Retention Capacity of Original Denture Adhesives and White Brands for Conventional Complete Dentures: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Materials Used in the Study
2.2. Methods
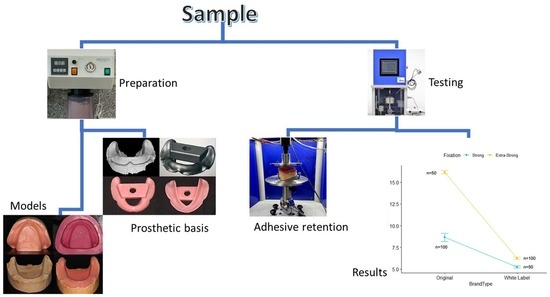
2.2.1. In Vitro Model Design
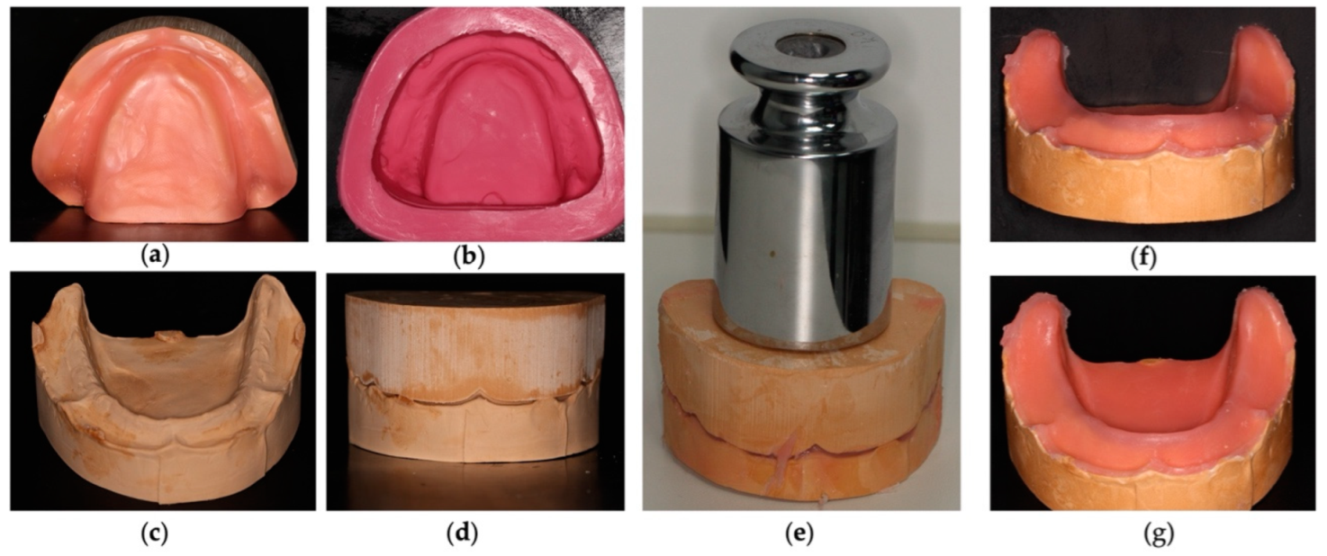
2.2.2. Preparation of the Base of Prosthetics
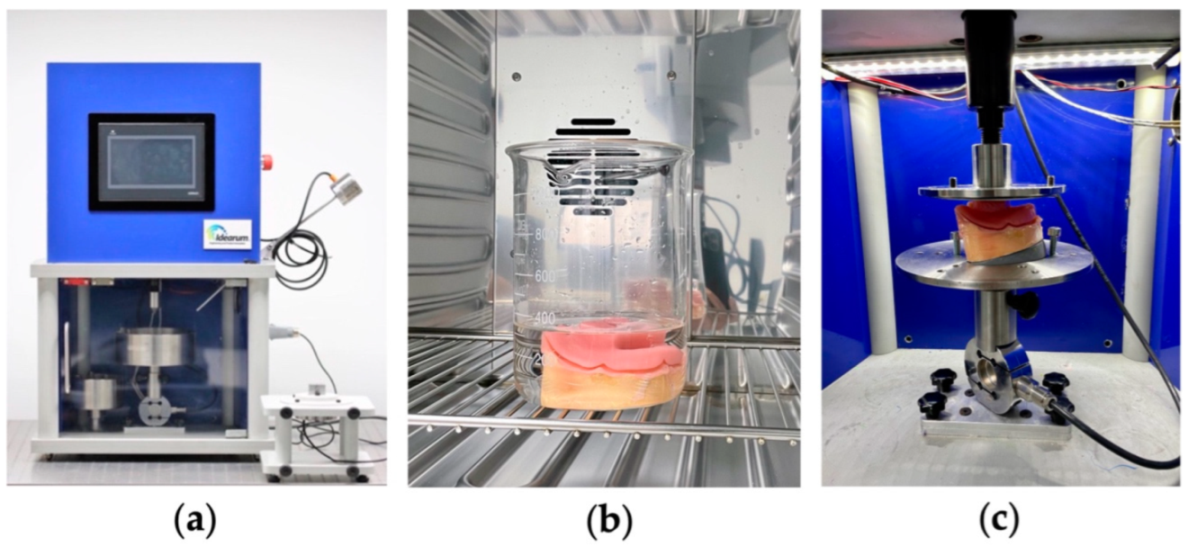
2.2.3. Traction Test to Measure Retention Strength of Adhesives for Dental Prosthetics
2.3. Statistical Analysis
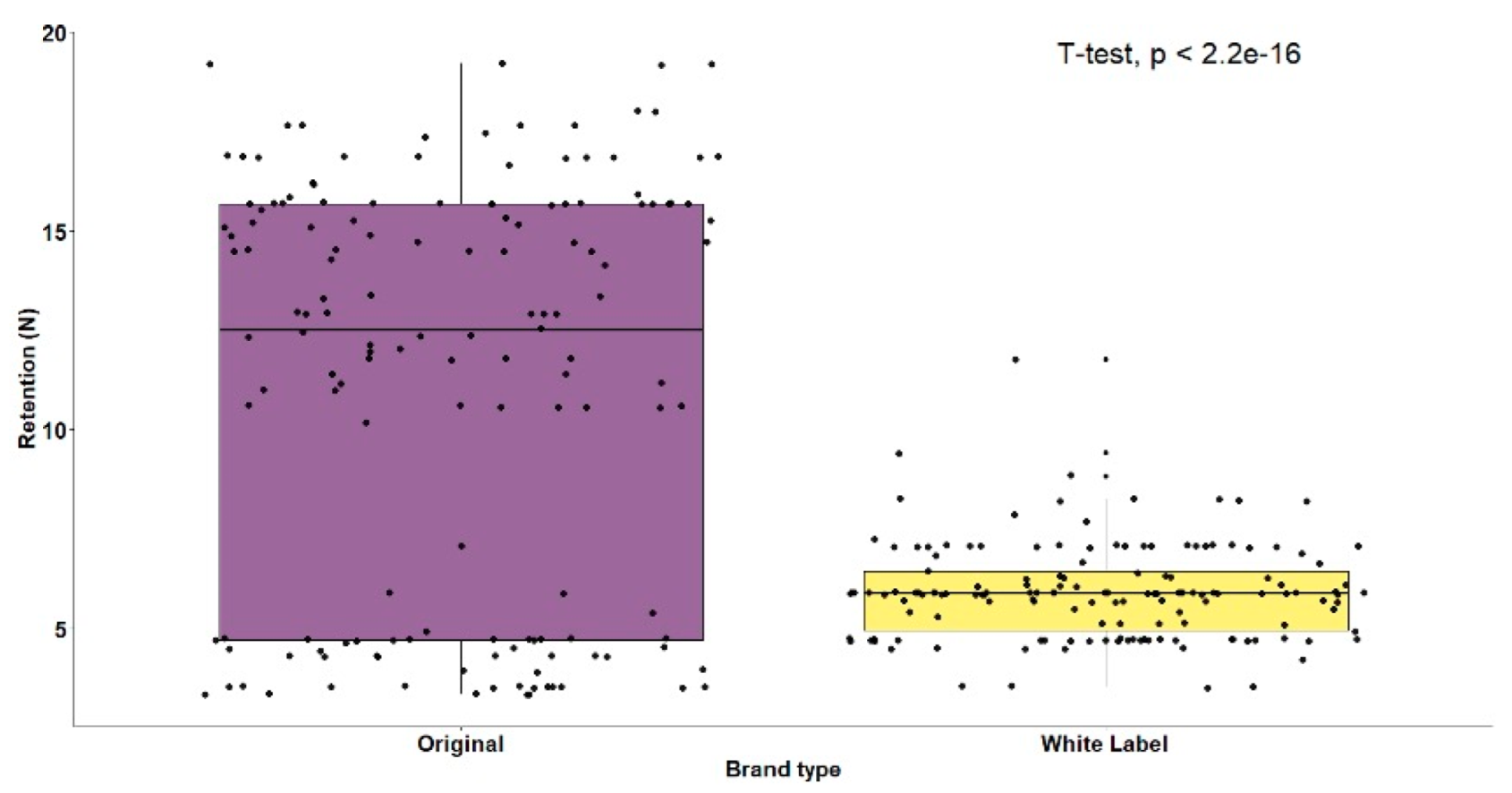
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petersen, P.E.; Kandelman, D.; Arpin, S.; Ogawa, H. Global oral health of older people--call for public health action. Community Dent Health 2010, 27, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Glick, M.; Williams, D.M.; Kleinman, D.V.; Vujicic, M.; Watt, R.G.; Weyant, R.J. A new definition for oral health developed by the FDI World Dental Federation opens the door to a universal definition of oral health. Br. Dent. J. 2016, 221, 792–793. [Google Scholar] [CrossRef]
- Jiang, C.M.; Chu, C.H.; Duangthip, D.; Ettinger, R.L.; Hugo, F.N.; Kettratad-Pruksapong, M.; Liu, J.; Marchini, L.; McKenna, G.; Ono, T.; et al. Global Perspectives of Oral Health Policies and Oral Healthcare Schemes for Older Adult Populations. Front. Oral Health 2021, 2, 703526. [Google Scholar] [CrossRef] [PubMed]
- Compagnoni, M.A.; Souza, R.F.; Leles, C.R. Kinesiographic Study of Complete Denture Movement Related to Mucosa Displacement in Edentulous Patients. Pesqui. Odontológica Bras. 2003, 17, 356–361. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Oral Disorders Collaborators; Bernabe, E.; Marcenes, W.; Hernandez, C.R.; Bailey, J.; Abreu, L.G.; Alipour, V.; Amini, S.; Arabloo, J.; Arefi, Z.; et al. Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease 2017 Study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, C.F.; Freilich, M.A.; Guckes, A.D.; Knoernschild, K.L.; Mcgarry, T.J.; Goldstein, G.; Goodacre, C.; Guckes, A.; Mor, S.; Rosenstiel, S.; et al. The Glossary of Prosthodontic Terms: Ninth Edition. J. Prosthet. Dent. 2017, 117, e1–e105. [Google Scholar] [CrossRef]
- Kanazawa, M.; Tanoue, M.; Miyayasu, A.; Takeshita, S.; Sato, D.; Asami, M.; Lam, T.V.; Thu, K.M.; Oda, K.; Komagamine, Y.; et al. The patient general satisfaction of mandibular single-implant overdentures and conventional complete dentures: Study protocol for a randomized crossover trial. Medicine 2018, 97, e10721. [Google Scholar] [CrossRef]
- Polychronakis, N.; Sykaras, N.; Polyzois, G.; Lagouvardos, P. Removal of denture adhesives from PMMA and Polyamide denture base materials. J. Appl. Oral Sci. 2021, 29, e20200448. [Google Scholar] [CrossRef]
- Munoz, C.A.; Gendreau, L.; Shanga, G.; Magnuszewski, T.; Fernandez, P.; Durocher, J. A Clinical Study to Evaluate Denture Adhesive Use in Well-Fitting Dentures. J. Prosthodont. 2012, 21, 123–129. [Google Scholar] [CrossRef]
- Żmudzki, J.; Chladek, G.; Krawczyk, C. Relevance of Tongue Force on Mandibular Denture Stabilization during Mastication. J. Prosthodont. 2019, 28, e27–e33. [Google Scholar] [CrossRef]
- Nogueira, T.E.; Schimmel, M.; Leles, C.R. Changes in masticatory performance of edentulous patients treated with single-implant mandibular overdentures and conventional complete dentures. J. Oral Rehabil. 2019, 46, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Kämmerer, P.W.; Wolf, J.M.; Buttchereit, I.; Frerich, B.; Ottl, P. Prospective clinical implementation of optional implant treatment into pregraduate dental education—mini implants for retention and support of mandibular overdentures. Int. J. Implant Dent. 2021, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Vissink, A.; Spijkervet, F.; Raghoebar, G. The medically compromised patient: Are dental implants a feasible option? Oral Dis. 2018, 24, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.D.D.; Viotto, H.; Policastro, V.B.; Leite, A.R.P.; Cassiano, A.F.; Marin, D.O.M.; Santana, T.D.S.; Mollo, F.; Pero, A.C. Influence of adhesives usage in complete dentures during adaptation period varying the degree of resorption of mandibular ridges. J. Prosthodont. Res. 2020, 64, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, S.; Kawai, Y.; Gunji, A.; Kondo, H.; Nomura, T.; Murakami, T.; Tsuboi, A.; Hong, G.; Minakuchi, S.; Sato, Y.; et al. Study protocol for a multi-center, randomized controlled trial to develop Japanese denture adhesive guidelines for patients with complete dentures: The Denture Adhesive Guideline trial: Study protocol for a randomized controlled trial. Trials 2016, 17, 506. [Google Scholar] [CrossRef]
- Ibraheem, E.M.A.; El-Sisy, A.M.E. Comparing the effect of three denture adhesives on the retention of mandibular complete dentures for diabetic patients (randomized clinical trial). Bull. Natl. Res. Cent. 2019, 43, 24. [Google Scholar] [CrossRef]
- Shu, X.; Fan, Y.; Lo, E.C.M.; Leung, K.C.M. A systematic review and meta-analysis to evaluate the efficacy of denture adhesives. J. Dent. 2021, 108, 103638. [Google Scholar] [CrossRef]
- Torres-Sánchez, C.; Montoya-Salazar, V.; Torres-Lagares, D.; Luis Gutierrez-Pérez, J.; Jimenez-Castellanos, E. Comparison of mas-ticatory efficacy among complete denture wearers with two adhesives and dentate individuals: A randomized, crossover, double-blind clinical trial. J. Prosthet. Dentistry. 2017, 177, 615–620. [Google Scholar]
- Bo, T.M.; Hama, Y.; Akiba, N.; Minakuchi, S. Utilization of denture adhesives and the factors associated with its use: A cross-sectional survey. BMC Oral Health 2020, 20, 194. [Google Scholar] [CrossRef]
- Fallahi, A.; Khadivi, N.; Roohpour, N.; Middleton, A.M.; Kazemzadeh-Narbat, M.; Annabi, N.; Khademhosseini, A.; Tamayol, A. Characterization, mechanistic analysis and improving the properties of denture adhesives. Dent. Mater. 2018, 34, 120–131. [Google Scholar] [CrossRef]
- Leles, C.R.; Oliveira, T.M.C.; De Araújo, S.C.; Nogueira, T.E.; Schimmel, M. Individual factors associated with masticatory performance of complete denture wearers: A cross-sectional study. J. Oral Rehabil. 2019, 46, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Darvell, B.; Clark, R. The physical mechanisms of complete denture retention. Br. Dent. J. 2000, 189, 248–252. [Google Scholar] [CrossRef]
- Alves, C.P.; Munhoz, M.F.V.; Nascimento, G.M.O.; Dds, G.A.N.; Paleari, A.G.; Camargos, G.D.V. The Influence of Age, Gender, Mandibular Bone Height, Previous Experience with Prostheses, and Fabrication Methods on Masticatory Performance of Complete Denture Wearers. J. Prosthodont. 2019, 28, e34–e40. [Google Scholar] [CrossRef] [PubMed]
- Deniz, D.A.; Ozkan, Y.K. The influence of occlusion on masticatory performance and satisfaction in complete denture wearers. J. Oral Rehabil. 2013, 40, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Shamsolketabi, S.; Nili, M. The effect of denture adhesive on the efficiency of complete denture in patients with different alve-olar ridges. Dent. Res. J. 2018, 15, 271–275. [Google Scholar]
- Choi, J.J.E.; Chen, S.; Waddell, J.N. Investigation of dental elastomers as oral mucosa simulant materials. Clin. Exp. Dent. Res. 2021, 7, 754–762. [Google Scholar] [CrossRef]
- Johnson, A. The Effect of Denture Design and Fixatives on the Retention of Mandibular Complete Dentures Tested on a Novel In-vitro Edentulous Model. Eur. J. Prosthodont. Restor. Dent. 2013, 21, 64–74. [Google Scholar] [CrossRef]
- Müller, H.; Schaller, N.; Eger, T.; Heinecke, A. Thickness of masticatory mucosa. J. Clin. Periodontol. 2000, 27, 431–436. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Qing, H.; Pei, X.; Chen, J.; Wang, J. Effect of implant number on the movement of mandibular implant-supported overdentures under biting force: An in-vitro study. Quintessence Int. 2018, 49, 709–717. [Google Scholar] [CrossRef]
- Ikemura, N.; Sato, Y.; Furuya, J.; Shimodaira, O.; Takeda, K.; Kakuta, T.; Yamane, K.; Kitagawa, N. Changes in denture retention with denture adhesives and oral moisturizers for the oral cavity: An in vitro study. BMC Oral Health 2021, 21, 438. [Google Scholar] [CrossRef]
- ISO 10873:2021; Dentistry—Denture Adhesives. Available online: https://www.iso.org/standard/72380.html (accessed on 16 March 2022).
- Pytko-Polonczyk, J.J.; Jakubik, A.; Przeklasa-Bierowiec, A.; Muszynska, B. Artificial saliva and its use in biological experiments. J. Physiol. Pharmacol. 2017, 68, 807–813. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2021. Available online: http://softlibre.unizar.es/manuales/aplicaciones/r/fullrefman.pdf (accessed on 16 March 2022).
- Kore, D.R.; Kattadiyil, M.T.; Hall, D.B.; Bahjri, K. In vitro comparison of the tensile bond strength of denture adhesives on denture bases. J. Prosthet. Dent. 2013, 110, 488–493. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mañes, J.; Selva, E.; De-Barutell, A.; Bouazza, K. Comparison of the retention strengths of three complete denture adhesives: An in vivo study. Med. Oral Patol. Oral Cir. Bucal. 2011, 16, 132–136. [Google Scholar] [CrossRef]
- Chowdhry, P.; Phukela, S.; Patil, R.; Yadav, H. A Study to Evaluate the Retentive Ability of Different Denture Adhesive Materi-als: An In Vitro Study. J. Indian Prosthodont. Soc. 2010, 10, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Flores-León, J.Z.J.; Domínguez-Pérez, R.A.; Ruíz-Valdez, H.E.; Sámano-Valencia, C.; Loyola-Rodríguez, J.P.; Castro-Ruiz, E. Comparison of the Retention of Conventional Dentures after the use of Common Adhesive Brands on Poor Denture Foundations. An in vitro Study. Int. J. Odontostomatol. 2020, 14, 236–241. [Google Scholar] [CrossRef]
- Quiney, D.; Ayre, W.; Milward, P. The effectiveness of adhesives on the retention of mandibular free end saddle partial den-tures: An in vitro study. J. Dentistery 2017, 62, 64–71. [Google Scholar] [CrossRef]


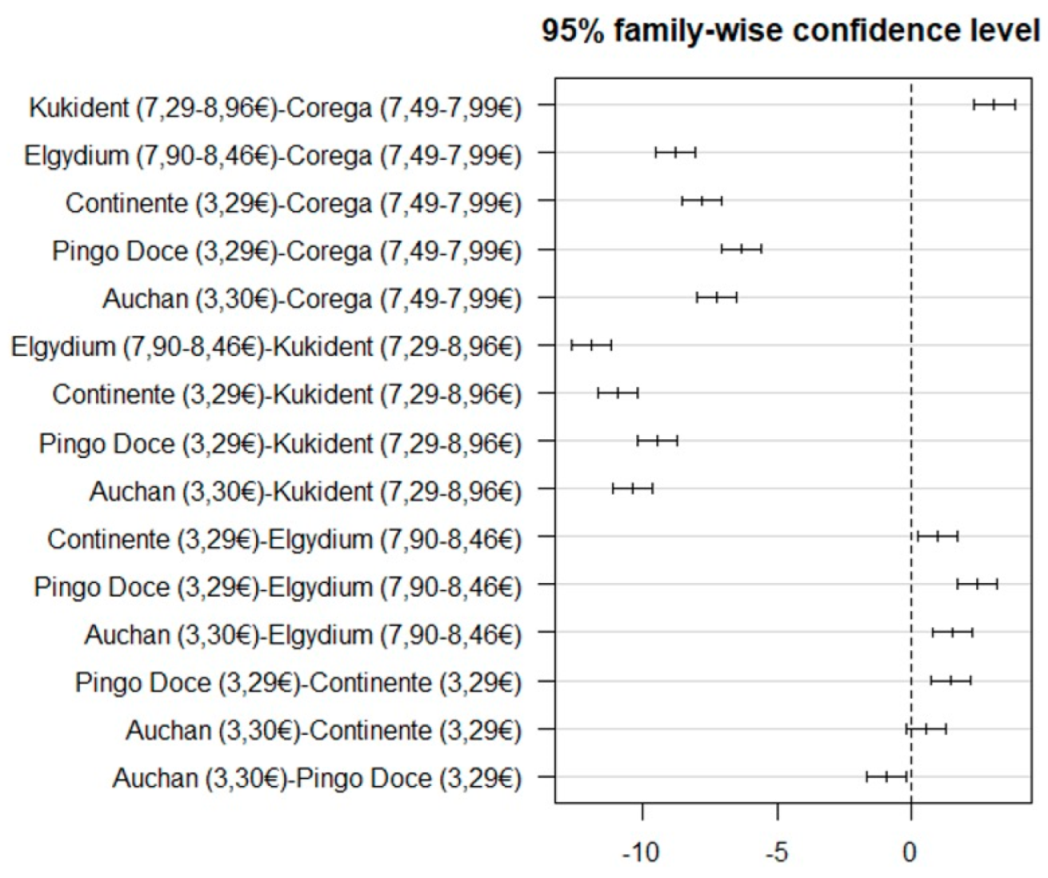

| Adhesive Name | Types and Classes | Fixation Type | Composition | Company |
|---|---|---|---|---|
| Corega® Fixação 3D | Type 1, Class 2-cream form | Strong | Calcium/Sodium PVM/MA Copolymer, Petrolatum, Cellulose Gum, Paraffinum Liquidum | GlaxoSmithKline®, Stafford Miller Ltd., Dungarvan Co. Waterford, Ireland) |
| KuKident® Pro | Type 1, Class 2-cream form | Extra-strong | Calcium/Zinc PVM/MA Copolymer, Paraffinum Liquidum, Cellulose Gum, Petrolatum, Sílica | Procter & Gamble Technical Centres®, Ltd., Whitehal Lane, Germany |
| Elgydium® Fix | Type 1, Class 2-cream form | Strong | Paraffinum Liquidum, Calcium/Sodium PVM/MA Copolymer, Cellulose Gum, Petrolatum, PVP, Isopropyl Palmitate, Isopropyl Myristate | Laboratórios URGO® SL, Florida, Spain |
| Fixador de próteses (Continente®) | Type 1, Class 2-cream form | Strong | Paraffinum Liquidum, Cellulose Gum, Calcium/Sodium PVM/MA Copolymer, Petrolatum, Aqua, Polyethylene, Mentha Piperita Oil, Menthol, Limonene | Propack®,Gmbh, Ladenburg, Germany |
| Fixação Extra forte (Pingo Doce®) | Type 1, Class 2-cream form | Extra-strong | Cellulose Gum, Calcium/Sodium PVM/MA Copolymer, Paraffinum Liquidum, Petrolatum | Laboratórios Cosmodent®, Cantabria, Spain |
| Creme Fixador de próteses (Auchan®) | Type 1, Class 2-cream form | Extra-strong | Paraffinum Liquidum, Cellulose Gum, Calcium/Sodium PVM/MA Copolymer, Petrolatum, Silica, Aroma Menthyl Lactate | Ellipse®, Roubaix, France |
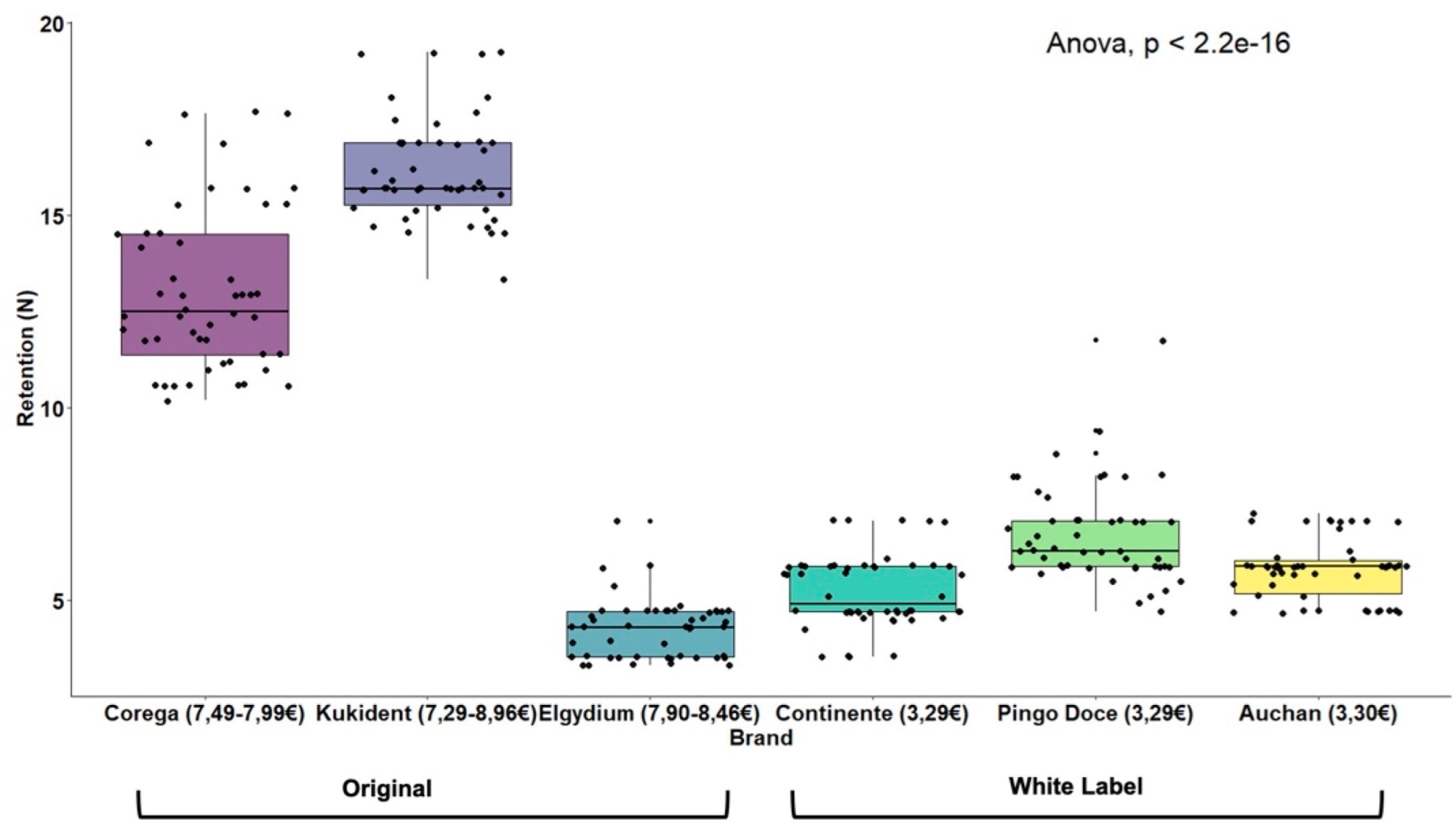
| Original Brand (n = 150) | White Label Brand (n = 150) | t-Test | ||||
|---|---|---|---|---|---|---|
| 11.16 (5.27) | 5.92 (1.18) | t(298) = 11.88 (p < 0.001) | ||||
| Corega® Fixação 3D (7.49–7.99) (n = 50) | KuKident® Pro (7.29–8.96 ) (n = 50) | Elgydium® Fix (7.90–8.46) (n = 50) | Continente® (3.29) (n = 50) | Pingo Doce® (3.29) (n = 50) | Auchan® (3.30) n = 50) | ANOVA |
| 13.05 (2.13) | 16.16 (1.34) | 4.27 (0.76) | 5.24 (0.94) | 6.71 (1.28) | 5.80 (0.79) | F(5.294) = 707.68 (p < 0.001) |
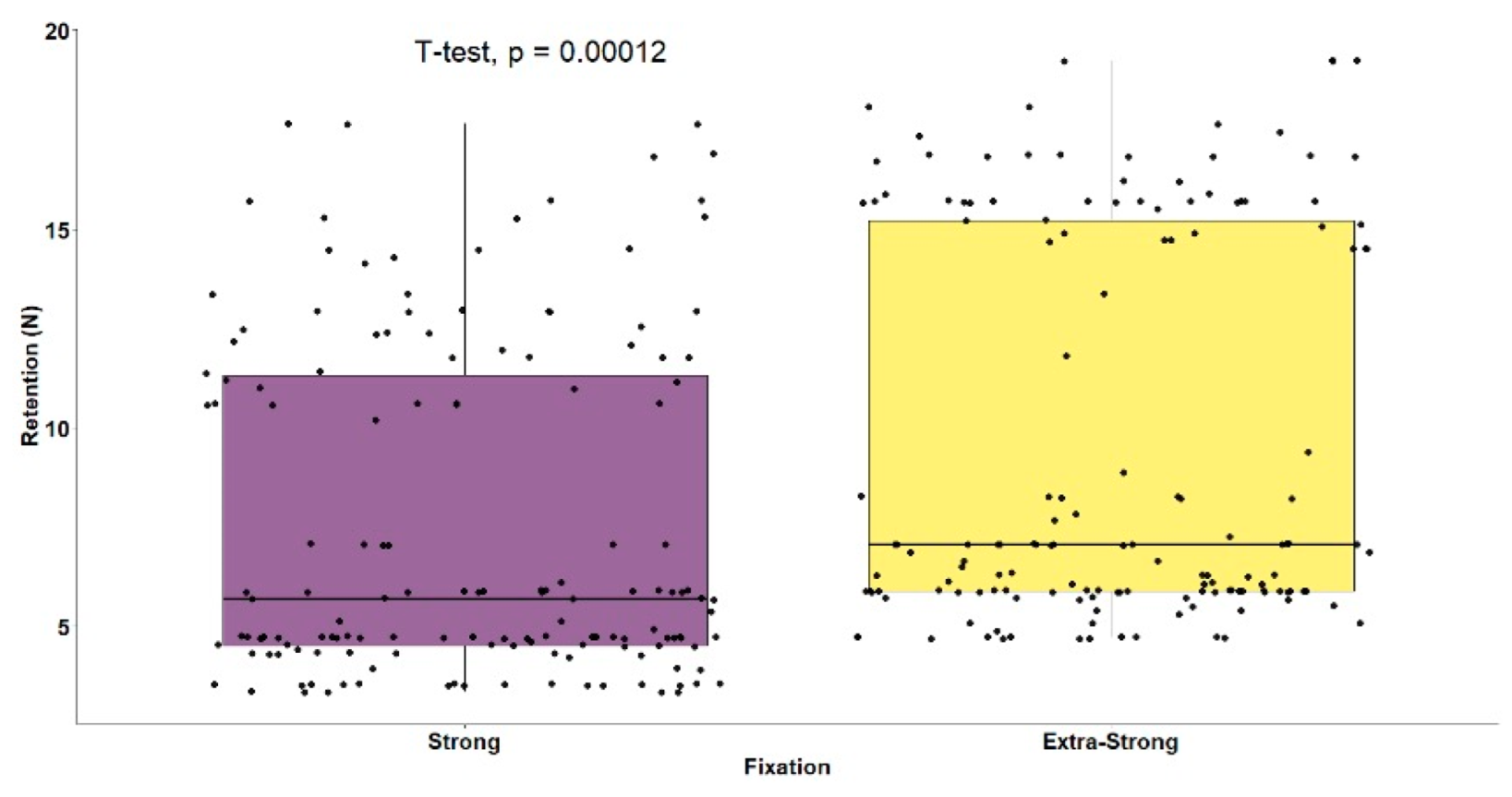
| Strong Fixation (n = 150) | Extra-Strong Fixation (n = 150) | t-Test | ||||
|---|---|---|---|---|---|---|
| 7.52 (4.19) | 9.57 (4.84) | t(298) = 3.90 (p < 0.001) | ||||
| Corega® Fixação 3D (7.49–7.99) (n = 50) | Elgydium® Fix (7.90–8.46) (n = 50) | Continente® (3.29) (n = 50) | KuKident® Pro (7.29–8.96) (n = 50) | Pingo® Doce (3.29) (n = 50) | Auchan® (3.30) (n = 50) | ANOVA |
| 13.05 (2.13) | 4.27 (0.76) | 5.24 (0.94) | 16.16 (1.34) | 6.71 (1.28) | 5.80 (0.79) | F(5.294) = 707.68 (p < 0.001) |
| Strong Fixation (n = 150) | Extra-Strong Fixation (n = 150) | |||||
|---|---|---|---|---|---|---|
| 7.52 (4.19) | 9.57 (4.84) | ANOVA | ||||
| Original Brand (n = 100) | White label Brand (n = 50) | Original Brand (n = 50) | White label Brand (n = 100) | Fixation | Brand type | Fixation x Brand type |
| 8.66 (4.69) | 5.24 (0.94) | 16.16 (1.34) | 6.26 (1.15) | F(1.296) = 37.65 (p < 0.001) | F(1.296) = 358.33 (p < 0.001) | F(1.296) = 84.83 (p < 0.001) |
| Retention | |||
|---|---|---|---|
| β | IC 95% | p-Value | |
| Price (in EUR) | |||
| ≈3.30 (White label brands) | 5.92 (a) | 5.70; 6.14 (a) | <0.001 (a) |
| Original Brands | |||
| ≈EUR7.74 (Corega® Fixação 3D) (+4.44) | 7.14 | 6.70; 7.57 | <0.001 |
| ≈EUR8.13 (KuKident® Pro) (+4.82) | 10.24 | 9.80; 10.67 | <0.001 |
| ≈EUR8.18 (Elgydium® Fix) (+4.88) | −1.65 | −2.09; −1.21 | <0.001 |
| Adhesive Name | Type of Brand | Fixation Type | Price (in EUR) |
|---|---|---|---|
| KuKident® Pro | Original Brand | Extra-strong | 8.13€ |
| Corega® Fixação 3D | Original Brand | Strong | 7.74€ |
| Fixação Extra forte (Pingo Doce®) | White label Brand | Extra-strong | 3.29€ |
| Creme Fixador de próteses (Auchan®) | White label Brand | Extra-strong | 3.30€ |
| Fixador de próteses (Continente®) | White label Brand | Strong | 3.29€ |
| Elgydium® Fix | Original Brand | Strong | 8.18€ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, J.; Mendes, J.M.; Barreiros, P.; Aroso, C.; Silva, A.S. Retention Capacity of Original Denture Adhesives and White Brands for Conventional Complete Dentures: An In Vitro Study. Polymers 2022, 14, 1749. https://doi.org/10.3390/polym14091749
Mendes J, Mendes JM, Barreiros P, Aroso C, Silva AS. Retention Capacity of Original Denture Adhesives and White Brands for Conventional Complete Dentures: An In Vitro Study. Polymers. 2022; 14(9):1749. https://doi.org/10.3390/polym14091749
Chicago/Turabian StyleMendes, Joana, José Manuel Mendes, Pedro Barreiros, Carlos Aroso, and António Sérgio Silva. 2022. "Retention Capacity of Original Denture Adhesives and White Brands for Conventional Complete Dentures: An In Vitro Study" Polymers 14, no. 9: 1749. https://doi.org/10.3390/polym14091749
APA StyleMendes, J., Mendes, J. M., Barreiros, P., Aroso, C., & Silva, A. S. (2022). Retention Capacity of Original Denture Adhesives and White Brands for Conventional Complete Dentures: An In Vitro Study. Polymers, 14(9), 1749. https://doi.org/10.3390/polym14091749