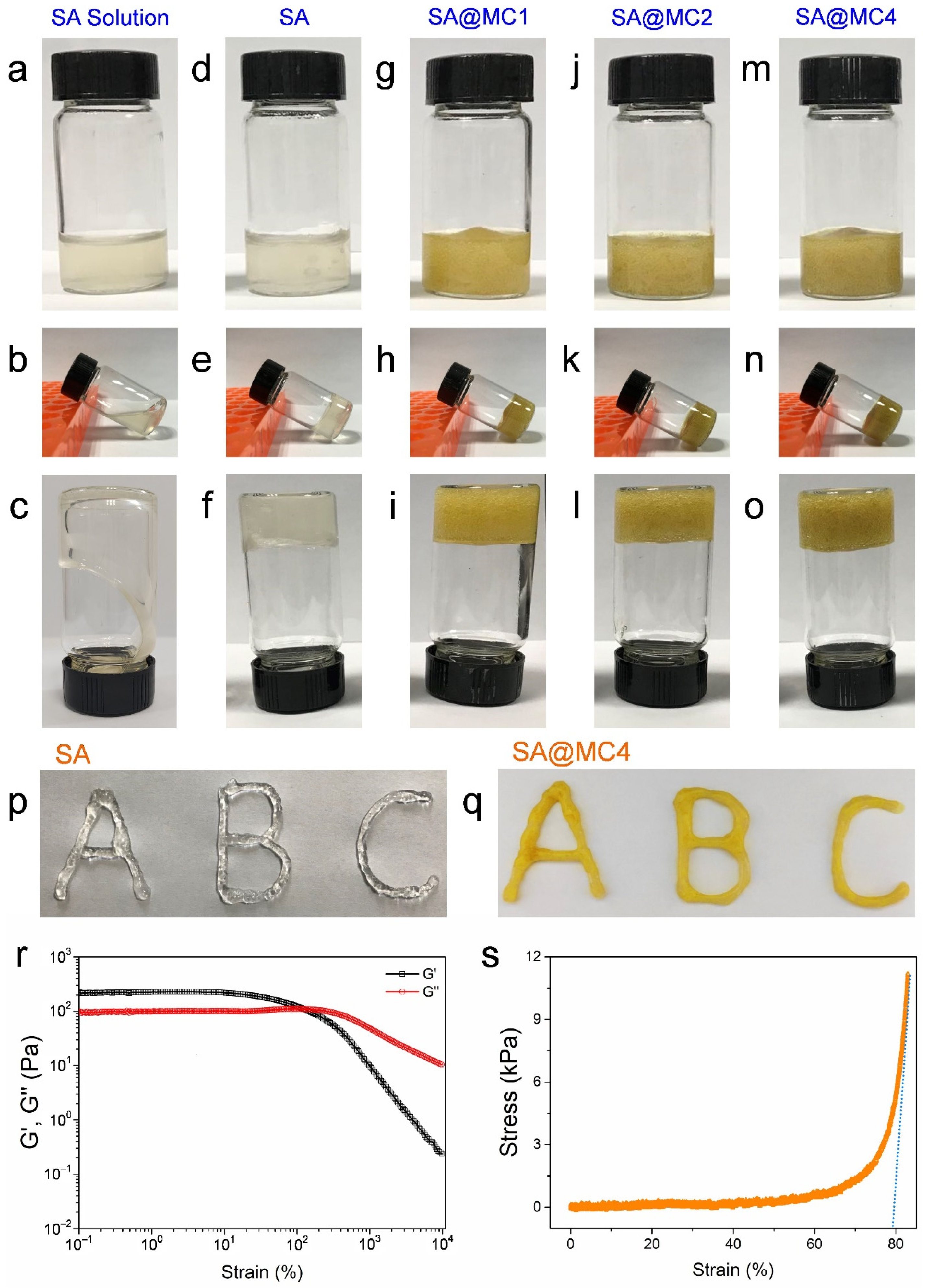
3.1. Injectable Property of SA@MC Injectable Hydrogels
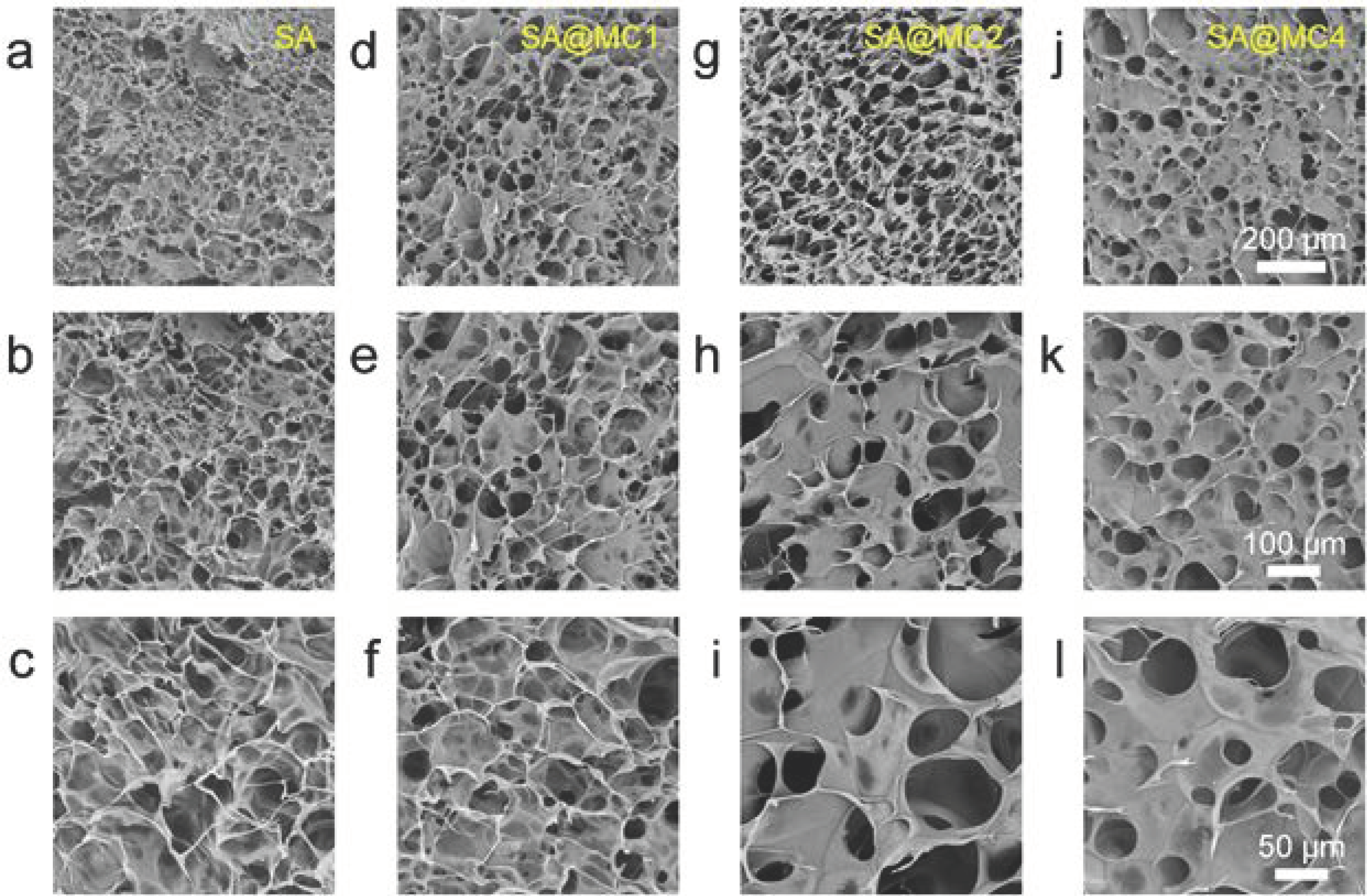
3.2. Characterization of SA@MC Injectable Hydrogels
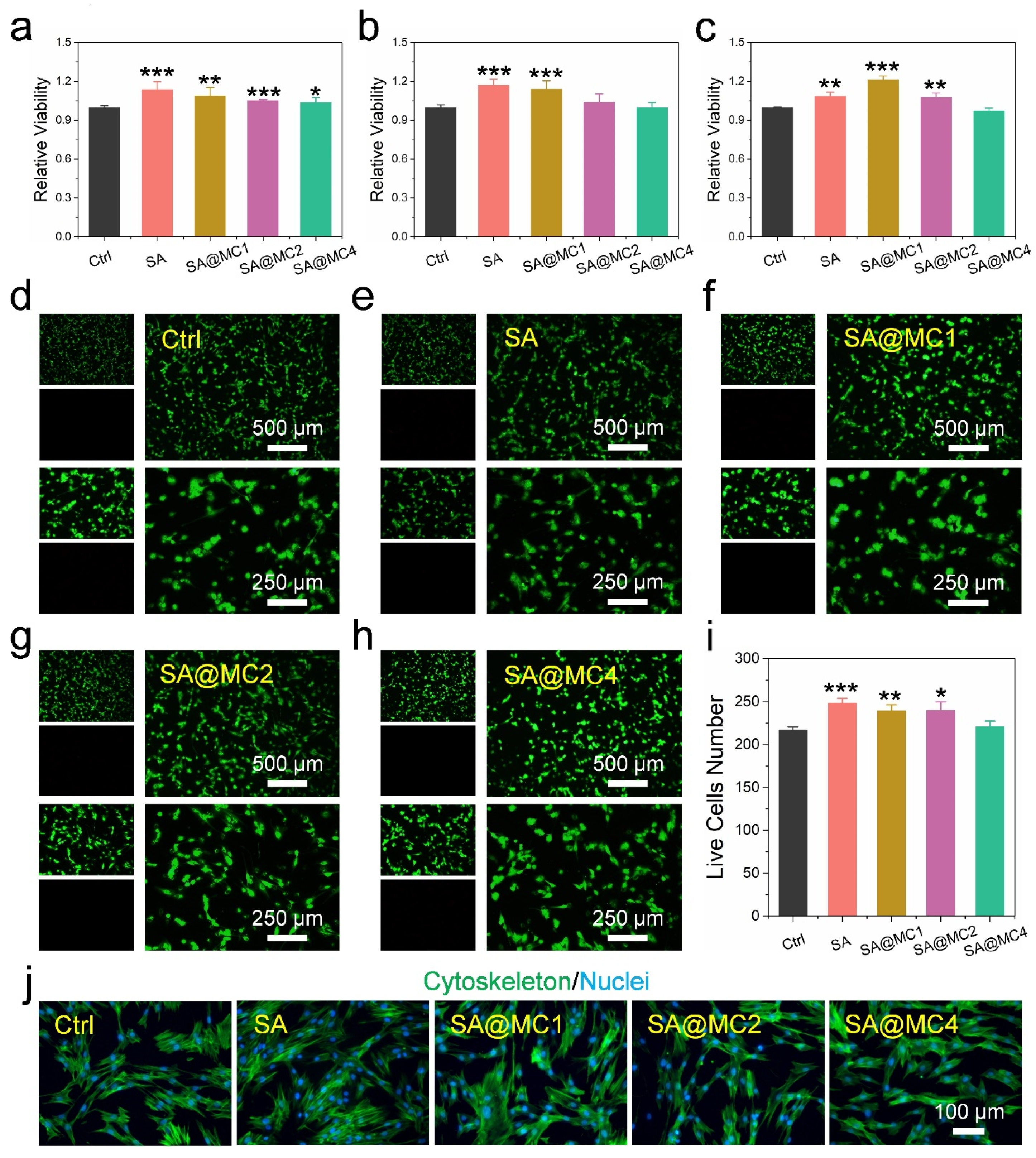
3.3. Biocompatibility of SA@MC Injectable Hydrogels
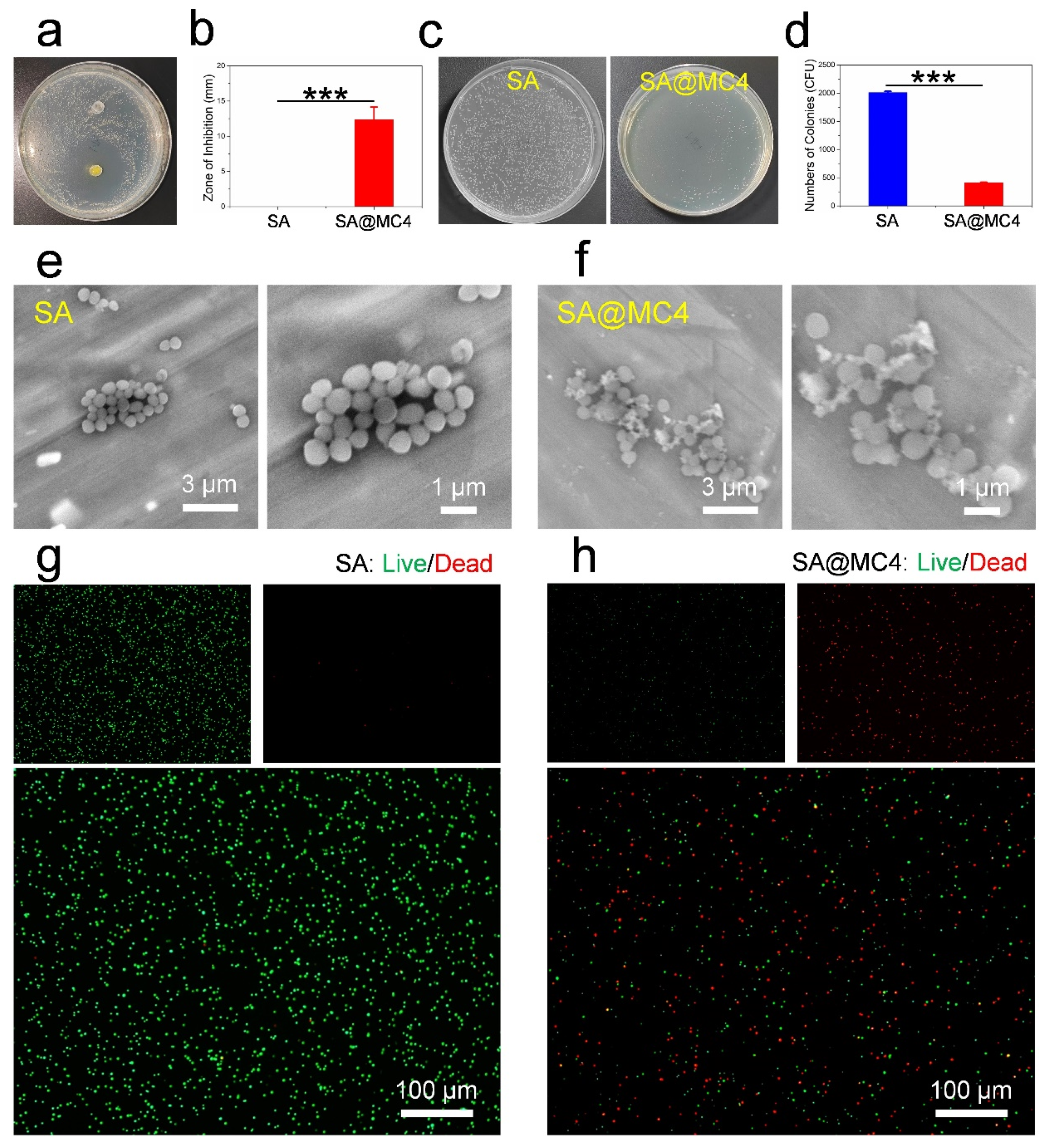
3.4. Antibacterial Activity of SA@MC Injectable Hydrogels
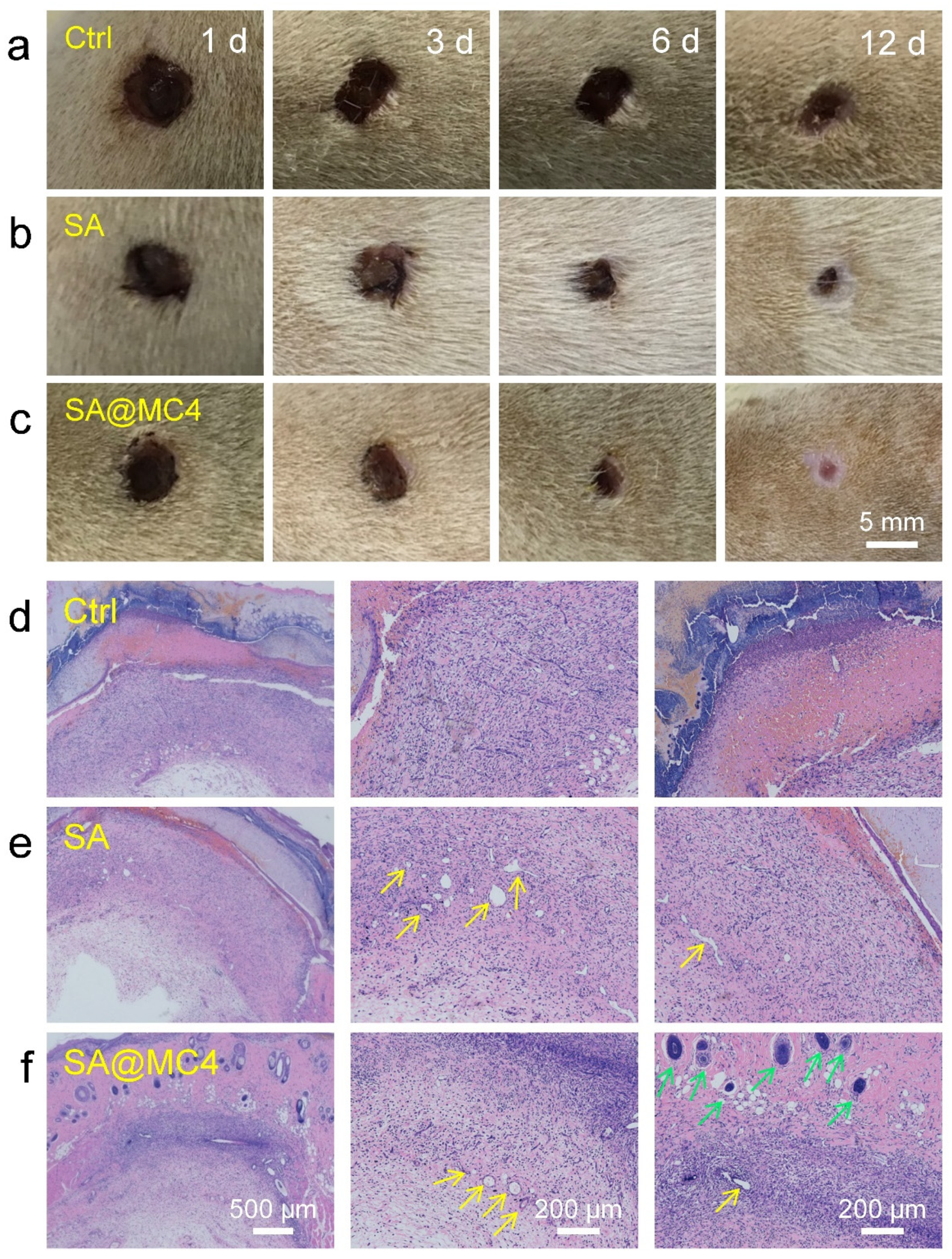
3.5. Wound Healing Ability In Vivo of SA@MC Injectable Hydrogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ming, Z.; Han, L.; Bao, M.; Zhu, H.; Qiang, S.; Xue, S.; Liu, W. Living Bacterial Hydrogels for Accelerated Infected Wound Healing. Adv. Sci. 2021, 8, 2102545. [Google Scholar] [CrossRef] [PubMed]
- Mohebali, A.; Abdouss, M.; Taromi, F.A. Fabrication of Biocompatible Antibacterial Nanowafers Based on Hnt/Pva Nanocomposites Loaded with Minocycline for Burn Wound Dressing. Mater. Sci. Eng. C 2020, 110, 110685. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, D.; Qian, Z.; Hou, S.; Li, L.; Jenkins, A.T.A.; Fan, Y. Bacteria-Responsive Intelligent Wound Dressing: Simultaneous in Situ Detection and Inhibition of Bacterial Infection for Accelerated Wound Healing. Biomaterials 2018, 161, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Couce, A.; Blázquez, J. Side Effects of Antibiotics on Genetic Variability. FEMS Microbiol. Rev. 2009, 33, 531–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heta, S.; Robo, I. The Side Effects of the Most Commonly Used Group of Antibiotics in Periodontal Treatments. Med. Sci. 2018, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- David, P. The Challenge of Antimicrobial Resistance. Lancet Gastroenterol Hepatol 2016, 1, 173. [Google Scholar]
- Kurt Yilmaz, N.; Schiffer, C.A. Introduction: Drug Resistance. Chem. Rev. 2021, 121, 3235–3237. [Google Scholar] [CrossRef]
- Smith, R.; Russo, J.; Fiegel, J.; Brogden, N. Antibiotic Delivery Strategies to Treat Skin Infections When Innate Antimicrobial Defense Fails. Antibiotics 2020, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Jepsen, K.; Jepsen, S. Antibiotics/Antimicrobials: Systemic and Local Administration in the Therapy of Mild to Moderately Advanced Periodontitis. Periodontol. 2000 2016, 71, 82–112. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial Anti-Oxidant Electroactive Injectable Hydrogel as Self-Healing Wound Dressing with Hemostasis and Adhesiveness for Cutaneous Wound Healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Wu, Q.; Wang, Y.; Zhang, D.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. A Biodegradable Hydrogel System Containing Curcumin Encapsulated in Micelles for Cutaneous Wound Healing. Biomaterials 2013, 34, 6377–6387. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.-a.; Muzzin, N.; Toufanian, S.; Slick, R.A.; Lawlor, M.W.; Seifried, B.; Moquin, P.; Latulippe, D.; Hoare, T. Drug-Impregnated, Pressurized Gas Expanded Liquid-Processed Alginate Hydrogel Scaffolds for Accelerated Burn Wound Healing. Acta Biomater. 2020, 112, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xing, X.; Tan, H.; Jia, Y.; Zhou, T.; Chen, Y.; Ling, Z.; Hu, X. Covalently Antibacterial Alginate-Chitosan Hydrogel Dressing Integrated Gelatin Microspheres Containing Tetracycline Hydrochloride for Wound Healing. Mater. Sci. Eng. C 2017, 70, 287–295. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable Conductive Injectable Hydrogels as Novel Antibacterial, Anti-Oxidant Wound Dressings for Wound Healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration during Wound Healing. Small 2019, 15, 1900046. [Google Scholar] [CrossRef]
- Zheng, K.; Tong, Y.; Zhang, S.; He, R.; Xiao, L.; Iqbal, Z.; Zhang, Y.; Gao, J.; Zhang, L.; Jiang, L. Flexible Bicolorimetric Polyacrylamide/Chitosan Hydrogels for Smart Real-Time Monitoring and Promotion of Wound Healing. Adv. Funct. Mater. 2021, 31, 2102599. [Google Scholar] [CrossRef]
- Güney, K.; Tatar, S.; Özel, B.; Seymen, C.; Elmas, Ç.; Tuncer, S.; Çenetoğlu, S. The Effect of Minocycline on Fat Graft Survival and Apoptotic Pathway. Facial Plast. Surg. 2019, 35, 096–102. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Wang, J.; Zhang, Y.; Dong, M.; Bu, T.; Li, L.; Liu, Y.; Wang, L. Multifunctional Injectable Hydrogel Dressings for Effectively Accelerating Wound Healing: Enhancing Biomineralization Strategy. Adv. Funct. Mater. 2021, 31, 2100093. [Google Scholar] [CrossRef]
- Morello, G.; Polini, A.; Scalera, F.; Rizzo, R.; Gigli, G.; Gervaso, F. Preparation and Characterization of Salt-Mediated Injectable Thermosensitive Chitosan/Pectin Hydrogels for Cell Embedding and Culturing. Polymers 2021, 13, 2674. [Google Scholar] [CrossRef]
- Parolo, M.E.; Avena, M.J.; Pettinari, G.; Zajonkovsky, I.; Valles, J.M.; Baschini, M.T. Antimicrobial Properties of Tetracycline and Minocycline-Montmorillonites. Appl. Clay Sci. 2010, 49, 194–199. [Google Scholar] [CrossRef]
- Zhang, T.; Nong, J.; Alzahrani, N.; Wang, Z.; Oh, S.W.; Meier, T.; Yang, D.G.; Ke, Y.; Zhong, Y.; Fu, J. Self-Assembly of DNA–Minocycline Complexes by Metal Ions with Controlled Drug Release. ACS Appl. Mater. Interfaces 2019, 11, 29512–29521. [Google Scholar] [CrossRef] [PubMed]
- Siboro, S.A.; Anugrah, D.S.; Ramesh, K.; Park, S.-H.; Kim, H.-R.; Lim, K.T. Tunable Porosity of Covalently Crosslinked Alginate-Based Hydrogels and Its Significance in Drug Release Behavior. Carbohydr. Polym. 2021, 260, 117779. [Google Scholar] [CrossRef]
- Dabiri, S.M.H.; Lagazzo, A.; Barberis, F.; Farokhi, M.; Finochio, E.; Pastorino, L. Characterization of Alginate-Brushite in-Situ Hydrogel Composites. Mater. Sci. Eng. C 2016, 67, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Raptopoulos, G.; Choinopoulos, I.; Kontoes-Georgoudakis, F.; Paraskevopoulou, P. Polylactide-Grafted Metal-Alginate Aerogels. Polymers 2022, 14, 1254. [Google Scholar] [CrossRef]
- Patwa, R.; Zandraa, O.; Capáková, Z.; Saha, N.; Sáha, P. Effect of Iron-Oxide Nanoparticles Impregnated Bacterial Cellulose on Overall Properties of Alginate/Casein Hydrogels: Potential Injectable Biomaterial for Wound Healing Applications. Polymers 2020, 12, 2690. [Google Scholar] [CrossRef]
- Abd El-Ghaffar, M.; Hashem, M.; El-Awady, M.; Rabie, A. Ph-Sensitive Sodium Alginate Hydrogels for Riboflavin Controlled Release. Carbohydr. Polym. 2012, 89, 667–675. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, C.; Zhang, Q.; Li, Z.; Ge, S.; Ma, B. Sustained and Microenvironment-Accelerated Release of Minocycline from Alginate Injectable Hydrogel for Bacteria-Infected Wound Healing. Polymers 2022, 14, 1816. https://doi.org/10.3390/polym14091816
Xie C, Zhang Q, Li Z, Ge S, Ma B. Sustained and Microenvironment-Accelerated Release of Minocycline from Alginate Injectable Hydrogel for Bacteria-Infected Wound Healing. Polymers. 2022; 14(9):1816. https://doi.org/10.3390/polym14091816
Chicago/Turabian StyleXie, Chengjia, Qun Zhang, Zhao Li, Shaohua Ge, and Baojin Ma. 2022. "Sustained and Microenvironment-Accelerated Release of Minocycline from Alginate Injectable Hydrogel for Bacteria-Infected Wound Healing" Polymers 14, no. 9: 1816. https://doi.org/10.3390/polym14091816
APA StyleXie, C., Zhang, Q., Li, Z., Ge, S., & Ma, B. (2022). Sustained and Microenvironment-Accelerated Release of Minocycline from Alginate Injectable Hydrogel for Bacteria-Infected Wound Healing. Polymers, 14(9), 1816. https://doi.org/10.3390/polym14091816





