Intravitreal Injectable Hydrogels for Sustained Drug Delivery in Glaucoma Treatment and Therapy
Abstract
1. Introduction
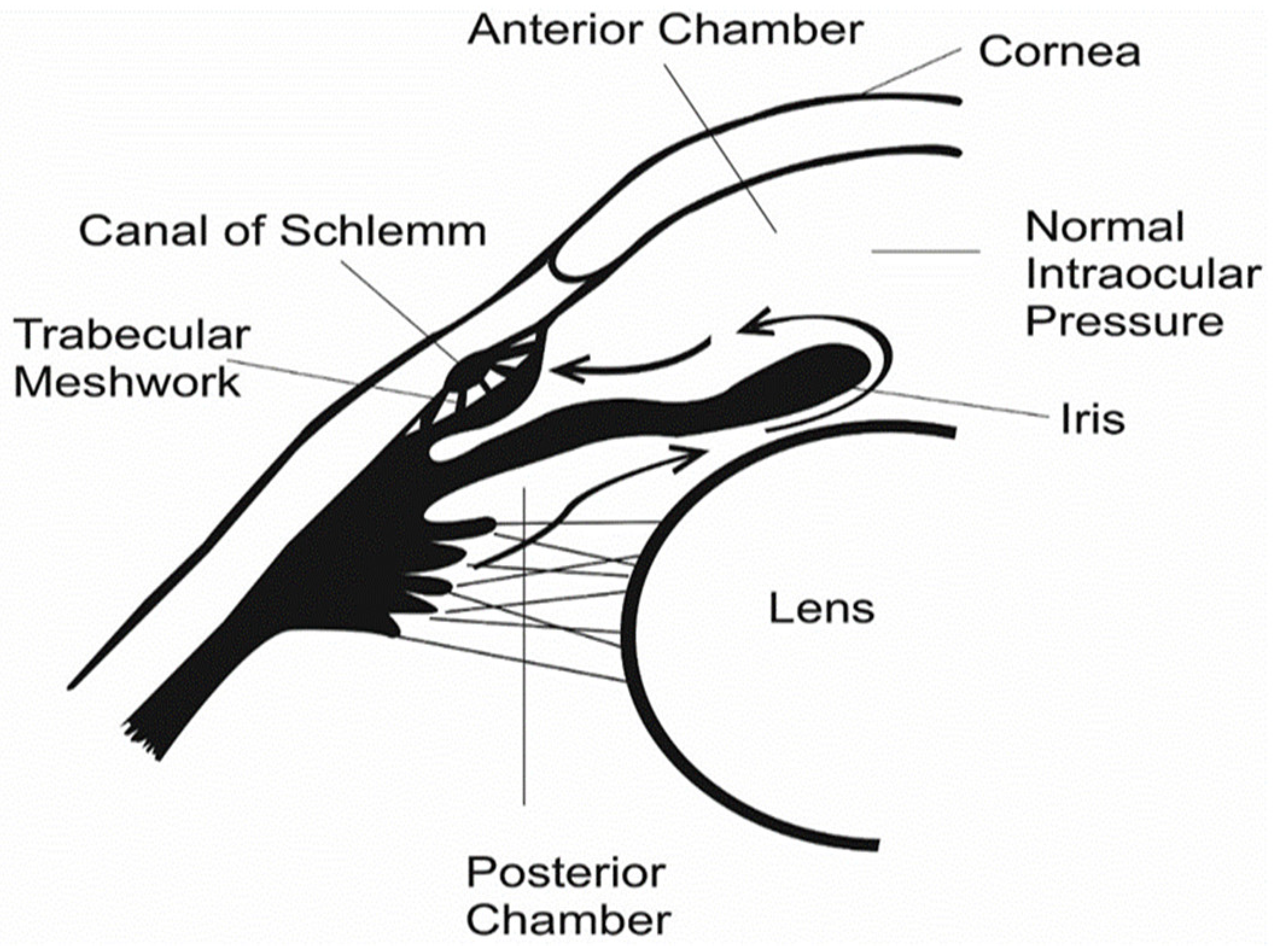
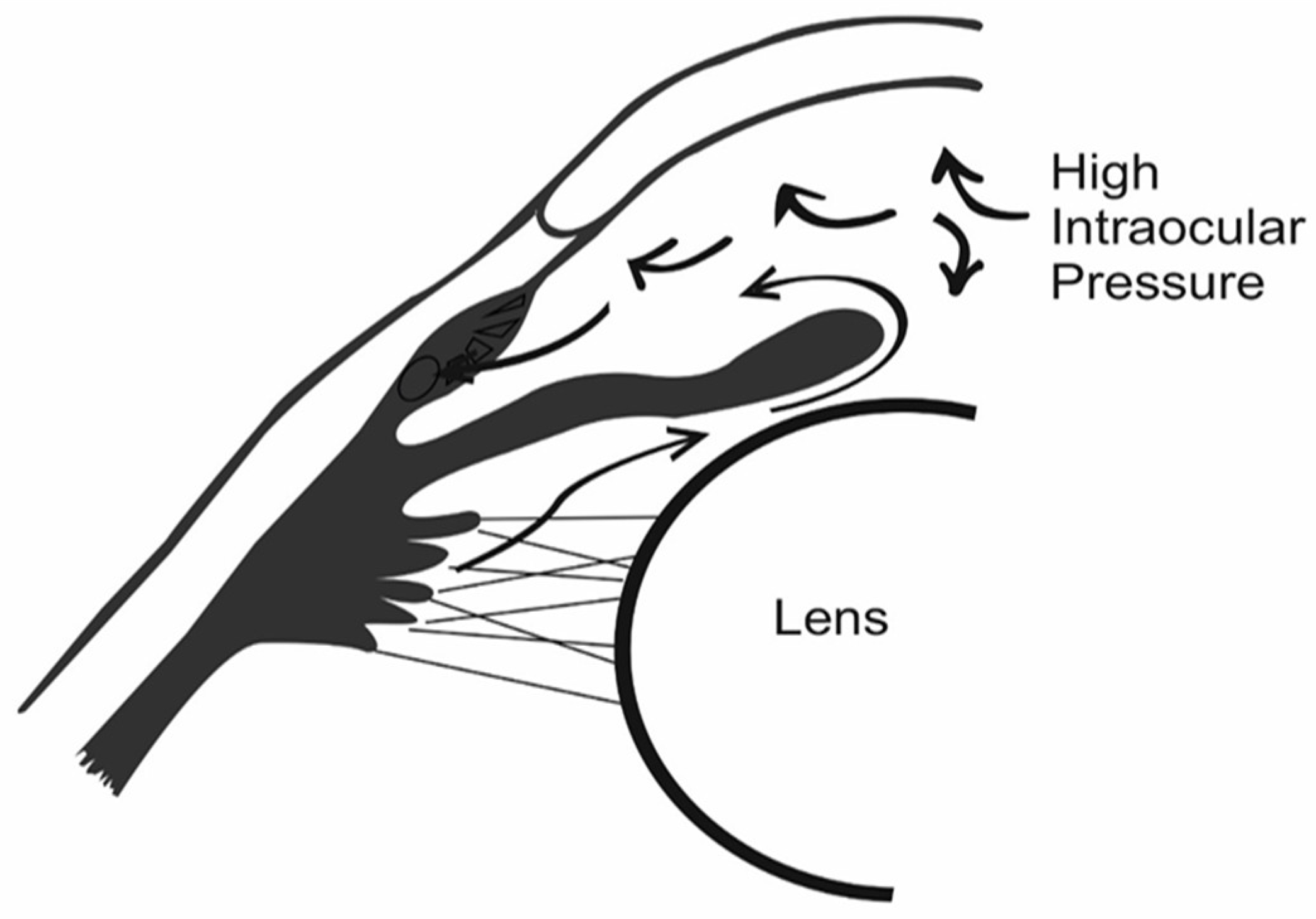
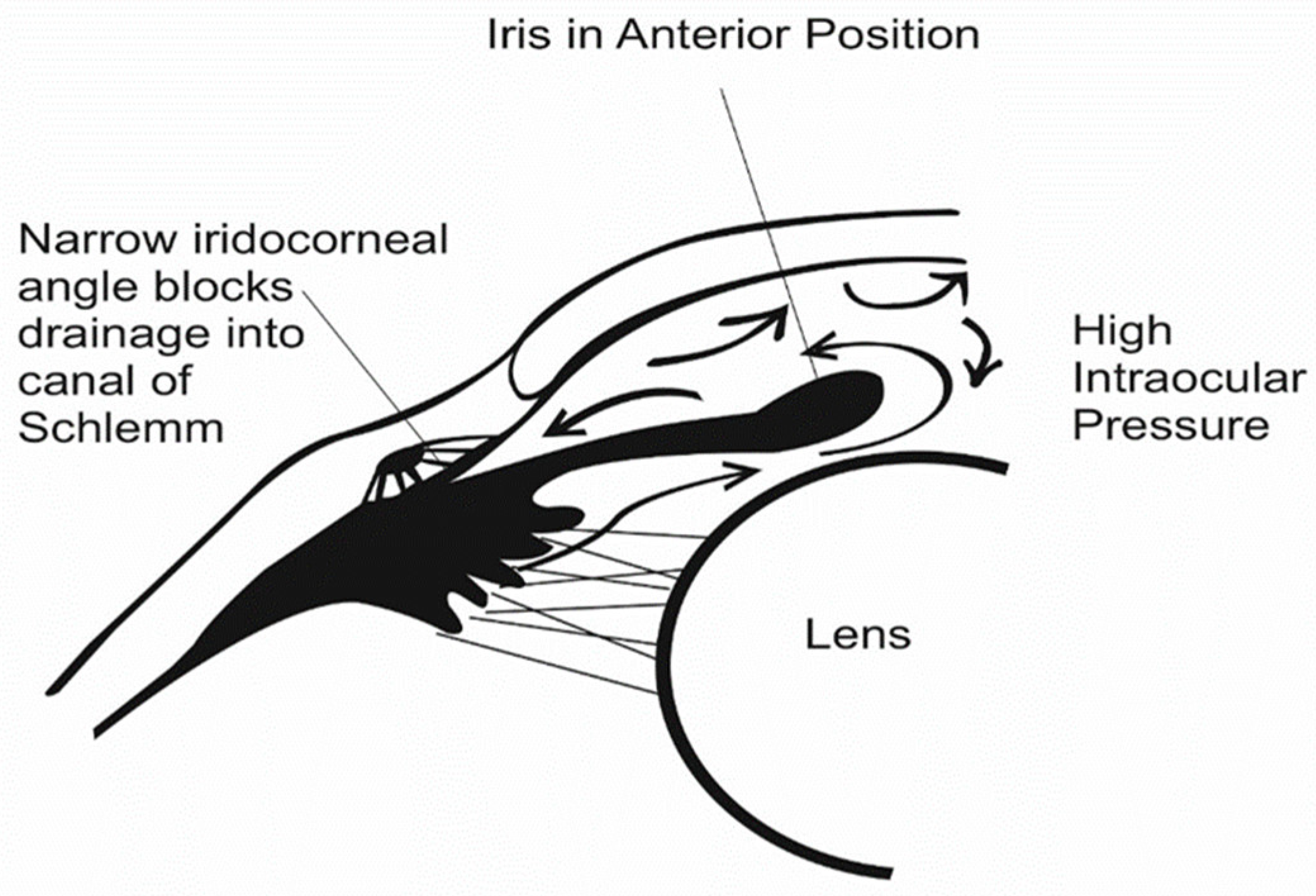
2. Glaucoma
3. Ocular Barriers
4. Current Therapies for Glaucoma
5. Constraints of Current Glaucoma Drug Delivery Treatment
5.1. Eye Drops and Eye Ointments
5.2. Trabeculectomy
5.3. Laser Treatment
5.4. Oral Medication
6. Current Pharmaceutical Interventions for the Treatment of Glaucoma
6.1. Beta-Adrenergic Blockers
6.2. PG Analogs
6.3. Alpha-Adrenergic Agonists
6.4. Carbonic Anhydrase Inhibitors
6.5. Miotic Agents
6.6. Hyperosmotic Agents
7. Natural Polymer-Based Hydrogels as Drug Delivery Vehicles for Glaucoma Therapy
7.1. Silk Fibroin
7.2. Chitosan
7.3. Alginic Acid
7.4. Pullulan
7.5. Hyaluronic Acid
7.6. Dextran
7.7. Methylcellulose
7.8. Gelatin
7.9. Collagen
8. Design of Hybrid Hydrogels for Injectable Drug Delivery in the Treatment of Glaucoma
8.1. Physicochemical, Pharmacokinetic, and Pharmacodynamic Properties of Ophthalmic Hydrogels
8.1.1. Drug Release Efficiency
8.1.2. Biocompatibility
8.1.3. Biodegradability
8.1.4. Porosity
8.1.5. Viscosity
8.1.6. Mechanical Strength
8.1.7. Swelling Properties
8.1.8. Rheology
8.1.9. Opacity and Transparency
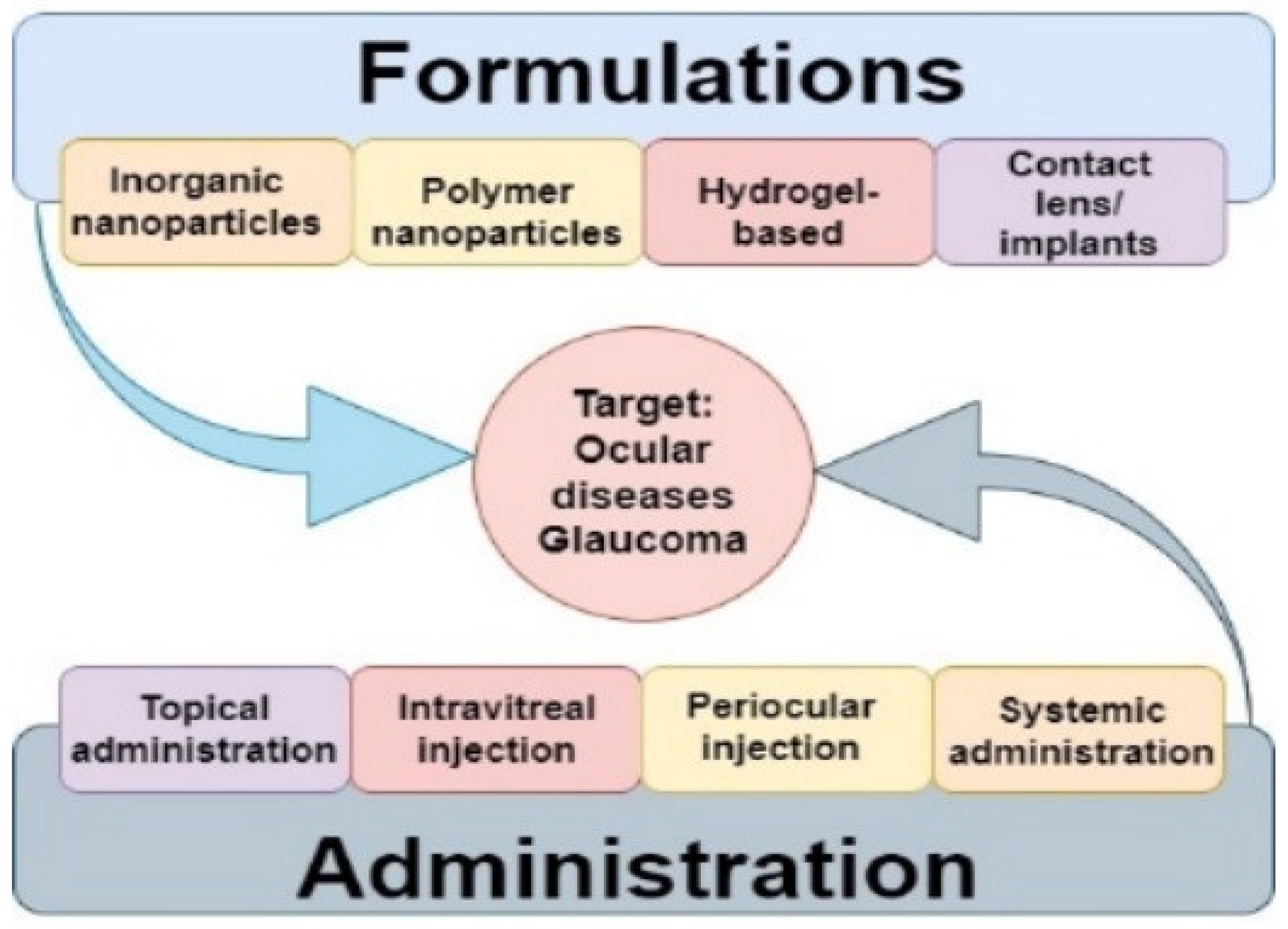
9. Intravitreal Administration of Injectable Drug-Loaded Hydrogels to The Eye
Alternative Injection Locations
10. Pharmacokinetics of Intravitreal Hydrogel Drug Release
11. Discussion
12. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bro, T.; Wickström, K.; Lindén, C. The future is old—Patients with topical ocular hypotensive treatment in the Nordic region between 2008 and 2017 with projections for 2040. Acta Ophthalmol. 2021, 99, e1442–e1448. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, G.M.; del Pozo, C.E.; Medina, J.R.; Naude, J.; Solorzano, A.B. Numerical simulation of the aqueous humor flow in the eye drainage system; a healthy and pathological condition comparison. Med. Eng. Phys. 2020, 83, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Cholkar, K.; Dasari, S.R.; Pal, D.; Mitra, A.K. Eye: Anatomy, physiology and barriers to drug delivery. In Ocular Transporters and Receptors; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–36. [Google Scholar]
- Modarreszadeh, S.; Abouali, O.; Ghaffarieh, A.; Ahmadi, G. Physiology of aqueous humor dynamic in the anterior chamber due to rapid eye movement. Physiol. Behav. 2014, 135, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Downie, L.E.; Bandlitz, S.; Bergmanson, J.P.; Craig, J.P.; Dutta, D.; Maldonado-Codina, C.; Ngo, W.; Siddireddy, J.S.; Wolffsohn, J.S. BCLA CLEAR—Anatomy and physiology of the anterior eye. Contact Lens Anterior Eye 2021, 44, 132–156. [Google Scholar] [CrossRef] [PubMed]
- Buffault, J.; Labbé, A.; Hamard, P.; Brignole-Baudouin, F.; Baudouin, C. The trabecular meshwork: Structure, function and clinical implications. A review of the literature. J. Fr. Ophtalmol. 2020, 43, e217–e230. [Google Scholar] [CrossRef]
- Silva, A.F.; Pimenta, F.; Alves, M.A.; Oliveira, M.S. Flow dynamics of vitreous humour during saccadic eye movements. J. Mech. Behav. Biomed. Mater. 2020, 110, 103860. [Google Scholar] [CrossRef]
- Yadav, K.S.; Rajpurohit, R.; Sharma, S. Glaucoma: Current treatment and impact of advanced drug delivery systems. Life Sci. 2019, 221, 362–376. [Google Scholar] [CrossRef]
- El Hoffy, N.M.; Azim, E.A.A.; Hathout, R.M.; Fouly, M.A.; Elkheshen, S.A. Glaucoma: Management and Future Perspectives for Nanotechnology-Based Treatment Modalities. Eur. J. Pharm. Sci. 2020, 158, 105648. [Google Scholar] [CrossRef]
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-Álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef]
- Baudouin, C.; Kolko, M.; Melik-Parsadaniantz, S.; Messmer, E.M. Inflammation in Glaucoma: From the back to the front of the eye, and beyond. Prog. Retin. Eye Res. 2020, 83, 100916. [Google Scholar] [CrossRef]
- Wu, A.; Khawaja, A.P.; Pasquale, L.R.; Stein, J.D. A review of systemic medications that may modulate the risk of glaucoma. Eye 2020, 34, 12–28. [Google Scholar] [CrossRef]
- Belay, D.B.; Derseh, M.; Damtie, D.; Shiferaw, Y.A.; Adigeh, S.C. Longitudinal analysis of intraocular pressure and its associated risk factors of glaucoma patients using Bayesian linear mixed model: A data from Felege Hiwot Hospital, Ethiopia. Sci. Afr. 2022, 16, e01160. [Google Scholar] [CrossRef]
- Yang, X.-L.; Van Der Merwe, Y.; Sims, J.; Parra, C.; Ho, L.C.; Schuman, J.S.; Wollstein, G.; Lathrop, K.L.; Chan, K.C. Age-related Changes in Eye, Brain and Visuomotor Behavior in the DBA/2J Mouse Model of Chronic Glaucoma. Sci. Rep. 2018, 8, 4643. [Google Scholar] [CrossRef] [PubMed]
- Haeck, I.M.; Rouwen, T.J.; Mik, L.T.-D.; de Bruin-Weller, M.S.; Bruijnzeel-Koomen, C.A. Topical corticosteroids in atopic dermatitis and the risk of glaucoma and cataracts. J. Am. Acad. Dermatol. 2011, 64, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, P.; Carradori, S.; Campestre, C.; Poce, G. Novel therapies for glaucoma: A patent review (2013–2019). Expert Opin. Ther. Patents 2019, 29, 769–780. [Google Scholar] [CrossRef]
- Singh, M.; Dev, D.; Prasad, D. A Recent Overview: In Situ Gel Smart Carriers for Ocular Drug Delivery. J. Drug Deliv. Ther. 2021, 11, 195–205. [Google Scholar] [CrossRef]
- Bahram, M.; Mohseni, N.; Moghtader, M. An Introduction to Hydrogels and Some Recent Applications. In Emerging Concepts in Analysis and Applications of Hydrogels; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Xi, L.; Wang, T.; Zhao, F.; Zheng, Q.; Li, X.; Luo, J.; Liu, J.; Quan, D.; Ge, J. Evaluation of an Injectable Thermosensitive Hydrogel As Drug Delivery Implant for Ocular Glaucoma Surgery. PLoS ONE 2014, 9, e100632. [Google Scholar] [CrossRef]
- Wang, K.; Han, Z. Injectable hydrogels for ophthalmic applications. J. Control. Release 2017, 268, 212–224. [Google Scholar] [CrossRef]
- Cordeiro, F.; Tian, K.; Shibata-Germanos, S.; Pahlitzsch, M. Current perspective of neuroprotection and glaucoma. Clin. Ophthalmol. 2015, 9, 2109–2118. [Google Scholar] [CrossRef]
- Pakravan, M.; Yazdani, S.; Javadi, M.-A.; Amini, H.; Behroozi, Z.; Ziaei, H.; Katibeh, M.; Solaimanizad, R.; Ghahari, E.; Yaseri, M. A Population-based Survey of the Prevalence and Types of Glaucoma in Central Iran. Ophthalmology 2013, 120, 1977–1984. [Google Scholar] [CrossRef]
- Bertaud, S.; Aragno, V.; Baudouin, C.; Labbé, A. Le glaucome primitif à angle ouvertPrimary open-angle glaucoma. Rev. Méd. Interne 2018, 40, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Artero-Castro, A.; Rodriguez-Jimenez, F.J.; Jendelova, P.; VanderWall, K.B.; Meyer, J.S.; Erceg, S. Glaucoma as a Neurodegenerative Disease Caused by Intrinsic Vulnerability Factors. Prog. Neurobiol. 2020, 193, 101817. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Dai, Y.; Chen, Y.; Yu, D.-Y.; Cringle, S.J.; Chen, J.; Kong, X.; Wang, X.; Jiang, C. Primary angle closure glaucoma: What we know and what we don’t know. Prog. Retin. Eye Res. 2017, 57, 26–45. [Google Scholar] [CrossRef]
- Kesav, N.; Palestine, A.G.; Kahook, M.Y.; Pantcheva, M.B. Current management of uveitis-associated ocular hypertension and glaucoma. Surv. Ophthalmol. 2019, 65, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Janagam, D.R.; Wu, L.; Lowe, T.L. Nanoparticles for drug delivery to the anterior segment of the eye. Adv. Drug Deliv. Rev. 2017, 122, 31–64. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Figueiras, A.; Veiga, F. Improvements in Topical Ocular Drug Delivery Systems: Hydrogels and Contact Lenses. J. Pharm. Pharm. Sci. 2015, 18, 683–695. [Google Scholar] [CrossRef]
- Kompella, U.B.; Hartman, R.R.; Patil, M.A. Extraocular, periocular, and intraocular routes for sustained drug delivery for glaucoma. Prog. Retin. Eye Res. 2020, 82, 100901. [Google Scholar] [CrossRef]
- Anjali, S.; Rameshwar, D.; Shivani, D.; Ranjit, S. Hydrogels in ophthalmic drug delivery system—A mini review. Asian Pac. J. Health Sci. 2018, 5, 96–104. [Google Scholar] [CrossRef]
- Wadhwa, A.; Jadhav, C.; Yadav, K.S. Bimatoprost: Promising novel drug delivery systems in treatment of glaucoma. J. Drug Deliv. Sci. Technol. 2022, 69, 103156. [Google Scholar] [CrossRef]
- Kumar, V.; Abu Zaalan, K.A.; Bezzabotnov, A.I.; Dushina, G.N.; Shradqa, A.S.S.; Rustamova, Z.S.; Frolov, M.A. Bleb-Independent Glaucoma Surgery to Activate the Uveolymphatic Route of Non-Trabecular Aqueous Humor Outflow: Short-Term Clinical and OCT Results. Vision 2022, 6, 4. [Google Scholar] [CrossRef]
- Yu, S.; Wang, Q.-M.; Wang, X.; Liu, D.; Zhang, W.; Ye, T.; Yang, X.; Pan, W. Liposome incorporated ion sensitive in situ gels for opthalmic delivery of timolol maleate. Int. J. Pharm. 2015, 480, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Occhiutto, M.L.; Maranhão, R.C.; Costa, V.P.; Konstas, A.G. Nanotechnology for Medical and Surgical Glaucoma Therapy—A Review. Adv. Ther. 2020, 37, 155–199. [Google Scholar] [CrossRef] [PubMed]
- Tabak, S.; Schreiber-Avissar, S.; Beit-Yannai, E. Influence of Anti-Glaucoma Drugs on Uptake of Extracellular Vesicles by Trabecular Meshwork Cells. Int. J. Nanomed. 2021, 16, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Ilochonwu, B.C.; Urtti, A.; Hennink, W.E.; Vermonden, T. Intravitreal hydrogels for sustained release of therapeutic proteins. J. Control. Release 2020, 326, 419–441. [Google Scholar] [CrossRef] [PubMed]
- Waduthanthri, K.D.; He, Y.; Montemagno, C.; Cetinel, S. An injectable peptide hydrogel for reconstruction of the human trabecular meshwork. Acta Biomater. 2019, 100, 244–254. [Google Scholar] [CrossRef]
- Jayanetti, V.; Sandhu, S.; Lusthaus, J.A. The Latest Drugs in Development That Reduce Intraocular Pressure in Ocular Hypertension and Glaucoma. J. Exp. Pharmacol. 2020, 12, 539–548. [Google Scholar] [CrossRef]
- Arimura, S.; Miyake, S.; Iwasaki, K.; Gozawa, M.; Matsumura, T.; Takamura, Y.; Inatani, M. Randomised Clinical Trial for Postoperative Complications after Ex-PRESS Implantation versus Trabeculectomy with 2-Year Follow-Up. Sci. Rep. 2018, 8, 16168. [Google Scholar] [CrossRef]
- Gedde, S.J.; Feuer, W.J.; Shi, W.; Lim, K.S.; Barton, K.; Goyal, S.; Ahmed, I.I.; Brandt, J.D.; Banitt, M.; Budenz, D.; et al. Treatment Outcomes in the Primary Tube Versus Trabeculectomy Study after 1 Year of Follow-up. Ophthalmology 2018, 125, 650–663. [Google Scholar] [CrossRef]
- Ma, A.; Yu, S.W.; Wong, J.K. Micropulse laser for the treatment of glaucoma: A literature review. Surv. Ophthalmol. 2019, 64, 486–497. [Google Scholar] [CrossRef]
- Lee, D.A.; Higginbotham, E.J. Glaucoma and its treatment: A review. Am. J. Health Syst. Pharm. 2005, 62, 691–699. [Google Scholar] [CrossRef]
- Miller, P.E.; Eaton, J.S. Medical anti-glaucoma therapy: Beyond the drop. Vet. Ophthalmol. 2020, 24, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Peng, T.; Li, Y.; Zhan, Z.; Zeng, Y.; Huang, Y.; Pan, X.; Wu, C.-Y.; Wu, C. Ocular Cubosome Drug Delivery System for Timolol Maleate: Preparation, Characterization, Cytotoxicity, Ex Vivo, and In Vivo Evaluation. AAPS PharmSciTech 2017, 18, 2919–2926. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Mercado, H.; Ivri, E.; Gonzalez-Salinas, R.; Kourtis, I.C.; Gilbert, J.; Pérez-Vázquez, J.F.; Blumenkranz, M.; Jiménez-Román, J.; Marcellino, G. Clinical Evaluation of a Novel Electromechanical Topical Ocular Drug Delivery System: Two Phase 1 Proof of Concept Studies. Clin. Ophthalmol. 2020, 14, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, A.; Ceruso, M.; Bua, S.; Lomelino, C.L.; Andring, J.T.; McKenna, R.; Lanzi, C.; Sgambellone, S.; Pecori, R.; Matucci, R.; et al. Discovery of β-Adrenergic Receptors Blocker–Carbonic Anhydrase Inhibitor Hybrids for Multitargeted Antiglaucoma Therapy. J. Med. Chem. 2018, 61, 5380–5394. [Google Scholar] [CrossRef] [PubMed]
- Holló, G.; Katsanos, A.; Boboridis, K.G.; Irkec, M.; Konstas, A.G.P. Preservative-Free Prostaglandin Analogs and Prostaglandin/Timolol Fixed Combinations in the Treatment of Glaucoma: Efficacy, Safety and Potential Advantages. Drugs 2017, 78, 39–64. [Google Scholar] [CrossRef]
- Lusthaus, J.; Goldberg, I. Current management of glaucoma. Med. J. Aust. 2019, 210, 180–187. [Google Scholar] [CrossRef]
- Chae, J.J.; Jung, J.H.; Zhu, W.; Gerberich, B.G.; Fard, M.R.B.; Grossniklaus, H.E.; Ethier, C.R.; Prausnitz, M.R. Drug-Free, Nonsurgical Reduction of Intraocular Pressure for Four Months after Suprachoroidal Injection of Hyaluronic Acid Hydrogel. Adv. Sci. 2020, 8, 2001908. [Google Scholar] [CrossRef]
- Figus, M.; Agnifili, L.; Lanzini, M.; Brescia, L.; Sartini, F.; Mastropasqua, L.; Posarelli, C. Topical preservative-free ophthalmic treatments: An unmet clinical need. Expert Opin. Drug Deliv. 2020, 18, 655–672. [Google Scholar] [CrossRef]
- Xu, E.; Campanella, O.H.; Ye, X.; Jin, Z.; Liu, D.; BeMiller, J.N. Advances in conversion of natural biopolymers: A reactive extrusion (REX)–enzyme-combined strategy for starch/protein-based food processing. Trends Food Sci. Technol. 2020, 99, 167–180. [Google Scholar] [CrossRef]
- Samadian, H.; Maleki, H.; Fathollahi, A.; Salehi, M.; Gholizadeh, S.; Derakhshankhah, H.; Allahyari, Z.; Jaymand, M. Naturally occurring biological macromolecules-based hydrogels: Potential biomaterials for peripheral nerve regeneration. Int. J. Biol. Macromol. 2020, 154, 795–817. [Google Scholar] [CrossRef]
- Meng, L.; Shao, C.; Cui, C.; Xu, F.; Lei, J.; Yang, J. Autonomous Self-Healing Silk Fibroin Injectable Hydrogels Formed via Surfactant-Free Hydrophobic Association. ACS Appl. Mater. Interfaces 2020, 12, 1628–1639. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Calado, R.; Marto, J.; Bettencourt, A.; Almeida, A.J.; Gonçalves, L.M.D. Chitosan Nanoparticles as a Mucoadhesive Drug Delivery System for Ocular Administration. Mar. Drugs 2017, 15, 370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, L.; Chen, J.; Wang, L.; Gui, X.; Ran, J.; Xu, G.; Zhao, H.; Zeng, M.; Ji, J.; et al. Silk Fibroin Biomaterial Shows Safe and Effective Wound Healing in Animal Models and a Randomized Controlled Clinical Trial. Adv. Healthc. Mater. 2017, 6, 1700121. [Google Scholar] [CrossRef]
- Suzuki, S.; Shadforth, A.; McLenachan, S.; Zhang, D.; Chen, S.-C.; Walshe, J.; Lidgerwood, G.; Pébay, A.; Chirila, T.V.; Chen, F.; et al. Optimization of silk fibroin membranes for retinal implantation. Mater. Sci. Eng. C 2019, 105, 110131. [Google Scholar] [CrossRef]
- Tulay, P.; Galam, N.; Adali, T. The Wonders of Silk Fibroin Biomaterials in the Treatment of Breast Cancer. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 129–134. [Google Scholar] [CrossRef]
- Galam, N.; Tulay, P.; Adali, T. In Vitro MCF-7 Cells Apoptosis Analysis of Carboplatin Loaded Silk Fibroin Particles. Molecules 2020, 25, 1110. [Google Scholar] [CrossRef]
- Abdel-Naby, W.; Cole, B.; Liu, A.; Liu, J.; Wan, P.; Guaiquil, V.H.; Schreiner, R.; Infanger, D.; Lawrence, B.D.; Rosenblatt, M.I. Silk-derived protein enhances corneal epithelial migration, adhesion, and proliferation. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1425–1433. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Fernández-Pérez, J.; Ahearne, M. Potential for combined delivery of riboflavin and all-trans retinoic acid, from silk fibroin for corneal bioengineering. Mater. Sci. Eng. C 2019, 105, 110093. [Google Scholar] [CrossRef]
- Applegate, M.B.; Partlow, B.P.; Coburn, J.; Marelli, B.; Pirie, C.; Pineda, R.; Kaplan, D.L.; Omenetto, F.G. Photocrosslinking of silk fibroin using riboflavin for ocular prostheses. Adv. Mater. 2016, 28, 2417–2420. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Kumorek, M.; Minisy, I.; Krunclová, T.; Voršiláková, M.; Venclíková, K.; Chánová, E.M.; Janoušková, O.; Kubies, D. pH-responsive and antibacterial properties of self-assembled multilayer films based on chitosan and tannic acid. Mater. Sci. Eng. C 2019, 109, 110493. [Google Scholar] [CrossRef] [PubMed]
- Highley, C.B.; Rodell, C.B.; Burdick, J.A. Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Adv. Mater. 2015, 27, 5075–5079. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, J.; Wang, Y.; Yin, Y.; Wang, L.; Liu, J.; Liu, Z.; Duan, C.; Zhu, P.; Wang, C. Promotion of cardiac differentiation of brown adipose derived stem cells by chitosan hydrogel for repair after myocardial infarction. Biomaterials 2014, 35, 3986–3998. [Google Scholar] [CrossRef]
- Cho, I.S.; Park, C.G.; Huh, B.K.; Cho, M.O.; Khatun, Z.; Li, Z.; Kang, S.-W.; Bin Choy, Y.; Huh, K.M. Thermosensitive hexanoyl glycol chitosan-based ocular delivery system for glaucoma therapy. Acta Biomater. 2016, 39, 124–132. [Google Scholar] [CrossRef]
- Skwarczynska, A.L.; Binias, D.; Maniukiewicz, W.; Modrzejewska, Z.; Douglas, T.E. The mineralization effect on chitosan hydrogel structure containing collagen and alkaline phosphatase. J. Mol. Struct. 2019, 1187, 86–97. [Google Scholar] [CrossRef]
- Franca, J.R.; Foureaux, G.; Fuscaldi, L.L.; Ribeiro, T.G.; Castilho, R.O.; Yoshida, M.I.; Cardoso, V.N.; Fernandes, S.O.; Cronemberger, S.; Nogueira, J.C.; et al. Chitosan/hydroxyethyl cellulose inserts for sustained-release of dorzolamide for glaucoma treatment: In vitro and in vivo evaluation. Int. J. Pharm. 2019, 570, 118662. [Google Scholar] [CrossRef]
- Kumirska, J.; Weinhold, M.X.; Thöming, J.; Stepnowski, P. Biomedical Activity of Chitin/Chitosan Based Materials—Influence of Physicochemical Properties Apart from Molecular Weight and Degree of N-Acetylation. Polymers 2011, 3, 1875–1901. [Google Scholar] [CrossRef]
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.M.; Xanthopoulou, E.; Bikiaris, D.N. Chitosan and its Derivatives for Ocular Delivery Formulations: Recent Advances and Developments. Polymers 2020, 12, 1519. [Google Scholar] [CrossRef]
- Popa, L.; Ghica, M.V.; Dinu-Pîrvu, C.E.; Irimia, T. Chitosan: A good candidate for sustained release ocular drug delivery systems. In Chitin-Chitosan—Myriad Functionalities in Science and Technology; InTech: London UK, 2018; pp. 283–310. [Google Scholar]
- Dekamin, M.G.; Karimi, Z.; Latifidoost, Z.; Ilkhanizadeh, S.; Daemi, H.; Naimi-Jamal, M.R.; Barikani, M. Alginic acid: A mild and renewable bifunctional heterogeneous biopolymeric organocatalyst for efficient and facile synthesis of polyhydroquinolines. Int. J. Biol. Macromol. 2018, 108, 1273–1280. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Ishii, D.; Iwata, T. Synthesis and characterization of alginic acid ester derivatives. Carbohydr. Polym. 2017, 171, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Manna, S.; Kabiraj, S.; Jana, S. Recent progress in alginate-based carriers for ocular targeting of therapeutics. Food Hydrocoll. Health 2022, 2, 100071. [Google Scholar] [CrossRef]
- Reddy, S.G. Alginates—A Seaweed Product: Its Properties and Applications; IntechOpen: Rijeka, Croatia, 2021; Chapter 2. [Google Scholar] [CrossRef]
- Panyamao, P.; Ruksiriwanich, W.; Sirisa-Ard, P.; Charumanee, S. Injectable Thermosensitive Chitosan/Pullulan-Based Hydrogels with Improved Mechanical Properties and Swelling Capacity. Polymers 2020, 12, 2514. [Google Scholar] [CrossRef] [PubMed]
- Saeaeh, K.; Thummarungsan, N.; Paradee, N.; Choeichom, P.; Phasuksom, K.; Lerdwijitjarud, W.; Sirivat, A. Soft and highly responsive multi-walled carbon nanotube/pullulan hydrogel composites as electroactive materials. Eur. Polym. J. 2019, 120, 109231. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Danti, S.; De Clerck, K.; Lazzeri, A.; Morganti, P. Pullulan for Advanced Sustainable Body- and Skin-Contact Applications. J. Funct. Biomater. 2020, 11, 20. [Google Scholar] [CrossRef]
- Larrañeta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent-free process for potential biomedical applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef]
- Egbu, R.; Brocchini, S.; Khaw, P.T.; Awwad, S. Antibody loaded collapsible hyaluronic acid hydrogels for intraocular delivery. Eur. J. Pharm. Biopharm. 2018, 124, 95–103. [Google Scholar] [CrossRef]
- Chang, W.-H.; Liu, P.-Y.; Lin, M.-H.; Lu, C.-J.; Chou, H.-Y.; Nian, C.-Y.; Jiang, Y.-T.; Hsu, Y.-H. Applications of Hyaluronic Acid in Ophthalmology and Contact Lenses. Molecules 2021, 26, 2485. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Ko, Y.-C.; Chang, Y.-F.; Huang, S.-H.; Liu, C.J.-L. Thermosensitive chitosan-gelatin-based hydrogel containing curcumin-loaded nanoparticles and latanoprost as a dual-drug delivery system for glaucoma treatment. Exp. Eye Res. 2019, 179, 179–187. [Google Scholar] [CrossRef]
- Campos, F.D.; Cassimiro, D.L.; Crespi, M.S.; Almeida, A.E.; Gremião, M.P. Preparation and characterisation of Dextran-70 hydrogel for controlled release of praziquantel. Braz. J. Pharm. Sci. 2013, 49, 75–83. [Google Scholar] [CrossRef]
- Yao, Y.; Saw, P.E.; Nie, Y.; Wong, P.-P.; Jiang, L.; Ye, X.; Chen, J.; Ding, T.; Xu, L.; Yao, H.; et al. Multifunctional sharp pH-responsive nanoparticles for targeted drug delivery and effective breast cancer therapy. J. Mater. Chem. B 2018, 7, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Kilic Bektas, C.; Burcu, A.; Gedikoglu, G.; Telek, H.H.; Ornek, F.; Hasirci, V. Methacrylated gelatin hydrogels as corneal stroma substitutes: In vivo study. J. Biomater. Sci. Polym. Ed. 2019, 30, 1803–1821. [Google Scholar] [CrossRef]
- Silva, S.S.; Fernandes, E.M.; Pina, S.; Silva-Correia, J.; Vieira, S.; Oliveira, J.M.; Reis, R.L. 2.11 Polymers of biological origin. Compr. Biomater. 2017, 2, 228–252. [Google Scholar]
- Bonetti, L.; De Nardo, L.; Farè, S. Thermo-Responsive Methylcellulose Hydrogels: From Design to Applications as Smart Biomaterials. Tissue Eng. Part B Rev. 2021, 27, 486–513. [Google Scholar] [CrossRef]
- Thrimawithana, T.; Young, S.; Bunt, C.; Green, C.; Alany, R. In-vitro and in-vivo evaluation of carrageenan/methylcellulose polymeric systems for transscleral delivery of macromolecules. Eur. J. Pharm. Sci. 2011, 44, 399–409. [Google Scholar] [CrossRef]
- Gupta, B.; Mishra, V.; Gharat, S.; Momin, M.; Omri, A. Cellulosic Polymers for Enhancing Drug Bioavailability in Ocular Drug Delivery Systems. Pharmaceuticals 2021, 14, 1201. [Google Scholar] [CrossRef]
- Kojima, N.; Tao, F.; Mihara, H.; Aoki, S. Methods for Engineering of Multicellular Spheroids to Reconstitute the Liver Tissue. In Stem Cells and Cancer in Hepatology; Academic Press: Cambridge, MA, USA, 2018; pp. 145–158. [Google Scholar] [CrossRef]
- El-Feky, G.S.; Zayed, G.; Elshaier, Y.; Alsharif, F.M. Chitosan-Gelatin Hydrogel Crosslinked with Oxidized Sucrose for the Ocular Delivery of Timolol Maleate. J. Pharm. Sci. 2018, 107, 3098–3104. [Google Scholar] [CrossRef]
- Wong, F.S.Y.; Tsang, K.K.; Chu, A.M.W.; Chan, B.; Yao, K.M.; Lo, A.C.Y. Injectable cell-encapsulating composite alginate-collagen platform with inducible termination switch for safer ocular drug delivery. Biomaterials 2019, 201, 53–67. [Google Scholar] [CrossRef]
- Sun, J.; Lei, Y.; Dai, Z.; Liu, X.; Huang, T.; Wu, J.; Xu, Z.P.; Sun, X. Sustained Release of Brimonidine from a New Composite Drug Delivery System for Treatment of Glaucoma. ACS Appl. Mater. Interfaces 2017, 9, 7990–7999. [Google Scholar] [CrossRef]
- Gaspar-Pintiliescu, A.; Stefan, L.M.; Anton, E.D.; Berger, D.; Matei, C.; Negreanu-Pirjol, T.; Moldovan, L. Physicochemical and Biological Properties of Gelatin Extracted from Marine Snail Rapana venosa. Mar. Drugs 2019, 17, 589. [Google Scholar] [CrossRef]
- He, Z.; Xiong, L. Evaluation of Biological Properties of Collagen/Hyaluronic Acid Composite Scaffolds. Polym. Polym. Compos. 2013, 21, 457–462. [Google Scholar] [CrossRef]
- Jain, E.; Hill, L.; Canning, E.; Sell, S.A.; Zustiak, S.P. Control of gelation, degradation and physical properties of polyethylene glycol hydrogels through the chemical and physical identity of the crosslinker. J. Mater. Chem. B 2017, 5, 2679–2691. [Google Scholar] [CrossRef] [PubMed]
- Peroglio, M.; Grad, S.; Mortisen, D.; Sprecher, C.M.; Illien-Junger, S.; Alini, M.; Eglin, D. Injectable thermoreversible hyaluronan-based hydrogels for nucleus pulposus cell encapsulation. Eur. Spine J. 2011, 21, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Ilka, R.; Mohseni, M.; Kianirad, M.; Naseripour, M.; Ashtari, K.; Mehravi, B. Nanogel-based natural polymers as smart carriers for the controlled delivery of Timolol Maleate through the cornea for glaucoma. Int. J. Biol. Macromol. 2018, 109, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Peng, X.; Qiao, J.; Jiang, Z.; Han, B.; Yang, C.; Liu, W. Evaluation of a photocrosslinkable hydroxyethyl chitosan hydrogel as a potential drug release system for glaucoma surgery. J. Mater. Sci. Mater. Med. 2017, 28, 149. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Hsieh, A.-C. A gelatin-g-poly(N-isopropylacrylamide) biodegradable in situ gelling delivery system for the intracameral administration of pilocarpine. Biomaterials 2011, 33, 2372–2387. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Liu, X.; Zhuang, X.; Liu, Y.; Li, S. Co-delivery of dexamethasone and melatonin by drugs laden PLGA nanoparticles for the treatment of glaucoma. J. Drug Deliv. Sci. Technol. 2020, 60, 102086. [Google Scholar] [CrossRef]
- Göttel, B.; Silva, J.M.D.S.E.; de Oliveira, C.S.; Syrowatka, F.; Fiorentzis, M.; Viestenz, A.; Viestenz, A.; Mäder, K. Electrospun nanofibers—A promising solid in-situ gelling alternative for ocular drug delivery. Eur. J. Pharm. Biopharm. 2020, 146, 125–132. [Google Scholar] [CrossRef]
- Liu, M.; Liu, N.L.M.; Zang, R.; Li, Y.; Yang, S.-T. Engineering stem cell niches in bioreactors. World J. Stem Cells 2013, 5, 124–135. [Google Scholar] [CrossRef]
- Anamizu, M.; Tabata, Y.C. Design of injectable hydrogels of gelatin and alginate with ferric ions for cell transplantation. Acta Biomater. 2019, 100, 184–190. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Stenekes, R.; Talsma, H.; Hennink, W. Formation of dextran hydrogels by crystallization. Biomaterials 2001, 22, 1891–1898. [Google Scholar] [CrossRef]
- Cooper, R.C.; Yang, H. Hydrogel-based ocular drug delivery systems: Emerging fabrication strategies, applications, and bench-to-bedside manufacturing considerations. J. Control. Release 2019, 306, 29–39. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef]
- Sharma; Deohra, A.; Reddy, K.R.; Sadhu, V. Biocompatible in-situ gelling polymer hydrogels for treating ocular infection. Methods Microbiol. 2019, 46, 93–114. [Google Scholar] [CrossRef]
- Zong, H.; Wang, B.; Li, G.; Yan, S.; Zhang, K.; Shou, Y.; Yin, J. Biodegradable High-Strength Hydrogels with Injectable Performance Based on Poly(l-Glutamic Acid) and Gellan Gum. ACS Biomater. Sci. Eng. 2020, 6, 4702–4713. [Google Scholar] [CrossRef]
- He, M.; Shi, L.; Wang, G.; Cheng, Z.; Han, L.; Zhang, X.; Wang, C.; Wang, J.; Zhou, P.; Wang, G. Biocompatible and biodegradable chitosan/sodium polyacrylate polyelectrolyte complex hydrogels with smart responsiveness. Int. J. Biol. Macromol. 2019, 155, 1245–1251. [Google Scholar] [CrossRef]
- Mallick, S.P.; Suman, D.K.; Singh, B.N.; Srivastava, P.; Siddiqui, N.; Yella, V.R.; Madhual, A.; Vemuri, P.K. Strategies toward development of biodegradable hydrogels for biomedical applications. Polym. Technol. Mater. 2020, 59, 911–927. [Google Scholar] [CrossRef]
- Bao, G.; Jiang, T.; Ravanbakhsh, H.; Reyes, A.; Ma, Z.; Strong, M.; Wang, H.; Kinsella, J.M.; Li, J.; Mongeau, L. Triggered micropore-forming bioprinting of porous viscoelastic hydrogels. Mater. Horizons 2020, 7, 2336–2347. [Google Scholar] [CrossRef] [PubMed]
- Dragon, E.S.; Dinu, M.V. Advances in porous chitosan-based composite hydrogels: Synthesis and applications. React. Funct. Polym. 2020, 146, 104372. [Google Scholar] [CrossRef]
- Champeau; Heinze, D.A.; Viana, T.N.; De Souza, E.R.; Chinellato, A.C.; Titotto, S. 4D Printing of Hydrogels: A Review. Adv. Funct. Mater. 2020, 30, 1910606. [Google Scholar] [CrossRef]
- Qin, Z.; Yu, X.; Wu, H.; Yang, L.; Lv, H.; Yang, X. Injectable and Cytocompatible Dual Cross-Linking Hydrogels with Enhanced Mechanical Strength and Stability. ACS Biomater. Sci. Eng. 2020, 6, 3529–3538. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.Y.; Lee, D.S. Advances in biodegradable and injectable hydrogels for biomedical applications. J. Control. Release 2021, 330, 151–160. [Google Scholar] [CrossRef]
- Sahranavard, M.; Zamanian, A.; Ghorbani, F.; Shahrezaee, M.H. A critical review on three dimensional-printed chitosan hydrogels for development of tissue engineering. Bioprinting 2020, 17, e00063. [Google Scholar] [CrossRef]
- Zhu, Z.; Ling, S.; Yeo, J.; Zhao, S.; Tozzi, L.; Buehler, M.J.; Omenetto, F.; Li, C.; Kaplan, D.L. High-Strength, Durable All-Silk Fibroin Hydrogels with Versatile Processability toward Multifunctional Applications. Adv. Funct. Mater. 2018, 28, 1704757. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, K.; Niu, L.; Zhang, Y.; Liu, Y.; Wang, C.; Chu, F. Highly mechanical properties nanocomposite hydrogels with biorenewable lignin nanoparticles. Int. J. Biol. Macromol. 2019, 128, 414–420. [Google Scholar] [CrossRef]
- Kowalski, G.; Kijowska, K.; Witczak, M.; Kuterasiński, Ł.; Łukasiewicz, M. Synthesis and Effect of Structure on Swelling Properties of Hydrogels Based on High Methylated Pectin and Acrylic Polymers. Polymers 2019, 11, 114. [Google Scholar] [CrossRef]
- Martin, N.; Youssef, G. Dynamic properties of hydrogels and fiber-reinforced hydrogels. J. Mech. Behav. Biomed. Mater. 2018, 85, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Kiyotake, E.A.; Douglas, A.W.; Thomas, E.E.; Nimmo, S.L.; Detamore, M.S. Development and quantitative characterization of the precursor rheology of hyaluronic acid hydrogels for bioprinting. Acta Biomater. 2019, 95, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Leone, G.; Consumi, M.; Pepi, S.; Pardinia, A.; Bonechiac, C.; Tamasiac, G.; Donatiac, A.; Lamponiab, S.; Rossiac, C.; Magnani, A. Enriched Gellan Gum hydrogel as visco-supplement. Carbohydr. Polyme. 2020, 227, 115–347. [Google Scholar]
- Luo, K.; Upadhyay, K.; Subhash, G.; Spearot, D.E. Transient-State Rheological Behavior of Poly(ethylene glycol) Diacrylate Hydrogels at High Shear Strain Rates. Macromolecules 2019, 52, 5860–5871. [Google Scholar] [CrossRef]
- Gyles, D.A.; Castro, L.D.; Silva, J.O.C.; Costa, R.M.R. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Sun, W.; Chen, G.; Wang, F.; Qin, Y.; Wang, Z.; Nie, J.; Ma, G. Polyelectrolyte-complex multilayer membrane with gradient porous structure based on natural polymers for wound care. Carbohydr. Polym. 2018, 181, 183–190. [Google Scholar] [CrossRef]
- Polat, T.G.; Duman, O.; Tunç, S. Preparation and characterization of environmentally friendly agar/κ-carrageenan/montmorillonite nanocomposite hydrogels. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 124987. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Luo, L.; Lai, J. Dendritic Effects of Injectable Biodegradable Thermogels on Pharmacotherapy of Inflammatory Glaucoma-Associated Degradation of Extracellular Matrix. Adv. Healthc. Mater. 2019, 8, e1900702. [Google Scholar] [CrossRef]
- Mealy, J.E.; Chung, J.J.; Jeong, H.; Issadore, D.; Lee, D.; Atluri, P.; Burdick, J.A. Injectable Granular Hydrogels with Multifunctional Properties for Biomedical Applications. Adv. Mater. 2018, 30, 1705912. [Google Scholar] [CrossRef]
- Vernon, R.B.; Gooden, M.D.; Preisinger, A.; Gebe, J.A. Controlled release of monoclonal antibodies from poly-l-lysine-coated alginate spheres within a scaffolded implant mitigates autoimmune responses to transplanted islets and limits systemic antibody toxicity. Mater. Sci. Eng. C 2018, 93, 390–398. [Google Scholar] [CrossRef]
- Zarembinski, T.I.; Doty, N.J.; Erickson, I.E.; Srinivas, R.; Wirostko, B.M.; Tew, W.P. Thiolated hyaluronan-based hydrogels crosslinked using oxidized glutathione: An injectable matrix designed for ophthalmic applications. Acta Biomater. 2014, 10, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Anwary, M.; Kumar, P.; du Toit, L.C.; Choonara, Y.E.; Pillay, V. Polymeric, injectable, intravitreal hydrogel devices for posterior segment applications and interventions. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Colino, A.; Quinteros, D.; Allemandi, D.A.; Girotti, A.; Palma, S.D.; Arias, F.J. Self-Assembling Elastin-Like Hydrogels for Timolol Delivery: Development of an Ophthalmic Formulation Against Glaucoma. Mol. Pharm. 2017, 14, 4498–4508. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Gooch, N.; Burr, R.M.; Holt, D.J.; Gale, B.; Ambati, B. Design and in Vitro Biocompatibility of a Novel Ocular Drug Delivery Device. J. Funct. Biomater. 2013, 4, 14–26. [Google Scholar] [CrossRef]
- Papadopoulos, Z. Recent Developments in the Treatment of Wet Age-related Macular Degeneration. Curr. Med. Sci. 2020, 40, 851–857. [Google Scholar] [CrossRef]
- Natu, M.V.; Gaspar, M.N.; Ribeiro, C.F.; Cabrita, A.; de Sousa, H.C.; Gil, M.H. In vitro and in vivo evaluation of an intraocular implant for glaucoma treatment. Int. J. Pharm. 2011, 415, 73–82. [Google Scholar] [CrossRef][Green Version]
- Tambe, S.; Jain, D.; Amin, P. Simultaneous determination of dorzolamide and timolol by first-order derivative UV spectroscopy in simulated biological fluid for in vitro drug release testing. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 255, 119682. [Google Scholar] [CrossRef]
- Sommersperger, M.; Weiss, J.; Nasseri, M.A.; Gehlbach, P.L.; Iordachita, I.; Navab, N. Real-time tool to layer distance estimation for robotic subretinal injection using intraoperative 4D OCT. Biomed. Opt. Express 2021, 12, 1085–1104. [Google Scholar] [CrossRef]
- Hwang, D.D.-J.; Kim, Y.W.; Woo, S.J.; Park, K.H. Comparison of Systemic Adverse Events Associated with Intravitreal Anti-VEGF Injection: Ranibizumab versus Bevacizumab. J. Korean Med. Sci. 2012, 27, 1580–1585. [Google Scholar] [CrossRef]
- Jonas, J.; Rensch, F. Intravitreal Steroid Slow-Release Device Replacing Repeated Intravitreal Triamcinolone Injections for Sympathetic Ophthalmia. Eur. J. Ophthalmol. 2008, 18, 834–836. [Google Scholar] [CrossRef] [PubMed]
- Nasef, M.M.; Aly, A.A.; Saidi, H.; Ahmad, A. Optimization of reaction parameters of radiation induced grafting of 1-vinylimidazole onto poly(ethylene-co-tetraflouroethene) using response surface method. Radiat. Phys. Chem. 2011, 80, 1222–1227. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, J.; Li, Y.; Huang, J.; Huang, Z.; Huang, Y.; Wu, C. Thermo-sensitive gel in glaucoma therapy for enhanced bioavailability: In vitro characterization, in vivo pharmacokinetics and pharmacodynamics study. Life Sci. 2018, 212, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Al-Kinani, A.A.; Zidan, G.; Elsaid, N.; Seyfoddin, A.; Alani, A.W.G.; Alany, R.G. Ophthalmic gels: Past, present and future. Adv. Drug Deliv. Rev. 2018, 126, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Voss, K.; Falke, K.; Bernsdorf, A.; Grabow, N.; Kastner, C.; Sternberg, K.; Hovakimyan, M. Development of a novel injectable drug delivery system for subconjunctival glaucoma treatment. J. Control. Release 2015, 214, 1–11. [Google Scholar] [CrossRef]
| Polymers | Delivery System | Drug Used | Feature | Reference |
|---|---|---|---|---|
| Silk fibroin | Nanoparticles | TM | TM caused a sustained and prolonged reduction in IOP without adverse effects on the physiology of the eye compared to conventional free drug use. | [99] |
| Hydroxyethyl chitosan | Hydrogel | Heparin | The heparin-loaded hydroxyethyl chitosan hydrogel was able to sustain and improve the reduction in the IOP after GFS for protracted periods of time. Clear inflammatory responses and results were not seen in the eye during the trial’s timeframe. | [100] |
| Gelatin-g-poly(N-isopropylacrylamide) | Hydrogel | Pilocarpine | Pilocarpine-loaded gelatin hydrogels were designed by grafting with carboxylic end-capped poly(N-isopropylacrylamide) for anti-glaucoma treatment by intracameral administration. | [101] |
| Poly (lactic-co-glycolic acid) (PLGA) | Nanoparticles | Dexamethasone and melatonin | A dual-loaded melatonin and dexamethasone poly(lactic-co-glycolic acid) nanoparticle system was designed as an anti-glaucoma treatment option. The in vitro release of the loaded drug from the nanoparticles revealed a supported delivery profile for the two medications, with no signs of burst discharge. | [102] |
| Gellan gum/pullulan | Nanofibers, in situ gel | Fluorescein sodium | Development of a novel fluorescein delivery system that is applied topically in dry nanofibrous form and gelates in situ immediately after administration guaranteed a solid match to the eye structure by the designed nanofibers, which were molded into conforming geometries. Prolongation of the ocular drugs’ residence time was achieved | [103] |
| Chtosan/hydroxyethyl cellulose | Ocular inserts | Dorzolamide | Dorzolamide-loaded ocular inserts were effective in glaucoma treatment. The ophthalmologic drug embedded in the polymeric matrix displayed a 3-h drug release efficiency, and released 75% of the loaded drug. | [104] |
| Alginate–chitosan | Nanoparticles/nanogels | TM | This study proposed that loading and delivering TM onto alginate–chitosan nanoparticles may be a suitable drug delivery approach for controlled delivery of TM through the cornea | [99] |
| Natural Biopolymer | Gelation | Strengths | Drawbacks | Reference |
|---|---|---|---|---|
| Silk fibroin | Ionic crosslinking, hydrophobic interactions | Easily modified | Low mechanical strength | [53] |
| Chitosan | Chemical crosslinking, pH gelation | Simple to adjust | Low dissolvability at neutral pH | [54] |
| Alginate | Chemical gelation, ionic crosslinking | Favorable mechanical properties, rapid gelation | Poor cytoadhesion | [55] |
| Gelatin | Chemical crosslinking | Hydrophilic, various responses available | Susceptible to degradation, poor mechanical properties, | [94] |
| Pullulan | Chemical crosslinking | Easily dissolvable | Undesirable swelling properties and mechanical properties | [77] |
| Methylcellulose | Hydrophobic, chemical, physical | Easy modification of physiochemical properties | Uncontrollable degradation, poor cell adhesion, poor mechanical properties | [74,89] |
| Dextran | Chemical crosslinking, physical crosslinking | Simple crosslinking, large capacity, hydrophilic, controlled drug release | Prone to causing in vivo side effects | [71,90] |
| Hyaluronic acid | High temperature (specific to contact with other polymers), chemical gelation | Simple modification, natural vitreous component (ECM), bioactive | High viscosity, susceptible to degradation | [80] |
| Collagen | Chemical crosslinking, high temperature | Natural ECM component, favorable cell adhesion, | Susceptible to degradation, strenuous dissolution | [85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akulo, K.A.; Adali, T.; Moyo, M.T.G.; Bodamyali, T. Intravitreal Injectable Hydrogels for Sustained Drug Delivery in Glaucoma Treatment and Therapy. Polymers 2022, 14, 2359. https://doi.org/10.3390/polym14122359
Akulo KA, Adali T, Moyo MTG, Bodamyali T. Intravitreal Injectable Hydrogels for Sustained Drug Delivery in Glaucoma Treatment and Therapy. Polymers. 2022; 14(12):2359. https://doi.org/10.3390/polym14122359
Chicago/Turabian StyleAkulo, Kassahun Alula, Terin Adali, Mthabisi Talent George Moyo, and Tulin Bodamyali. 2022. "Intravitreal Injectable Hydrogels for Sustained Drug Delivery in Glaucoma Treatment and Therapy" Polymers 14, no. 12: 2359. https://doi.org/10.3390/polym14122359
APA StyleAkulo, K. A., Adali, T., Moyo, M. T. G., & Bodamyali, T. (2022). Intravitreal Injectable Hydrogels for Sustained Drug Delivery in Glaucoma Treatment and Therapy. Polymers, 14(12), 2359. https://doi.org/10.3390/polym14122359







