Slow Release and Water Retention Performance of Poly(acrylic acid-co-acrylamide)/Fulvic Acid/Oil Shale Semicoke Superabsorbent Composites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
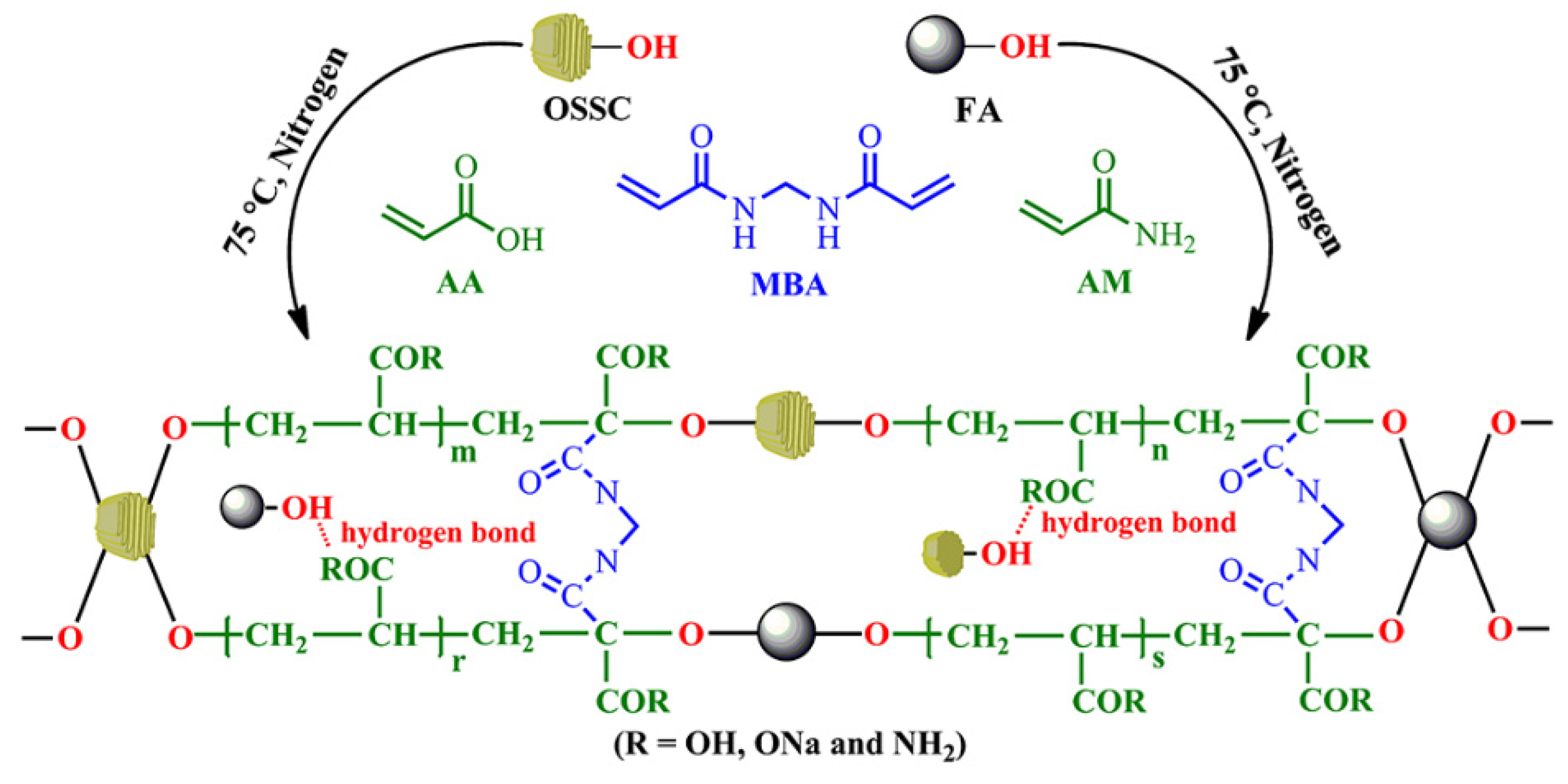
2.2. Synthesis of Superabsorbent Composites
2.3. Characterization
2.4. Swelling Kinetics of Superabsorbent Composites in Water
2.5. Reswelling Capability and Retention Behavior in Distilled Water and Soil
2.6. Slow Release Behavior in Water and Different Salt Concentrations
2.7. Soilcolumn Leaching Experiment
2.8. Pot Culture Experiment
3. Results and Discussion
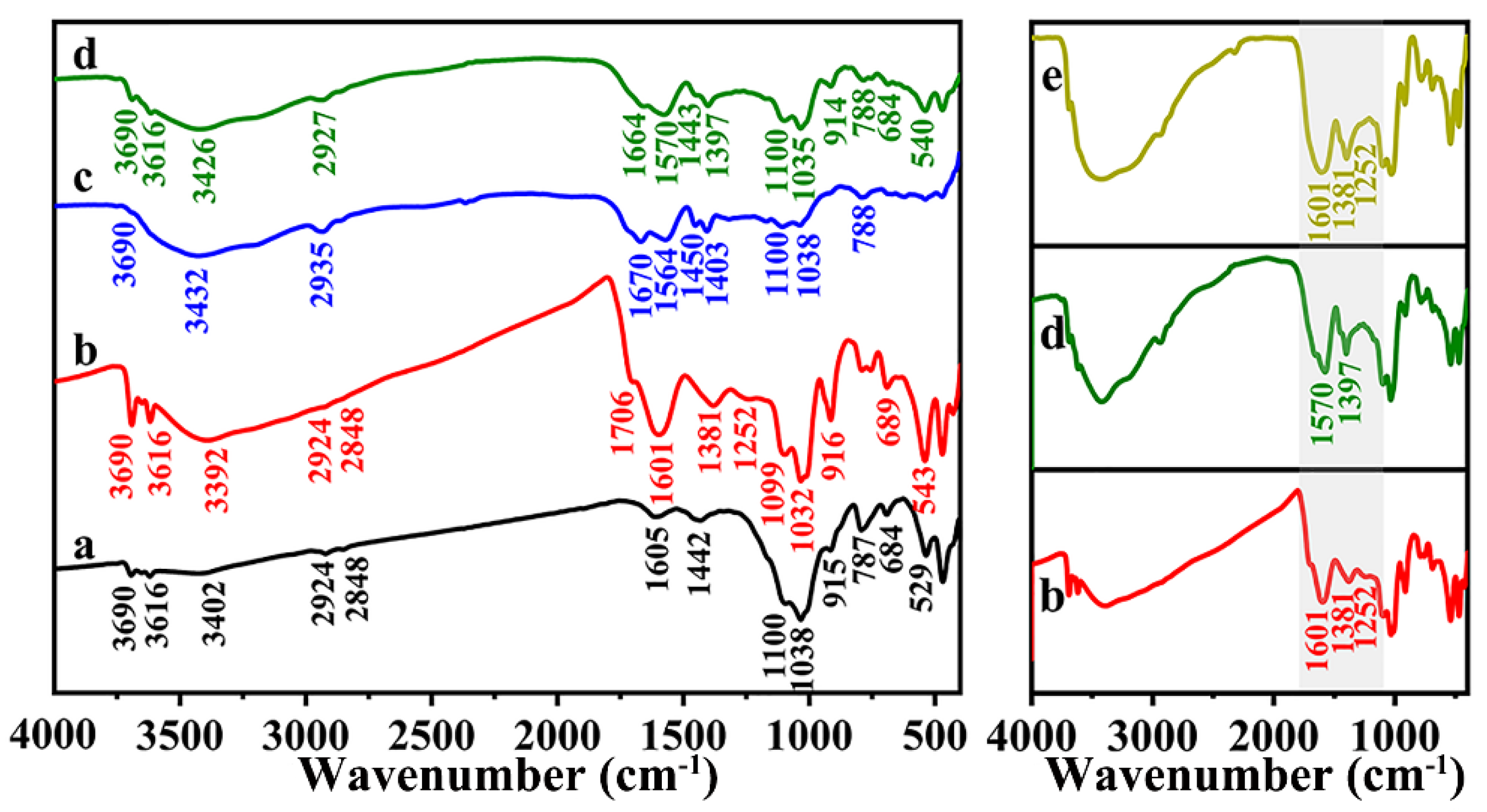
3.1. FTIR Analysis
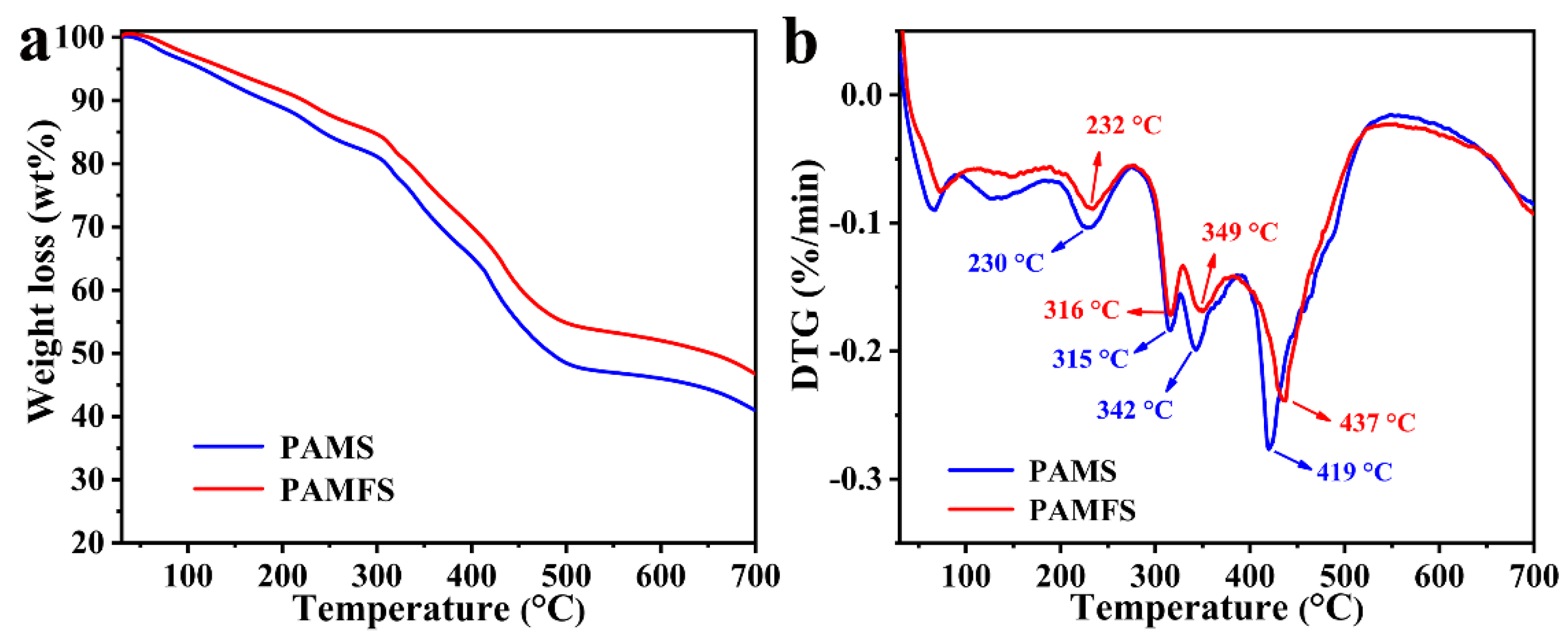
3.2. Thermogravimetric Analysis
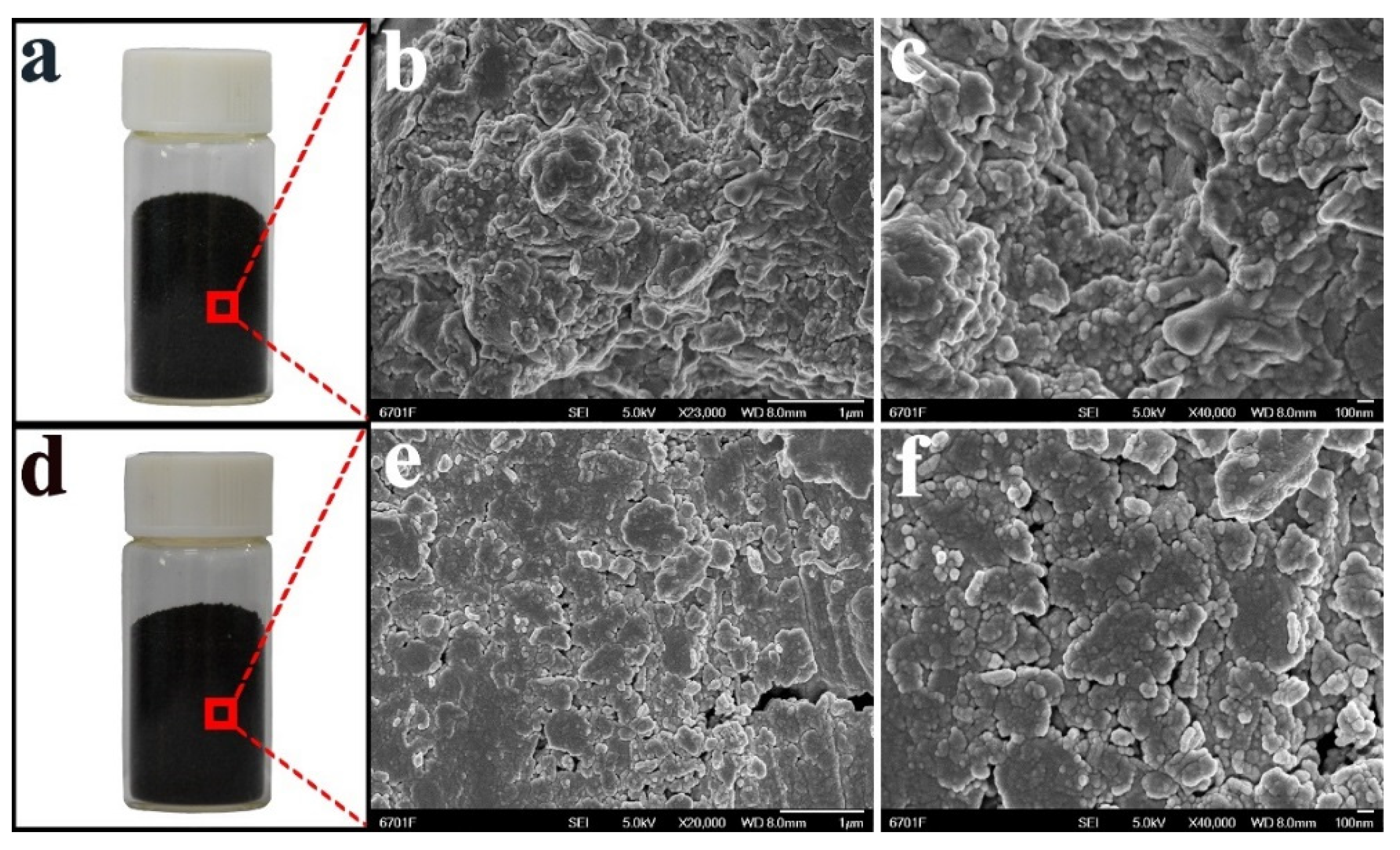
3.3. SEM Characterization
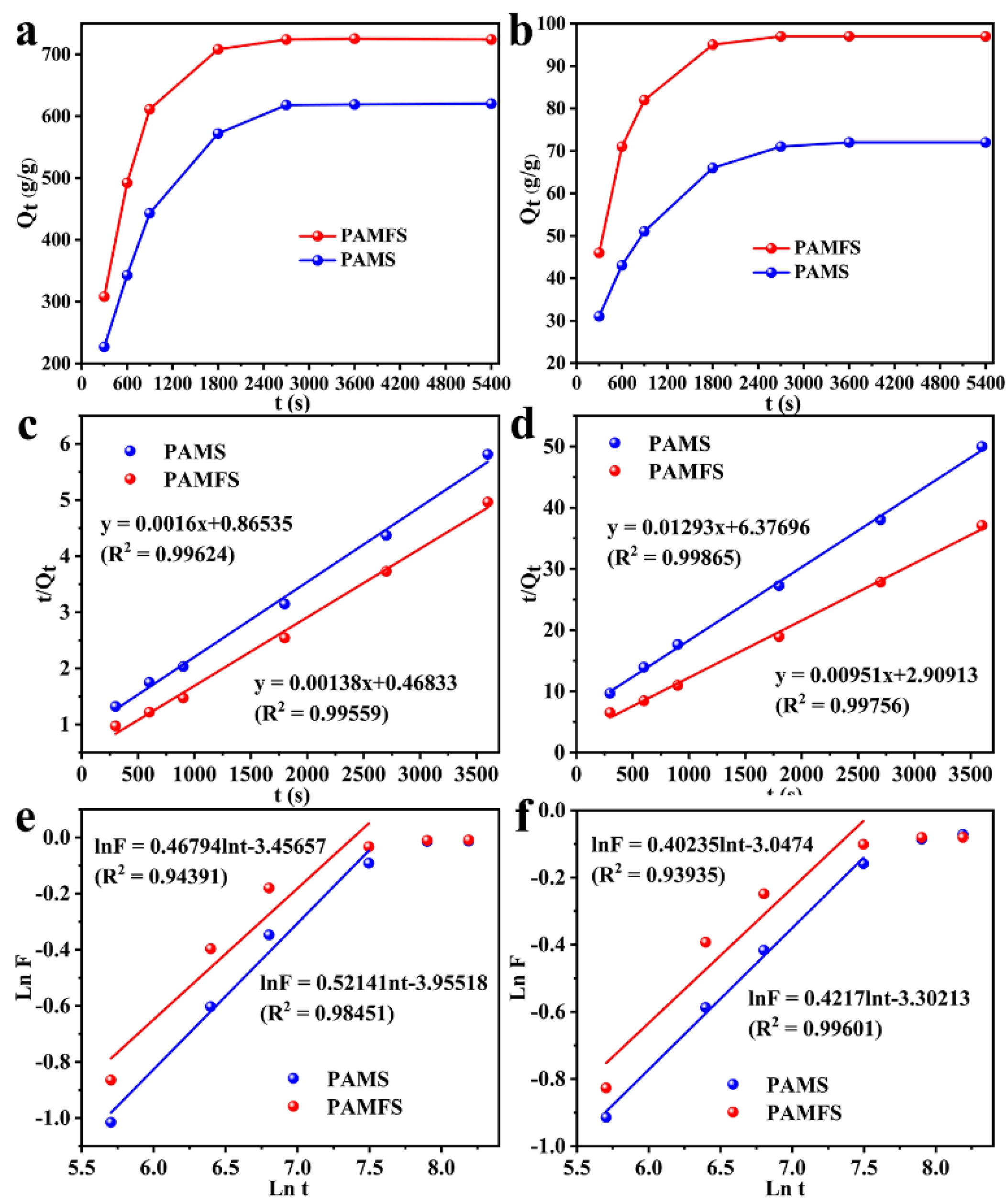
3.4. Swelling Kinetics of PAMFS
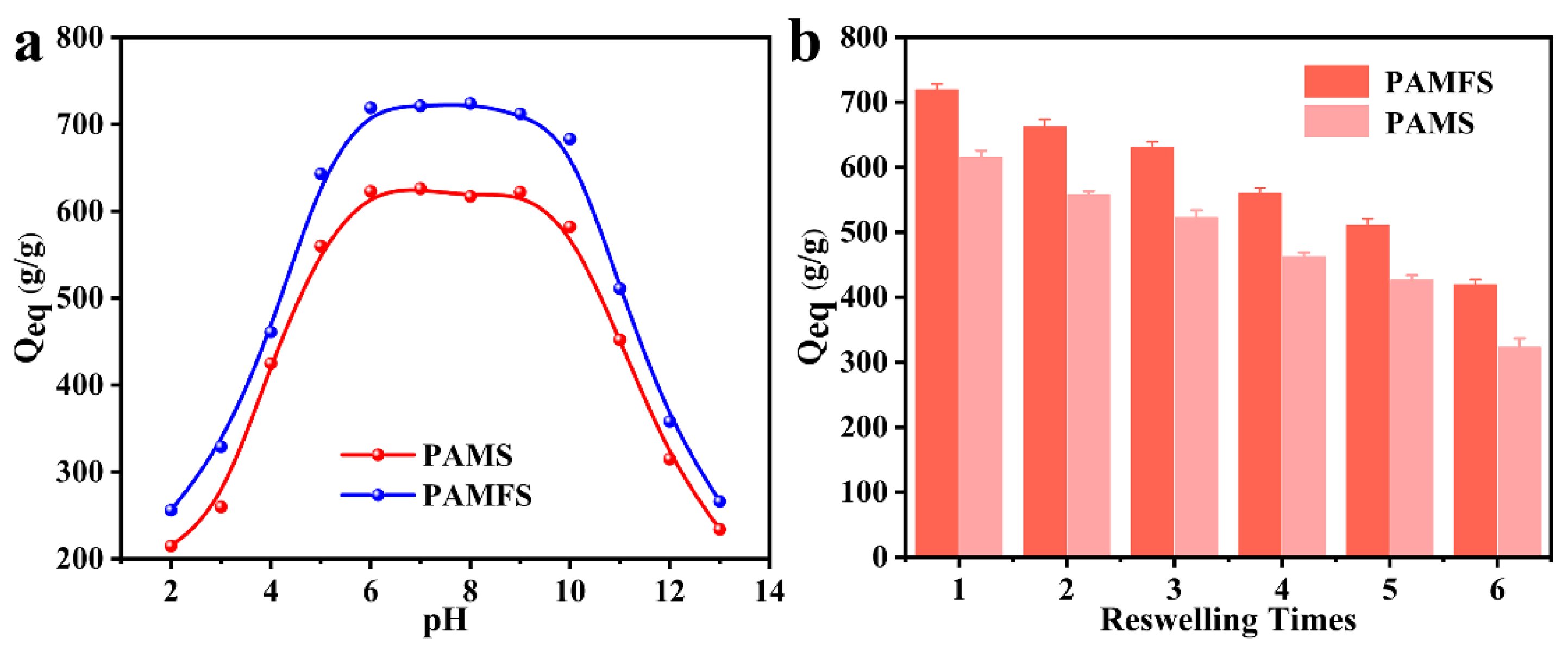
3.5. Effects of pH on Water Absorbency and Reswelling Capability of PAMFS
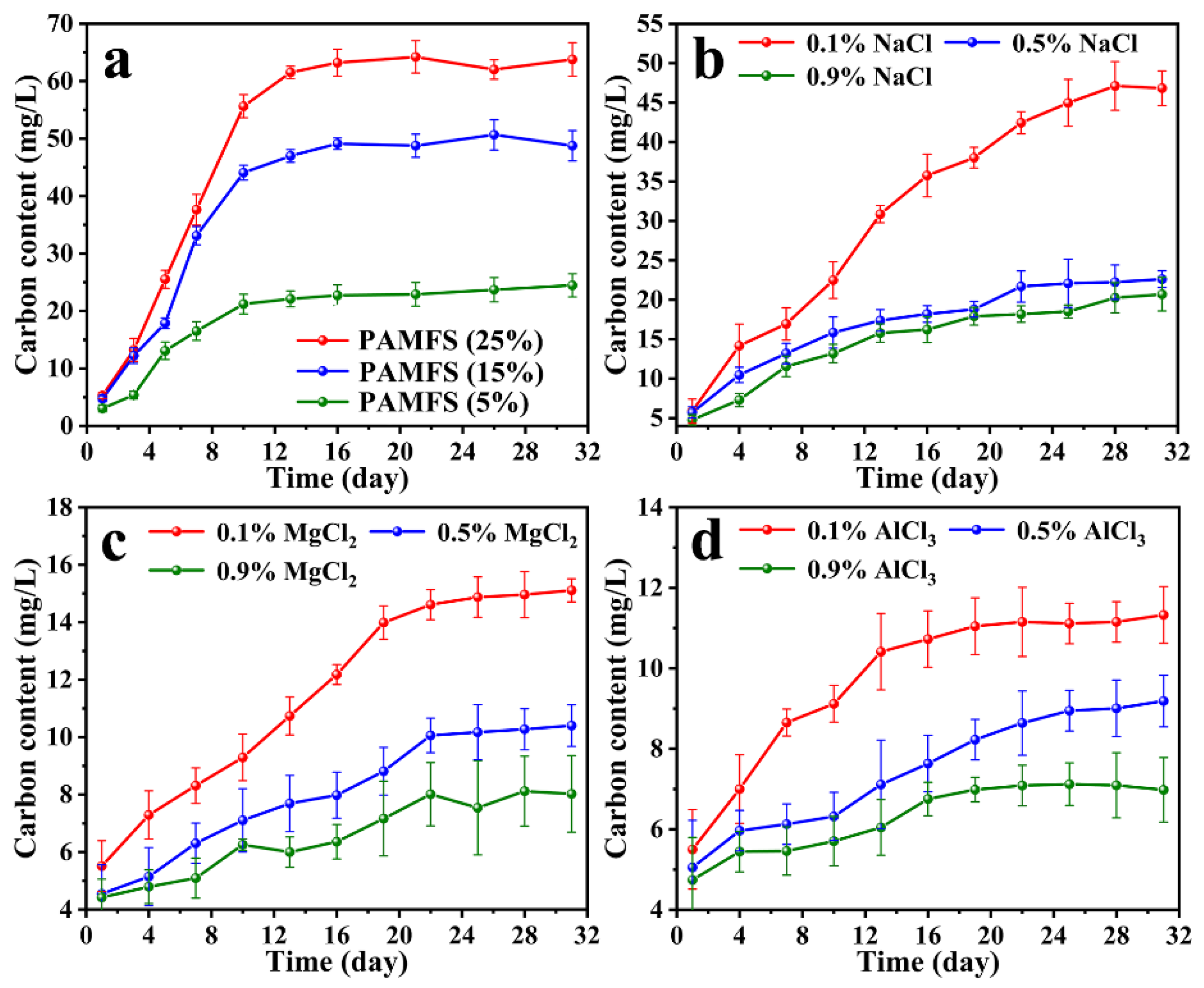
3.6. Slow Release of FA in Distilled Water and Salt Solutions
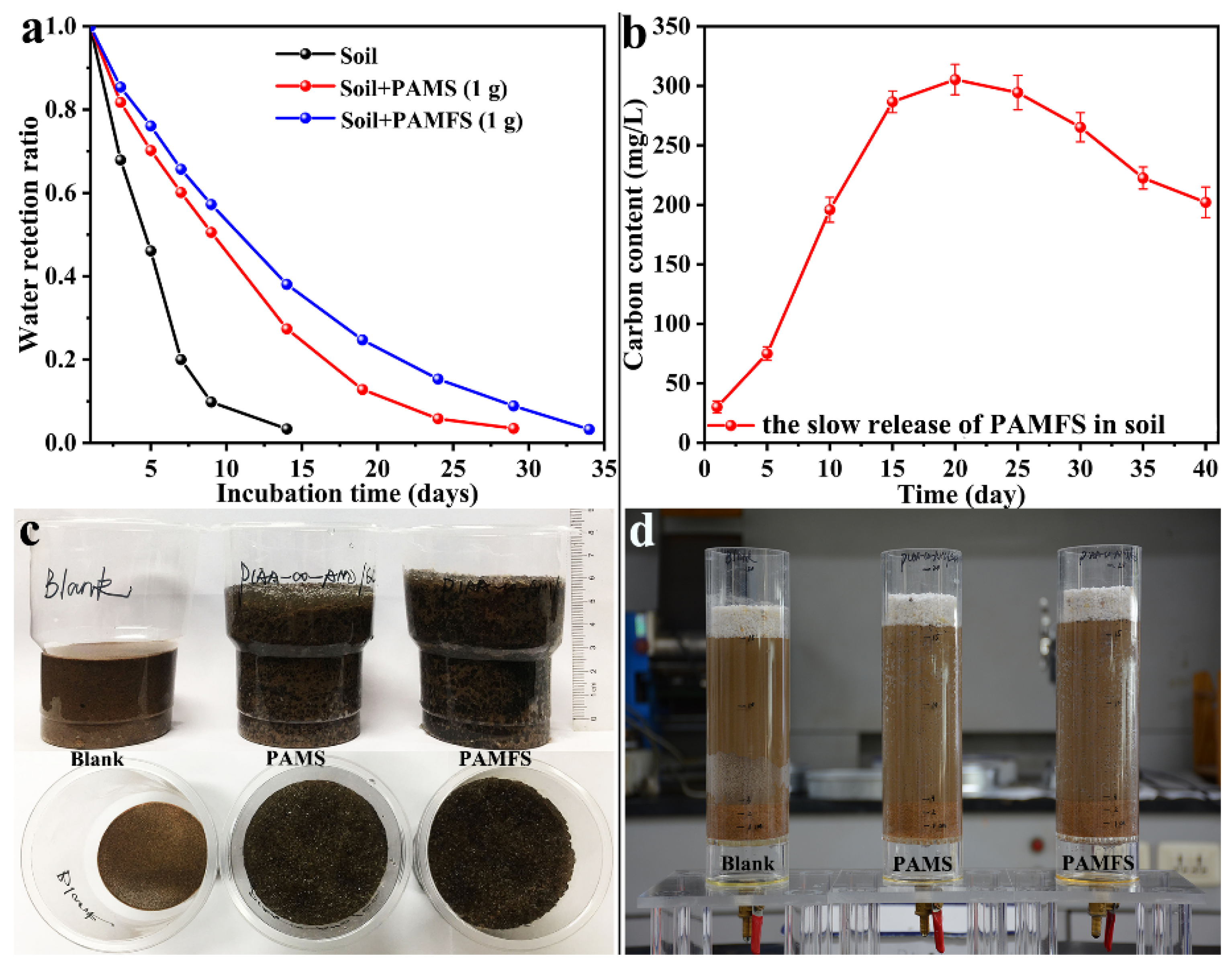
3.7. Water Retention Capacity and Slow Release of Superabsorbent Composites in Soil
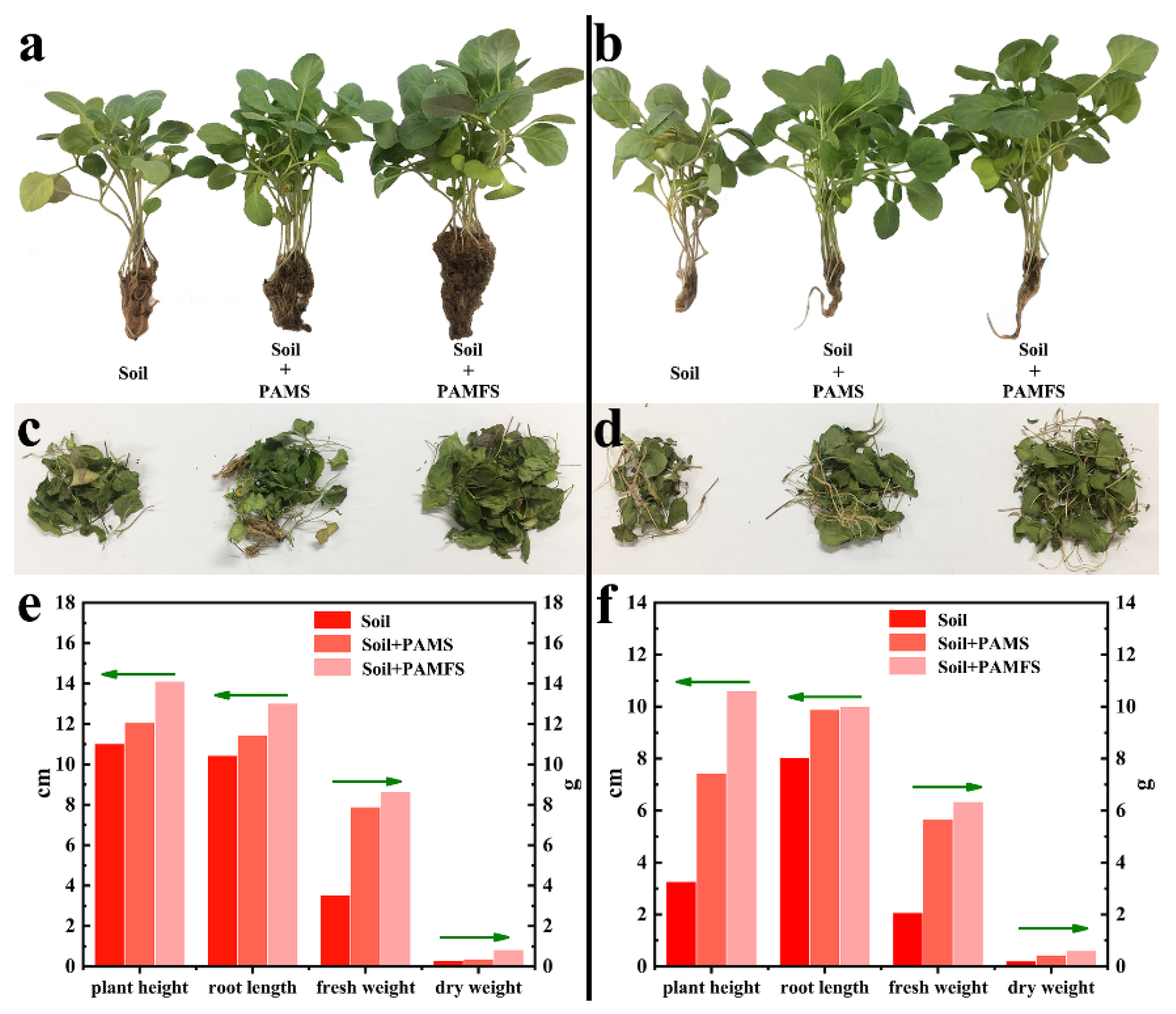
3.8. Pot Culture Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Yang, Z.; Zhang, A.; Yang, S. Water retention and fertilizer slow release integrated superabsorbent synthesized from millet straw and applied in agriculture. Ind. Crops Prod. 2021, 163, 113126. [Google Scholar] [CrossRef]
- Rockstrom, J.; Williams, J.; Daily, G.; Noble, A.; Matthews, N.; Gordon, L.; Wetterstrand, H.; DeClerck, F.; Shah, M.; Steduto, P.; et al. Sustainable intensification of agriculture for human prosperity and global sustainability. Ambio 2017, 46, 4–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essawy, H.A.; Ghazy, M.B.M.; El-Hai, F.A.; Mohamed, M.F. Superabsorbent hydrogels via graft polymerization of acrylic acid from chitosan-cellulose hybrid and their potential in controlled release of soil nutrients. Int. J. Biol. Macromol. 2016, 89, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Cai, D.; He, L.; Zhong, N.; Yu, M.; Zhang, X.; Wu, Z. Fabrication of a high-performance fertilizer to control the loss of water and nutrient using micro/nano networks. ACS Sustain. Chem. Eng. 2015, 3, 645–653. [Google Scholar] [CrossRef]
- Ganguly, S.; Maity, T.; Mondal, S.; Das, P.; Das, N.C. Starch functionalized biodegradable semi-IPN as a pH-tunable controlled release platform for memantine. Int. J. Biol. Macromol. 2017, 95, 185–198. [Google Scholar] [CrossRef]
- Chen, M.; Chen, X.; Zhang, C.; Cui, B.; Li, Z.; Zhao, D.; Wang, Z. Kaolin-enhanced superabsorbent composites: Synthesis, characterization and swelling behaviors. Polymers 2021, 13, 1204. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, N.C. Water uptake kinetics and control release of agrochemical fertilizers from nanoclay-assisted semi-interpenetrating sodium acrylate-based hydrogel. Polym-Plast. Technol. 2017, 7, 744–761. [Google Scholar] [CrossRef]
- Santos, A.C.S.D.; Henrique, H.M.; Cardoso, V.L.; Reis, M.H.M. Slow release fertilizer prepared with lignin and poly(vinylacetate) bioblend. Int. J. Biol. Macromol. 2021, 185, 543–550. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, J.; Sun, Y.; Xu, H.; Yang, Z.; Liu, S.; Jia, X.; Zheng, H. The effects of different water and nitrogen managements on yield and nitrogen use efficiency in hybrid rice of China. Field Crop. Res. 2012, 127, 85–98. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Ma, R.; Shao, J.; Huang, S.; Liu, Y.; Jiang, Y.; Cheng, D. Novel water- and fertilizer-management strategy: Nutrient-water carrier. J. Clean. Prod. 2021, 291, 125961. [Google Scholar] [CrossRef]
- Xie, L.; Liu, M.; Ni, B.; Wang, Y. Utilization of wheat straw for the preparation of coated controlled-release fertilizer with the function of water retention. J. Agric. Food Chem. 2012, 60, 6921–6928. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Ru, X.; Shi, J.; Song, J.; Zhao, H.; Liu, Y.; Guo, D.; Lu, X. Preparation and properties of a novel semi-IPN slow-release fertilizer with the function of water retention. J. Agric. Food Chem. 2017, 65, 10851–10858. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Antonietti, M. The sleeping giant: A polymer view on humic matter in synthesis and application. Prog. Polym. Sci. 2020, 100, 101182. [Google Scholar] [CrossRef]
- Li, H.; Yang, L.; Cao, J.; Nie, C.; Liu, H.; Tian, J.; Chen, W.; Geng, P.; Xie, G. Water-preserving and salt-resistant slow-release fertilizers of polyacrylic acid-potassium humate coated ammonium dihydrogen phosphate. Polymers 2021, 13, 2844. [Google Scholar] [CrossRef] [PubMed]
- Araújo, B.R.; Romão, L.P.C.; Doumer, M.E. Evaluation of the interactions between chitosan and humics in media for the controlled release of nitrogen fertilizer. J. Environ. Manag. 2017, 190, 122–131. [Google Scholar] [CrossRef]
- Shen, Y.; Jiao, S.; Ma, Z.; Li, H.; Chen, J. Humic acid-modified bentonite composite material enhances urea-nitrogen use efficiency. Chemosphere 2020, 255, 126976. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Liu, Y.; Mu, B.; Wang, A. Research on preparation and properties of a multifunctional superabsorbent based on semicoke and humic acid. Eur. Polym. J. 2021, 159, 110750. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, J.; Du, C.; Zhou, Z. Hydrophobic modification of waterborne polymer slows urea release and improves nitrogen use efficiency in rice. Sci. Total Environ. 2021, 794, 148612. [Google Scholar] [CrossRef]
- Martino, A.D.; Drannikov, A.; Surgutskaia, N.S.; Ozaltin, K.; Postnikov, P.S.; Marina, T.E.; Sedlarik, V. Chitosan-collagen based film for controlled delivery of a combination of short life anesthetics. Int. J. Biol. Macromol. 2019, 140, 1183–1193. [Google Scholar] [CrossRef]
- Xie, L.; Lu, S.; Liu, M.; Gao, C.; Wang, X.; Wu, L. Recovery of ammonium onto wheat straw to be reused as a slow-release fertilizer. J. Agric. Food Chem. 2013, 61, 3382–3388. [Google Scholar] [CrossRef]
- Sarkar, D.; De, D.K.; Das, R.; Mandal, B. Removal of organic matter and oxides of iron and manganese from soil influences boron adsorption in soil. Geoderma 2014, 214–215, 213–216. [Google Scholar] [CrossRef]
- Martínez-Gallegos, S.; Rosano-Ortega, G.; González-Juárez, J.; Pérez-Armendáriz, B.; Vega-Lebrún, C.A.; Macedo, G.; Illescas, J. A simulated column packed with soil-activated carbon for organic matter removal. Soil Till. Res. 2017, 170, 130–135. [Google Scholar] [CrossRef]
- Rabat, N.E.; Hashim, S.; Majid, R.A. Effect of different monomers on water retention properties of slow release fertilizer hydrogel. Procedia Eng. 2016, 148, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhu, Y.; Liu, Y.; Wang, A. Fabrication of eco-friendly superabsorbent composites based on waste semicoke. Polymers 2020, 12, 2347. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, J.; Wang, W.; Wang, T.; Zong, L.; Wang, A. Synthesis of iron red hybrid pigments from oil shale semi-coke waste. Adv. Powder Technol. 2020, 31, 2276–2284. [Google Scholar] [CrossRef]
- Dong, Y.; Lin, H.; Zhao, Y.; Menzembere, E.R.G.Y. Remediation of vanadium-contaminated soils by the combination ofnatural clay mineral and humic acid. J. Clean. Prod. 2021, 279, 123874. [Google Scholar] [CrossRef]
- Amir, S.; Jouraiphy, A.; Meddich, A.; Gharous, M.E.I.; Winterton, P.; Mohamed, H. Structural study of humic acids during composting of activated sludge-green waste: Elemental analysis, FTIR and 13C NMR. J. Hazard. Mater. 2010, 177, 524–529. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I. Physical and chemical characterisation of acrylamide-based hydrogels, Aam, Aam/NaCMC and Aam/NaCMC/MgO. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1439–1449. [Google Scholar] [CrossRef]
- Sabbagh, F.; Khatir, N.M.; Karim, A.K.; Omidvar, A.; Nazari, Z.; Jaberi, R. Mechanical properties and swelling behavior of acrylamide hydrogels using montmorillonite and kaolinite as clays. J. Environ. Treat. Tech. 2019, 7, 211–219. [Google Scholar]
- Feng, Y.; Wu, D.; Zhou, Y.; Kaimin, S. A metal-free method of generating sulfate radicals through direct interaction of hydroxylamine and peroxymonosulfate: Mechanisms, kinetics, and implications. Chem. Eng. J. 2017, 330, 906–913. [Google Scholar] [CrossRef]
- Spagnol, C.; Rodrigues, F.H.A.; Neto, A.G.V.C.; Pereira, A.G.B.; Fajardo, A.R.; Radovanovic, E.; Rubira, A.F.; Muniz, E.C. Nanocomposites based on poly(acrylamide-co-acrylate) and cellulose nanowhiskers. Eur. Polym. J. 2012, 48, 454–463. [Google Scholar] [CrossRef]
- Olad, A.; Doustdar, F.; Gharekhani, H. Fabrication and characterization of a starch-based superabsorbent hydrogel composite reinforced with cellulose nanocrystals from potato peel waste. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 124962. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Mu, B.; Liu, Y.; Wang, A. From the waste semicoke to superabsorbent composite: Synthesis, characterization and performance evaluation. J. Polym. Environ. 2021, 29, 4017–4026. [Google Scholar] [CrossRef]
- Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Tabar, A.R. Slow-release NPK fertilizer encapsulated by carboxymethyl cellulose-based nanocomposite with the function of water retention in soil. Mater. Sci. Eng. 2018, 90, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Luo, Y.; Fu, F.; Zhang, Y.; Ma, R. Synthesis, characterization, and swelling kinetics of pH-responsive and temperature-responsive carboxymethyl chitosan/polyacrylamide hydrogels. J. Appl. Polym. Sci. 2013, 129, 806–814. [Google Scholar] [CrossRef]
- Rashidzadeh, A.; Olad, A.; Salari, D.; Reyhanitabar, A. On the preparation and swelling properties of hydrogel nanocomposite based on sodium alginate-g-poly(acrylic acid-co-acrylamide)/clinoptilolite and its application as slow release fertilizer. J. Polym. Res. 2014, 21, 344–358. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Salimi, H. New protein-based hydrogel with superabsorbing properties: Effect of monomer ratio on swelling behavior and kinetics. Ind. Eng. Chem. Res. 2008, 47, 9206–9213. [Google Scholar] [CrossRef]
- Wu, L.; Liu, M. Slow-release potassium silicate fertilizer with the function of superabsorbent and water retention. Ind. Eng. Chem. Res. 2007, 46, 6494–6500. [Google Scholar] [CrossRef]
- Ye, H.; Li, H.; Wang, C.; Yang, J.; Huang, G.; Meng, X.; Zhou, Q. Degradable polyester/urea inclusion complex applied as a facile and environment-friendly strategy for slow-release fertilizer: Performance and mechanism. Chem. Eng. J. 2020, 381, 122704. [Google Scholar] [CrossRef]
- Murray, H.H. Traditional and new applications for kaolin, smectite, and palygorskite: A general overview. Appl. Clay Sci. 2000, 17, 207–221. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Q.; Liu, F.; Chen, J.; Guo, W.P.; Lei, Z. Study on seed emergence and seedling growth of artemisia desertorum with superabsorbent polymers. Polymers 2020, 12, 2873. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhu, Y.; Liu, Y.; Mu, B.; Wang, A. Slow Release and Water Retention Performance of Poly(acrylic acid-co-acrylamide)/Fulvic Acid/Oil Shale Semicoke Superabsorbent Composites. Polymers 2022, 14, 1719. https://doi.org/10.3390/polym14091719
Wang Y, Zhu Y, Liu Y, Mu B, Wang A. Slow Release and Water Retention Performance of Poly(acrylic acid-co-acrylamide)/Fulvic Acid/Oil Shale Semicoke Superabsorbent Composites. Polymers. 2022; 14(9):1719. https://doi.org/10.3390/polym14091719
Chicago/Turabian StyleWang, Yongsheng, Yongfeng Zhu, Yan Liu, Bin Mu, and Aiqin Wang. 2022. "Slow Release and Water Retention Performance of Poly(acrylic acid-co-acrylamide)/Fulvic Acid/Oil Shale Semicoke Superabsorbent Composites" Polymers 14, no. 9: 1719. https://doi.org/10.3390/polym14091719
APA StyleWang, Y., Zhu, Y., Liu, Y., Mu, B., & Wang, A. (2022). Slow Release and Water Retention Performance of Poly(acrylic acid-co-acrylamide)/Fulvic Acid/Oil Shale Semicoke Superabsorbent Composites. Polymers, 14(9), 1719. https://doi.org/10.3390/polym14091719







