Synthesis and In Vitro Characterization of Ascorbyl Palmitate-Loaded Solid Lipid Nanoparticles
Abstract
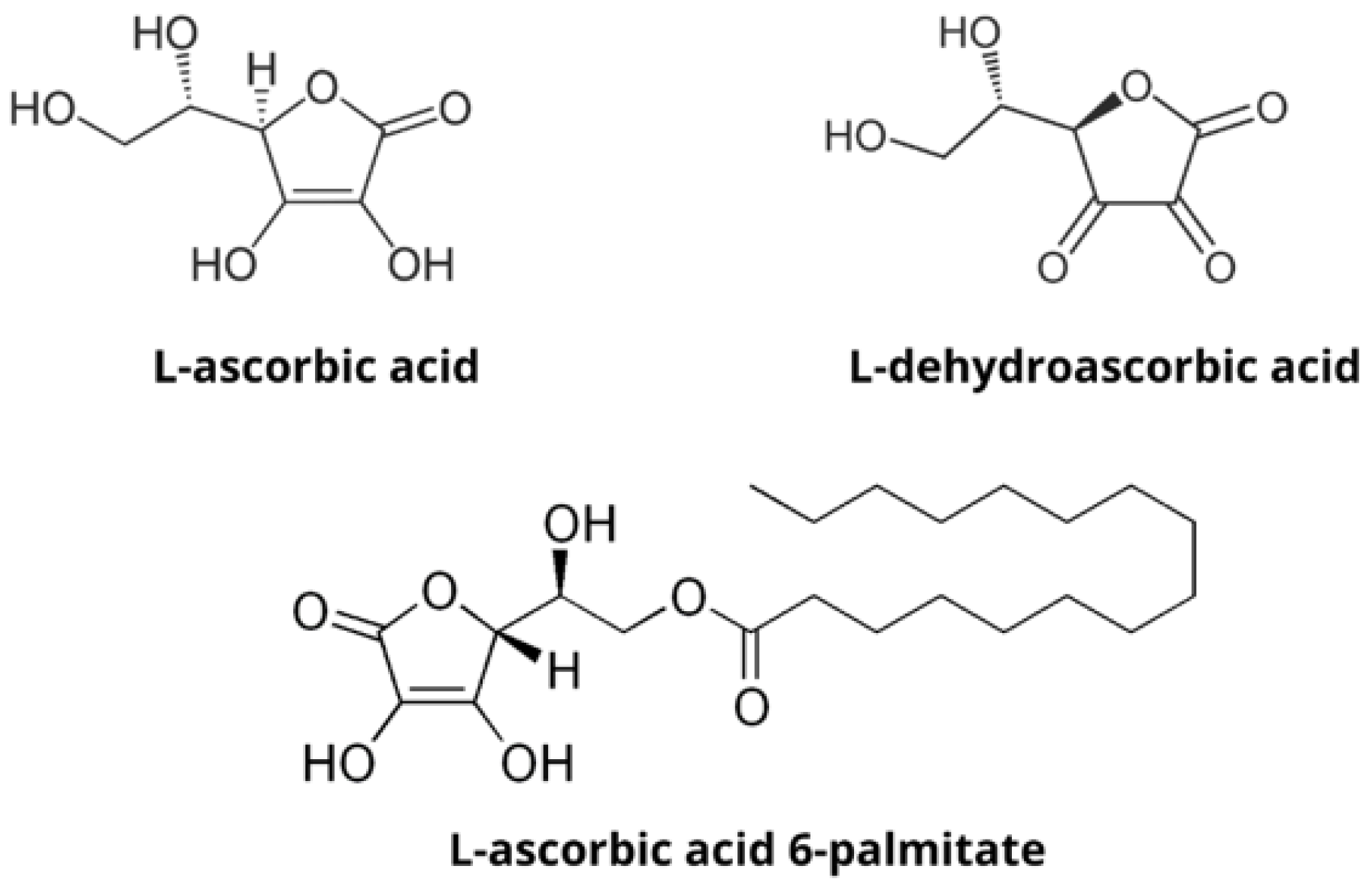
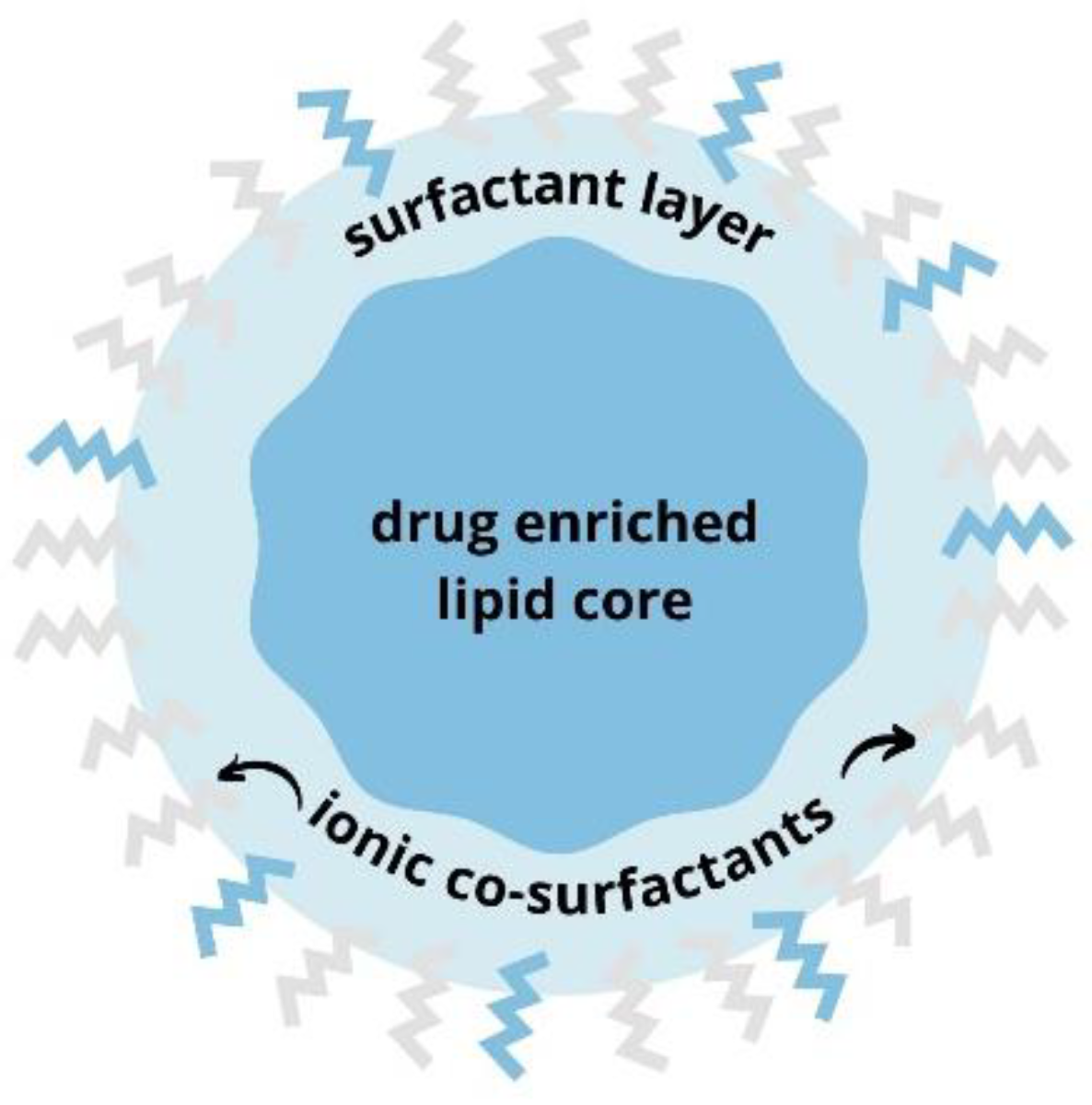
:1. Introduction
2. Materials and Methods
2.1. Materials
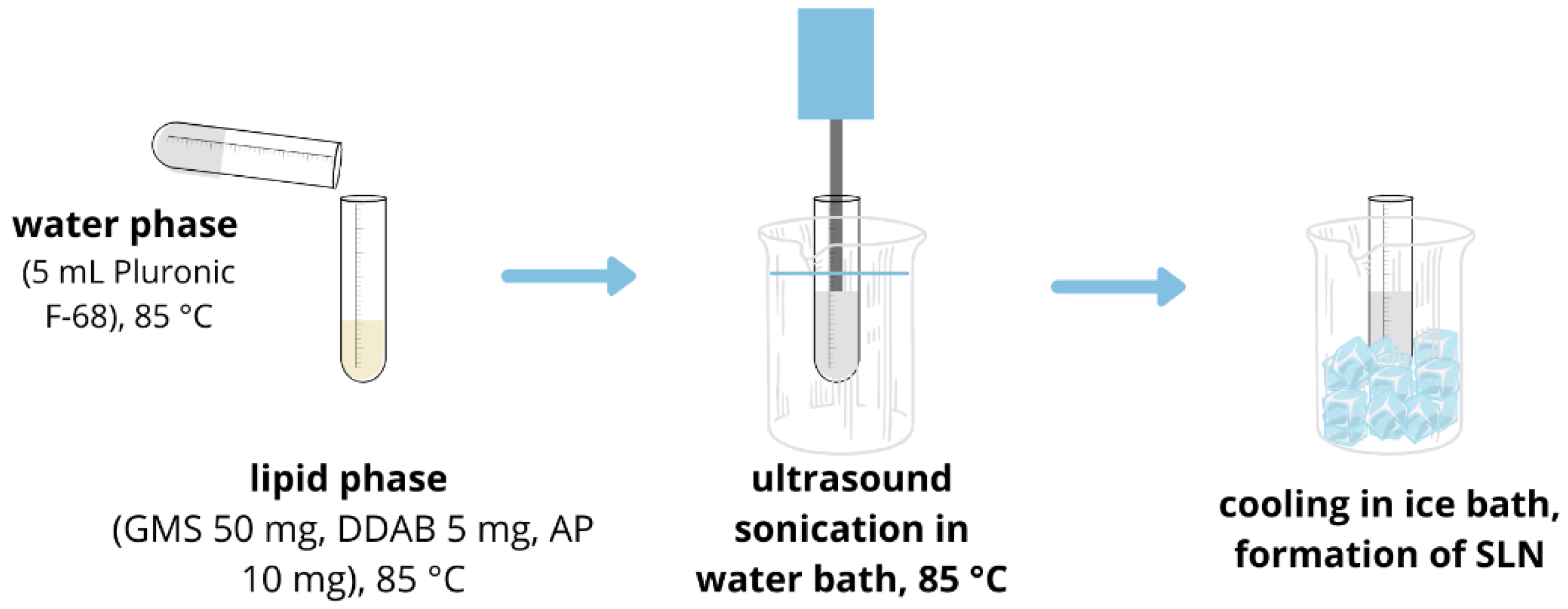
2.2. SLN Synthesis and Characterization
2.3. Cell Culture
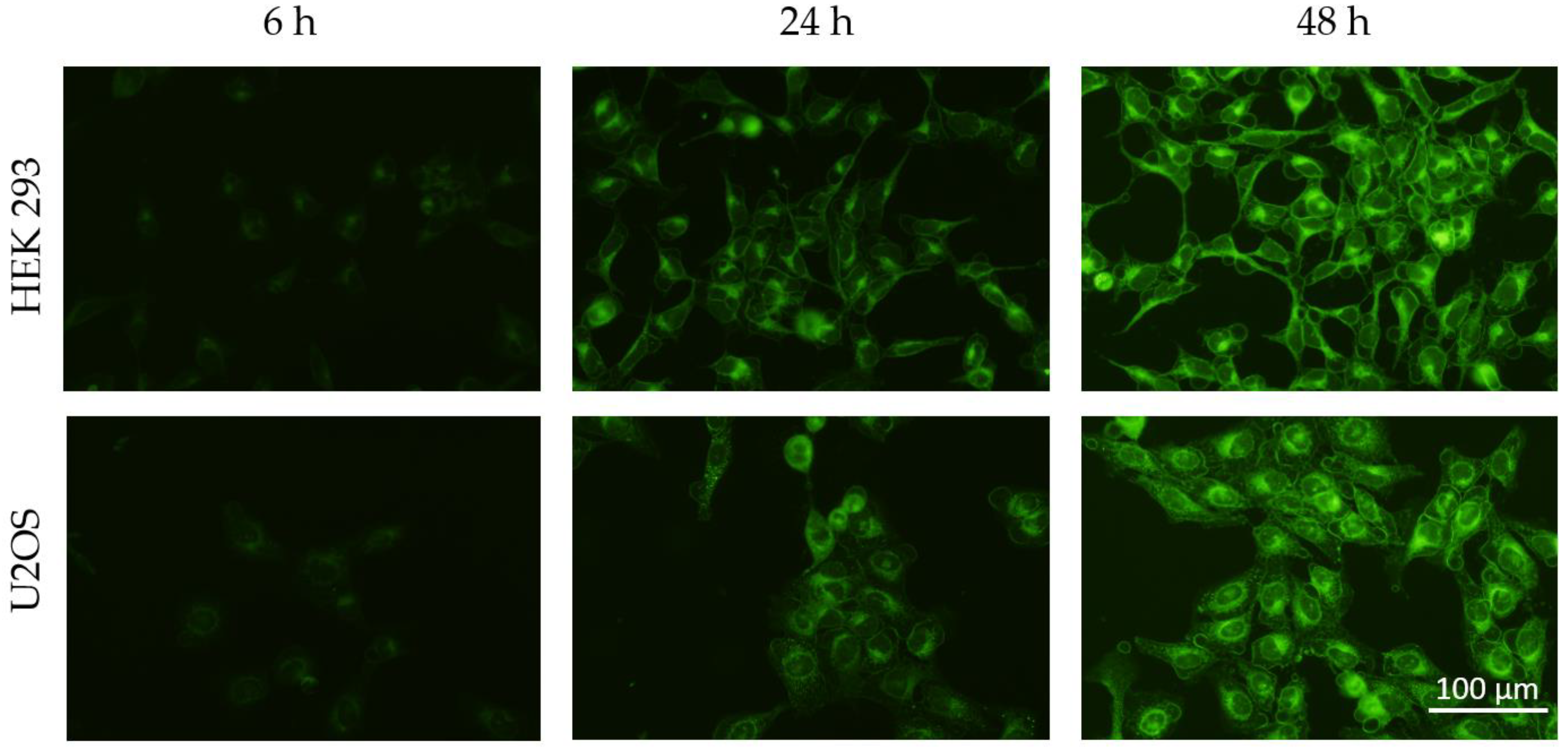
2.4. Cellular Uptake of Solid Lipid Nanoparticles
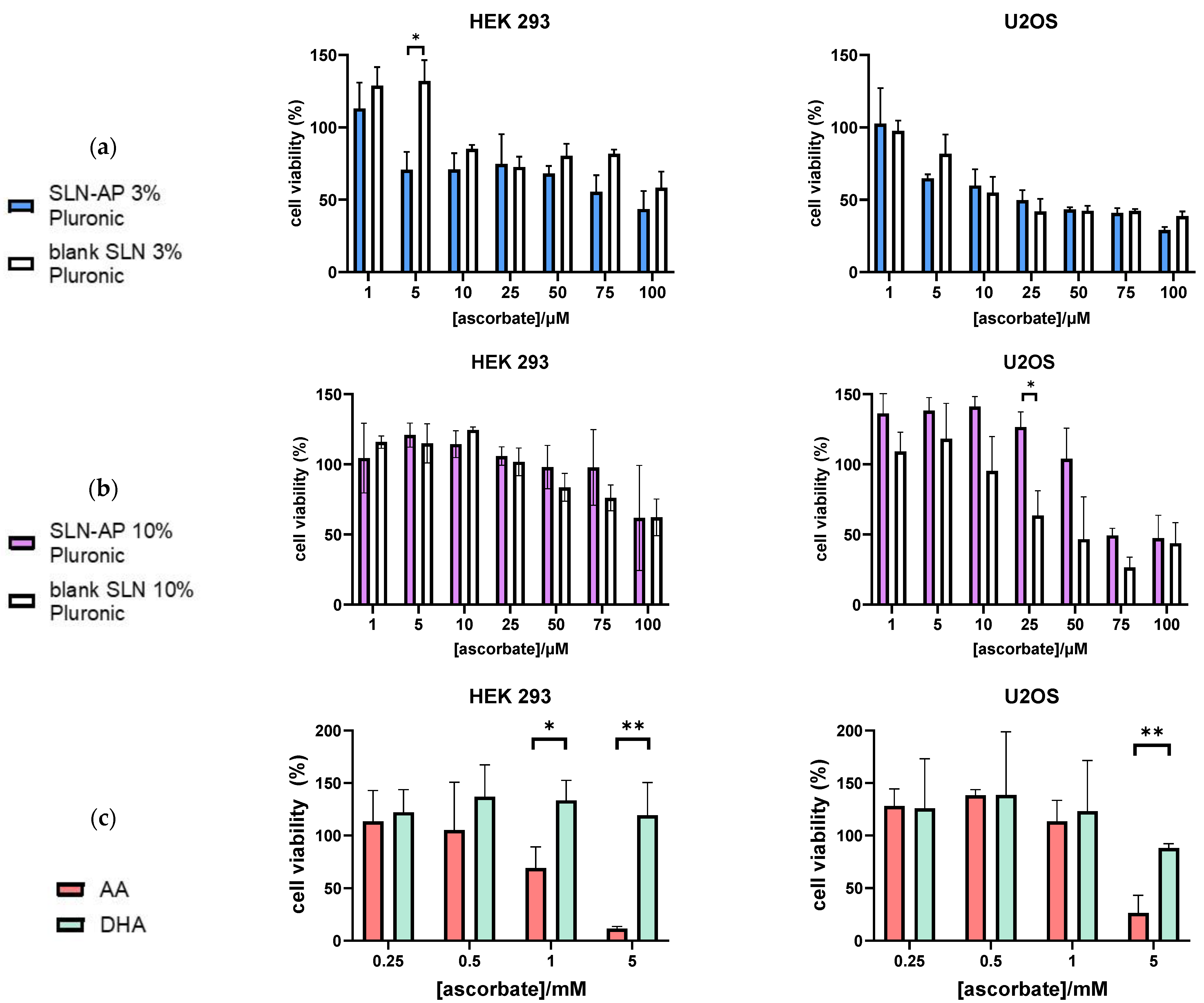
2.5. Cytotoxicity Analysis Using MTT Test
2.6. Analysis of Reactive Oxygen Species
3. Results
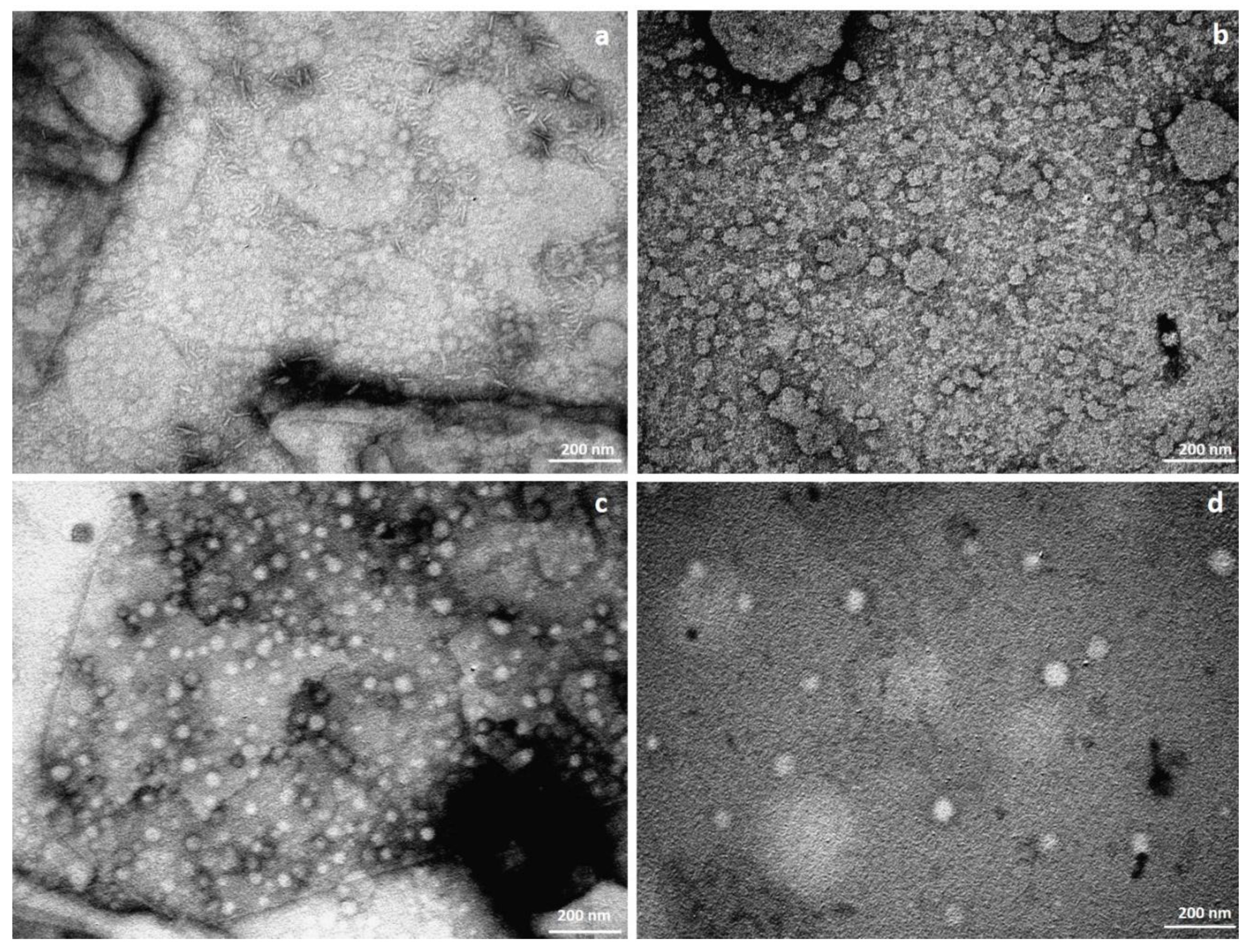
3.1. SLN Synthesis and Characterization
3.2. Cellular Uptake of SLN
3.3. Cytotoxicity Analysis
3.4. Analysis of Reactive Oxygen Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caritá, A.C.; Fonseca-Santos, B.; Shultz, J.D.; Michniak-Kohn, B.; Chorilli, M.; Leonardi, G.R. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102117. [Google Scholar] [CrossRef] [PubMed]
- Moribe, K.; Limwikrant, W.; Higashi, K.; Yamamoto, K. Drug Nanoparticle Formulation Using Ascorbic Acid Derivatives. J. Drug Deliv. 2011, 2011, 138929. [Google Scholar] [CrossRef] [PubMed]
- Pokorski, M.; Marczak, M.; Dymecka, A.; Suchocki, P. Ascorbyl palmitate as a carrier of ascorbate into neural tissues. J. Biomed. Sci. 2003, 10, 193–198. [Google Scholar] [CrossRef]
- Kristl, J.; Volk, B.; Gasperlin, M.; Sentjurc, M.; Jurkovic, P. E ffect of colloidal carriers on ascorbyl palmitate stability. Eur. J. Pharm. Sci. 2003, 19, 181–189. [Google Scholar] [CrossRef]
- Gopinath, D.; Ravi, D.; Rao, B.R.; Apte, S.S.; Renuka, D.; Rambhau, D. Ascorbyl palmitate vesicles (Aspasomes): Formation, characterization and applications. Int. J. Pharm. 2004, 271, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Teeranachaideekul, V.; Souto, E.B.; Müller, R.H.; Junyaprasert, B. Release studies of ascorbyl palmitate-loaded semi- solid nanostructured lipid carriers ( NLC gels). J. Microencapsul. 2008, 2048, 110–120. [Google Scholar] [CrossRef]
- Vissers, M.C.M.; Das, A.B. Potential mechanisms of action for vitamin C in cancer: Reviewing the evidence. Front. Physiol. 2018, 9, 809. [Google Scholar] [CrossRef] [Green Version]
- Ngo, B.; Van Riper, J.M.; Cantley, L.C.; Yun, J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat. Rev. Cancer 2019, 19, 271–282. [Google Scholar] [CrossRef]
- Zhou, M.; Li, X.; Li, Y.; Yao, Q.; Ming, Y.; Li, Z.; Lu, L.; Shi, S. Ascorbyl palmitate-incorporated paclitaxel-loaded composite nanoparticles for synergistic anti-tumoral therapy. Drug Deliv. 2017, 24, 1230–1242. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Guo, C.; Feng, F.; Fan, A.; Dai, Y.; Li, N.; Zhao, D.; Chen, X.; Lu, Y. Co-delivery of docetaxel and palmitoyl ascorbate by liposome for enhanced synergistic antitumor efficacy. Nat. Publ. Gr. 2016, 6, 38787. [Google Scholar] [CrossRef] [Green Version]
- Jukanti, R.; Devraj, G.; Shashank, A.S.; Devraj, R. Biodistribution of ascorbyl palmitate loaded doxorubicin pegylated liposomes in solid tumor bearing mice. J. Microencapsul. 2011, 28, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Qiu, H.; Yin, S.; Wang, H.; Li, Y. Polymeric drug delivery system based on pluronics for cancer treatment. Molecules 2021, 26, 3610. [Google Scholar] [CrossRef]
- Ascorbyl Palmitate|C22H38O7–PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ascorbyl-palmitate (accessed on 10 August 2021).
- Hallan, S.S.; Sguizzato, M.; Esposito, E.; Cortesi, R. Challenges in the physical characterization of lipid nanoparticles. Pharmaceutics 2021, 13, 549. [Google Scholar] [CrossRef] [PubMed]
- Yakimova, L.S.; Guralnik, E.G.; Shurpik, D.N.; Evtugyn, V.G.; Osin, Y.N.; Subakaeva, E.V.; Sokolova, E.A.; Zelenikhin, P.V.; Stoikov, I.I. Morphology, structure and cytotoxicity of dye-loaded lipid nanoparticles based on monoamine pillar[5]arenes. Mater. Chem. Front. 2020, 4, 2962–2970. [Google Scholar] [CrossRef]
- Rivolta, I.; Panariti, A.; Lettiero, B.; Sesana, S.; Gasco, P.; Gasco, M.R.; Masserini, M.; Miserocchi, G. Cellular uptake of coumarin-6 as a model drug loaded in solid lipid nanoparticles. J. Physiol. Pharmacol. 2011, 62, 45–53. [Google Scholar] [PubMed]
- Finke, J.H.; Richter, C.; Gothsch, T.; Kwade, A.; Büttgenbach, S.; Müller-Goymann, C.C. Coumarin 6 as a fluorescent model drug: How to identify properties of lipid colloidal drug delivery systems via fluorescence spectroscopy? Eur. J. Lipid Sci. Technol. 2014, 116, 1234–1246. [Google Scholar] [CrossRef]
- Shi, S.; Yang, L.; Yao, Q.; Li, X.; Ming, Y.; Zhao, Y. Ascorbic palmitate as a bifunctional drug and nanocarrier of paclitaxel for synergistic anti-tumor therapy. J. Biomed. Nanotechnol. 2018, 14, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, J.; Mullarky, E.; Lu, C.; Bosch, K.N.; Kavalier, A.; Rivera, K.; Roper, J.; Chio, I.I.C.; Giannopoulou, E.G.; Rago, C.; et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 2015, 350, 1391–1396. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.W.; Jin, D.H.; Hahm, E.S.; Yim, S.H.; Lim, J.S.; Kim, K.I.; Yang, Y.; Lee, S.S.; Kang, J.S.; Lee, W.J.; et al. Ascorbate (vitamin C) induces cell death through the apoptosis-inducing factor in human breast cancer cells. Oncol. Rep. 2007, 18, 811–815. [Google Scholar] [CrossRef] [Green Version]
- Satheesh, N.J.; Samuel, S.M.; Büsselberg, D. Combination Therapy with Vitamin C Could Eradicate Cancer Stem Cells. Biomolecules 2020, 10, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, A.C.; Cook, J.; Carr, A.C. Intravenous Vitamin C for Cancer Therapy–Identifying the Current Gaps in Our Knowledge. Front. Physiol. 2018, 9, 1182. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.R.; Vaze, O.; Souza, G.G.M.D.; Rockwell, K.; Vladimir, P. Palmitoyl Ascorbate-Loaded Polymeric Micelles: Cancer Cell Targeting and Cytotoxicity. Pharm. Res. 2011, 28, 301–308. [Google Scholar] [CrossRef]
- Armstrong, D. Advanced protocols in oxidative stress III, Methods in Molecular Biology. Adv. Protoc. Oxidative Stress III 2014, 594, 1–477. [Google Scholar] [CrossRef]
- Sawant, R.R.; Vaze, O.S.; Rockwell, K.; Torchilin, V.P. Palmitoyl ascorbate-modified liposomes as nanoparticle platform for ascorbate-mediated cytotoxicity and paclitaxel co-delivery. Eur. J. Pharm. Biopharm. 2010, 75, 321–326. [Google Scholar] [CrossRef]
- Vaiserman, A.; Koliada, A.; Zayachkivska, A.; Lushchak, O. Nanodelivery of Natural Antioxidants: An Anti-aging Perspective. Front. Bioeng. Biotechnol. 2020, 7, 447. [Google Scholar] [CrossRef] [Green Version]

| 3% Pluronic F-68 | 10% Pluronic F-68 | |||
|---|---|---|---|---|
| Measurement | Blank SLN | SLN-AP | Blank SLN | SLN-AP |
| Diameter/nm | 18.2 ± 3.21 125 ± 4.52 | 337 ± 8.52 | 445 ± 14.3 | 414 ± 32.1 |
| PDI | 0.291 ± 0.0224 | 0.137 ± 0.0336 | 0.182 ± 0.0219 | 0.198 ± 0.0451 |
| zeta potential/mV | +38.1 ± 3.36 | +1.45 ± 0.337 | +21.5 ± 0.308 | −4.18 ± 0.251 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ledinski, M.; Marić, I.; Peharec Štefanić, P.; Ladan, I.; Caput Mihalić, K.; Jurkin, T.; Gotić, M.; Urlić, I. Synthesis and In Vitro Characterization of Ascorbyl Palmitate-Loaded Solid Lipid Nanoparticles. Polymers 2022, 14, 1751. https://doi.org/10.3390/polym14091751
Ledinski M, Marić I, Peharec Štefanić P, Ladan I, Caput Mihalić K, Jurkin T, Gotić M, Urlić I. Synthesis and In Vitro Characterization of Ascorbyl Palmitate-Loaded Solid Lipid Nanoparticles. Polymers. 2022; 14(9):1751. https://doi.org/10.3390/polym14091751
Chicago/Turabian StyleLedinski, Maja, Ivan Marić, Petra Peharec Štefanić, Iva Ladan, Katarina Caput Mihalić, Tanja Jurkin, Marijan Gotić, and Inga Urlić. 2022. "Synthesis and In Vitro Characterization of Ascorbyl Palmitate-Loaded Solid Lipid Nanoparticles" Polymers 14, no. 9: 1751. https://doi.org/10.3390/polym14091751
APA StyleLedinski, M., Marić, I., Peharec Štefanić, P., Ladan, I., Caput Mihalić, K., Jurkin, T., Gotić, M., & Urlić, I. (2022). Synthesis and In Vitro Characterization of Ascorbyl Palmitate-Loaded Solid Lipid Nanoparticles. Polymers, 14(9), 1751. https://doi.org/10.3390/polym14091751







