Formulation and Characterization of Carbopol-934 Based Kojic Acid-Loaded Smart Nanocrystals: A Solubility Enhancement Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of KA Nano-Suspension
2.3. Stability Studies of Nano-Suspensions
2.4. Lyophilization of Nano-Suspensions
2.5. Characterization of Nanocrystals
2.6. Particle Size, Zeta Potential and Dispersity Index (DI) of Nanocrystals
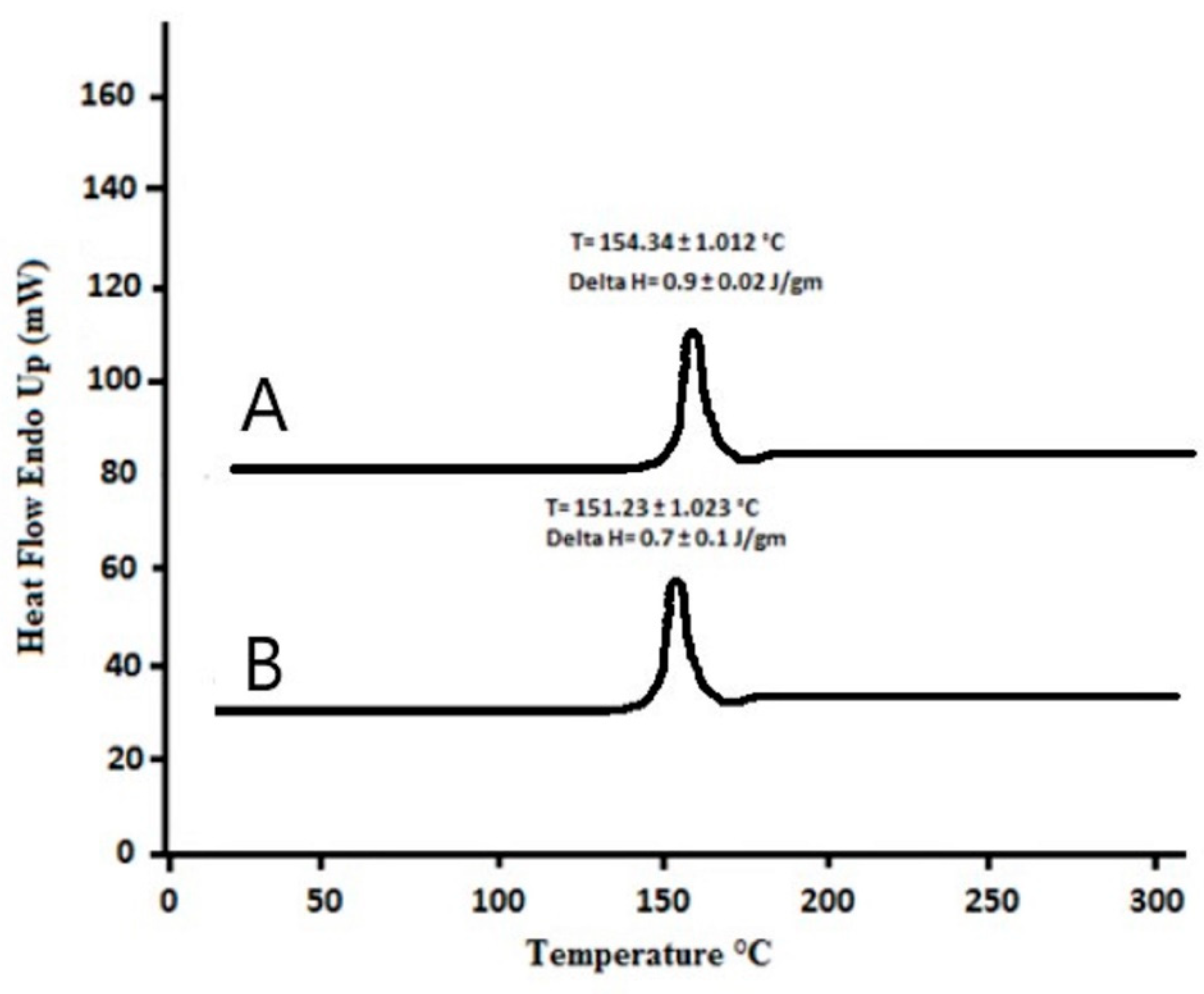
2.7. Differential Scanning Calorimetry Studies
2.8. Scanning Electron Microscopy
2.9. X-ray Diffraction Studies
2.10. Drug Content of Nanocrystals
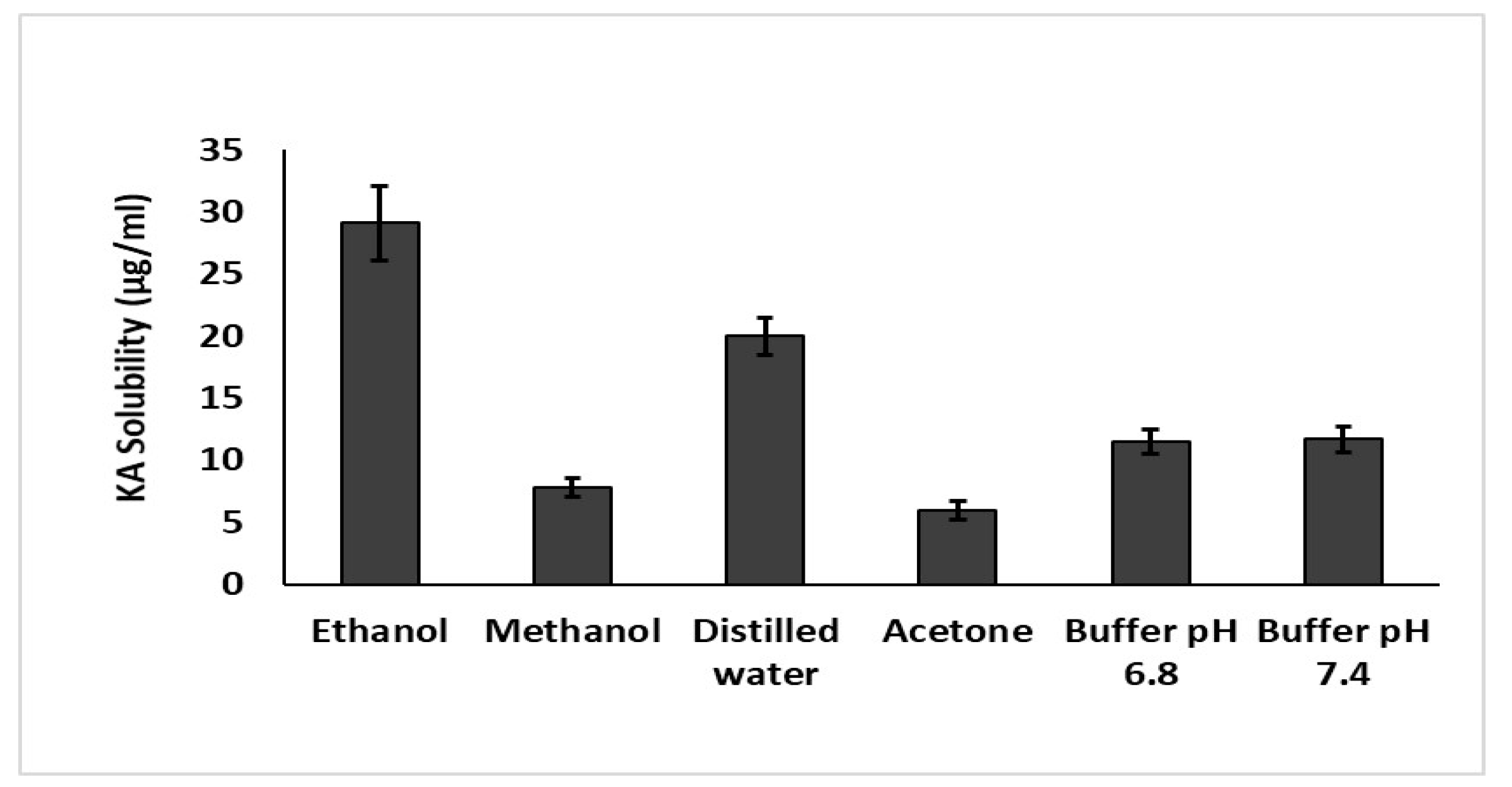
2.11. Solubility Studies
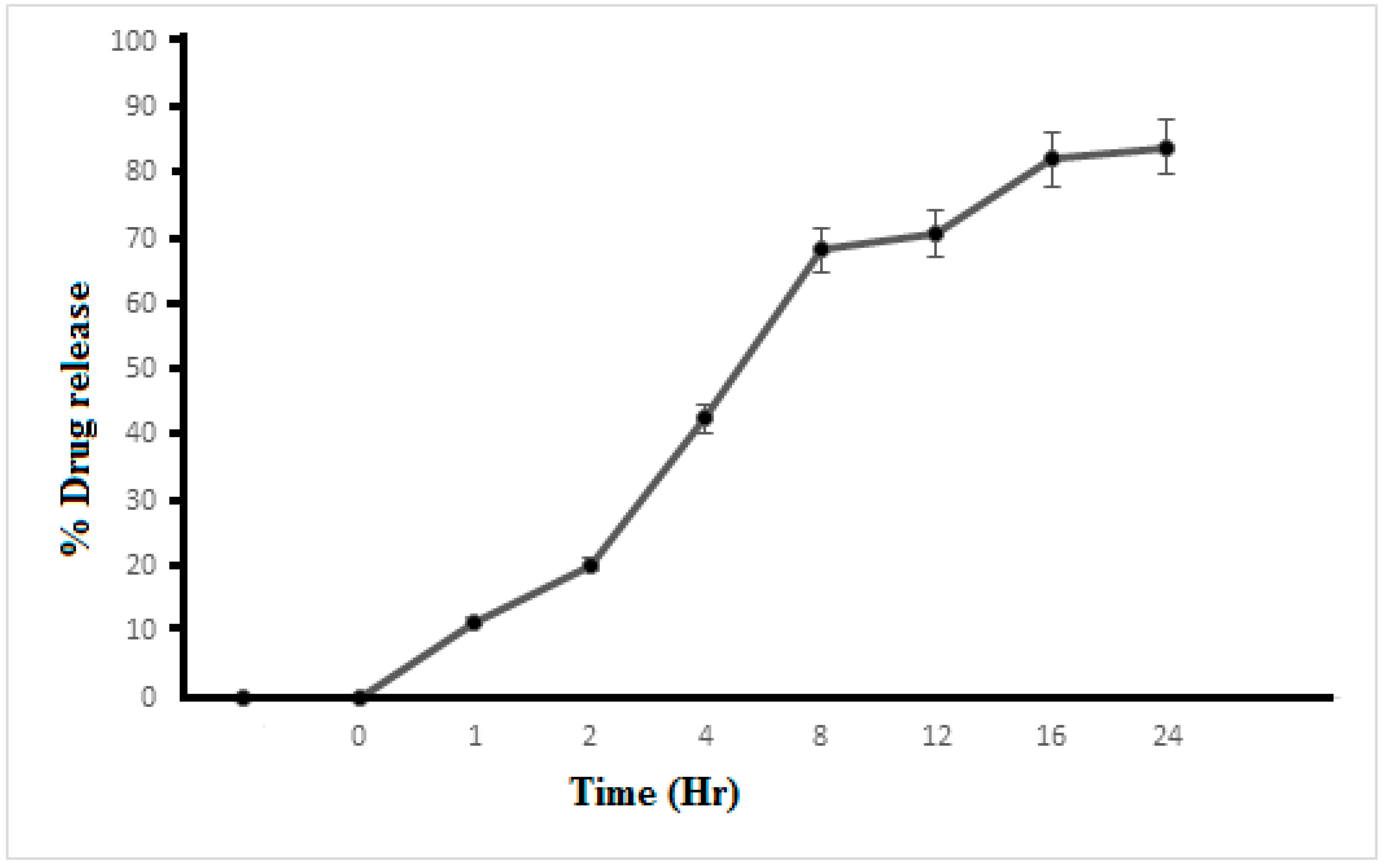
2.12. In Vitro Release Profile
2.13. Vibrational Analysis
2.14. Statistical Analysis
3. Results and Discussion
3.1. Stability Studies
3.2. Particle Size, Zeta Potential and DI of KA Nanocrystals
3.3. Drug Content and Entrapment Efficiency
3.4. Differential Scanning Calorimetry
3.5. Scanning Electron Microscopy
3.6. X-ray Diffraction Studies
3.7. Solubility Studies
3.8. In Vitro Drug Release
3.9. ATR-FTIR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palakkot, A.; Sadeghi, F.; Varshosaz, J.; Garekani, H.A.; Nokhodchi, A. Promising dissolution enhancement effect of soluplus on crystallized celecoxib obtained through antisolvent precipitation and high pressure homogenization techniques. Colloids Surf. B Biointerfaces 2014, 122, 591–600. [Google Scholar]
- Han, F.; Yin, R.; Che, X.; Yuan, J.; Cui, Y.; Yin, H.; Li, S. Nanostructured lipid carriers (NLC) based topical gel of flurbiprofen: Design, characterization and in vivo evaluation. Int. J. Pharm. 2012, 439, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Hanna, P.A.; Ghorab, M.M.; Gad, S. Development of Betamethasone Dipropionate-Loaded Nanostructured Lipid Carriers for Topical and Transdermal Delivery. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2019, 18, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Reddy, V.A.; Arora, S.; Patel, K. Development of surface stabilized candesartan cilexetil nanocrystals with enhanced dissolution rate, permeation rate across CaCo-2, and oral bioavailability. Drug Deliv. Transl. Res. 2016, 6, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Akbari, J.; Saeedi, M.; Farzin, D.; Morteza-Semnani, K.; Esmaili, Z. Transdermal absorption enhancing effect of the essential oil of Rosmarinus officinalis on percutaneous absorption of Na diclofenac from topical gel. Pharm. Biol. 2015, 53, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.I.; Larregieu, C.A.; Benet, L.Z. Classification of natural products as sources of drugs according to the biopharmaceutics drug disposition classification system (BDDCS). Chin. J. Nat. Med. 2016, 14, 888–897. [Google Scholar]
- Jadhav, S.M.; Morey, P.; Karpe, M.; Kadam, V. Novel vesicular system: An overview. J. Appl. Pharm. Sci. 2012, 2, 193–202. [Google Scholar]
- Noh, J.-M.; Kwak, S.-Y.; Seo, H.-S.; Seo, J.-H.; Kim, B.-G.; Lee, Y.-S. Kojic acid-amino acid conjugates as tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 5586–5589. [Google Scholar] [CrossRef] [PubMed]
- Junghanns, J.-U.A.H.; Müller, R.H. Nanocrystal technology, drug delivery and clinical applications. Int. J. Nanomed. 2008, 3, 295–309. [Google Scholar]
- Jones, D.S. FASTtrack Pharmaceutics Dosage Form and Design; Pharmaceutical Press: London, UK, 2016. [Google Scholar]
- Lademann, J.; Richter, H.; Meinke, M.C.; Lange-Asschenfeldt, B.; Antoniou, C.; Mak, W.C.; Renneberg, R.; Sterry, W.; Patzelt, A. Drug delivery with topically applied nanoparticles: Science fiction or reality. Skin Pharmacol. Physiol. 2013, 26, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Goindi, S.; Katare, O.P. Formulation, characterisation and in vivo evaluation of lipid-based nanocarrier for topical delivery of diflunisal. J. Microencapsul. 2016, 33, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Kahn, V.; Lindner, P.; Zakin, Y. Effect of kojic acid on the oxidation of dihydroxyphenols by mushroom tryosinase. J. Food Biochem. 1994, 18, 253–271. [Google Scholar] [CrossRef]
- Walters, K.A.; Roberts, M.S. Dermatologic, Cosmeceutic, and Cosmetic Development: Therapeutic and Novel Approaches; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Lewis, K.M.; Robkin, N.; Gaska, K.; Njoki, L.C. Investigating motivations for women’s skin bleaching in Tanzania. Psychol. Women Q. 2011, 35, 29–37. [Google Scholar] [CrossRef]
- Suresh, S.; Singh, C.; Jena, S.K.; Bagri, S.; Ahuja, B.K. Nanocrystals: A Novel Approach for Drug Delivery. Nanotechnology 2014, 1, 97–104. [Google Scholar]
- Shafie Pour, N.; Saeedi, M.; Morteza Semnani, K.; Akbari, J. Sun protection for children: A review. J. Pediatr. Rev. 2015, 3, e155. [Google Scholar] [CrossRef]
- Sheng, H.; Zhang, Y.; Nai, J.; Wang, S.; Dai, M.; Lin GZhang, Q. Preparation of oridonin nanocrystals and study of their endocytosis and transcytosis behaviours on MDCK polarized epithelial cells. Pharm. Biol. 2020, 58, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Shafiu Kamba, A.; Ismail, M.; Tengku Ibrahim, T.A.; Zakaria, Z.A.B. A pH-sensitive, biobased calcium carbonate aragonite nanocrystal as a novel anticancer delivery system. BioMed Res. Int. 2013, 2013, 587451. [Google Scholar] [CrossRef] [PubMed]
- Shahidan, N.S.; Salim, N.; Ashari, S.E. Preparation and Optimization of Ibuprofen-Loaded Nanoemulsion Formulation. J. Multidiscip. Eng. Sci. Technol. 2019, 6, 89–96. [Google Scholar]
- Jacob, S.; Nair, A.B.; Shah, J. Emerging role of nanosuspensions in drug delivery systems. Biomater. Res. 2020, 24, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takamizawa, K.; Nakashima, S.; Yahashi, Y.; Kubata, K.B.; Suzuki, T.; Kawai, K.; Horitsu, H. Optimization of kojic acid production rate using the Box-Wilson method. J. Ferment. Bioeng. 1996, 82, 414–416. [Google Scholar] [CrossRef]



| Formulation Code | Carbopol-934 (w/w) | Tween-80 (w/w) | Kojic Acid (w/w) | Water (w/w) |
|---|---|---|---|---|
| F1 | 0.1 | 0.5 | 49.4 | |
| F2 | 0.5 | 0.5 | 50 | 49.0 |
| F3 | 1.0 | 0.5 | 50 | 48.5 |
| F4 | 1.5 | 0.5 | 50 | 48.0 |
| F5 | 2.0 | 0.5 | 50 | 47.5 |
| Formulations | Particle Size (nm) | Zeta Potential (mV) | DI |
|---|---|---|---|
| F1 | 136.5 ± 1.8 | ‒12.5 ± 2.3 | 0.44 ± 0.1 |
| F2 | 150 ± 2.8 | ‒15.2 ± 4.1 | 0.36 ± 0.4 |
| F3 | 110 ± 3.2 | ‒20.7 ± 3.5 | 0.41 ± 1.5 |
| Formulation | Kojic Acid Concentration (mg) | Kojic Acid Obtained (mg) | Kojic Acid Content (%) | Percentage Entrapment Efficiency ± SD |
|---|---|---|---|---|
| F2 | 200 | 165 | 82.5 | 82.5 ± 3.4 |
| F3 | 200 | 170 | 85.0 | 85.0 ± 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, B.A.; Waheed, M.; Hosny, K.M.; Rizg, W.Y.; Murshid, S.S.; Alharbi, M.; Khan, M.K. Formulation and Characterization of Carbopol-934 Based Kojic Acid-Loaded Smart Nanocrystals: A Solubility Enhancement Approach. Polymers 2022, 14, 1489. https://doi.org/10.3390/polym14071489
Khan BA, Waheed M, Hosny KM, Rizg WY, Murshid SS, Alharbi M, Khan MK. Formulation and Characterization of Carbopol-934 Based Kojic Acid-Loaded Smart Nanocrystals: A Solubility Enhancement Approach. Polymers. 2022; 14(7):1489. https://doi.org/10.3390/polym14071489
Chicago/Turabian StyleKhan, Barkat Ali, Maryam Waheed, Khaled M. Hosny, Waleed Y. Rizg, Samar S. Murshid, Majed Alharbi, and Muhammad Khalid Khan. 2022. "Formulation and Characterization of Carbopol-934 Based Kojic Acid-Loaded Smart Nanocrystals: A Solubility Enhancement Approach" Polymers 14, no. 7: 1489. https://doi.org/10.3390/polym14071489
APA StyleKhan, B. A., Waheed, M., Hosny, K. M., Rizg, W. Y., Murshid, S. S., Alharbi, M., & Khan, M. K. (2022). Formulation and Characterization of Carbopol-934 Based Kojic Acid-Loaded Smart Nanocrystals: A Solubility Enhancement Approach. Polymers, 14(7), 1489. https://doi.org/10.3390/polym14071489







