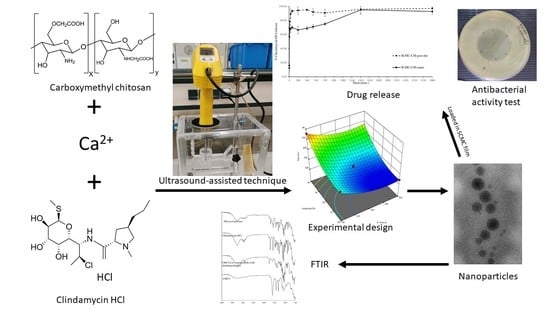
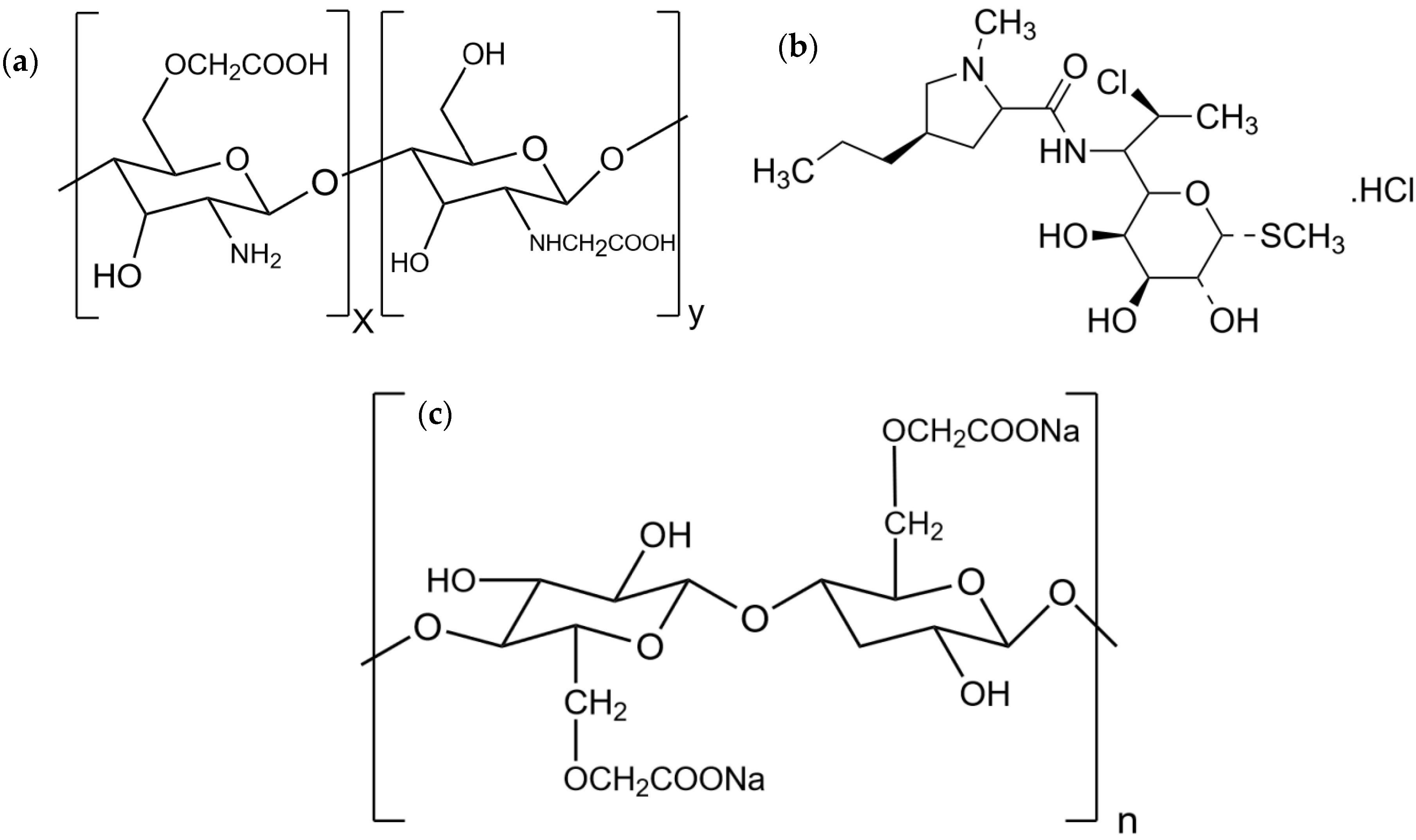
Development of Carboxymethyl Chitosan Nanoparticles Prepared by Ultrasound-Assisted Technique for a Clindamycin HCl Carrier
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CMCS-Ca2+ Nanoparticles
2.3. Experimental Design
2.4. Particle Size Measurement
2.5. Lyophilization
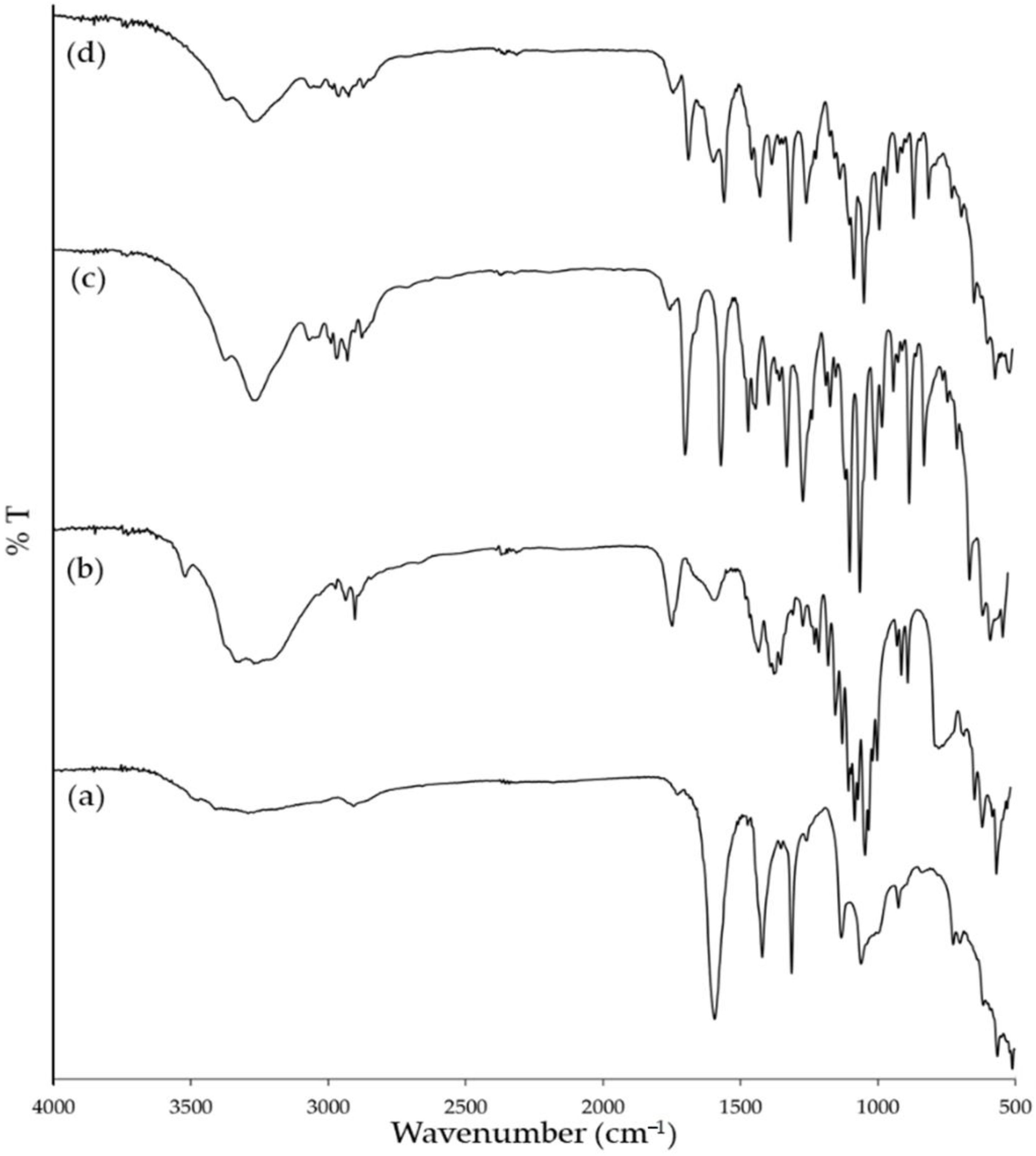
2.6. Fourier-Transform Infrared Spectroscopy
2.7. Drug Loading Content in CMCS-Ca2+ Nanoparticles
2.8. Transmission Electron Microscope (TEM) Imaging of CMCS-Ca2+ Nanoparticles
2.9. Preparation of Sodium Carboxy Methylcellulose (SCMC) Film Loading CMCS-Ca2+ Nanoparticles
2.10. Scanning Electron Microscope (SEM) Imaging of SCMC film loading CMCS-Ca2+ Nanoparticles
2.11. Clindamycin HCl Loading Content in the SCMC Film Containing CMCS- Ca2+ Nanoparticles
2.12. Drug Release Profile
2.13. Antibacterial Activity
2.14. Statistic Analysis
3. Results and Discussion
3.1. Nanoparticle Preparation Using the Ultrasound-Assisted Technique
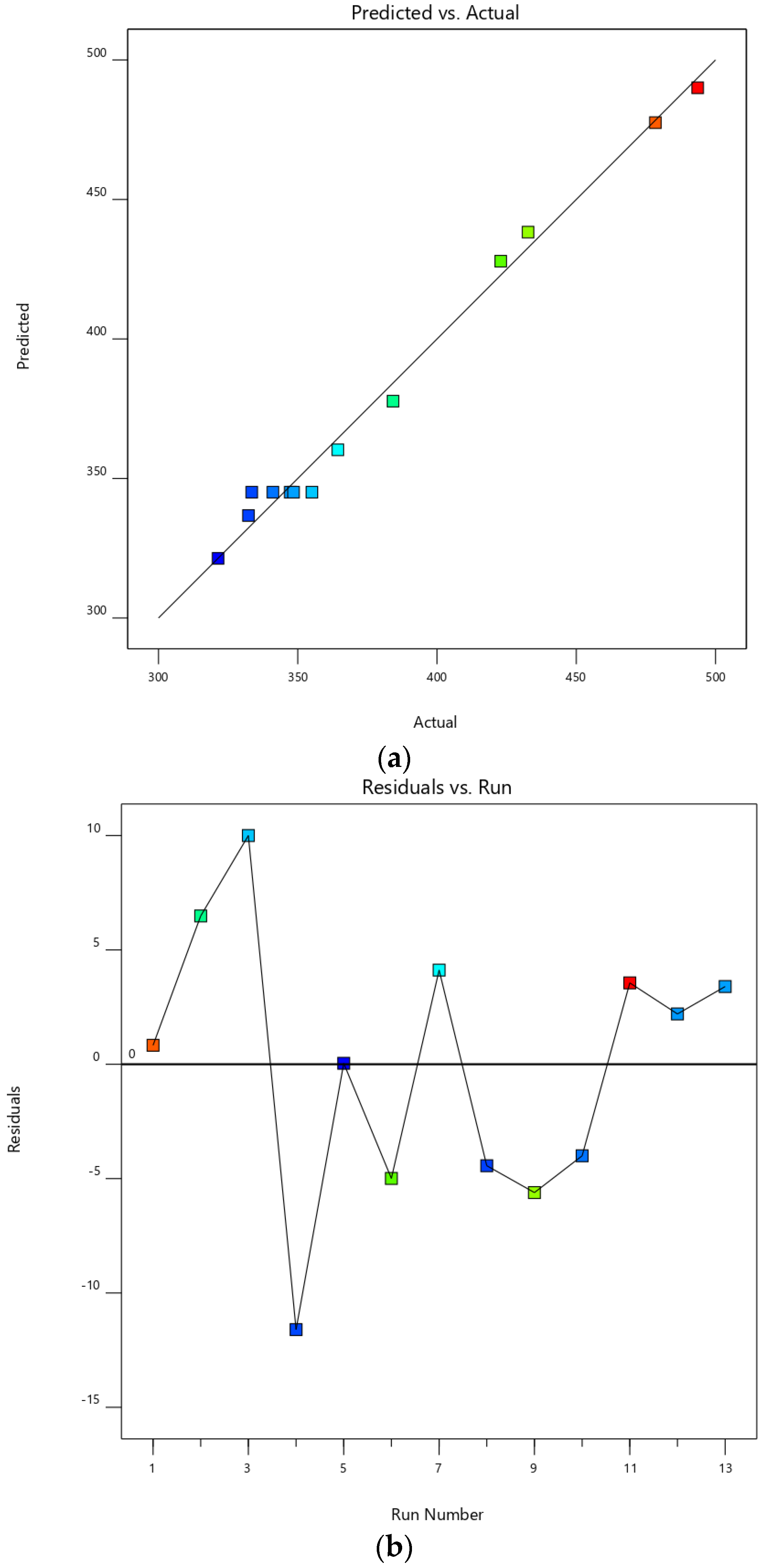
3.2. Experimental Design
3.3. Particle Size of CMCS-Ca2+ Nanoparticles
3.4. Fourier Transform Infrared Spectroscopy
3.5. Clindamycin HCl Content in CMCS-Ca2+ Nanoparticles
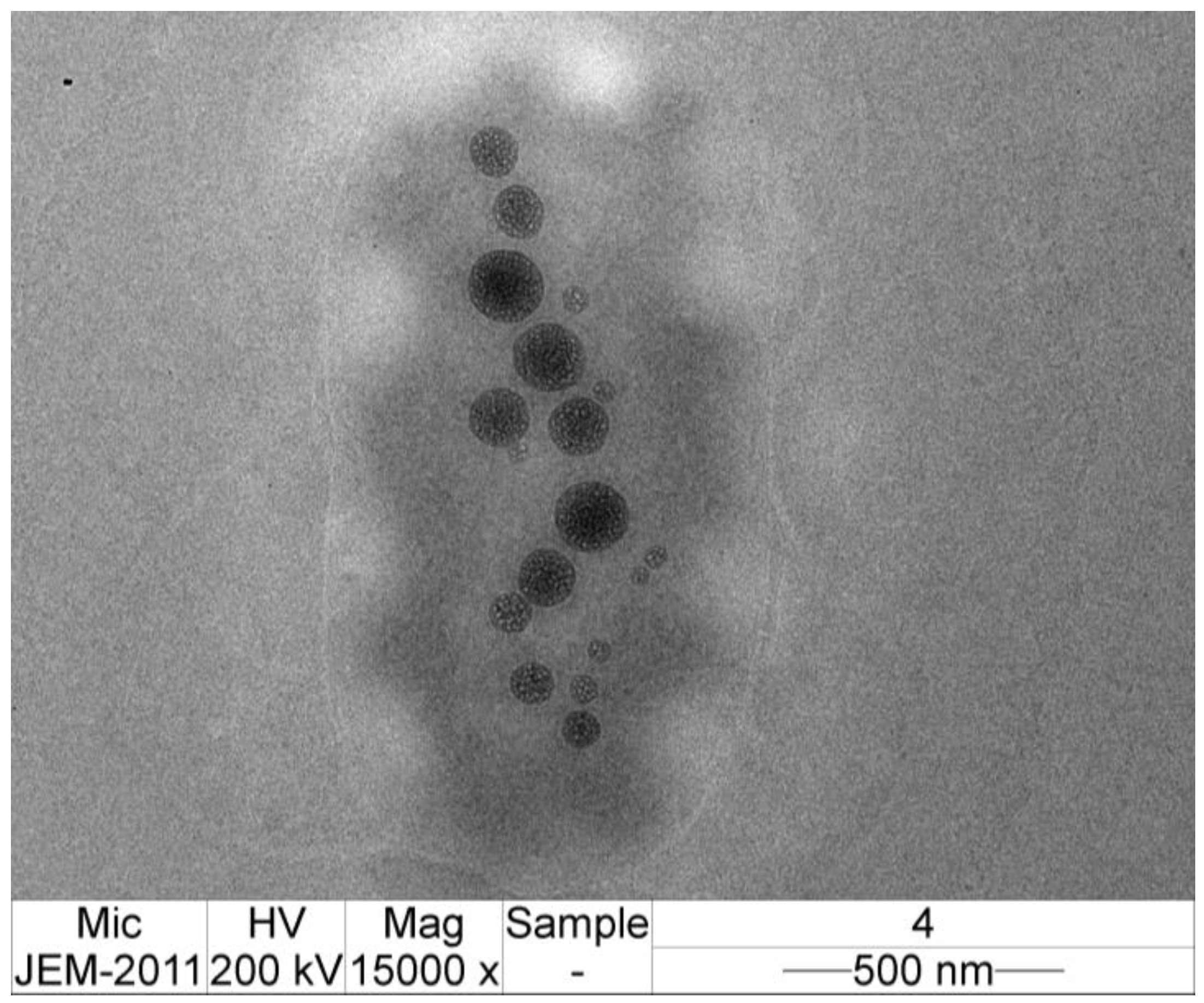
3.6. TEM Image of CMCS-Ca2+ Nanoparticles
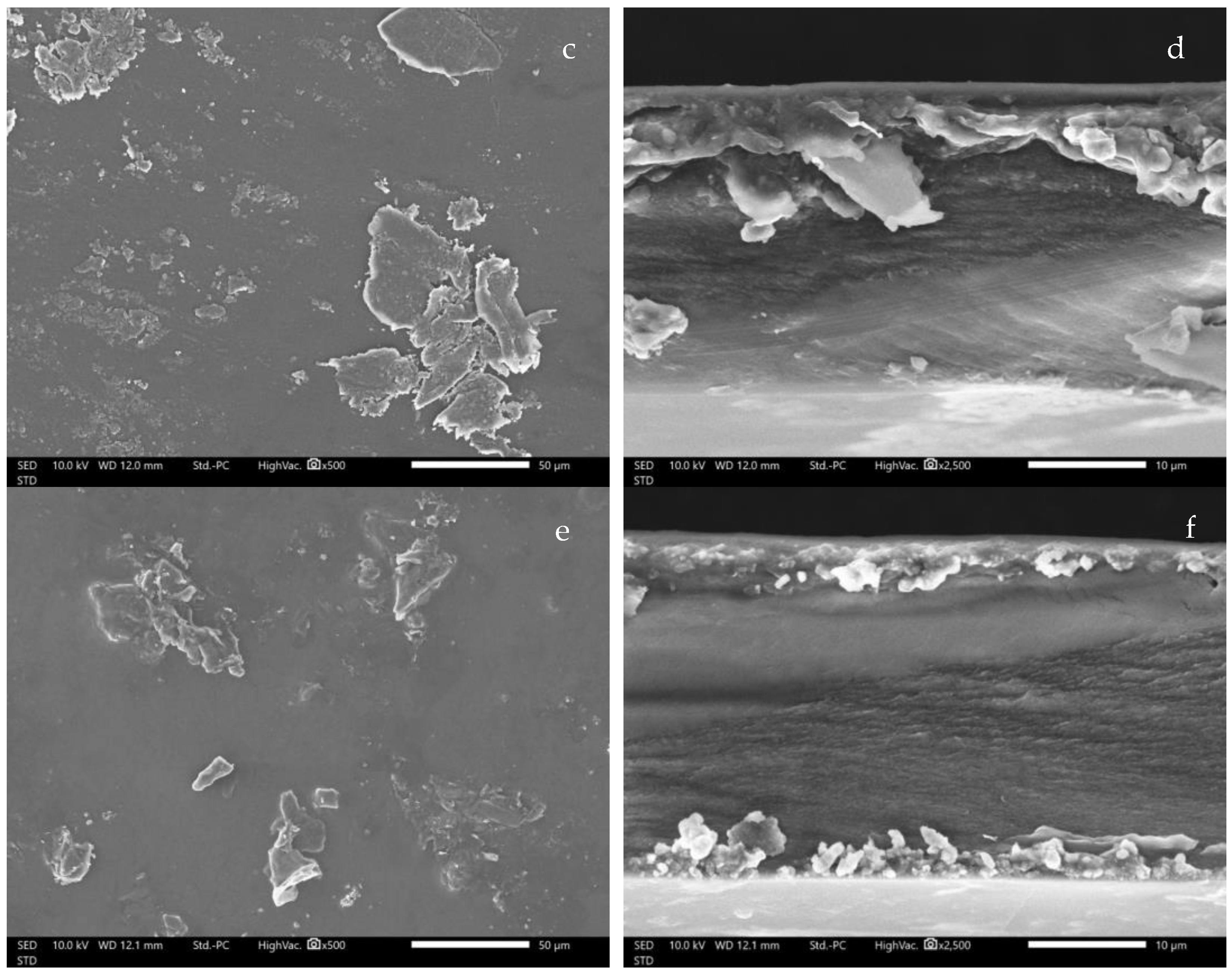
3.7. SEM image of CMCS-Ca2+ Nanoparticles Film
3.8. Clindamycin HCl Content in CMCS-Ca2+ Nanoparticles Film
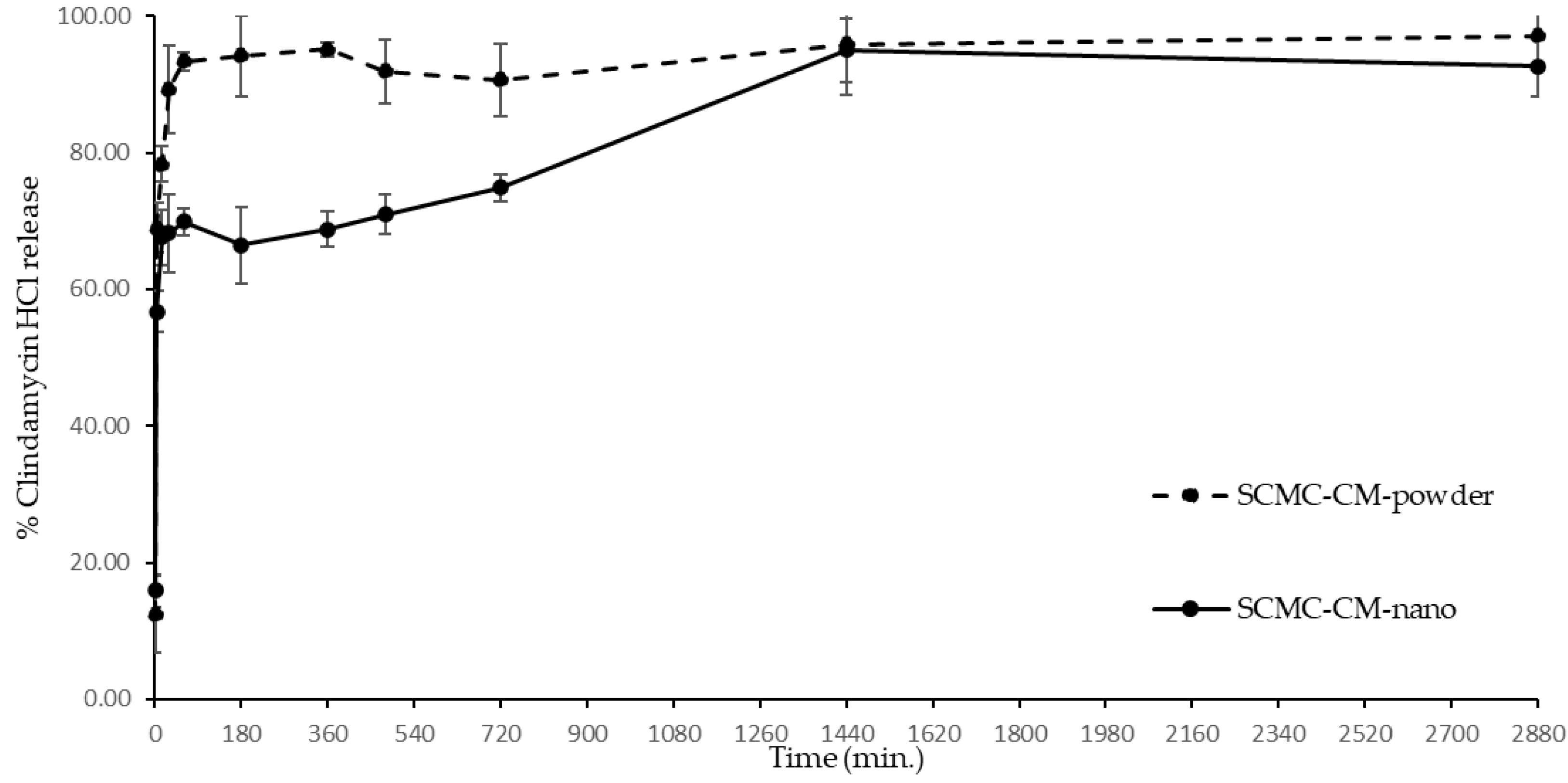
3.9. Clindamycin HCl Release
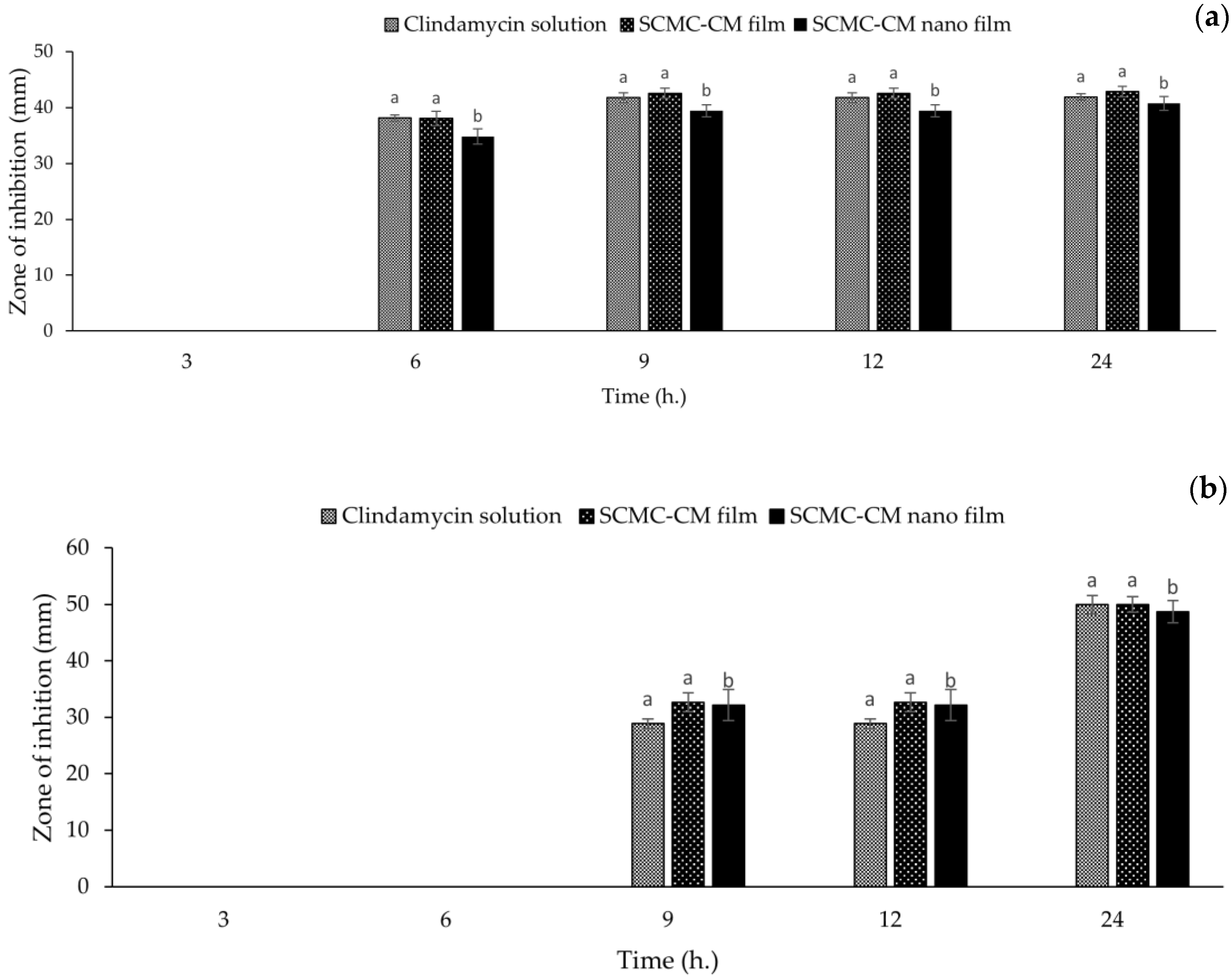
3.10. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M. Transdermal and Intravenous Nano Drug Delivery Systems: Present and Future. In Applications of Targeted Nano Drugs and Delivery Systems; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Oxford, UK, 2019; pp. 499–550. [Google Scholar]
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Couvreur, P. Nanoparticles in drug delivery: Past, present and future. Adv. Drug Deliv. Rev. 2013, 65, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Tajdini, F.; Amini, M.A.; Nafissi-Varcheh, N.; Faramarzi, M.A. Production, physiochemical and antimicrobial properties of fungal chitosan from Rhizomucor miehei and Mucor racemosus. Int. J. Biol. Macromol. 2010, 47, 180–183. [Google Scholar] [CrossRef]
- Sugimoto, M.; Morimoto, M.; Sashiwa, H.; Saimoto, H.; Shigemasa, Y. Preparation and characterization of water-soluble chitin and chitosan derivatives. Carbohydr. Polym. 1998, 36, 49–59. [Google Scholar] [CrossRef]
- Chen, X.G.; Park, H.J. Chemical characteristics of O-carboxymethyl chitosans related to the preparation conditions. Carbohydr. Polym. 2003, 53, 355–359. [Google Scholar] [CrossRef]
- Kalliola, S.; Repo, E.; Srivastava, V.; Heiskanen, J.P.; Sirviö, J.A.; Liimatainen, H.; Sillanpää, M. The pH sensitive properties of carboxymethyl chitosan nanoparticles cross-linked with calcium ions. Colloids. Surf. B Biointerfaces 2017, 153, 229–236. [Google Scholar] [CrossRef]
- Shi, X.; Du, Y.; Yang, J.; Zhang, B.; Sun, L. Effect of degree of substitution and molecular weight of carboxymethyl chitosan nanoparticles on doxorubicin delivery. J. Appl. Polym. Sci. 2006, 100, 4689–4696. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Naghibzadeh, M.; Amani, A.; Amini, M.; Esmaeilzadeh, E.; Mottaghi-Dastjerdi, N.; Faramarzi, M.A. An insight into the interactions between α-tocopherol and chitosan in ultrasound-prepared nanoparticles. J. Nanomater. 2010, 44, 1–7. [Google Scholar] [CrossRef]
- Barber, B.P.; Putterman, S.J. Observation of synchronous picosecond sonoluminescence. Nature 1991, 352, 318–320. [Google Scholar] [CrossRef]
- Sauter, C.; Emin, M.A.; Schuchmann, H.P.; Tavman, S. Influence of hydrostatic pressure and sound amplitude on the ultrasound induced dispersion and de-agglomeration of nanoparticles. Ultrason. Sonochem. 2008, 15, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Gani, A.; Hassan, I.; Huang, Q.; Shabbir, H. Production and characterization of starch nanoparticles by mild alkali hydrolysis and ultra-sonication process. Sci. Rep. 2020, 10, 3533. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.S.K.; Huang, M.; Lim, L.Y. Ultrasonication of chitosan and chitosan nanoparticles. Int. J. Pharm. 2003, 265, 103–114. [Google Scholar] [CrossRef]
- Tsai, M.L.; Bai, S.W.; Chen, R.H. Cavitation effects versus stretch effects resulted in different size and polydispersity of ionotropic gelation chitosan–sodium tripolyphosphate nanoparticle. Carbohydr. Polym. 2008, 71, 448–457. [Google Scholar] [CrossRef]
- Esmaeilzadeh-Gharedaghi, E.; Faramarzi, M.A.; Amini, M.A.; Rouholamini Najafabadi, A.; Rezayat, S.M.; Amani, A. Effects of processing parameters on particle size of ultrasound prepared chitosan nanoparticles: An artificial neural networks study. Pharm. Dev. Technol. 2012, 17, 638–647. [Google Scholar] [CrossRef]
- Smieja, M. Current indications for the use of clindamycin: A critical review. Can. J. Infect. Dis. 1998, 9, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Li, X.; Zhao, C. Strategies to Obtain Encapsulation and Controlled Release of Small Hydrophilic Molecules. Front. Bioeng. Biotechnol. 2020, 8, 437. [Google Scholar] [CrossRef]
- Gao, P.; Nie, X.; Zou, M.; Shi, Y.; Cheng, G. Recent advances in materials for extended-release antibiotic delivery system. J. Antibiot. Res. 2011, 64, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Hasan, N.; Cao, J.; Lee, J.; Hlaing, S.P.; Oshi, M.A.; Naeem, M.; Ki, M.-H.; Lee, B.L.; Jung, Y.; Yoo, J.-W. Bacteria-Targeted Clindamycin Loaded Polymeric Nanoparticles: Effect of Surface Charge on Nanoparticle Adhesion to MRSA, Antibacterial Activity, and Wound Healing. Pharmaceutics 2019, 11, 236. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Chen, Y.; Jiang, X.; Gui, Z.; Zhang, L. Development of Hydrophilic Drug Encapsulation and Controlled Release Using a Modified Nanoprecipitation Method. Processes 2019, 7, 331. [Google Scholar] [CrossRef] [Green Version]
- Donnadio, A.; Ambrogi, V.; Pietrella, D.; Pica, M.; Sorrentino, G.; Casciola, M. Carboxymethylcellulose films containing chlorhexidine–zirconium phosphate nanoparticles: Antibiofilm activity and cytotoxicity. RSC Adv. 2016, 6, 46249–46257. [Google Scholar] [CrossRef]
- Ng, S.-F.; Jumaat, N. Carboxymethyl cellulose wafers containing antimicrobials: A modern drug delivery system for wound infections. Eur. J. Pharm. Sci. 2014, 51, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Vimala, K.; Ravindra, S.; Narayana Reddy, N.; Venkata Subba Reddy, G.; Mohana Raju, K. Fabrication of silver nanocomposite films impregnated with curcumin for superior antibacterial applications. J. Mater. Sci. Mater. Med. 2011, 22, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Ghorpade, V.S.; Yadav, A.V.; Dias, R.J.; Mali, K.K.; Pargaonkar, S.S.; Shinde, P.V.; Dhane, N.S. Citric acid crosslinked carboxymethylcellulose-poly (ethylene glycol) hydrogel films for delivery of poorly soluble drugs. Int. J. Biol. Macromol. 2018, 118, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Junmahasathien, T.; Panraksa, P.; Protiarn, P.; Hormdee, D.; Noisombut, R.; Kantrong, N.; Jantrawut, P. Preparation and evaluation of metronidazole-loaded pectin films for potentially targeting a microbial infection associated with periodontal disease. Polymers 2018, 10, 1021. [Google Scholar] [CrossRef] [Green Version]
- Chaiwarit, T.; Rachtanapun, P.; Kantrong, N.; Jantrawut, P. Preparation of Clindamycin Hydrochloride Loaded De-Esterified Low-Methoxyl Mango Peel Pectin Film Used as a Topical Drug Delivery System. Polymers 2020, 12, 1006. [Google Scholar] [CrossRef]
- Popa-Nita, S.; Lucas, J.-M.; Ladavière, C.; David, L.; Domard, A. Mechanisms involved during the ultrasonically induced depolymerization of chitosan: Characterization and control. Biomacromolecules 2009, 10, 1203–1211. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Hickey, J.W.; Santos, J.L.; Williford, J.-M.; Mao, H.-Q. Control of polymeric nanoparticle size to improve therapeutic delivery. J. Control. Release 2015, 219, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, Y.; Lim, D.W.; Choi, J. Assessment of size-dependent antimicrobial and cytotoxic properties of silver nanoparticles. Adv. Mater. Sci. Eng. 2014, 2014, 763807. [Google Scholar] [CrossRef] [Green Version]
- Doshi, B.; Repo, E.; Heiskanen, J.P.; Sirviö, J.A.; Sillanpää, M. Effectiveness of N,O-carboxymethyl chitosan on destabilization of Marine Diesel, Diesel and Marine-2T oil for oil spill treatment. Carbohydr. Polym. 2017, 167, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Bidgoli, H.; Zamani, A.; Taherzadeh, M.J. Effect of carboxymethylation conditions on the water-binding capacity of chitosan-based superabsorbents. Carbohydr. Res. 2010, 345, 2683–2689. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Hassabo, A. Composite material based on pullulan/silane/ZnO-NPs as pH, thermo-sensitive and antibacterial agent for cellulosic fabrics. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 045005. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, S.; Wang, H.; Li, Y.; Kishen, A.; Deng, X.; Yang, X.; Wang, Y.; Cong, C.; Wang, H.; et al. Biomimetic Remineralization of Demineralized Dentine Using Scaffold of CMC/ACP Nanocomplexes in an In Vitro Tooth Model of Deep Caries. PLoS ONE 2015, 10, e0116553. [Google Scholar] [CrossRef]
- Sangnim, T.; Limmatvapirat, S.; Nunthanid, J.; Sriamornsak, P.; Sittikijyothin, W.; Wannachaiyasit, S.; Huanbutta, K. Design and characterization of clindamycin-loaded nanofiber patches composed of polyvinyl alcohol and tamarind seed gum and fabricated by electrohydrodynamic atomization. Asian J. Pharm. Sci. 2018, 13, 450–458. [Google Scholar] [CrossRef]
- Patel, P.; Patel, P. Formulation and evaluation of clindamycin HCL in situ gel for vaginal application. Int. J. Pharm. Investig. 2015, 5, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Rauta, P.R.; Das, N.M.; Nayak, D.; Ashe, S.; Nayak, B. Enhanced efficacy of clindamycin hydrochloride encapsulated in PLA/PLGA based nanoparticle system for oral delivery. IET Nanobiotechnol. 2016, 10, 254–261. [Google Scholar] [CrossRef]
- Ouazib, F.; Mokhnachi, N.B.; Haddadine, N.; Barille, R. Role of polymer/polymer and polymer/drug specific interactions in drug delivery systems. J. Polym. Eng. 2019, 39, 534–544. [Google Scholar] [CrossRef]
- Tolentino, S.; Pereira, M.N.; Cunha-Filho, M.; Gratieri, T.; Gelfuso, G.M. Targeted clindamycin delivery to pilosebaceous units by chitosan or hyaluronic acid nanoparticles for improved topical treatment of acne vulgaris. Carbohydr. Polym. 2021, 253, 117295. [Google Scholar] [CrossRef] [PubMed]
- Mohod, A.V.; Gogate, P.R. Ultrasonic degradation of polymers: Effect of operating parameters and intensification using additives for carboxymethyl cellulose (CMC) and polyvinyl alcohol (PVA). Ultrason. Sonochem. 2011, 18, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Samsi, M.S.; Kamari, A.; Din, S.M.; Lazar, G. Synthesis, characterization and application of gelatin–carboxymethyl cellulose blend films for preservation of cherry tomatoes and grapes. J. Food Sci. Technol. 2019, 56, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Kamaci, M.; Kaya, İ. Biodegradable and antibacterial poly(azomethine-urethane)-chitosan hydrogels for potential drug delivery application. Polym. Adv. Technol. 2020, 31, 898–908. [Google Scholar] [CrossRef]
- Kamaci, M.; Kaya, I. Melamine-based poly(azomethine) hydrogels: Mechanical, biodegradability, drug loading and antibacterial properties. Eur. Polym. J. 2018, 108, 107–115. [Google Scholar] [CrossRef]
- Kilicarslan, M.; Ilhan, M.; Inal, O.; Orhan, K. Preparation and evaluation of clindamycin phosphate loaded chitosan/alginate polyelectrolyte complex film as mucoadhesive drug delivery system for periodontal therapy. Eur. J. Pharm. Sci. 2018, 123, 441–451. [Google Scholar] [CrossRef]
- Sungkhaphan, P.; Thavornyutikarn, B.; Kaewkong, P.; Pongkittiphan, V.; Pornsuwan, S.; Singhatanadgit, W.; Janvikul, W. Antibacterial and osteogenic activities of clindamycin-releasing mesoporous silica/carboxymethyl chitosan composite hydrogels. R. Soc. Open Sci. 2021, 8, 210808. [Google Scholar] [CrossRef]
- Son, G.H.; Lee, B.J.; Cho, C.W. Mechanisms of drug release from advanced drug formulations such as polymeric-based drug-delivery systems and lipid nanoparticles. J. Pharm. Investig. 2017, 47, 287–296. [Google Scholar] [CrossRef]
- Lee, J.H.; Yeo, Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Kamaci, M.; Kaya, I. Preparation of biodegradable, and pH-sensitive poly(azomethine)-chitosan hydrogels for potential application of 5-fluoro uracil delivery. Eur. Polym. J. 2021, 158, 110680. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T.; Elliott, A.G.; Kavanagh, A.M.; Ramu, S.; Cooper, M.A. In vitro Antimicrobial Activity of Acne Drugs Against Skin-Associated Bacteria. Sci. Rep. 2019, 9, 14658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, S.; Liu, X.; Zhou, J.; Zhang, J.; Dong, A.; Huang, P.; Wang, W.; Deng, L. Dual-crosslinked nanocomposite hydrogels based on quaternized chitosan and clindamycin-loaded hyperbranched nanoparticles for potential antibacterial applications. Int. J. Biol. Macromol. 2020, 155, 153–162. [Google Scholar] [CrossRef] [PubMed]


| Variables | Level | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | α | |
| Amplitude (%) | 31.71 | 40 | 60 | 80 | 88.28 |
| Time (s) | 10.29 | 60 | 180 | 300 | 349.71 |
| Source | Sum of Squares | Mean Square | F-Value | p-Value |
|---|---|---|---|---|
| Model | 39,098.83 | 7819.77 | 131.77 | <0.0001 |
| A-Amplitude | 23,502.49 | 23,502.49 | 396.04 | <0.0001 |
| B-Time | 4571.19 | 4571.19 | 77.03 | <0.0001 |
| AB | 73.10 | 73.10 | 1.23 | 0.3037 |
| A2 | 8109.88 | 8109.88 | 136.66 | <0.0001 |
| B2 | 4173.52 | 4173.52 | 70.33 | <0.0001 |
| Residual | 415.41 | 59.34 | ||
| Lack of Fit | 148.45 | 49.48 | 0.7414 | 0.5804 |
| Pure error | 266.96 | 66.74 |
| Exp. Order | Variables | Response | PDI | ||
|---|---|---|---|---|---|
| Mean Diameter (nm) | |||||
| Amplitude (%) | Time (s) | Actual Value | Predicted Value | ||
| 1 | 40 | 60 | 478.40 ± 4.12 * | 477.57 * | 0.442 ± 0.025 |
| 2 | 80 | 60 | 384.20 ± 14.38 * | 377.71 * | 0.515 ± 0.094 |
| 3 | 40 | 300 | 432.70 ± 4.89 * | 438.31 * | 0.456 ± 0.093 |
| 4 | 80 | 300 | 321.40 ± 15.03 * | 321.36 * | 0.394 ± 0.040 |
| 5 | 31.72 | 180 | 493.57 ± 5.76 * | 490.04 * | 0.594 ± 0.147 |
| 6 | 88.28 | 180 | 323.30 ± 8.40 * | 336.73 * | 0.372 ± 0.044 |
| 7 | 60 | 10.29 | 422.87 ± 17.42 * | 427.89 * | 0.439 ± 0.101 |
| 8 | 60 | 349.71 | 364.42 ± 16.80 * | 360.28 * | 0.422 ± 0.065 |
| 9 | 60 | 180 | 341.11 ± 13.72 * | 345.10 * | 0.551 ± 0.106 |
| 10 | 60 | 180 | 333.50 ± 6.45 * | 345.10 * | 0.513 ± 0.185 |
| 11 | 60 | 180 | 347.31 ± 15.97 * | 345.10 * | 0.455 ± 0.042 |
| 12 | 60 | 180 | 355.12 ± 5.54 * | 345.10 * | 0.407 ± 0.011 |
| 13 | 60 | 180 | 348.47 ± 10.08 * | 345.10 * | 0.328 ± 0.030 |
| Test data | |||||
| 14 | 50 | 150 | 394.30 ± 4.50 * | 387.71 * | 0.316 ± 0.057 |
| 15 | 65 | 200 | 335.90 ± 6.71 * | 330.20 * | 0.236 ± 0.025 |
| 16 | 75 | 180 | 318.40 ± 7.56 * | 323.65 * | 0.289 ± 0.064 |
| 17 | 50 | 80 | 421.22 ± 10.45 * | 415.89 * | 0.489 ±0.164 |
| Exp. Order | Mean Diameter (nm) | Drug Content (%) |
|---|---|---|
| 1 | 478.40 ± 4.12 a | 46.88 ± 4.86 * |
| 2 | 384.20 ± 14.38 b | 41.45 ± 2.60 #◊ |
| 3 | 432.70 ± 4.89 c | 47.66 ± 3.51 * |
| 4 | 321.40 ± 15.03 d | 33.30 ± 2.04 † |
| 5 | 493.57 ± 5.76 a | 46.52 ± 2.91 * |
| 6 | 323.30 ± 8.40 df | 34.44 ± 1.71 † |
| 7 | 422.87 ± 17.42 c | 41.63 ± 1.53 #∆◊ |
| 8 | 364.42 ± 16.80 bhk | 31.93 ± 3.20 † |
| 9 | 341.11 ± 13.72 di | 34.26 ± 3.34 † |
| 10 | 333.50 ± 6.45 djm | 33.12 ± 1.77 † |
| 11 | 347.31 ± 15.97 fhijk | 33.54 ± 1.63 † |
| 12 | 355.12 ± 5.54 djkl | 31.63 ± 2.55 † |
| 13 | 348.47 ± 10.08 hilmn | 40.03 ± 2.91 ∆◊ |
| 14 | 394.30 ± 4.50 b | 31.99 ± 2.75 † |
| 15 | 335.90 ± 6.71 djn | 34.50 ± 1.17 † |
| 16 | 318.40 ± 7.56 dj | 34.68 ± 2.45 † |
| 17 | 421.22 ± 10.45 c | 41.02 ± 2.27 #◊ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiwarit, T.; Sommano, S.R.; Rachtanapun, P.; Kantrong, N.; Ruksiriwanich, W.; Kumpugdee-Vollrath, M.; Jantrawut, P. Development of Carboxymethyl Chitosan Nanoparticles Prepared by Ultrasound-Assisted Technique for a Clindamycin HCl Carrier. Polymers 2022, 14, 1736. https://doi.org/10.3390/polym14091736
Chaiwarit T, Sommano SR, Rachtanapun P, Kantrong N, Ruksiriwanich W, Kumpugdee-Vollrath M, Jantrawut P. Development of Carboxymethyl Chitosan Nanoparticles Prepared by Ultrasound-Assisted Technique for a Clindamycin HCl Carrier. Polymers. 2022; 14(9):1736. https://doi.org/10.3390/polym14091736
Chicago/Turabian StyleChaiwarit, Tanpong, Sarana Rose Sommano, Pornchai Rachtanapun, Nutthapong Kantrong, Warintorn Ruksiriwanich, Mont Kumpugdee-Vollrath, and Pensak Jantrawut. 2022. "Development of Carboxymethyl Chitosan Nanoparticles Prepared by Ultrasound-Assisted Technique for a Clindamycin HCl Carrier" Polymers 14, no. 9: 1736. https://doi.org/10.3390/polym14091736
APA StyleChaiwarit, T., Sommano, S. R., Rachtanapun, P., Kantrong, N., Ruksiriwanich, W., Kumpugdee-Vollrath, M., & Jantrawut, P. (2022). Development of Carboxymethyl Chitosan Nanoparticles Prepared by Ultrasound-Assisted Technique for a Clindamycin HCl Carrier. Polymers, 14(9), 1736. https://doi.org/10.3390/polym14091736