Assessment of Physicochemical and In Vivo Biological Properties of Polymeric Nanocapsules Based on Chitosan and Poly(N-vinyl pyrrolidone-alt-itaconic anhydride)
Abstract
1. Introduction
2. Experimental Part (Materials and Methods)
2.1. Materials
2.2. Preparation Method of Nanocapsules
2.3. Characterization Methods
2.3.1. Structural Characterization
2.3.2. Size, Morphology and Zeta Potential of NCs
2.3.3. Thermal Properties
2.3.4. Swelling Behaviour in Aqueous Solutions
2.3.5. Drug Encapsulating Studies
2.3.6. In Vitro Drug Release
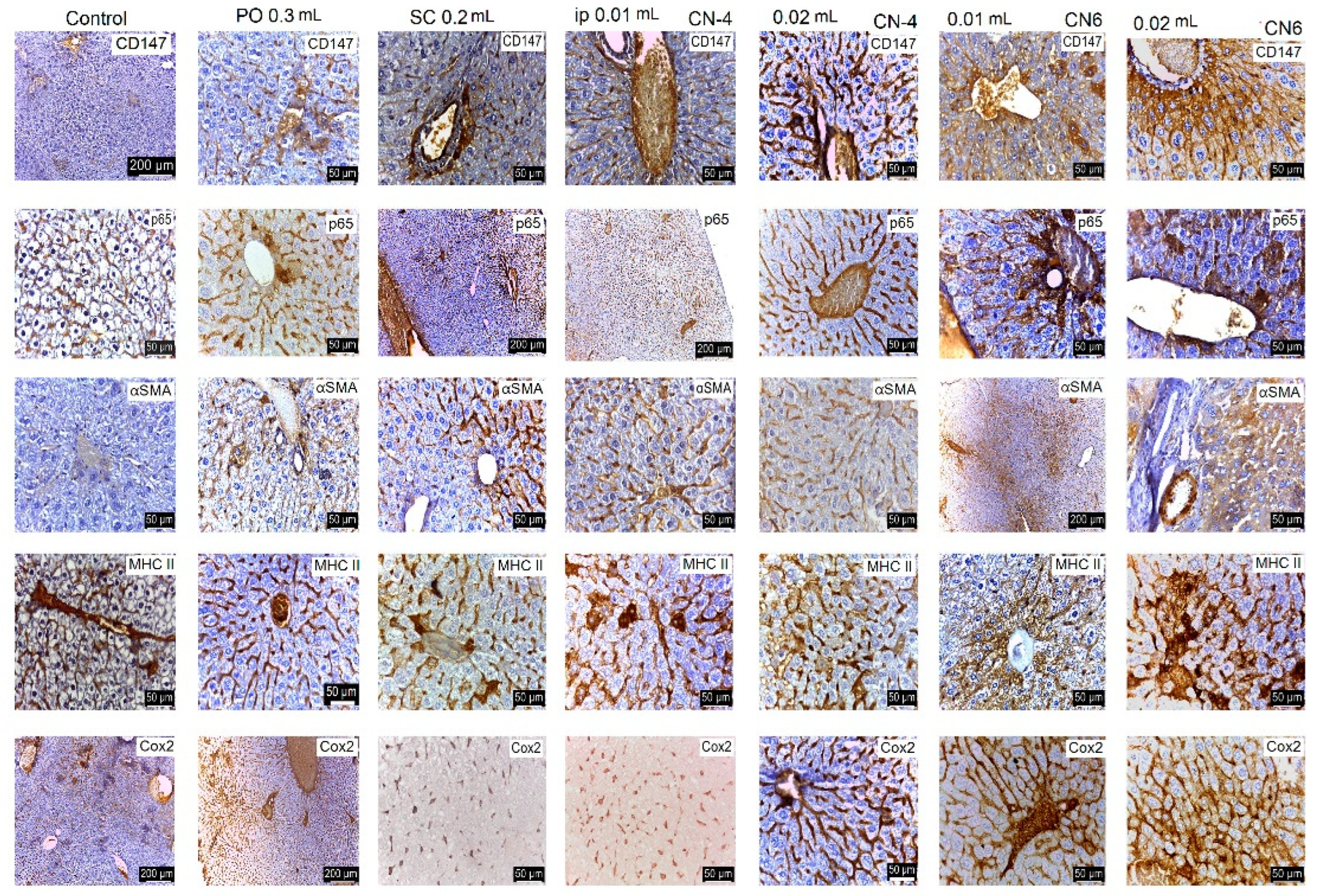
2.3.7. In Vivo Testing
2.3.8. Histological and Immunohistological Analysis
2.4. Statistical Analysis
3. Results and Discussion
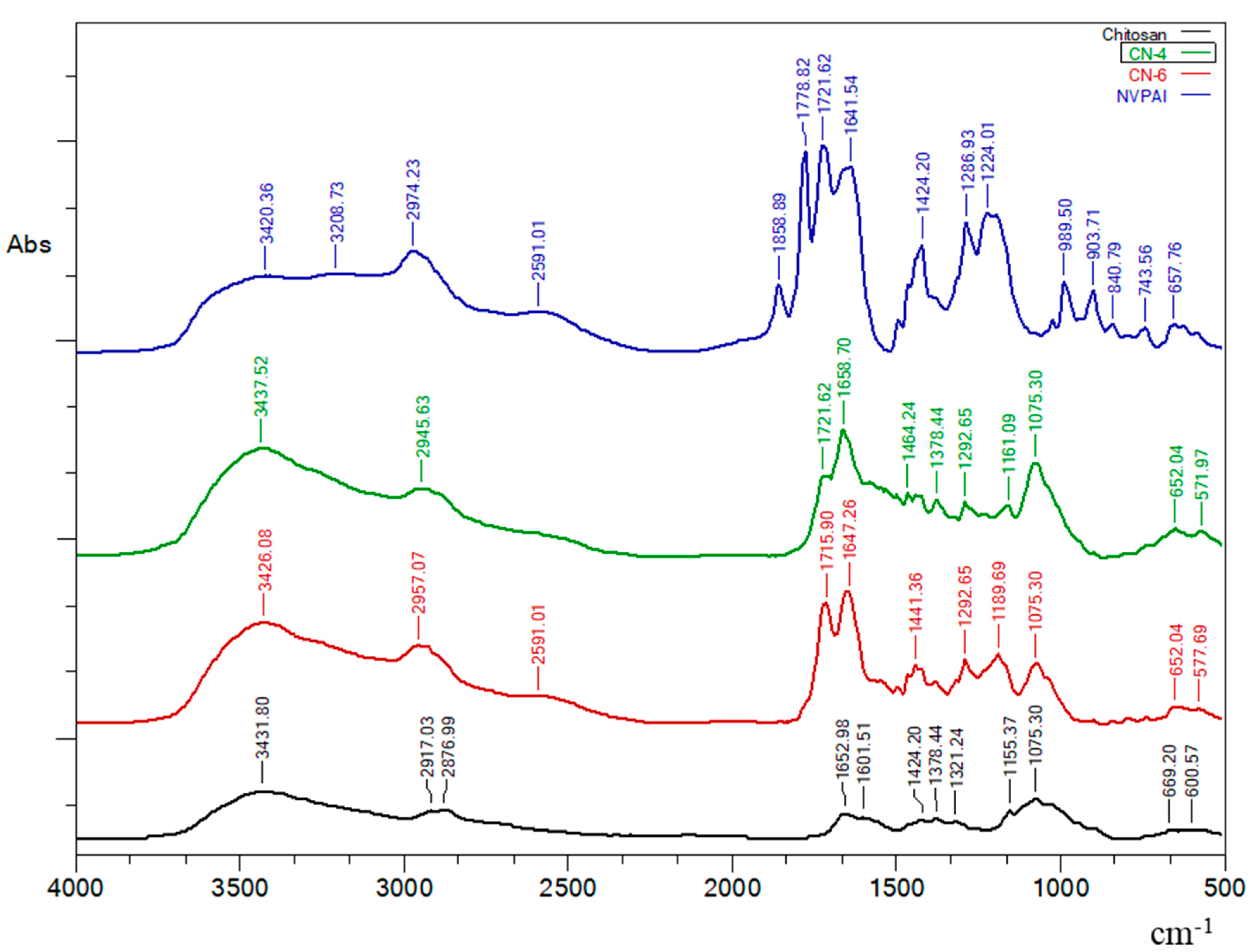
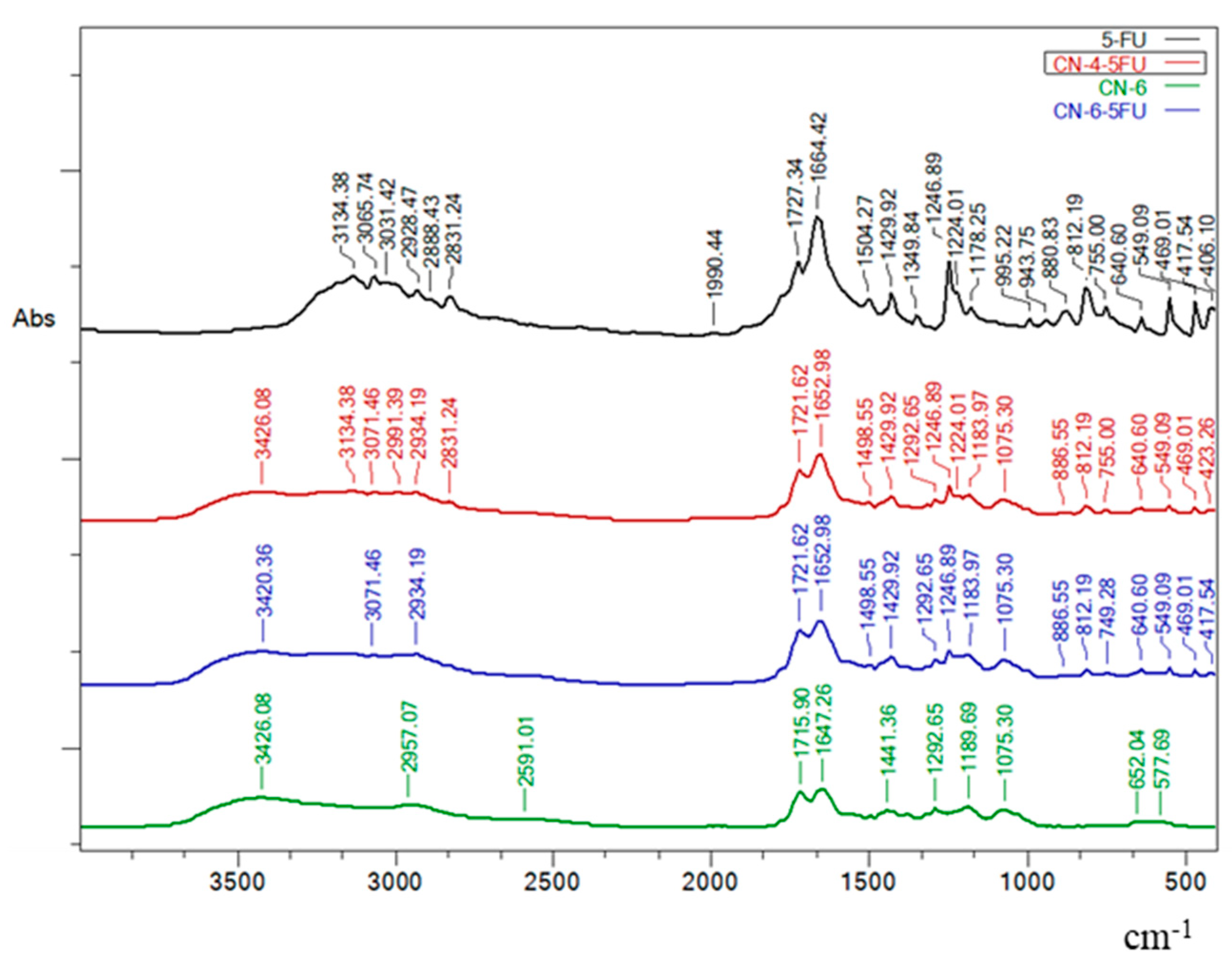
3.1. FTIR Spectroscopy
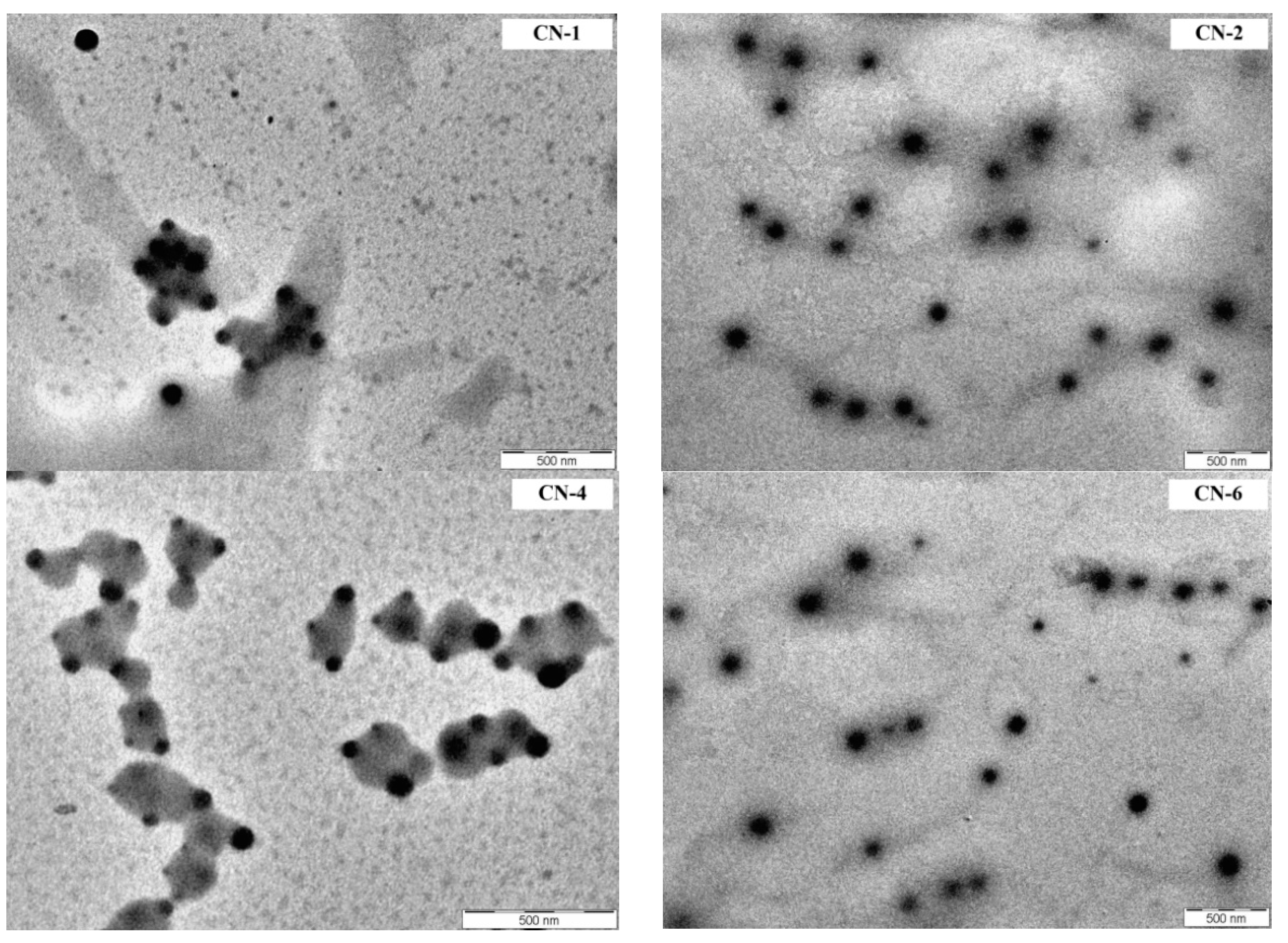
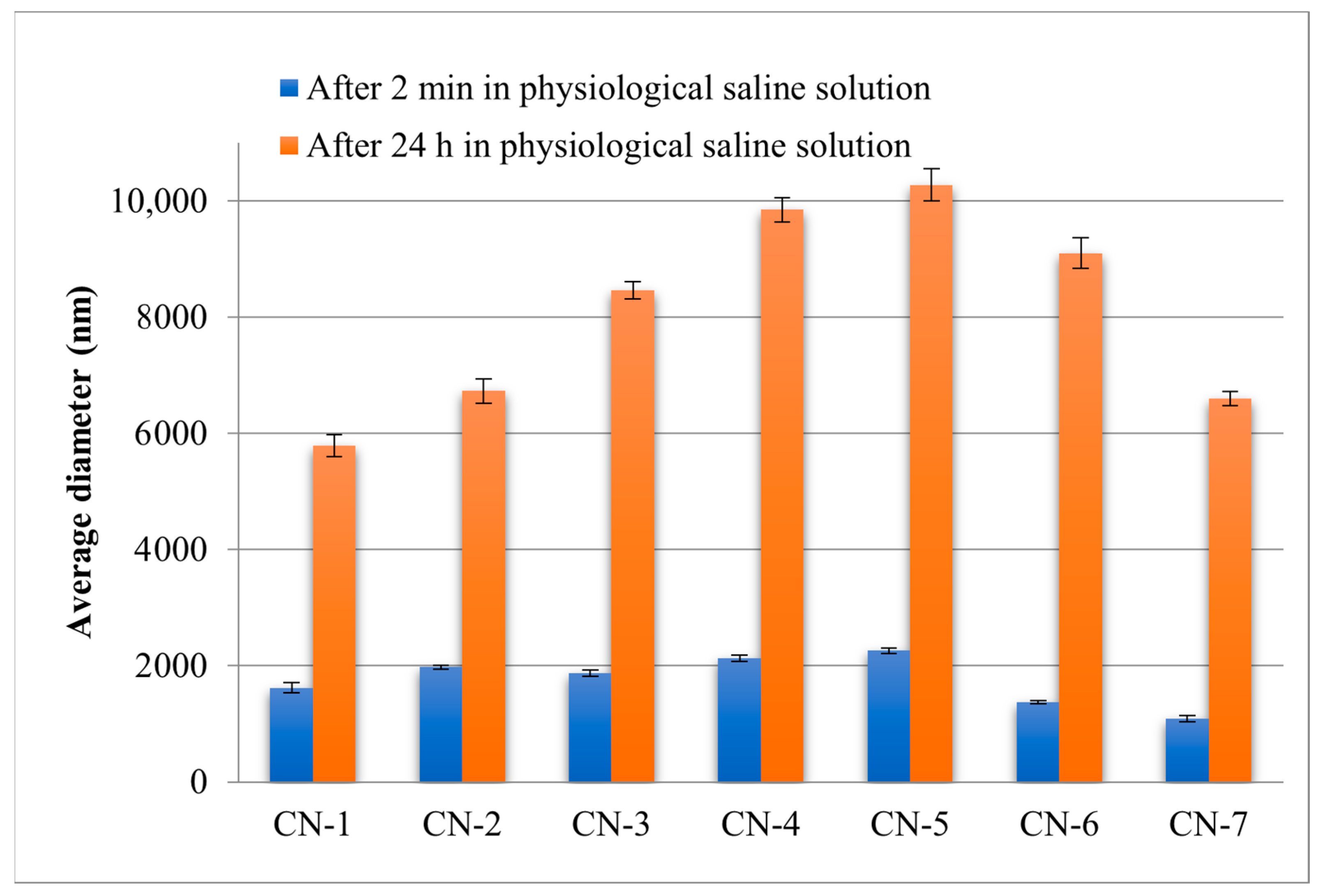
3.2. Size, Morphology and Zeta Potential of NCs
3.3. Thermal Behaviour
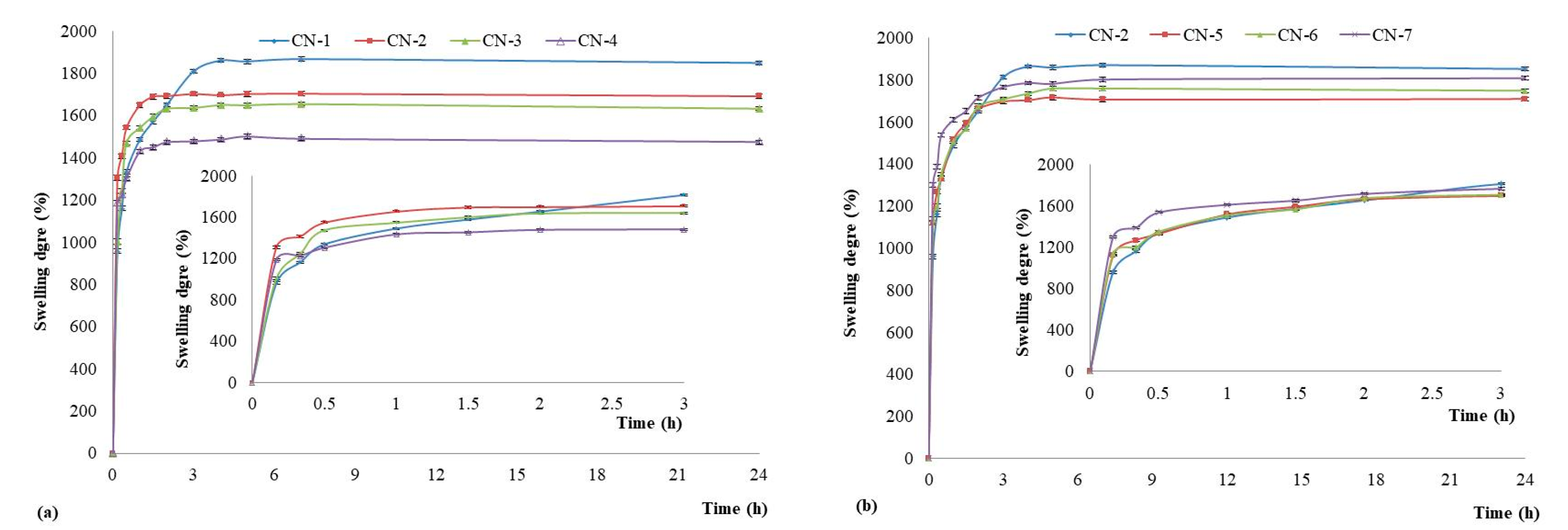
3.4. Swelling Degree of NCs
3.5. Encapsulation Efficiency of a Model Drug
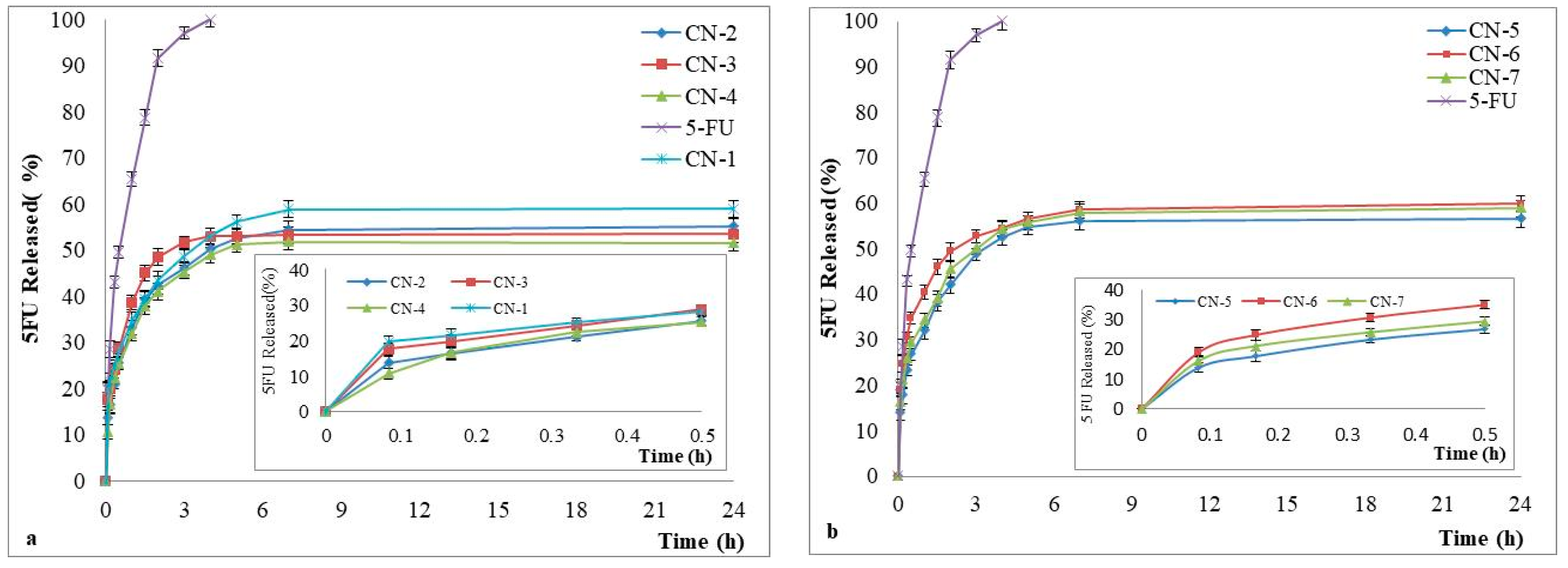
3.6. Drug Release Kinetics
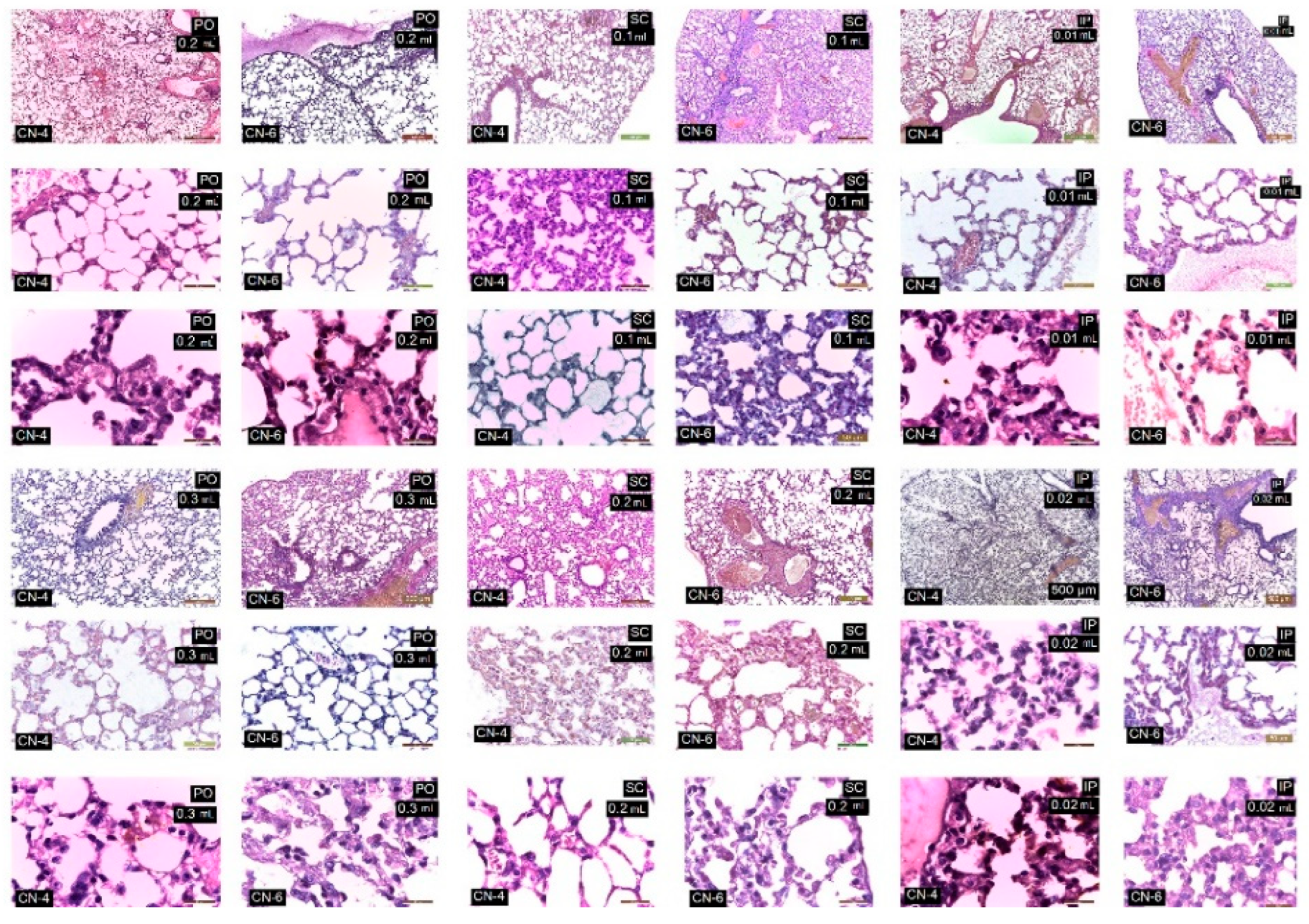
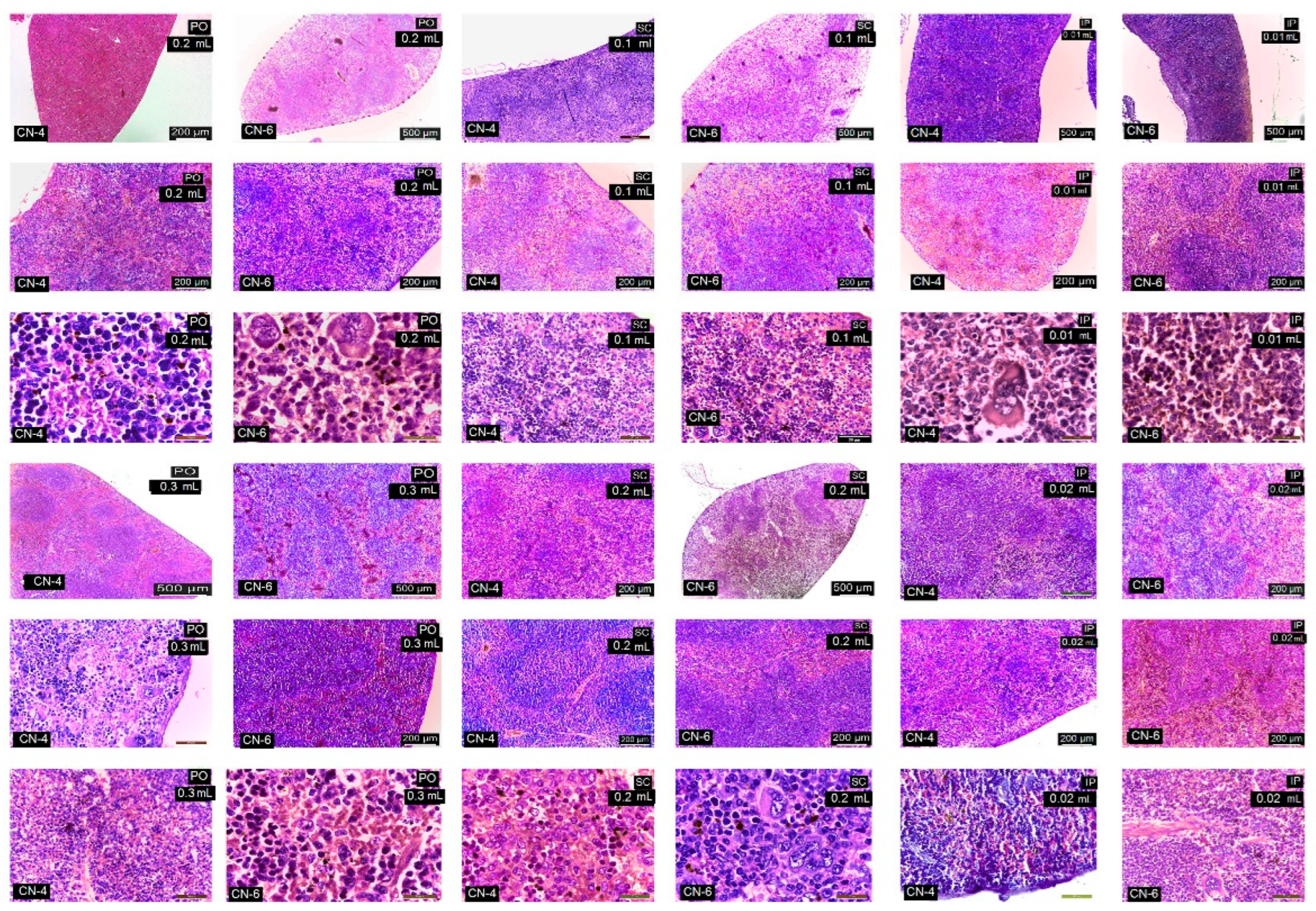
3.7. In Vivo Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Hano, C.; Abbasi, B.H. Plant-based green synthesis of nanoparticles: Production, characterization and applications. Biomolecules 2022, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, Y.; Tang, W.; Zhao, J.; Jing, L.; McHugh, K.J. Theranostic nanoparticles with disease-specific administration strategies. Nano Today 2022, 42, 101335. [Google Scholar] [CrossRef]
- Vasant, V.R.; Mannfred, A.H. Drug Delivery Systems, 2nd ed.; Lewis Publisher: Boca Raton, FL, USA, 2003. [Google Scholar]
- Babu, A.; Templeton, A.K.; Munshi, A.; Ramesh, R. Nanoparticle-based drug delivery for therapy of lung cancer: Progress and challenges. J. Nanomater. 2013, 2013, 863951. [Google Scholar] [CrossRef]
- Daraba, O.M.; Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Vochita, G. Antitumoral drug-loaded biocompatible polymeric nanoparticles obtained by non-aqueous emulsion polymerization. Polymers 2020, 12, 1018. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.E.; Cretan, M.S.; Purcar, V.; Popa, M.; Daraba, O.M.; Atanase, L.I.; Ochiuz, L. Drug delivery system based on pH-sensitive biocompatible poly(2-vinyl pyridine)-b-poly(ethylene oxide) nanomicelles loaded with curcumin and 5-Fluorouracil. Polymers 2020, 12, 1450. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.E.; Atanase, L.I.; Jerome, C.; Sol, V.; Martin, P.; Popa, M.; Ochiuz, L. Polysaccharides-based complex particles’ protective role on the stability and bioactivity of immobilized Curcumin. Int. J. Mol. Sci. 2021, 22, 3075. [Google Scholar] [CrossRef]
- Jesus, S.; Schmutz, M.; Som, C.; Borchard, G.; Wick, P.; Borges, O. Hazard assessment of polymeric nanobiomaterials for drug delivery: What can we learn from literature so far. Front. Bioeng. Biotechnol. 2019, 7, 261. [Google Scholar] [CrossRef]
- Couvreur, P.; Barratt, G.; Fattal, E.; Legrand, P.; Vauthier, C. Nanocapsule technology: A review. Crit. Rev. Ther. Drug Carrier Syst. 2002, 19, 99–134. [Google Scholar] [CrossRef] [PubMed]
- Rata, D.M.; Chailan, J.F.; Peptu, C.A.; Costuleanu, M.; Popa, M. Chitosan:poly(N-vinylpyrrolidone-alt-itaconic anhydride) nanocapsules—A promising alternative for the lung cancer treatment. J. Nanopart. Res. 2015, 17, 316–327. [Google Scholar] [CrossRef]
- Jou, S.; Peters, L.; Mucalo, M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar]
- Raţă, D.M.; Popa, M.; Chailan, J.F.; Zamfir, C.L.; Peptu, C.A. Biomaterial properties evaluation of poly(vinyl acetate-alt-maleic anhydride)/chitosan nanocapsules. J. Nanopart. Res. 2014, 16, 2569. [Google Scholar] [CrossRef]
- Alupei, L.; Lisa, G.; Butnariu, A.; Desbrieres, J.; Cadinoiu, A.N.; Peptu, C.A.; Calin, G.; Popa, M. New Folic Acid-Chitosan Derivative Based Nanoparticles—Potential Applications in Cancer Therapy. Cellulose Chem. Technol. 2017, 51, 631–648. [Google Scholar]
- Iurea, D.M.; Peptu, C.A.; Chailan, J.F.; Carriere, P.; Popa, M. Sub-micronic capsules based on gelatin and poly(maleic anhydride-alt-vinylacetate) obtained by interfacial condensation with potential biomedical applications. J. Nanosci. Nanotechnol. 2013, 13, 3841–3850. [Google Scholar] [CrossRef]
- Dellali, K.Z.; Rata, D.M.; Popa, M.; Djennad, M.; Ouagued, A.; Gherghel, D. Antitumoral drug: Loaded hybrid nanocapsules based on chitosan with potential effects in breast cancer therapy. Int. J. Mol. Sci. 2020, 21, 5659. [Google Scholar] [CrossRef]
- Zhou, C.; Hao, G.; Thomas, P.; Liu, J.; Yu, M.; Sun, S.; Öz, O.K.; Sun, X.; Zheng, J. Near-infrared emitting radioactive gold nanoparticles with molecular pharmacokinetics. Angew. Chem. 2012, 51, 10118–10122. [Google Scholar] [CrossRef]
- Burns, A.A.; Vider, J.; Ow, H.; Herz, E.; Penate-Medina, O.; Baumgart, M.; Larson, S.M.; Wiesner, U.; Bradbury, M. Fluorescent silica nanoparticles with efficient urinary excretion for nanomedicine. Nano Lett. 2009, 9, 442–448. [Google Scholar] [CrossRef]
- Hajam, I.A.; Senevirathne, A.; Hewawaduge, C.; Kim, J.; Lee, J.H. Intranasally administered protein coated chitosan nanoparticles encapsulating influenza H9N2 HA2 and M2e mRNA molecules elicit protective immunity against avian influenza viruses in chickens. Vet. Res. 2020, 51, 37. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Ong, Z.Y.; Xu, K.; Ee, P.L.R.; Zheng, S.; Hedrick, J.L.; Yang, Y.Y. Polymer- and lipidbased nanoparticle therapeutics for the treatment of liver diseases. Nano Today 2010, 5, 296–312. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, M.; He, Z.; Wei, Q.; Li, J. The biological function of kupffer cells in liver disease. In Biology of Myelomonocytic Cells; Ghosh, A., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Dobrovolskaia, M.A.; McNeil, S.E. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007, 2, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Li, Y.; Luo, J.; Lee, J.S.; Xiao, W.; Gonik, A.M.; Agarwal, R.; Lama, K.S. The effect of surface charge on in vivo biodistribution of PEGoligocholic acid based micellar nanoparticles. Biomaterials 2011, 32, 3435–3446. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G. Caveolae meet endosomes: A stable relationship? Dev. Cell. 2004, 7, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Pelkmans, L.; Burli, T.; Zerial, M.; Helenius, A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell 2004, 118, 767–780. [Google Scholar] [CrossRef]
- Gratton, S.E.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef]
- Yan, C.; Gu, J.; Guo, Y.; Chen, D. In vivo biodistribution for tumor targeting of 5-fluorouracil (5-FU) loaded N-succinyl-chitosan (Suc-Chi) nanoparticles. Yakugaku Zasshi 2010, 130, 801–804. [Google Scholar] [CrossRef]
- Zhang, C.; Qu, G.; Sun, Y.; Wu, X.; Yao, Z.; Guo, Q.; Ding, Q.; Yuan, S.; Shen, Z.; Ping, Q.; et al. Pharmacokinetics, biodistribution, efficacy and safety of N-octyl-O-sulfate chitosan micelles loaded with paclitaxel. Biomaterials 2008, 29, 1233–1241. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Sonin, D.; Pochkaeva, E.; Zhuravskii, S.; Postnov, V.; Korolev, D.; Vasina, L.; Kostina, D.; Mukhametdinova, D.; Zelinskaya, I.; Skorik, Y.; et al. Biological Safety and Biodistribution of Chitosan Nanoparticles. Nanomaterials 2020, 10, 810. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, D.H.; Son, J.H.; Park, J.K.; Kim, S.K. Optimization of chitosan-alginate encapsulation process using pig hepatocytes for development of bioartificial liver. J. Microbiol. Biotechnol. 2005, 15, 7–13. [Google Scholar]
- Chu, X.H.; Shi, X.L.; Feng, Z.Q.; Gu, Z.Z.; Ding, Y.T. Chitosan nanofiber scaffold enhances hepatocyte adhesion and function. Biotechnol. Lett. 2009, 31, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Carreno-Gómez, B.; Duncan, R. Valuation of the biological properties of soluble chitosan and chitosan microspheres. Int. J. Pharm. 1997, 148, 231–240. [Google Scholar] [CrossRef]
- Li, X.; Radomski, A.; Corrigan, O.I.; Tajber, L.; De Sousa Menezes, F.; Endter, S.; Medina, C.; Radomski, M.W. Platelet compatibility of PLGA, chitosan and PLGA-chitosan nanoparticles. Nanomedicine 2009, 4, 735–746. [Google Scholar] [CrossRef]
- Nadesh, R.; Narayanan, D.; Sreerekha, P.R.; Vadakumpully, S.; Mony, U.; Koyakkutty, M.; Nair, S.V.; Menon, D. Hematotoxicological analysis of surface-modified and -unmodified chitosan nanoparticles. J. Biomed. Mater. Res. A 2013, 101, 2957–2966. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Zhou, J.; Li, L. An investigation of chitosan and its derivatives on red blood cell agglutination. RSC Adv. 2017, 7, 12247–12254. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Poon, W.; Tavares, A.J.; Mcgilvray, I.D.; Chan, W.C.W. Nanoparticle-liver interactions: Cellular uptake and hepatobiliary elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef]
- Hirano, S.; Seino, H.; Akiyama, Y.; Nonaka, I. Chitosan: A biocompatible material for oral and intravenous administrations. In Progress in Biomedical Polymers; Gebelein, C.G., Dunn, R.L., Eds.; Springer: Boston, MA, USA, 1990; pp. 283–290. [Google Scholar]
- Banerjee, T.; Singh, A.K.; Sharma, R.K.; Maitra, A.N. Labeling efficiency and biodistribution of Technetium-99m labeled nanoparticles: Interference by colloidal tin oxide particles. Int. J. Pharm. 2005, 289, 189–195. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jung, E.; Park, J.H.; Park, J.W.; Shim, C.K.; Kim, D.D.; Yoon, I.S.; Cho, H.J. Transient aggregation of chitosan-modified poly(D,L-lactic-co-glycolic) acid nanoparticles in the blood stream and improved lung targeting efficiency. J. Colloid. Interface Sci. 2016, 480, 102–108. [Google Scholar] [CrossRef]
- Walkey, C.D.; Olsen, J.B.; Guo, H.; Emili, A.; Chan, W.C. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 2012, 134, 2139–2147. [Google Scholar] [CrossRef] [PubMed]
- Sadauskas, E.; Danscher, G.; Stoltenberg, M.; Vogel, U.; Larsen, A.; Wallin, H. Protracted elimination of gold nanoparticles from mouse liver. Nanomedicine 2009, 5, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Lacava, L.M.; Garcia, V.A.P.; Kückelhaus, S.; Azevedo, R.B.; Sadeghiani, N.; Buske, N.; Morais, P.C.; Lacava, Z.G.M. Long-term retention of dextran-coated magnetite nanoparticles in the liver and spleen. J. Magn. Magn. Mater. 2004, 272–276, 2434–2435. [Google Scholar] [CrossRef]
- Levy, M.; Luciani, N.; Alloyeau, D.; Elgrabli, D.; Deveaux, V.; Pechoux, C.; Chat, S.; Wang, G.; Vats, N.; Gendron, F.; et al. Long term in vivo biotransformation of iron oxide nanoparticles. Biomaterials 2011, 32, 3988–3999. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, M.S.; Lee, D.; Kwon, T.K.; Khang, D.; Yun, H.S.; Kim, S.H. The comparative immunotoxicity of mesoporous silica nanoparticles and colloidal silica nanoparticles in mice. Int. J. Nanomed. 2013, 8, 147–158. [Google Scholar]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef]
- Muramatsu, T. Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. J. Biochem. 2016, 159, 481–490. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Stefan, J.; Lorkowska-Zawicka, B.; Kaminski, K.; Szczubialka, K.; Nowakowska, M.; Korbut, R. The current view on biological potency of cationically modified chitosan. J. Physiol. Pharmacol. 2014, 65, 341–347. [Google Scholar]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Dorrington, M.G.; Fraser, I.D.C. NF-κB signaling in macrophages: Dynamics, crosstalk, and signal integration. Front. Immunol. 2019, 10, 705. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Sig. Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of human macrophage polarization in inflammation during infectious diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Orekhova, V.A.; Nikiforov, N.G.; Myasoedova, V.A.; Grechko, A.V.; Romanenko, E.B.; Zhang, D.; Chistiakov, D.A. Monocyte differentiation and macrophage polarization. Vessel Plus 2019, 3, 10. [Google Scholar] [CrossRef]
- Wosen, J.E.; Mukhopadhyay, D.; Macaubas, C.; Mellins, E.D. Epithelial MHC class II expression and its role in antigen presentation in the gastrointestinal and respiratory tracts. Front. Immunol. 2018, 25, 2144. [Google Scholar] [CrossRef] [PubMed]
- Barbatis, C.; Kelly, P.; Greveson, J.; Heryet, A.; McGee, J.O. Immunocytochemical analysis of HLA class II (DR) antigens in liver disease in man. J. Clin. Pathol. 1987, 40, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Heymann, F.; Peusquens, J.; Ludwig-Portugall, I.; Kohlhepp, M.; Ergen, C.; Niemietz, P.; Martin, C.; van Rooijen, N.; Ochando, J.C.; Randolph, G.J.; et al. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology 2015, 62, 279–291. [Google Scholar] [CrossRef]
- Bartneck, M.; Warzecha, K.T.; Tacke, F. Therapeutic targeting of liver inflammation and fibrosis by nanomedicine. Hepatobiliary Surg. Nutr. 2014, 3, 364–376. [Google Scholar]
- Franco-Molina, M.A.; Coronado-Cerda, E.E.; López-Pacheco, E.; Zarate-Triviño, D.G.; Galindo-Rodríguez, S.A.; Salazar-Rodríguez, M.C.; Ramos-Zayas, Y.; Tamez-Guerra, R.; Rodríguez-Padilla, C. Chitosan nanoparticles plus KLH adjuvant as an alternative for human dendritic cell differentiation. Curr. Nanosci. 2019, 15, 532–540. [Google Scholar] [CrossRef]
- Harris, R.C. An update on cyclooxygenase-2 expression and metabolites in the kidney. Curr. Opin. Nephrol. Hypertens. 2008, 17, 64–69. [Google Scholar] [CrossRef]
- Shinohara, T.; Pantuso, T.; Shinohara, S.; Kogiso, M.; Myrvik, Q.N.; Henriksen, R.A.; Shibata, Y. Persistent inactivation of macrophage cyclooxygenase-2 in mycobacterial pulmonary inflammation. Am. J. Respir. Cell Mol. Biol. 2008, 41, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Nag, M.; Lahiri, D.; Mukherjee, D.; Banerjee, R.; Garai, S.; Sarkar, T.; Ghosh, S.; Dey, A.; Ghosh, S.; Pattnaik, S.; et al. Functionalized chitosan nanomaterials: A jammer for quorum sensing. Polymers 2021, 13, 2533. [Google Scholar] [CrossRef] [PubMed]
- Bannunah, M.; Vllasaliu, D.; Lord, J.; Stolnik, S. Mechanisms of nanoparticle internalization and transport across an intestinal epithelial cell model: Effect of size and surface charge. Mol. Pharm. 2014, 11, 4363–4373. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.; Park, J.H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9, 51–63. [Google Scholar]
- Saraf, S.; Jain, S.; Sahoo, R.N.; Mallick, S. Present scenario of m-cell targeting ligands for oral mucosal immunization. Curr. Drug Targets 2020, 21, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Nawroth, I.; Alsner, J.; Deleuran, B.W.; Dagnaes-Hansen, F.; Yang, C.; Horsman, M.R.; Overgaard, J.; Howard, K.A.; Kjems, J.; Gao, S. Peritoneal macrophages mediated delivery of chitosan/siRNA nanoparticle to the lesion site in a murine radiation-induced fibrosis model. Acta Oncol. 2013, 52, 1730–1738. [Google Scholar] [CrossRef]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanoparticles. Small 2004, 4, 26–49. [Google Scholar] [CrossRef]
- Hussein, H.; Kishen, A. Engineered chitosan-based nanoparticles modulate macrophage–periodontal ligament fibroblast interactions in biofilm-mediated inflammation. J. Endod. 2021, 47, 1435–1444. [Google Scholar] [CrossRef]
- Yan, C.; Chen, D.; Gu, J.; Hu, H.; Zhao, X.; Qiao, M. Preparation of N-succinyl-chitosan and its physical-chemical properties as a novel excipient. Yakugaku Zasshi 2006, 126, 789–793. [Google Scholar] [CrossRef]
- Kato, Y.; Onishi, H.; Machida, Y. Biological fate of highly-succinylated N-succinyl-chitosan and antitumor characteristics of its water-soluble conjugate with mitomycin C at i.v. and i.p. administration into tumor-bearing mice. Biol. Pharm. Bull. 2000, 23, 1497–1503. [Google Scholar] [CrossRef][Green Version]
- Wilson, B.; Samanta, M.K.; Muthu, M.S.; Vinothapooshan, G. Design and evaluation of chitosan nanoparticles as novel drug carrier for the delivery of rivastigmine to treat. Ther. Deliv. 2011, 2, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, F.; Guo, J.; Xue, J.; Qian, Z.; Gu, Y. Folate-modified chitosan micelles with enhanced tumor targeting evaluated by near infrared imaging system. Carbohydr. Polym. 2011, 86, 1118–1129. [Google Scholar] [CrossRef]









| Sample Code | CS/Poly(NVPAI) (mol/mol) | % CS (w/v) | Aqueous Phase/Organic Phase Ratio (v/v) |
|---|---|---|---|
| CN-1 | 0.2/1 | 0.50 | 1:2.0 |
| CN-2 | 0.3/1 | 0.75 | |
| CN-3 | 0.4/1 | 1.00 | |
| CN-4 | 0.5/1 | 1.25 | |
| CN-5 | 0.3/1 | 0.75 | 1:2.5 |
| CN-6 | 1:3.0 | ||
| CN-7 | 1:3.5 |
| Sample Code | CN-1 | CN-2 | CN-3 | CN-4 | CN-5 | CN-6 | CN-7 |
|---|---|---|---|---|---|---|---|
| Yield (%) | 28 | 39 | 45 | 49 | 62 | 77 | 83 |
| Samples Code | Dv (nm) in Acetone | PDI | Dv (nm) in Physiological Saline Solution | PDI | ZP (mV) in PBS (pH = 7.4) |
|---|---|---|---|---|---|
| CN-1 | 107 ± 0.11 | 0.96 ± 0.04 | 1621 ± 84.42 | 0.43 ± 0.12 | −19.8 ± 0.01 |
| CN-2 | 188 ± 0.23 | 0.73 ± 0.02 | 1976 ± 34.51 | 0.42 ± 0.09 | −18.4 ± 0.04 |
| CN-3 | 192 ± 0.51 | 0.83 ± 0.04 | 1869 ± 57.84 | 0.42 ± 0.04 | −18.0 ± 0.05 |
| CN-4 | 220 ± 0.53 | 0.64 ± 0.03 | 2131 ± 51.61 | 0.41 ± 0.09 | −16.8 ± 0.01 |
| CN-5 | 250 ± 0.30 | 0.94 ± 0.03 | 2256 ± 43.82 | 0.31 ± 0.09 | −18.4 ± 0.04 |
| CN-6 | 150 ± 0.22 | 0.68 ± 0.06 | 1371 ± 30.91 | 0.23 ± 0.02 | −8.5 ± 0.02 |
| CN-7 | 114 ± 0.21 | 0.94 ± 0.04 | 1089 ± 53.84 | 0.46 ± 0.03 | −11.06 ± 0.07 |
| CN-1-5FU | 169 ± 0.73 | 1.22 ± 0.07 | 2263 ± 157.62 | 1.30 ± 0.24 | −20.07 ± 0.24 |
| CN-2-5FU | 226 ± 0.63 | 1.02 ± 0.10 | 2156 ± 203.41 | 1.27 ± 0.39 | −20.57 ± 0.38 |
| CN-3-5FU | 254 ± 0.42 | 1.04 ± 0.04 | 2396 ± 107.04 | 1.07 ± 0.68 | −21.1 ± 0.32 |
| CN-4-5FU | 240 ± 0.87 | 0.92 ± 0.08 | 2421 ± 124.95 | 1.12 ± 0.60 | −21.52 ± 0.17 |
| CN-5-5FU | 297 ± 0.43 | 1.00 ± 0.12 | 2947 ± 191.39 | 1.10 ± 0.35 | −22.22 ± 0.04 |
| CN-6-5FU | 227 ± 0.44 | 0.89 ± 0.05 | 1887 ± 54.22 | 0.54 ± 0.23 | −23.21 ± 0.34 |
| CN-7-5FU | 181 ± 0.69 | 0.93 ± 0.24 | 1594 ± 79.73 | 0.81 ± 0.16 | −22.8 ± 0.18 |
| Sample Code | Encapsulated Drug g/g NCs | The Encapsulation Efficiency (%) |
|---|---|---|
| CN-1 | 0.317 ± 0.001 | 42.3 ± 0.173 |
| CN-2 | 0.244 ± 0.003 | 32.6 ± 0.333 |
| CN-3 | 0.219 ± 0.001 | 29.3 ± 0.120 |
| CN-4 | 0.198 ± 0.002 | 26.5 ± 0.289 |
| CN-5 | 0.249 ± 0.001 | 33.2 ± 0.120 |
| CN-6 | 0.258 ± 0.006 | 34.5 ± 0.866 |
| CN-7 | 0.266 ± 0.004 | 36.0 ± 0.577 |
| Organ/Lesions | Control | PO 0.3 mL | SC 0.2 mL | Ip 0.01 mL CN-4 | Ip 0.02 mL CN-4 | Ip 0.01 mL CN-6 | Ip 0.02 mL CN-6 |
|---|---|---|---|---|---|---|---|
| Liver | |||||||
| -cords are radially arranged from the centrilobular venule to the periphery of the lobule | + | + | + | + | + | + | + |
| No of Kupffer cells/12,879.8 mm2 | 3.5 | 6–7 | 6–7.5 | 7–8 | 8–9.3 | 9–10.5 | 9–11 |
| Vascular congestion | − | − | − | − | ++ | ++ | ++ |
| Kidney | |||||||
| Malpighian corpuscles and urinary tubules show changes | − | 1 | 1 | 1 | 1 | 3 | 3 |
| No of. Interstitial macrophages/12,879.8 mm2 | − | 2–4 | 2–5 | 4–6 | 4–6.6 | 5–6 | 5–7.5 |
| Increased in volume of nephrocytes of the proximal convoluted tubules | − | − | − | + | + | ++ | ++ |
| Vascular congestion | − | − | − | + | + | + | ++ |
| Lungs | |||||||
| thin-walled alveoli | + | + | + | + | + | + | + |
| No of alveolar macrophages/12,879.8 mm2 | 3–4 | 14–22 | 14–24 | 9–11 | 9–13 | 9.5–17 | 9–18.5 |
| Vascular congestion | − | − | − | + | + | ++ | ++ |
| Spleen | |||||||
| Lymphoid follicles and lymphatic cords | − | − | ++ | ++ | ++ | ++ | ++ |
| Vascular congestion | − | − | + | + | + | ++ | ++ |
| Megakaryocytes and pigment cells | − | − | + | + | + | ++ | ++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dellali, K.Z.; Dellali, M.; Raţă, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Popa, M.; Spataru, M.-C.; Solcan, C. Assessment of Physicochemical and In Vivo Biological Properties of Polymeric Nanocapsules Based on Chitosan and Poly(N-vinyl pyrrolidone-alt-itaconic anhydride). Polymers 2022, 14, 1811. https://doi.org/10.3390/polym14091811
Dellali KZ, Dellali M, Raţă DM, Cadinoiu AN, Atanase LI, Popa M, Spataru M-C, Solcan C. Assessment of Physicochemical and In Vivo Biological Properties of Polymeric Nanocapsules Based on Chitosan and Poly(N-vinyl pyrrolidone-alt-itaconic anhydride). Polymers. 2022; 14(9):1811. https://doi.org/10.3390/polym14091811
Chicago/Turabian StyleDellali, Kheira Zanoune, Mohammed Dellali, Delia Mihaela Raţă, Anca Niculina Cadinoiu, Leonard Ionut Atanase, Marcel Popa, Mihaela-Claudia Spataru, and Carmen Solcan. 2022. "Assessment of Physicochemical and In Vivo Biological Properties of Polymeric Nanocapsules Based on Chitosan and Poly(N-vinyl pyrrolidone-alt-itaconic anhydride)" Polymers 14, no. 9: 1811. https://doi.org/10.3390/polym14091811
APA StyleDellali, K. Z., Dellali, M., Raţă, D. M., Cadinoiu, A. N., Atanase, L. I., Popa, M., Spataru, M.-C., & Solcan, C. (2022). Assessment of Physicochemical and In Vivo Biological Properties of Polymeric Nanocapsules Based on Chitosan and Poly(N-vinyl pyrrolidone-alt-itaconic anhydride). Polymers, 14(9), 1811. https://doi.org/10.3390/polym14091811









