In Vitro Biocompatibility and Degradation Analysis of Mass-Produced Collagen Fibers
Abstract
:1. Introduction
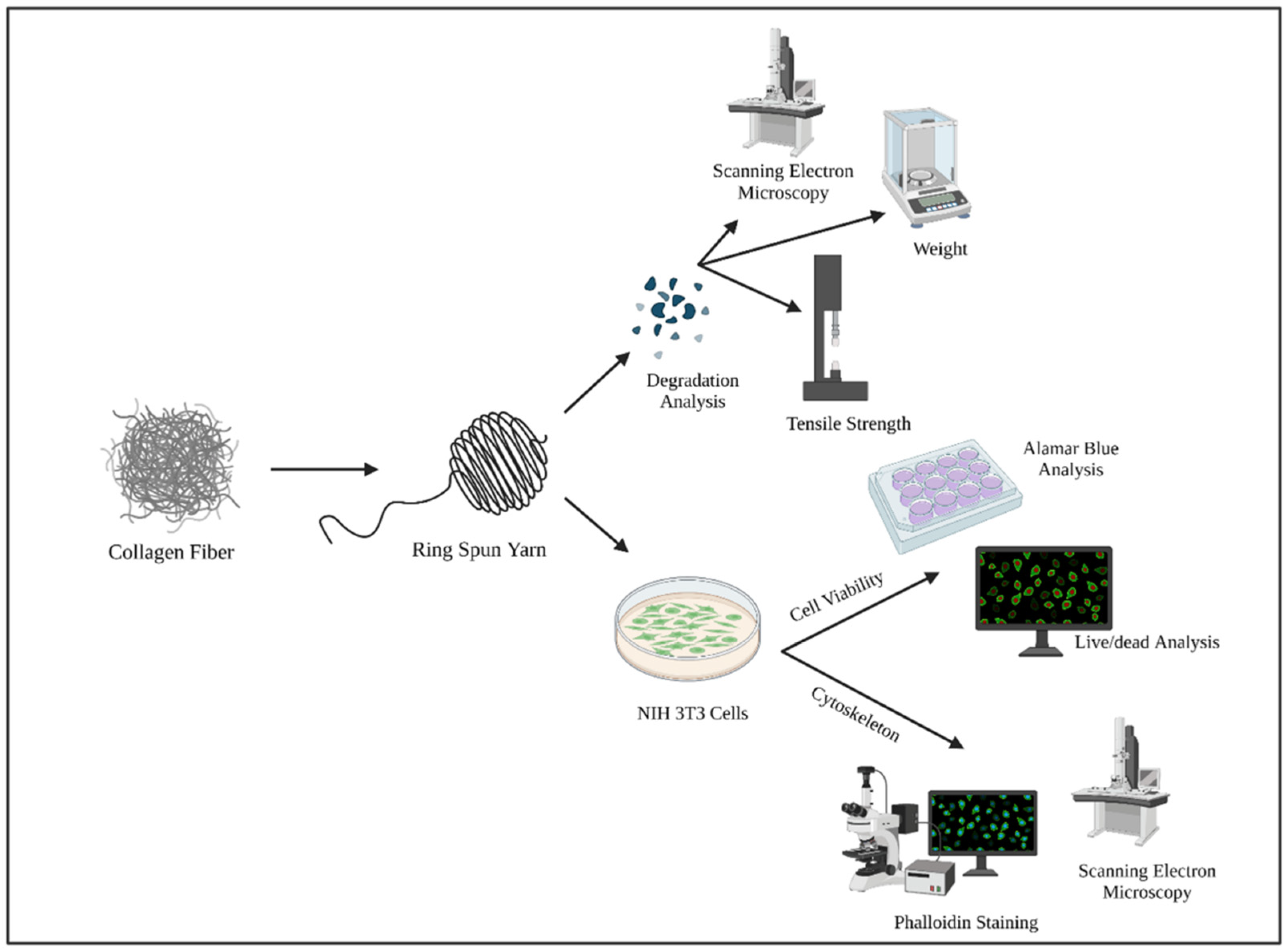
2. Materials and Methods
2.1. Degradation Study
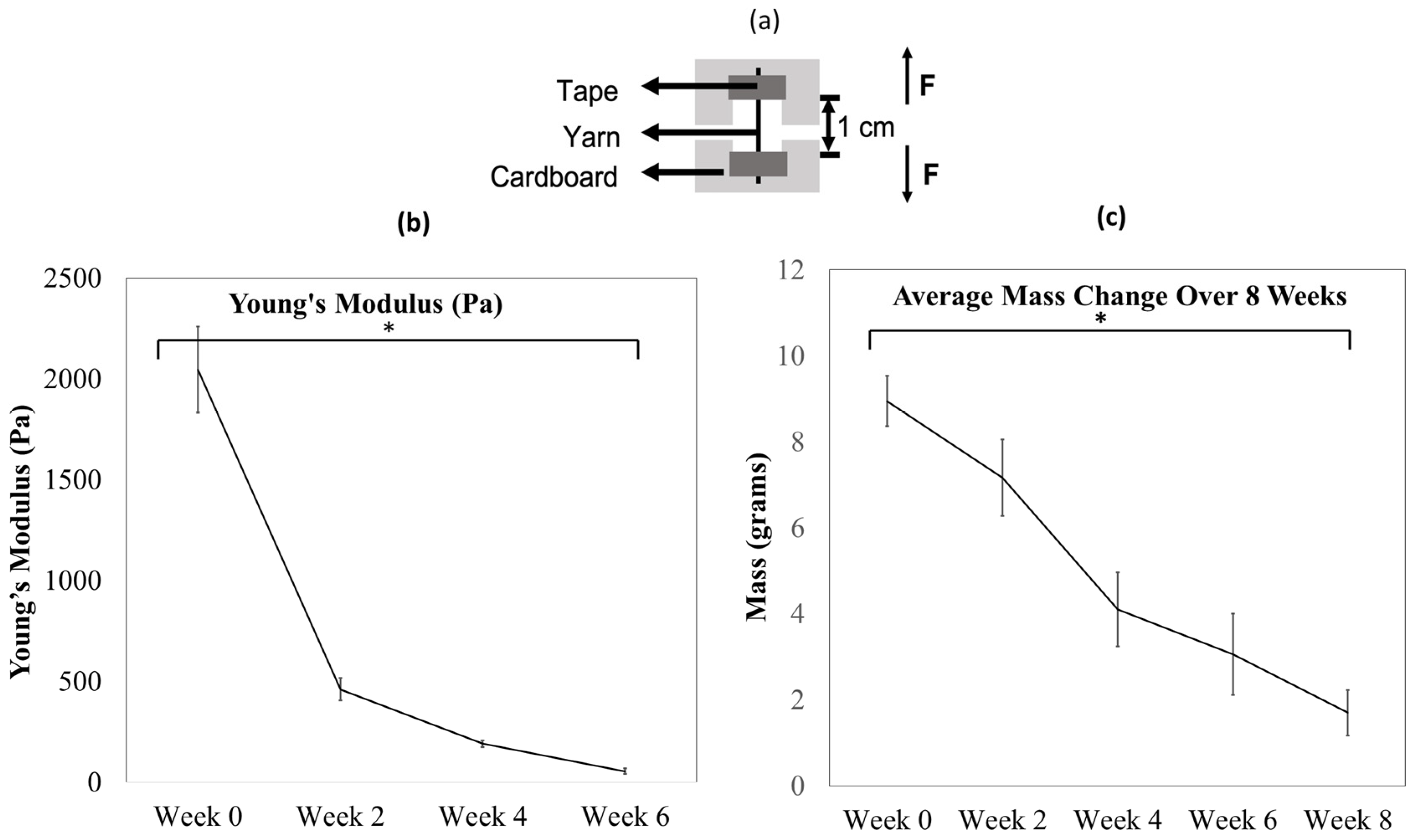
2.1.1. Weight Change
2.1.2. Tensile Test
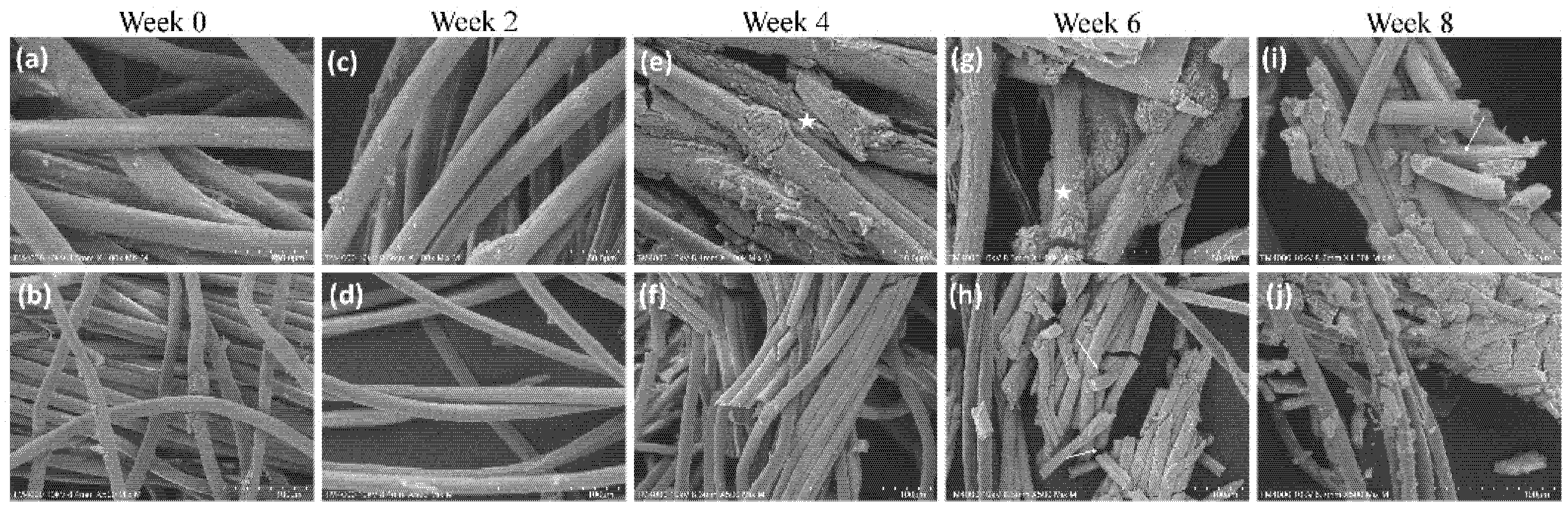
2.1.3. Scanning Electron Microscopy
2.2. Biocompatibility
2.2.1. Sample Preparation
2.2.2. Cell Culture
2.2.3. Cell Proliferation
2.2.4. Phalloidin Staining
2.2.5. Ultrastructure Analysis (Scanning Electron Microscopy)
2.3. Statistical Analysis
3. Results
3.1. Collagen Yarns Degrade in the Presence of Enzymes in an 8-Week Study
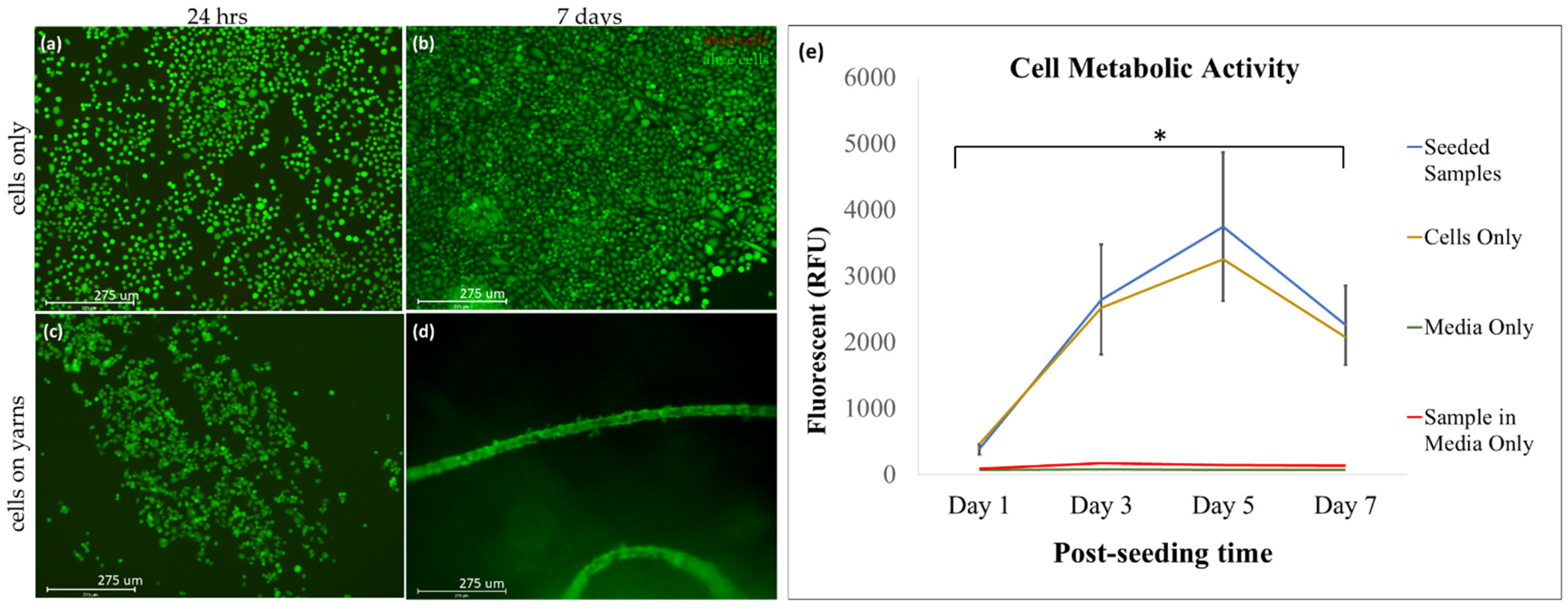
3.2. The Collagen Material Is Biocompatible with Mouse Fibroblast (NIH 3T3) Cell Line
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Zileli, M.; Benzel, E.C.; Bell, G.R. Chapter 93—Bone graft harvesting. In Spine Surgery, 3rd ed.; Benzel, E.C., Ed.; Churchill Livingstone: Philadelphia, PA, USA, 2005; pp. 1253–1261. [Google Scholar] [CrossRef]
- Transplanting Human Tissue: Ethics, Policy, and Practice; Youngner, S.J.; Youngner, S.J.; Anderson, M.W.; Schapiro, R. (Eds.) Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Atala, A. Tissue engineering and regenerative medicine: Concepts for clinical application. Rejuvenation Res. 2004, 7, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ushida, T.; Tateishi, T. Scaffold design for tissue engineering. Macromol. Biosci. 2002, 2, 67–77. [Google Scholar] [CrossRef]
- Rahmanian-Schwarz, A.; Held, M.; Knoeller, T.; Stachon, S.; Schmidt, T.; Schaller, H.E.; Just, L. In vivo biocompatibility and biodegradation of a novel thin and mechanically stable collagen scaffold. J. Biomed. Mater. Res. 2014, 102, 1173–1179. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [Green Version]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolym. Orig. Res. Biomol. 2008, 89, 338–344. [Google Scholar] [CrossRef]
- Cen, L.; Liu, W.; Cui, L.; Zhang, W.; Cao, Y. Collagen tissue engineering: Development of novel biomaterials and applications. Pediatr. Res. 2008, 63, 492–496. [Google Scholar] [CrossRef]
- George, J.; Onodera, J.; Miyata, T. Biodegradable honeycomb collagen scaffold for dermal tissue engineering. J. Biomed. Mater. Res. 2008, 87, 1103–1111. [Google Scholar] [CrossRef]
- Roßbach, B.P.; Gülecyüz, M.F.; Kempfert, L.; Pietschmann, M.F.; Ullamann, T.; Ficklscherer, A.; Niethammer, T.R.; Zhang, A.; Klar, R.M.; Müller, P.E. Rotator cuff repair with autologous tenocytes and biodegradable collagen scaffold: A histological and biomechanical study in sheep. Am. J. Sports Med. 2020, 48, 450–459. [Google Scholar] [CrossRef]
- Khan, R.; Khan, M.H. Use of collagen as a biomaterial: An update. J. Indian Soc. Periodontol. 2013, 17, 539–542. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.; Xu, L.; Gu, X.; Ma, X. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Charriere, G.; Bejot, M.; Schnitzler, L.; Ville, G.; Hartmann, D.J. Reactions to a bovine collagen implant: Clinical and immunologic study in 705 patients. J. Am. Acad. Dermatol. 1989, 21, 1203–1208. [Google Scholar] [CrossRef]
- Keefe, J.; Wauk, L.; Chu, S.; DeLustro, F. Clinical use of injectable bovine collagen: A decade of experience. Clin. Mater. 1992, 9, 155–162. [Google Scholar] [CrossRef]
- Dutta, R.C.; Dey, M.; Dutta, A.K.; Basu, B. Competent processing techniques for scaffolds in tissue engineering. Biotechnol. Adv. 2017, 35, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Leong, K.; Du, Z.; Chua, C. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Y.; Li, C.; Liu, L.; Wang, F.; Liu, X.; Mao, J.; Wang, L. Construction and application of textile-based tissue engineering scaffolds: A review. Biomater. Sci. 2020, 8, 3574–3600. [Google Scholar] [CrossRef]
- Nivedhitha Sundaram, M.; Deepthi, S.; Mony, U.; Shalumon, K.T.; Chen, J.P.; Jayakumar, R. Chitosan hydrogel scaffold reinforced with twisted poly(l lactic acid) aligned microfibrous bundle to mimic tendon extracellular matrix. Int. J. Biol. Macromol. 2019, 122, 37–44. [Google Scholar] [CrossRef]
- Benjamin, M.; Kaiser, E.; Milz, S. Structure-function relationships in tendons: A review. J. Anat. 2008, 212, 211–228. [Google Scholar] [CrossRef]
- Badekila, A.K.; Kini, S.; Jaiswal, A.K. Fabrication techniques of biomimetic scaffolds in three-dimensional cell culture: A review. J. Cell. Physiol. 2021, 236, 741–762. [Google Scholar] [CrossRef]
- Alagirusamy, R.; Das, A. Chapter 8—Conversion of fibre to yarn: An overview. In Textiles and Fashion; Sinclair, R., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 159–189. [Google Scholar] [CrossRef]
- Lawrence, C.A. Advances in Yarn Spinning Technology; Elsevier Science: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Subramaniam, V.; Mohammad, P. Wrap spinning technology—A critical review of yarn properties. Indian J. Fibre Text. Res. 1992, 17, 252–254. [Google Scholar]
- Das, A.; Alagirusamy, R. 3—Fundamental principles of open end yarn spinning. In Advances in Yarn Spinning Technology; Lawrence, C.A., Ed.; Woodhead Publishing: Sawston, UK, 2010; pp. 79–101. [Google Scholar] [CrossRef]
- Gilmore, J.; Yin, F.; Burg, K.J.L. Evaluation of permeability and fluid wicking in woven fiber bone scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Van Lieshout, M.I.; Vaz, C.M.; Rutten, M.C.M.; Peters, G.W.M.; Baaijens, F.P.T. Electrospinning versus knitting: Two scaffolds for tissue engineering of the aortic valve. J. Biomater. Sci. Polym. Ed. 2006, 17, 77–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Bambharoliya, T.; Xie, Y.; Liu, L.; Celik, H.; Wang, L.; Akkus, O.; King, M.W. A hybrid vascular graft harnessing the superior mechanical properties of synthetic fibers and the biological performance of collagen filaments. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111418. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Hu, X.; Ma, L.; You, C.; Zheng, Y.; Sun, H.; Han, C.; Gao, C. Fabrication and characterization of poly(l-lactide-co-glycolide) knitted mesh-reinforced collagen–chitosan hybrid scaffolds for dermal tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 8, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Learn, G.D.; McClellan, P.E.; Knapik, D.M.; Cumsky, J.L.; Webster-Wood, V.; Anderson, J.M.; Gillespie, R.J.; Akkus, O. Woven collagen biotextiles enable mechanically functional rotator cuff tendon regeneration during repair of segmental tendon defects in vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1864–1876. [Google Scholar] [CrossRef]
- Alberti, K.A.; Xu, Q. Biocompatibility and degradation of tendon-derived scaffolds. Regen. Biomater. 2016, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Von Burkersroda, F.; Schedl, L.; Göpferich, A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials 2002, 23, 4221–4231. [Google Scholar] [CrossRef]
- Streichan, S.J.; Hoerner, C.R.; Schneidt, T.; Holzer, D.; Hufnagel, L. Spatial constraints control cell proliferation in tissues. Proc. Natl. Acad. Sci. USA 2014, 111, 5586–5591. [Google Scholar] [CrossRef] [Green Version]
- Rico-Llanos, G.; Borrego-González, S.; Moncayo-Donoso, M.; Becerra, J.; Visser, R. Collagen type I biomaterials as scaffolds for bone tissue engineering. Polymers 2021, 13, 599. [Google Scholar] [CrossRef]
- Cvrček, L.; Horáková, M. Chapter 14—Plasma modified polymeric materials for implant applications. In Non-Thermal Plasma Technology for Polymeric Materials; Thomas, S., Mozetič, M., Cvelbar, U., Špatenka, P., Praveen, K.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 367–407. [Google Scholar] [CrossRef]
- Ge, Z.; Jin, Z.; Cao, T. Manufacture of degradable polymeric scaffolds for bone regeneration. Biomed. Mater. 2008, 3, 022001. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of collagen scaffold in tissue engineering: Recent advances and new perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonndorf, R.; Aibibu, D.; Cherif, C. Isotropic and anisotropic scaffolds for tissue engineering: Collagen, conventional, and textile fabrication technologies and properties. Int. J. Mol. Sci. 2021, 22, 9561. [Google Scholar] [CrossRef] [PubMed]
- Saidy, N.T.; Wolf, F.; Bas, O.; Keijdener, H.; Hutmacher, D.W.; Mela, P.; De-Juan-Pardo, E.M. Biologically inspired scaffolds for heart valve tissue engineering via melt electrowriting. Small 2019, 15, 1900873. [Google Scholar] [CrossRef] [PubMed]
- Roshanbinfar, K.; Vogt, L.; Ruther, F.; Roether, J.A.; Boccaccini, A.R.; Engel, F.B. Nanofibrous composite with tailorable electrical and mechanical properties for cardiac tissue engineering. Adv. Funct. Mater. 2020, 30, 1908612. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Wang, H.; Chi, Y.; Wang, Y.; Jiang, L.; Xu, N.; Wu, Q.; Feng, Q.; Sun, X. Preparation of oriented collagen fiber scaffolds and its application in bone tissue engineering. Appl. Mater. Today 2021, 22, 100902. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, J.; Celik, H.; Akkus, O.; King, M.W. Evaluation of an electrochemically aligned collagen yarn for textile scaffold fabrication. Biomed. Mater. 2021, 16, 025001. [Google Scholar] [CrossRef]
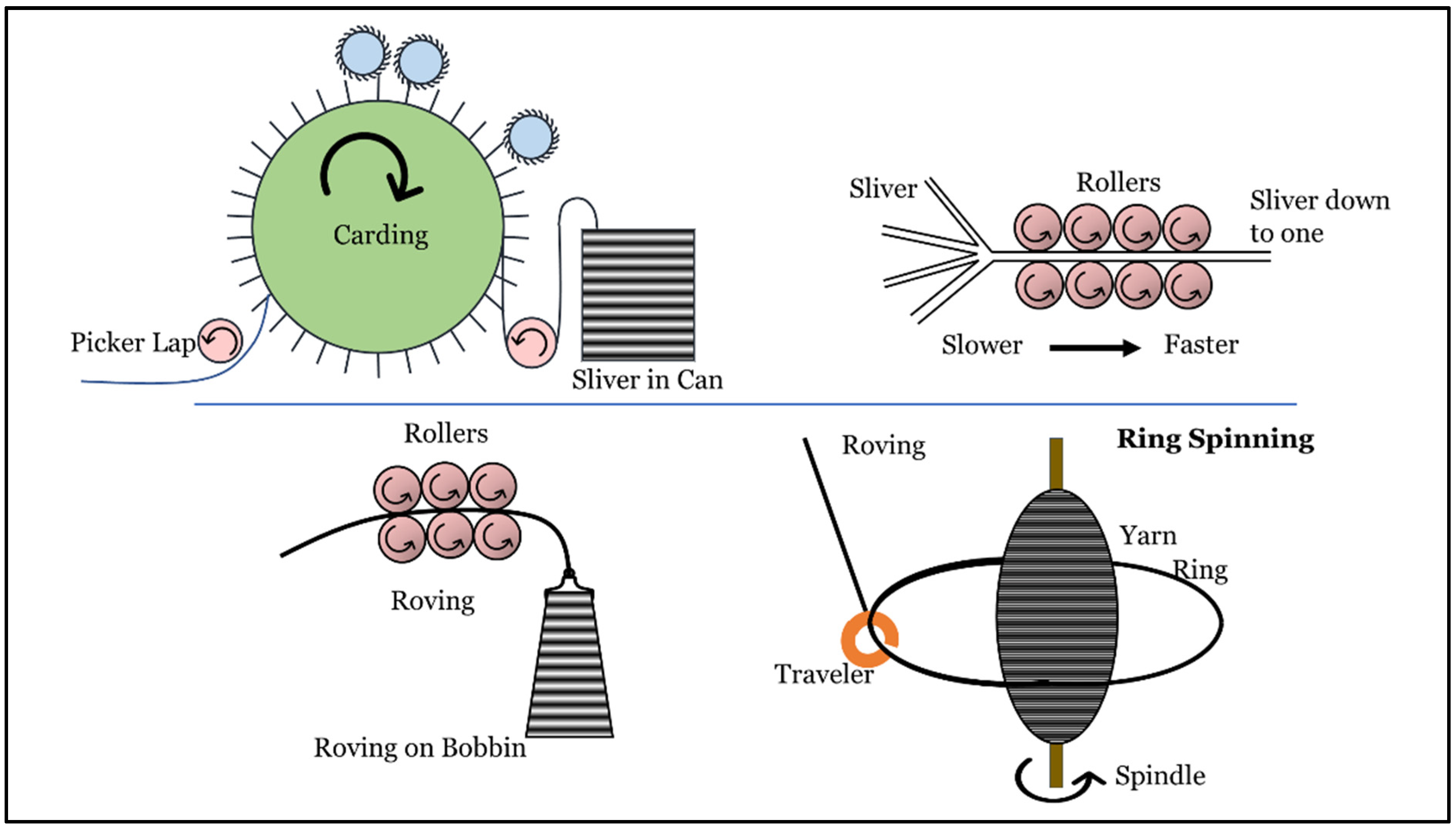

| Method | Advantages | Disadvantages | Ref |
|---|---|---|---|
| Ring Spinning | The yarns that are produced have high strength. This method is applicable to a wide variety of fibers. | The method consumes high amounts of energy, and therefore, production costs are also high. | [22,23] |
| Rotor Spinning | The production costs of using this method are relatively low. | The resultant strength of the yarns is low. | [22,23] |
| Wrap-Spinning | The yarns that are produced through this method are highly absorbent. The method has high production efficiency. | The yarns have low strength. | [22,23,24] |
| Core-Spinning | This method uses two or more fibers, providing excellent properties to the resultant composite yarn. | There are limited applications for this method. | [22,23,25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, K.M.; Huang, Y.; Amanah, A.Y.; Mahmood, N.; Suh, T.C.; Gluck, J.M. In Vitro Biocompatibility and Degradation Analysis of Mass-Produced Collagen Fibers. Polymers 2022, 14, 2100. https://doi.org/10.3390/polym14102100
Ali KM, Huang Y, Amanah AY, Mahmood N, Suh TC, Gluck JM. In Vitro Biocompatibility and Degradation Analysis of Mass-Produced Collagen Fibers. Polymers. 2022; 14(10):2100. https://doi.org/10.3390/polym14102100
Chicago/Turabian StyleAli, Kiran M., Yihan Huang, Alaowei Y. Amanah, Nasif Mahmood, Taylor C. Suh, and Jessica M. Gluck. 2022. "In Vitro Biocompatibility and Degradation Analysis of Mass-Produced Collagen Fibers" Polymers 14, no. 10: 2100. https://doi.org/10.3390/polym14102100
APA StyleAli, K. M., Huang, Y., Amanah, A. Y., Mahmood, N., Suh, T. C., & Gluck, J. M. (2022). In Vitro Biocompatibility and Degradation Analysis of Mass-Produced Collagen Fibers. Polymers, 14(10), 2100. https://doi.org/10.3390/polym14102100






