Electrospun Polymer Nanofibers with Antimicrobial Activity
Abstract
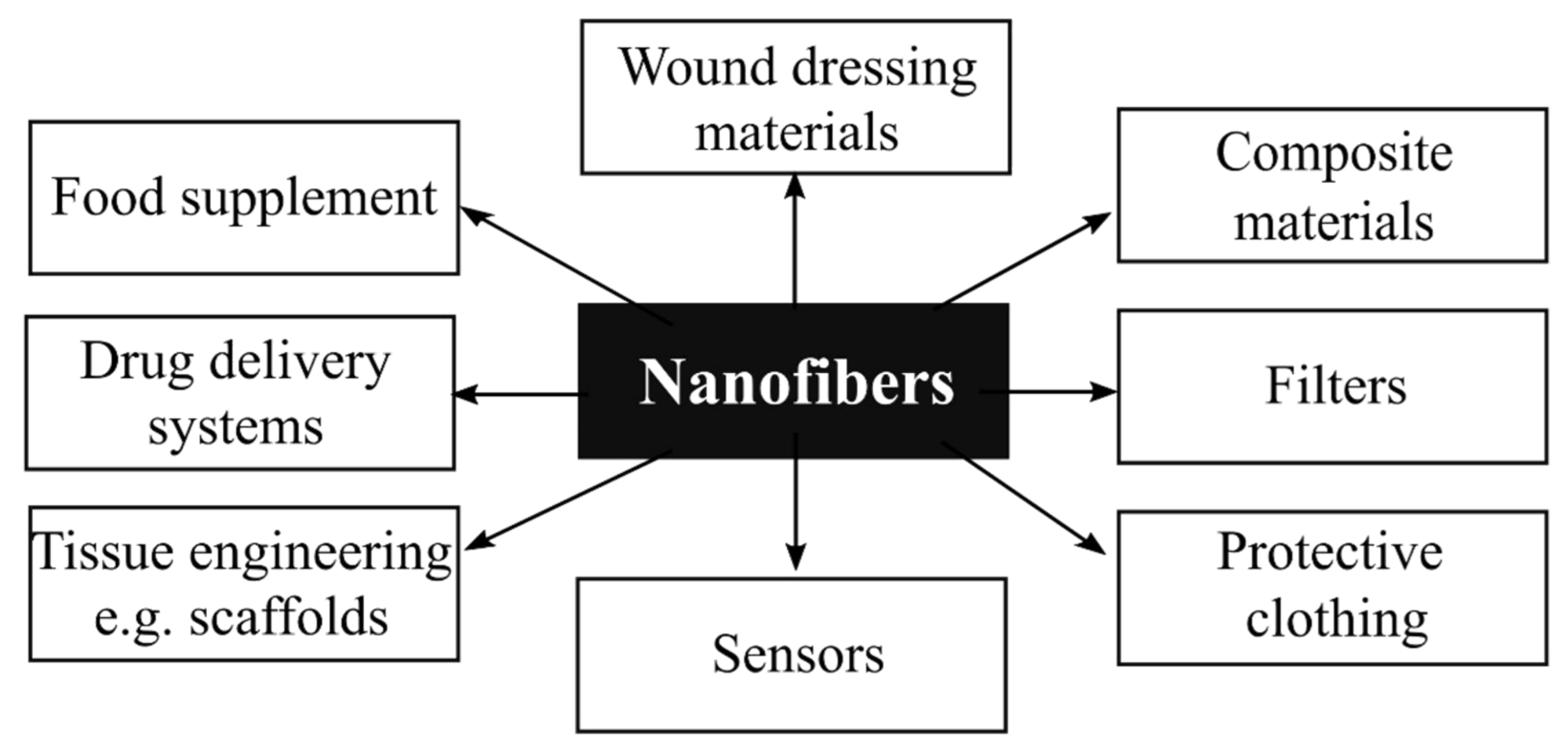
1. Introduction
- (1)
- Drawing is the process that requires a viscoelastic material that can be severely deformed but is still cohesive sufficiently to withstand the stresses arising during pulling.
- (2)
- Template synthesis involves using a template or mold to create the required material or structure.
- (3)
- Phase separation is the method, the polymer is first mixed with a solvent and then subjected to gelation. the key mechanism of this process is to split the phases owing to physical incompatibilities. The solvent is then extracted, and the polymeric material is left.
- (4)
- Self-assembly deals with the formation of nanofibers using smaller molecules as the basic building blocks. The intermolecular forces that occur in the process allow the smaller units to be brought together. The final shape of the fiber is strictly related to the shape of the constituent molecules.
- (5)
- Electrospinning is the process of converting a liquid polymer solution into solid nanofibers by applying an electrical force in the presence of a strong electric field.
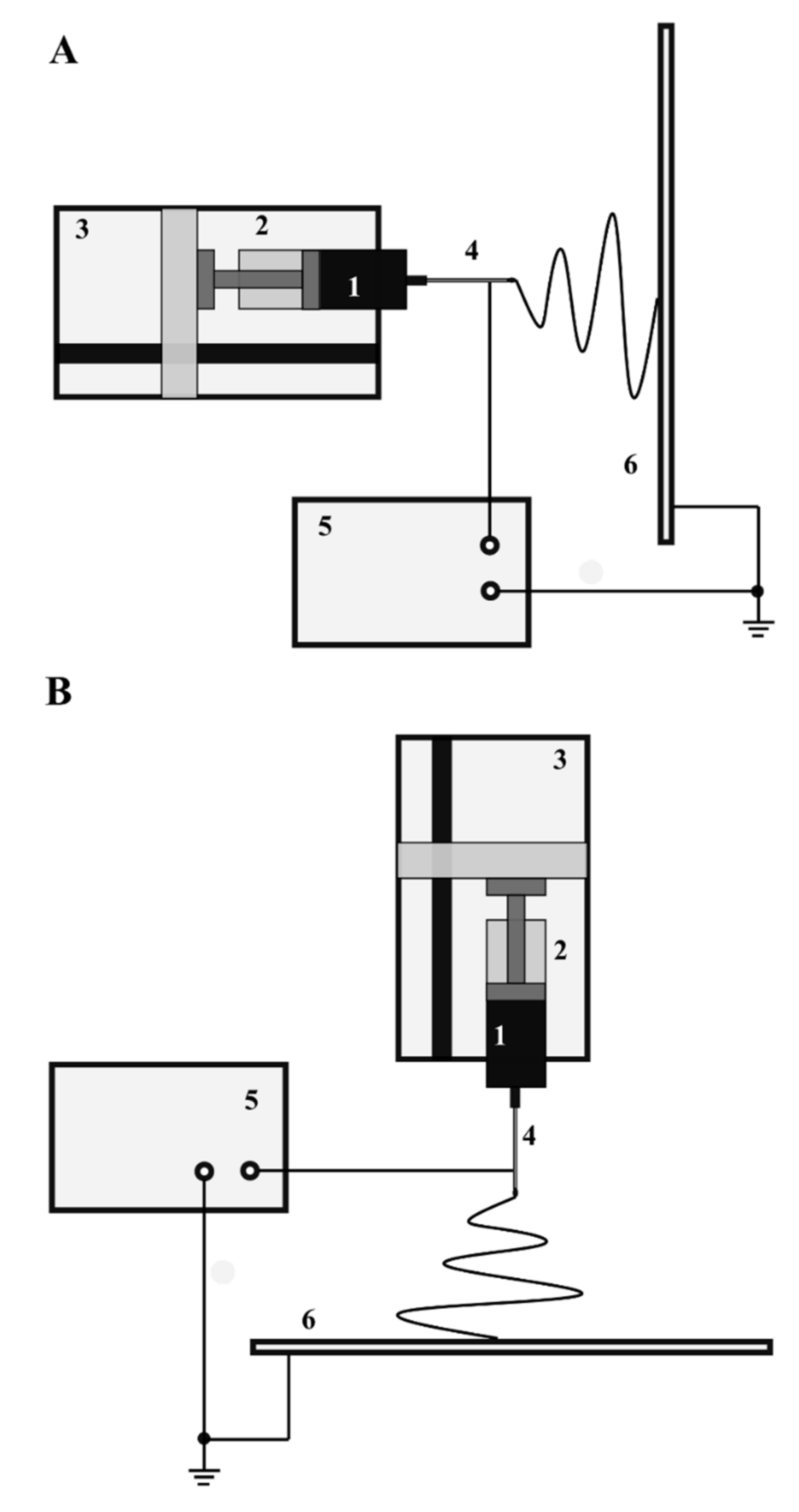
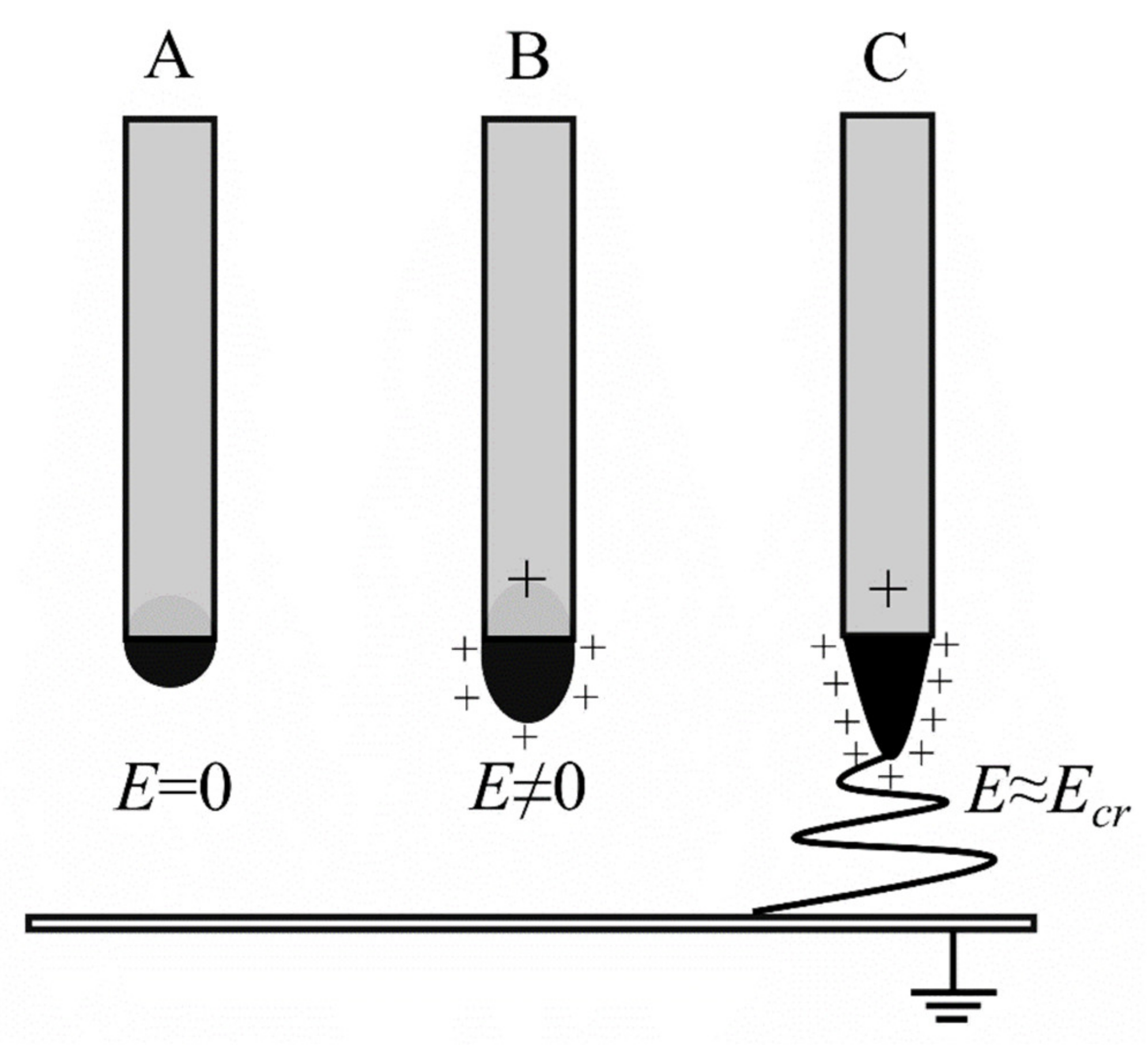
2. Electrospinning as a Process for Nanofiber Fabrication
3. Factors Influencing the Electrospinning Process
4. Electrospun Fibers Based on Natural Antibacterial Polymers
5. Electrospun Fibers Based on Synthetic Polymers
6. Antimicrobial Effect of Electrospun Nanofibers Loaded with Metallic Nanoparticles
6.1. Silver Nanoparticles
6.2. Metallic Oxides
7. Electrospun Nanofibers Containing Antimicrobial Plant Extracts
7.1. Crude Plant Extracts
7.2. Encapsulation of Essential Oils (EO) into Electrospun Polymeric Fibers
8. Other Chemical Components
9. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bu, N.; Huang, Y.; Wang, X.; Yin, Z. Continuously tunable and oriented nanofiber direct-written by mechano-electrospinning. Mater. Manuf. Process. 2012, 27, 1318–1323. [Google Scholar] [CrossRef]
- Tao, S.L.; Desai, T.A. Aligned arrays of biodegradable poly(ε-caprolactone) nanowires and nanofibers by template synthesis. Nano Lett. 2007, 7, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.S.; Lin, J.; Liu, Y.; Huang, P.; Jin, A.; Chen, X. Self-assembly mechanisms of nanofibers from peptide amphiphiles in solution and on substrate surfaces. Nanoscale 2016, 8, 14814–14820. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Wang, L.; Guo, B.; Ma, P.X. Electroactive nanofibrous biomimetic scaffolds by thermally induced phase separation. J. Mater. Chem. B 2014, 2, 6119–6130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Wang, P.; Zhang, H. Electrospinning of nanofibers: Potentials and perspectives for active food packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 479–502. [Google Scholar] [CrossRef] [PubMed]
- Irawati, N.; Suthaskumar, M.; John, V.; Ali, N.M.; Ahmad, H.; Harun, S.W. Fabrication of polymer microfiber by direct drawing. Microw. Opt. Technol. Lett. 2015, 57, 820–823. [Google Scholar] [CrossRef]
- Liang, H.W.; Guan, Q.F.; Chen, L.F.; Zhu, Z.; Zhang, W.J.; Yu, S.H. Macroscopic-scale template synthesis of robust carbonaceous nanofiber hydrogels and aerogels and their applications. Angew. Chemie Int. Ed. 2012, 51, 5101–5105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, M.; Lu, H.; Feng, S.; Ji, G.; Cao, J. Template Synthesis of Carbon Nanofibers Containing Linear Mesocage Arrays. Nanoscale Res. Lett. 2010, 5, 913. [Google Scholar] [CrossRef][Green Version]
- Shi, G.; Li, J.; Sang, X.; Wang, L.; Ni, C.; Li, Y. Micro-nano fabrication of hierarchical PPy/TiO 2/Si by continuous self-assembly technology. Mater. Manuf. Process. 2017, 33, 1–5. [Google Scholar] [CrossRef]
- Yang, Z.; Shen, C.; Zou, Y.; Wu, D.; Zhang, H.; Chen, K.; Yang, Z.; Shen, C.; Zou, Y.; Wu, D.; et al. Application of Solution Blow Spinning for Rapid Fabrication of Gelatin/Nylon 66 Nanofibrous Film. Foods 2021, 10, 2339. [Google Scholar] [CrossRef]
- Himmler, M.; Schubert, D.W.; Dähne, L.; Egri, G.; Fuchsluger, T.A. Electrospun PCL Scaffolds as Drug Carrier for Corneal Wound Dressing Using Layer-by-Layer Coating of Hyaluronic Acid and Heparin. Int. J. Mol. Sci. 2022, 23, 2765. [Google Scholar] [CrossRef]
- Miao, F.; Shao, C.; Li, X.; Wang, K.; Lu, N.; Liu, Y. Electrospun Carbon Nanofibers/Carbon Nanotubes/Polyaniline Ternary Composites with Enhanced Electrochemical Performance for Flexible Solid-State Supercapacitors. ACS Sustain. Chem. Eng. 2016, 4, 1689–1696. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Lai, C.C.; Ho, C.L.; Lo, C.T. Preparation of interconnected carbon nanofibers as electrodes for supercapacitors. Electrochim. Acta 2014, 127, 369–376. [Google Scholar] [CrossRef]
- Jin, J.; Shi, Z.Q.; Wang, C.Y. Electrochemical Performance of Electrospun carbon nanofibers as free-standing and binder-free anodes for Sodium-Ion and Lithium-Ion Batteries. Electrochim. Acta 2014, 141, 302–310. [Google Scholar] [CrossRef]
- Bognitzki, M.; Hou, H.; Ishaque, M.; Frese, T.; Hellwig, M.; Schwarte, C.; Schaper, A.; Wendorff, J.H.; Greiner, A. Polymer, metal, and hybrid nano- and mesotubes by coating degradable polymer template fibers (TUFT process). Adv. Mater. 2000, 12, 637–640. [Google Scholar] [CrossRef]
- Liu, W.; Graham, M.; Evans, E.A.; Reneker, D.H. Poly(meta-phenylene isophthalamide) nanofibers: Coating and post processing. J. Mater. Res. 2002, 17, 3206–3212. [Google Scholar] [CrossRef]
- Hou, H.; Jun, Z.; Reuning, A.; Schaper, A.; Wendorff, J.H.; Greiner, A. Poly(p-xylylene) Nanotubes by Coating and Removal of Ultrathin Polymer Template Fibers. Macromolecules 2002, 35, 2429–2431. [Google Scholar] [CrossRef]
- Song, J.; Deng, Q.; Huang, M.; Kong, Z. Carbon nanotube enhanced membrane distillation for salty and dyeing wastewater treatment by electrospinning technology. Environ. Res. 2022, 204, 111892. [Google Scholar] [CrossRef]
- Xu, D.; Chen, Y.; Qiu, T.; Qi, S.; Zhang, L.; Yin, M.; Ge, K.; Wei, X.; Tian, X.; Wang, P.; et al. Hierarchical mesoporous SnO2 nanotube templated by staphylococcus aureus through electrospinning for highly sensitive detection of triethylamine. Mater. Sci. Semicond. Process. 2021, 136, 106129. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, M.A.; Nazir, A.; Arshad, S.N.; Qadir, M.B.; Khaliq, Z.; Khan, Z.S.; Satti, A.N.; Mushtaq, B.; Shahzad, A. Triaxial electrospun mixed-phased TiO2 nanofiber-in-nanotube structure with enhanced photocatalytic activity. Microporous Mesoporous Mater. 2021, 320, 111104. [Google Scholar] [CrossRef]
- Chang, C.; Tran, V.H.; Wang, J.; Fuh, Y.-K.; Lin, L. Direct-Write Piezoelectric Polymeric Nanogenerator with High Energy Conversion Efficiency. Nano Lett. 2010, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, S.; Sheikholeslami, T.F.; Behzadmehr, A. Investigation on the electrospun PVDF/NP-ZnO nanofibers for application in environmental energy harvesting. J. Mater. Res. Technol. 2019, 8, 1608–1615. [Google Scholar] [CrossRef]
- MacDiarmid, A.G.; Jones, W.E.; Norris, I.D.; Gao, J.; Johnson, A.T.; Pinto, N.J.; Hone, J.; Han, B.; Ko, F.K.; Okuzaki, H.; et al. Electrostatically-generated nanofibers of electronic polymers. Synth. Met. 2001, 119, 27–30. [Google Scholar] [CrossRef]
- Gandavadi, D.; Sundarrajan, S.; Ramakrishna, S. Bio-Based Nanofibers Involved in Wastewater Treatment. Macromol. Mater. Eng. 2019, 304, 1–15. [Google Scholar] [CrossRef]
- Wassel, A.R.; El-Naggar, M.E.; Shoueir, K. Recent advances in polymer/metal/metal oxide hybrid nanostructures for catalytic applications: A review. J. Environ. Chem. Eng. 2020, 8, 104175. [Google Scholar] [CrossRef]
- Sharma, D.; Satapathy, B.K. Optimization and physical performance evaluation of electrospun nanofibrous mats of PLA, PCL and their blends. J. Ind. Text. 2020. [Google Scholar] [CrossRef]
- Kim, B.J.; Cheong, H.; Choi, E.S.; Yun, S.H.; Choi, B.H.; Park, K.S.; Kim, I.S.; Park, D.H.; Cha, H.J. Accelerated skin wound healing using electrospun nanofibrous mats blended with mussel adhesive protein and polycaprolactone. J. Biomed. Mater. Res. Part A 2017, 105, 218–225. [Google Scholar] [CrossRef]
- De Carvalho, L.D.; Peres, B.U.; Maezono, H.; Shen, Y.; Haapasalo, M.; Jackson, J.; Carvalho, R.M.; Manso, A.P. Doxycycline release and antibacterial activity from PMMA/PEO electrospun fiber mats. J. Appl. Oral Sci. 2019, 27, 1–10. [Google Scholar] [CrossRef]
- Kurpinski, K.; Patel, S. Dura mater regeneration with a novel synthetic, bilayered nanofibrous dural substitute: An experimental study. Nanomedicine 2011, 6, 325–337. [Google Scholar] [CrossRef]
- Shoueir, K.; Kandil, S.; El-hosainy, H.; El-Kemary, M. Tailoring the surface reactivity of plasmonic Au@TiO2 photocatalyst bio-based chitosan fiber towards cleaner of harmful water pollutants under visible-light irradiation. J. Clean. Prod. 2019, 230, 383–393. [Google Scholar] [CrossRef]
- Al-Ahmed, Z.A.; Al Jahdaly, B.A.; Radwan, H.A.; Hassana, A.A.; Almahri, A.; Ahmed, M.K.; Taher, M.M. Electrospun nanofibrous scaffolds of ϵ-polycaprolactone containing graphene oxide and encapsulated with magnetite nanoparticles for wound healing utilizations. Mater. Res. Express 2021, 8, 025013. [Google Scholar] [CrossRef]
- Farboudi, A.; Mahboobnia, K.; Chogan, F.; Karimi, M.; Askari, A.; Banihashem, S.; Davaran, S.; Irani, M. UiO-66 metal organic framework nanoparticles loaded carboxymethyl chitosan/poly ethylene oxide/polyurethane core-shell nanofibers for controlled release of doxorubicin and folic acid. Int. J. Biol. Macromol. 2020, 150, 178–188. [Google Scholar] [CrossRef]
- Sofi, H.S.; Akram, T.; Tamboli, A.H.; Majeed, A.; Shabir, N.; Sheikh, F.A. Novel lavender oil and silver nanoparticles simultaneously loaded onto polyurethane nanofibers for wound-healing applications. Int. J. Pharm. 2019, 569, 118590. [Google Scholar] [CrossRef]
- Ullah, A.; Ullah, S.; Khan, M.Q.; Hashmi, M.; Nam, P.D.; Kato, Y.; Tamada, Y.; Kim, I.S. Manuka honey incorporated cellulose acetate nanofibrous mats: Fabrication and in vitro evaluation as a potential wound dressing. Int. J. Biol. Macromol. 2020, 155, 479–489. [Google Scholar] [CrossRef]
- Lee, C.H.; Liu, K.S.; Cheng, C.W.; Chan, E.C.; Hung, K.C.; Hsieh, M.J.; Chang, S.H.; Fu, X.; Juang, J.H.; Hsieh, I.C.; et al. Codelivery of Sustainable Antimicrobial Agents and Platelet-Derived Growth Factor via Biodegradable Nanofibers for Repair of Diabetic Infectious Wounds. ACS Infect. Dis. 2020, 6, 2688–2697. [Google Scholar] [CrossRef]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer—Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef]
- Cordoba, A.; Saldias, C.; Urz, M.; Montalti, M.; Guernelli, M.; Focarete, M.L.; Leiva, A. On the Versatile Role of Electrospun Polymer Nanofibers as Photocatalytic Hybrid Materials Applied to Contaminated Water Remediation: A Brief Review. Nanomaterials 2022, 12, 756. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chemie-Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Cheng, T.; Hund, R.D.; Aibibu, D.; Horakova, J.; Cherif, C. Pure chitosan and chitsoan/chitosan lactate blended nanofibres made by single step electrospinning. Autex Res. J. 2013, 13, 128–133. [Google Scholar] [CrossRef]
- Steyaert, I.; Van Der Schueren, L.; Rahier, H.; De Clerck, K. An alternative solvent system for blend electrospinning of polycaprolactone/chitosan nanofibres. Macromol. Symp. 2012, 321–322, 71–75. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Gao, W.; Liang, H.; Wang, H.; Li, J. Preparation of chitosan/PLA blend micro/nanofibers by electrospinning. Mater. Lett. 2009, 63, 658–660. [Google Scholar] [CrossRef]
- Pakravan, M.; Heuzey, M.C.; Ajji, A. A fundamental study of chitosan/PEO electrospinning. Polymer 2011, 52, 4813–4824. [Google Scholar] [CrossRef]
- Chen, Z.; Mo, X.; Qing, F. Electrospinning of collagen-chitosan complex. Mater. Lett. 2007, 61, 3490–3494. [Google Scholar] [CrossRef]
- Homayoni, H.; Ravandi, S.A.H.; Valizadeh, M. Electrospinning of chitosan nanofibers: Processing optimization. Carbohydr. Polym. 2009, 77, 656–661. [Google Scholar] [CrossRef]
- Peesan, M.; Rujiravanit, R.; Supaphol, P. Electrospinning of hexanoyl chitosan/polylactide blends. J. Biomater. Sci. Polym. Ed. 2006, 17, 547–565. [Google Scholar] [CrossRef]
- Kriegel, C.; Arrechi, A.; Kit, K.; Mcclements, D.J.; Weiss, J. Fabrication, Functionalization, and Application of Electrospun Biopolymer Nanofibers. Crit. Rev. Food Sci. Nutr. 2008, 48, 775–797. [Google Scholar] [CrossRef]
- Sharma, D.; Saha, S.; Satapathy, B.K. Recent advances in polymer scaffolds for biomedical applications. J. Biomater. Sci. Polym. Ed. 2022, 33, 342–408. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrostat. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Yu, J. Electrospun Nanofibers for Energy and Environmental Applications; Springer-Verlag: Berlin/Heidelberg, Germany, 2014; ISBN 9783642541599. [Google Scholar]
- Balgis, R.; Kartikowati, C.W.; Ogi, T.; Gradon, L.; Bao, L.; Seki, K.; Okuyama, K. Synthesis and evaluation of straight and bead-free nanofibers for improved aerosol filtration. Chem. Eng. Sci. 2015, 137, 947–954. [Google Scholar] [CrossRef]
- Megelski, S.; Stephens, J.S.; Bruce Chase, D.; Rabolt, J.F. Micro- and nanostructured surface morphology on electrospun polymer fibers. Macromolecules 2002, 35, 8456–8466. [Google Scholar] [CrossRef]
- Chan, Z.; Chen, Z.; Zhang, A.; Hu, J.; Wang, X.; Yang, Z. Electrospun nanofibers for cancer diagnosis and therapy. Biomater. Sci. 2016, 4, 922–932. [Google Scholar] [CrossRef]
- Nam, J.; Huang, Y.; Agarwal, S.; Lannutti, J. Materials selection and residual solvent retention in biodegradable electrospun fibers. J. Appl. Polym. Sci. 2008, 107, 1547–1554. [Google Scholar] [CrossRef]
- D’Amato, A.R.; Schaub, N.J.; Cardenas, J.M.; Franz, E.; Rende, D.; Ziemba, A.M.; Gilbert, R.J. Evaluation of procedures to quantify solvent retention in electrospun fibers and facilitate solvent removal. Fibers Polym. 2017, 18, 483–492. [Google Scholar] [CrossRef]
- D’Amato, A.R.; Schaub, N.J.; Cardenas, J.M.; Fiumara, A.S.; Troiano, P.M.; Fischetti, A.; Gilbert, R.J. Removal of retained electrospinning solvent prolongs drug release from electrospun PLLA fibers. Polymer 2017, 123, 121–127. [Google Scholar] [CrossRef]
- Puertas-Bartolomé, M.; Mora-Boza, A.; García-Fernández, L. Emerging Biofabrication Techniques: A Review on Natural Polymers for Biomedical Applications. Polymers 2021, 13, 1209. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Hamdan, N.; Yamin, A.; Hamid, S.A.; Khodir, W.K.W.A.; Guarino, V. Functionalized Antimicrobial Nanofibers: Design Criteria and Recent Advances. J. Funct. Biomater. 2021, 12, 59. [Google Scholar] [CrossRef]
- Sorlier, P.; Denuzière, A.; Viton, C.; Domard, A. Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan. Biomacromolecules 2001, 2, 765–772. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Confederat, L.G.; Tuchilus, C.G.; Dragan, M.; Sha’at, M.; Dragostin, O.M. Preparation and Antimicrobial Activity of Chitosan and Its Derivatives: A Concise Review. Molecules 2021, 26, 3694. [Google Scholar] [CrossRef] [PubMed]
- Neamnark, A.; Rujiravanit, R.; Supaphol, P. Electrospinning of hexanoyl chitosan. Carbohydr. Polym. 2006, 66, 298–305. [Google Scholar] [CrossRef]
- Antaby, E.; Klinkhammer, K.; Sabantina, L. Electrospinning of chitosan for antibacterial applications—Current trends. Appl. Sci. 2021, 11, 11937. [Google Scholar] [CrossRef]
- Burger, C.; Hsiao, B.S.; Chu, B. Nanofibrous materials and their applications. Annu. Rev. Mater. Res. 2006, 36, 333–368. [Google Scholar] [CrossRef]
- Cui, C.; Sun, S.; Wu, S.; Chen, S.; Ma, J.; Zhou, F. Electrospun chitosan nanofibers for wound healing application. Eng. Regen. 2021, 2, 82–90. [Google Scholar] [CrossRef]
- Keirouz, A.; Radacsi, N.; Ren, Q.; Dommann, A.; Beldi, G.; Maniura-Weber, K.; Rossi, R.M.; Fortunato, G. Nylon-6/chitosan core/shell antimicrobial nanofibers for the prevention of mesh-associated surgical site infection. J. Nanobiotechnology 2020, 18, 1–17. [Google Scholar] [CrossRef]
- Barzegar, S.; Zare, M.R.; Shojaei, F.; Zareshahrabadi, Z.; Koohi-Hosseinabadi, O.; Saharkhiz, M.J.; Iraji, A.; Zomorodian, K.; Khorram, M. Core-shell chitosan/PVA-based nanofibrous scaffolds loaded with Satureja mutica or Oliveria decumbens essential oils as enhanced antimicrobial wound dressing. Int. J. Pharm. 2021, 597, 120288. [Google Scholar] [CrossRef]
- Li, B.; Shan, C.L.; Zhou, Q.; Fang, Y.; Wang, Y.L.; Xu, F.; Han, L.R.; Ibrahim, M.; Guo, L.B.; Xie, G.L.; et al. Synthesis, characterization, and antibacterial activity of cross-linked chitosan-glutaraldehyde. Mar. Drugs 2013, 11, 1534–1552. [Google Scholar] [CrossRef]
- Grkovic, M.; Stojanovic, D.B.; Pavlovic, V.B.; Rajilic-Stojanovic, M.; Bjelovic, M.; Uskokovic, P.S. Improvement of mechanical properties and antibacterial activity of crosslinked electrospun chitosan/poly (ethylene oxide) nanofibers. Compos. Part B Eng. 2017, 121, 58–67. [Google Scholar] [CrossRef]
- Naz, M.; Jabeen, S.; Gull, N.; Ghaffar, A.; Islam, A.; Rizwan, M.; Abdullah, H.; Rasool, A.; Khan, S.; Khan, R. Novel Silane Crosslinked Chitosan Based Electrospun Nanofiber for Controlled Release of Benzocaine. Front. Mater. 2022, 9, 1–7. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, D.; Chen, X.; Xu, Q.; Lu, F.; Nie, J. Electrospun water-soluble carboxyethyl chitosan/poly(vinyl alcohol) nanofibrous membrane as potential wound dressing for skin regeneration. Biomacromolecules 2008, 9, 349–354. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun nanofibers as dressings for chronic wound care: Advances, challenges, and future prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef]
- Woo, C.H.; Choi, Y.C.; Choi, J.S.; Lee, H.Y.; Cho, Y.W. A bilayer composite composed of TiO2-incorporated electrospun chitosan membrane and human extracellular matrix sheet as a wound dressing. J. Biomater. Sci. Polym. Ed. 2015, 26, 841–854. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Zhang, C.L.; Li, P. Characterisation and cooperative antimicrobial properties of chitosan/nano-ZnO composite nanofibrous membranes. Food Chem. 2012, 132, 419–427. [Google Scholar] [CrossRef]
- Suwantong, O.; Pankongadisak, P.; Deachathai, S.; Supaphol, P. Electrospun poly(l-lactic acid) fiber mats containing crude Garcinia mangostana extracts for use as wound dressings. Polym. Bull. 2014, 71, 925–949. [Google Scholar] [CrossRef]
- Zupančič, Š.; Potrč, T.; Baumgartner, S.; Kocbek, P.; Kristl, J. Formulation and evaluation of chitosan/polyethylene oxide nanofibers loaded with metronidazole for local infections. Eur. J. Pharm. Sci. 2016, 95, 152–160. [Google Scholar] [CrossRef]
- Sadri, M.; Sorkhi, S.A. Preparation and characterization of CS/PEO/cefazolin nanofibers with in vitro and in vivo testing. Nanomedicine Res. J. 2017, 2, 100–110. [Google Scholar] [CrossRef]
- Sadri, M.; Arab-Sorkhi, S.; Vatani, H.; Bagheri-Pebdeni, A. New wound dressing polymeric nanofiber containing green tea extract prepared by electrospinning method. Fibers Polym. 2015, 16, 1742–1750. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Cui, H. Novel electrospun gelatin-glycerin-ε-Poly-lysine nanofibers for controlling Listeria monocytogenes on beef. Food Packag. Shelf Life 2018, 18, 21–30. [Google Scholar] [CrossRef]
- Kikionis, S.; Ioannou, E.; Konstantopoulou, M.; Roussis, V. Electrospun Micro/Nanofibers as Controlled Release Systems for Pheromones of Bactrocera oleae and Prays oleae. J. Chem. Ecol. 2017, 43, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ma, Q.; Wang, S.D.; Liu, H.; Zhang, S.Z.; Bao, W.; Zhang, K.Q.; Ling, L.Z. Electrospinning of silver nanoparticles loaded highly porous cellulose acetate nanofibrous membrane for treatment of dye wastewater. Appl. Phys. A Mater. Sci. Process. 2016, 122, 1–10. [Google Scholar] [CrossRef]
- Kalwar, K.; Shen, M. Electrospun cellulose acetate nanofibers and Au@AgNPs for antimicrobial activity-A mini review. Nanotechnol. Rev. 2019, 8, 246–257. [Google Scholar] [CrossRef]
- Shi, D.; WANG, F.; Lan, T.; Zhang, Y.; SHAO, Z. Convenient fabrication of carboxymethyl cellulose electrospun nanofibers functionalized with silver nanoparticles. Cellulose 2016, 23, 1899–1909. [Google Scholar] [CrossRef]
- Kalwar, K.; Hu, L.; Li, D.L.; Shan, D. AgNPs incorporated on deacetylated electrospun cellulose nanofibers and their effect on the antimicrobial activity. Polym. Adv. Technol. 2018, 29, 394–400. [Google Scholar] [CrossRef]
- Czapka, T.; Winkler, A.; Maliszewska, I.; Kacprzyk, R. Fabrication of photoactive electrospun cellulose acetate nanofibers for antibacterial applications. Energies 2021, 14, 2598. [Google Scholar] [CrossRef]
- Abdul Khodir, W.; Abdul Razak, A.; Ng, M.; Guarino, V.; Susanti, D. Encapsulation and Characterization of Gentamicin Sulfate in the Collagen Added Electrospun Nanofibers for Skin Regeneration. J. Funct. Biomater. 2018, 9, 36. [Google Scholar] [CrossRef]
- Thenmozhi, S.; Dharmaraj, N.; Kadirvelu, K.; Kim, H.Y. Electrospun nanofibers: New generation materials for advanced applications. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2017, 217, 36–48. [Google Scholar] [CrossRef]
- Kim, K.; Luu, Y.K.; Chang, C.; Fang, D.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J. Control. Release 2004, 98, 47–56. [Google Scholar] [CrossRef]
- Bhattacharjee, S. Understanding the burst release phenomenon: Toward designing effective nanoparticulate drug-delivery systems. Ther. Deliv. 2021, 12, 21–36. [Google Scholar] [CrossRef]
- Del Valle, L.J.; Franco, L.; Katsarava, R.; Puiggalí, J. Electrospun biodegradable polymers loaded with bactericide agents. AIMS Mol. Sci. 2016, 3, 52–87. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; Bromberg, L.; Hatton, T.A.; Rutledge, G.C. Electrospun cellulose acetate fibers containing chlorhexidine as a bactericide. Polymer 2008, 49, 1266–1275. [Google Scholar] [CrossRef]
- Stevenson, C.L.; Santini, J.T.; Langer, R. Reservoir-based drug delivery systems utilizing microtechnology. Adv. Drug Deliv. Rev. 2012, 64, 1590–1602. [Google Scholar] [CrossRef]
- Zhang, C.L.; Yu, S.H. Nanoparticles meet electrospinning: Recent advances and future prospects. Chem. Soc. Rev. 2014, 43, 4423–4448. [Google Scholar] [CrossRef]
- Mahanta, N.; Valiyaveettil, S. Surface modified electrospun poly(vinyl alcohol) membranes for extracting nanoparticles from water. Nanoscale 2011, 3, 4625–4631. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, S.; Han, G.; Dong, J. Fabrication of the silver/polypyrrole/polyacrylonitrile composite nanofibrous mats. Mater. Lett. 2008, 62, 4031–4034. [Google Scholar] [CrossRef]
- Shi, W.; Song, S.; Zhang, H. Hydrothermal synthetic strategies of inorganic semiconducting nanostructures. Chem. Soc. Rev. 2013, 42, 5714–5743. [Google Scholar] [CrossRef]
- Wu, H.; Kong, D.; Ruan, Z.; Hsu, P.C.; Wang, S.; Yu, Z.; Carney, T.J.; Hu, L.; Fan, S.; Cui, Y. A transparent electrode based on a metal nanotrough network. Nat. Nanotechnol. 2013, 8, 421–425. [Google Scholar] [CrossRef]
- Prasher, P.; Singh, M.; Mudila, H. ·Silver nanoparticles as antimicrobial therapeutics: Current perspectives and future challenges. 3 Biotech 2018, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Abdullah, C.A.C.; Ahmad, S.A. Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials 2022, 15, 427. [Google Scholar] [CrossRef]
- Duval, R.E.; Gouyau, J.; Lamouroux, E. Limitations of recent studies dealing with the antibacterial properties of silver nanoparticles: Fact and opinion. Nanomaterials 2019, 9, 1775. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Park, W.H. Antimicrobial cellulose acetate nanofibers containing silver nanoparticles. Carbohydr. Polym. 2006, 65, 430–434. [Google Scholar] [CrossRef]
- Rujitanaroj, P.O.; Pimpha, N.; Supaphol, P. Preparation, characterization, and antibacterial properties of electrospun polyacrylonitrile fibrous membranes containing silver nanoparticles. J. Appl. Polym. Sci. 2010, 116, 1967–1976. [Google Scholar] [CrossRef]
- Phan, D.N.; Dorjjugder, N.; Saito, Y.; Taguchi, G.; Ullah, A.; Kharaghani, D.; Kim, I.S. The synthesis of silver-nanoparticle-anchored electrospun polyacrylonitrile nanofibers and a comparison with as-spun silver/polyacrylonitrile nanocomposite membranes upon antibacterial activity. Polym. Bull. 2020, 77, 4197–4212. [Google Scholar] [CrossRef]
- Jatoi, A.W.; Kim, I.S.; Ni, Q.Q. A comparative study on synthesis of AgNPs on cellulose nanofibers by thermal treatment and DMF for antibacterial activities. Mater. Sci. Eng. C 2019, 98, 1179–1195. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Zhang, J.; Yu, Z.; Yang, D. Electrospun polyacrylonitrile nanofibers loaded with silver nanoparticles by silver mirror reaction. Mater. Sci. Eng. C 2015, 51, 346–355. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, G.; Min, J.; Ding, J.; Jiang, X. Synthesis and antibacterial testing of silver/poly (ether amide) composite nanofibers with ultralow silver content. J. Nanomater. 2014, 2014, 684251. [Google Scholar] [CrossRef]
- Du, L.; Li, T.; Wu, S.; Zhu, H.F.; Zou, F.Y. Electrospun composite nanofibre fabrics containing green reduced Ag nanoparticles as an innovative type of antimicrobial insole. RSC Adv. 2019, 9, 2244–2251. [Google Scholar] [CrossRef]
- Yuan, J.; Geng, J.; Xing, Z.; Shen, J.; Kang, I.K.; Byun, H. Electrospinning of antibacterial poly(vinylidene fluoride) nanofibers containing silver nanoparticles. J. Appl. Polym. Sci. 2010, 116, 668–672. [Google Scholar] [CrossRef]
- Jesús Villarreal-Gómez, L.; Cornejo-Bravo, M.; Vera-Graziano, R.; Grande, D. Electrospinning as a powerful technique for biomedical applications: A critically selected survey. J. Biomater. Sci. 2016, 27, 157–176. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Z.; Lin, S.; Deng, C.; Li, F.; Chen, Z.; He, H. Green fabrication of antibacterial polymer/silver nanoparticle nanohybrids by dual-spinneret electrospinning. RSC Adv. 2015, 5, 40141–40147. [Google Scholar] [CrossRef]
- Shi, Q.; Vitchuli, N.; Nowak, J.; Noar, J.; Caldwell, J.M.; Breidt, F.; Bourham, M.; McCord, M.; Zhang, X. One-step synthesis of silver nanoparticle-filled nylon 6 nanofibers and their antibacterial properties. J. Mater. Chem. 2011, 21, 10330–10335. [Google Scholar] [CrossRef]
- Tucker, N.; Stanger, J.J.; Staiger, M.P.; Razzaq, H.; Hofman, K. The History of the Science and Technology of Electrospinning from 1600 to 1995. J. Eng. Fibers Fabr. 2012, 63. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, L.; Li, M.C.; Wu, Q.; Kojima, Y.; Zhou, D. Preparation and properties of electrospun poly (vinyl pyrrolidone)/cellulose nanocrystal/silver nanoparticle composite fibers. Materials 2016, 9, 523. [Google Scholar] [CrossRef]
- He, M.; Chen, M.; Dou, Y.; Ding, J.; Yue, H.; Yin, G.; Chen, X.; Cui, Y. Electrospun silver nanoparticles-embedded feather keratin/poly(vinyl alcohol)/poly(ethylene oxide) antibacterial composite nanofibers. Polymers 2020, 12, 305. [Google Scholar] [CrossRef]
- Rzayev, Z.M.O.; Erdönmez, D.; Erkan, K.; Şimşek, M.; Bunyatova, U. Functional copolymer/Organo-MMT nanoarchitectures. XXII. Fabrication and characterization of antifungal and antibacterial poly (vinyl alcohol-co-vinyl acetate/ODA-MMT/AgNPs nanofibers and nanocoatings by e-spinning and c-spinning methods. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 267–278. [Google Scholar] [CrossRef]
- Martínez-Abad, A.; Sanchez, G.; Lagaron, J.M.; Ocio, M.J. Influence of speciation in the release profiles and antimicrobial performance of electrospun ethylene vinyl alcohol copolymer (EVOH) fibers containing ionic silver ions and silver nanoparticles. Colloid Polym. Sci. 2013, 291, 1381–1392. [Google Scholar] [CrossRef]
- Song, J.; Wang, C.; Chen, M.; Regina, V.R.; Wang, C.; Meyer, R.L.; Xie, E.; Dong, M.; Besenbacher, F. Safe and effective Ag nanoparticles immobilized antimicrobial nanononwovens. Adv. Eng. Mater. 2012, 14, B240–B246. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Chi, H.; Wei, G.; Zhang, J.; Dai, L. Preparation and antibacterial activity of polyvinyl alcohol/regenerated silk fibroin composite fibers containing Ag nanoparticles. J. Appl. Polym. Sci. 2012, 123, 20–25. [Google Scholar] [CrossRef]
- Paneva, D.; Manolova, N.; Argirova, M.; Rashkov, I. Antibacterial electrospun poly(ε-caprolactone)/ascorbyl palmitate nanofibrous materials. Int. J. Pharm. 2011, 416, 346–355. [Google Scholar] [CrossRef]
- Tian, L.; Wang, P.; Zhao, Z.; Ji, J. Antimicrobial activity of electrospun poly(butylenes succinate) fiber mats containing PVP-capped silver nanoparticles. Appl. Biochem. Biotechnol. 2013, 171, 1890–1899. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Hudecki, A.; Chladek, G.; Król, W.; Mertas, A. Surface properties and antimicrobial activity of composite nanofibers of polycaprolactone with silver precipitations. Arch. Mater. Sci. Eng. 2014, 70, 53–60. [Google Scholar]
- Hafez, E.E.; El-Aassar, M.R.; Khalil, K.A.; Al-Deyab, S.S.; Taha, T.H. Poly (acrylonitrile-co-methyl methacrylate) nanofibers grafted with bio-nanosilver particles as antimicrobial against multidrug resistant bacteria. African J. Biotechnol. 2011, 10, 19658–19669. [Google Scholar] [CrossRef]
- Gao, Y.; Truong, Y.B.; Zhu, Y.; Louis Kyratzis, I. Electrospun antibacterial nanofibers: Production, activity, and in vivo applications. J. Appl. Polym. Sci. 2014, 131, 9041–9053. [Google Scholar] [CrossRef]
- Lopez-Esparza, J.; Francisco Espinosa-Cristobal, L.; Donohue-Cornejo, A.; Reyes-Lopez, S.Y. Antimicrobial activity of silver nanoparticles in polycaprolactone nanofibers against gram-positive and gram-negative bacteria. Ind. Eng. Chem. Res. 2016, 55, 12532–12538. [Google Scholar] [CrossRef]
- Bhullar, S.K.; Ruzgar, D.G.; Fortunato, G.; Aneja, G.K.; Orhan, M.; Saber-Samandari, S.; Sadighi, M.; Ahadian, S.; Ramalingam, M. A Facile Method for Controlled Fabrication of Hybrid Silver Nanoparticle-Poly(-caprolactone) Fibrous Constructs with Antimicrobial Properties. J. Nanosci. Nanotechnol. 2019, 19, 6949–6955. [Google Scholar] [CrossRef]
- Lala, N.L.; Ramaseshan, R.; Bojun, L.; Sundarrajan, S.; Barhate, R.S.; Liu, Y.J.; Ramakrishna, S. Fabrication of nanofibers with antimicrobial functionality used as filters: Protection against bacterial contaminants. Biotechnol. Bioeng. 2007, 97, 1357–1365. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Fabra, M.J.; Cabedo, L.; Lagaron, J.M. On the use of the electrospinning coating technique to produce antimicrobial polyhydroxyalkanoate materials containing in situ-stabilized silver nanoparticles. Nanomaterials 2017, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Dolina, J.; Dvořák, L.; Lederer, T.; Vacková, T.; Mikmeková, Š.; Šlouf, M.; Černík, M. Characterisation of morphological, antimicrobial and leaching properties of in situ prepared polyurethane nanofibres doped with silver behenate. RSC Adv. 2016, 6, 23816–23826. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Masheane, M.L.; Malinga, S.P.; Barnard, T.G.; Nxumalo, E.N.; Mamba, B.B.; Mhlanga, S.D. UV-assisted reduction of in situ electrospun antibacterial chitosan-based nanofibres for removal of bacteria from water. RSC Adv. 2016, 6, 95936–95943. [Google Scholar] [CrossRef]
- Ansari, M.A.; Albetran, H.M.; Alheshibri, M.H.; Timoumi, A.; Algarou, N.A.; Akhtar, S.; Slimani, Y.; Almessiere, M.A.; Alahmari, F.S.; Baykal, A.; et al. Synthesis of electrospun TiO2 nanofibers and characterization of their antibacterial and antibiofilm potential against gram-positive and gram-negative bacteria. Antibiotics 2020, 9, 572. [Google Scholar] [CrossRef]
- Kudhier, M.A.; Sabry, R.S.; Al-Haidarie, Y.K.; All-Marjani, M.F. Significantly enhanced antibacterial activity of Ag-doped TiO2 nanofibers synthesized by electrospinning. Mater. Technol. 2018, 33, 220–226. [Google Scholar] [CrossRef]
- Jatoi, A.W. Polyurethane nanofibers incorporated with ZnAg composite nanoparticles for antibacterial wound dressing applications. Compos. Commun. 2020, 19, 103–107. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Ehsani, A.; Divband, B.; Alizadeh-Sani, M. Antimicrobial activity of Titanium dioxide and Zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Pascariu, P.; Cojocaru, C.; Airinei, A.; Olaru, N.; Rosca, I.; Koudoumas, E.; Suchea, M.P. Innovative ag–tio2 nanofibers with excellent photocatalytic and antibacterial actions. Catalysts 2021, 11, 1234. [Google Scholar] [CrossRef]
- Shi, J.; Li, J.; Wang, Y.; Zhang, C.Y. TiO2-based nanosystem for cancer therapy and antimicrobial treatment: A review. Chem. Eng. J. 2022, 431, 133714. [Google Scholar] [CrossRef]
- Anjusree, G.S.; Bhupathi, A.; Balakrishnan, A.; Vadukumpully, S.; Subramanian, K.R.V.; Sivakumar, N.; Ramakrishna, S.; Nair, S.V.; Nair, A.S. Fabricating fiber, rice and leaf-shaped TiO2 by tuning the chemistry between TiO2 and the polymer during electrospinning. RSC Adv. 2013, 3, 16720–16727. [Google Scholar] [CrossRef]
- Gupta, K.K.; Mishra, P.K.; Srivastava, P.; Gangwar, M.; Nath, G.; Maiti, P. Hydrothermal in situ preparation of TiO 2 particles onto poly(lactic acid) electrospun nanofibres. Appl. Surf. Sci. 2013, 264, 375–382. [Google Scholar] [CrossRef]
- Lee, K.; Lee, S. Multifunctionality of poly(vinyl alcohol) nanofiber webs containing titanium dioxide. J. Appl. Polym. Sci. 2012, 124, 4038–4046. [Google Scholar] [CrossRef]
- Korina, E.; Stoilova, O.; Manolova, N.; Rashkov, I. Multifunctional hybrid materials from poly(3-hydroxybutyrate), TiO2 nanoparticles, and chitosan oligomers by combining electrospinning/electrospraying and impregnation. Macromol. Biosci. 2013, 13, 707–716. [Google Scholar] [CrossRef]
- Braga, N.F.; Vital, D.A.; Guerrini, L.M.; Lemes, A.P.; Formaggio, D.M.D.; Tada, D.B.; Arantes, T.M.; Cristovan, F.H. PHBV-TiO2 mats prepared by electrospinning technique: Physico-chemical properties and cytocompatibility. Biopolymers 2018, 109, 1–12. [Google Scholar] [CrossRef]
- Sundarrajan, S.; Venkatesan, A.; Ramakrishna, S. Fabrication of nanostructured self-detoxifying nanofiber membranes that contain active polymeric functional groupsa. Macromol. Rapid Commun. 2009, 30, 1769–1774. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; de Soares, N.F.F.; dos Coimbra, J.S.R.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Jesline, A.; John, N.P.; Narayanan, P.M.; Vani, C.; Murugan, S. Antimicrobial activity of zinc and titanium dioxide nanoparticles against biofilm-producing methicillin-resistant Staphylococcus aureus. Appl. Nanosci. 2015, 5, 157–162. [Google Scholar] [CrossRef]
- Lubasova, D.; Barbora, S. Antibacterial Efficiency of Nanofiber Membranes with Biologically Active Nanoparticles. In Proceedings of the International Conference on Agriculture, Biology and Environmental Sciences, Bali, Indonesia, 8–9 December 2014. [Google Scholar]
- Virovska, D.; Paneva, D.; Manolova, N.; Rashkov, I.; Karashanova, D. Electrospinning/electrospraying vs. electrospinning: A comparative study on the design of poly(l-lactide)/zinc oxide non-woven textile. Appl. Surf. Sci. 2014, 311, 842–850. [Google Scholar] [CrossRef]
- Augustine, R.; Dominic, E.A.; Reju, I.; Kaimal, B.; Kalarikkal, N.; Thomas, S. Electrospun polycaprolactone membranes incorporated with ZnO nanoparticles as skin substitutes with enhanced fibroblast proliferation and wound healing. RSC Adv. 2014, 4, 24777–24785. [Google Scholar] [CrossRef]
- Augustine, R.; Malik, H.N.; Singhal, D.K.; Mukherjee, A.; Malakar, D.; Kalarikkal, N.; Thomas, S. Electrospun polycaprolactone/ZnO nanocomposite membranes as biomaterials with antibacterial and cell adhesion properties. J. Polym. Res. 2014, 21, 347. [Google Scholar] [CrossRef]
- Haider, A.; Kwak, S.; Gupta, K.C.; Kang, I.K. Antibacterial activity and cytocompatibility of PLGA/CuO hybrid nanofiber scaffolds prepared by electrospinning. J. Nanomater. 2015, 2015, 832762. [Google Scholar] [CrossRef]
- Ungur, G.; Hrůza, J. Influence of copper oxide on the formation of polyurethane nanofibers via electrospinning. Fibers Polym. 2015, 16, 621–628. [Google Scholar] [CrossRef]
- Castro Mayorga, J.L.; Fabra Rovira, M.J.; Cabedo Mas, L.; Sánchez Moragas, G.; Lagarón Cabello, J.M. Antimicrobial nanocomposites and electrospun coatings based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and copper oxide nanoparticles for active packaging and coating applications. J. Appl. Polym. Sci. 2018, 135, 1–11. [Google Scholar] [CrossRef]
- Ahire, J.J.; Neveling, D.P.; Dicks, L.M.T. Polyacrylonitrile (PAN) nanofibres spun with copper nanoparticles: An anti- Escherichia coli membrane for water treatment. Appl. Microbiol. Biotechnol. 2018, 102, 7171–7181. [Google Scholar] [CrossRef]
- Chan, W.P.; Huang, K.C.; Bai, M.Y. Silk fibroin protein-based nonwoven mats incorporating baicalein Chinese herbal extract: Preparation, characterizations, and in vivo evaluation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 420–430. [Google Scholar] [CrossRef]
- Yao, C.H.; Yeh, J.Y.; Chen, Y.S.; Li, M.H.; Huang, C.H. Wound-healing effect of electrospun gelatin nanofibres containing Centella asiatica extract in a rat model. J. Tissue Eng. Regen. Med. 2017, 11, 905–915. [Google Scholar] [CrossRef]
- Suganya, S.; Senthil Ram, T.; Lakshmi, B.S.; Giridev, V.R. Herbal drug incorporated antibacterial nanofibrous mat fabricated by electrospinning: An excellent matrix for wound dressings. J. Appl. Polym. Sci. 2011, 121, 2893–2899. [Google Scholar] [CrossRef]
- Agnes Mary, S.; Giri Dev, V.R. Electrospun herbal nanofibrous wound dressings for skin tissue engineering. J. Text. Inst. 2015, 106, 886–895. [Google Scholar] [CrossRef]
- Al-Youssef, H.M.; Amina, M.; Hassan, S.; Amna, T.; Jeong, J.W.; Nam, K.T.; Kim, H.Y. Herbal drug loaded poly(D,L-lactide-co-glycolide) ultrafine fibers: Interaction with pathogenic bacteria. Macromol. Res. 2013, 21, 589–598. [Google Scholar] [CrossRef]
- Motealleh, B.; Zahedi, P.; Rezaeian, I.; Moghimi, M.; Abdolghaffari, A.H.; Zarandi, M.A. Morphology, drug release, antibacterial, cell proliferation, and histology studies of chamomile-loaded wound dressing mats based on electrospun nanofibrous poly(ε-caprolactone)/polystyrene blends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 977–987. [Google Scholar] [CrossRef]
- Lin, S.; Chen, M.; Jiang, H.; Fan, L.; Sun, B.; Yu, F.; Yang, X.; Lou, X.; He, C.; Wang, H. Green electrospun grape seed extract-loaded silk fibroin nanofibrous mats with excellent cytocompatibility and antioxidant effect. Colloids Surf. B Biointerfaces 2016, 139, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Prabhakaran, M.P.; Kai, D.; Annamalai, S.K.; Arunachalam, K.D.; Ramakrishna, S. Tissue engineered plant extracts as nanofibrous wound dressing. Biomaterials 2013, 34, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ronca, S.; Mele, E. Electrospun nanofibres containing antimicrobial plant extracts. Nanomaterials 2017, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Correia, I.J. Electrospun polycaprolactone/Aloe Vera_chitosan nanofibrous asymmetric membranes aimed for wound healing applications. Polymers 2017, 9, 183. [Google Scholar] [CrossRef]
- Rehman, R.; Hanif, M.A.; Mushtaq, Z.; Al-Sadi, A.M. Biosynthesis of essential oils in aromatic plants: A review. Food Rev. Int. 2016, 32, 117–160. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.H.; Wu, H.; Zong, M.H.; Jing, Y.R.; Han, S.Y. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Control 2016, 59, 366–376. [Google Scholar] [CrossRef]
- Feng, K.; Wen, P.; Yang, H.; Li, N.; Lou, W.Y.; Zong, M.H.; Wu, H. Enhancement of the antimicrobial activity of cinnamon essential oil-loaded electrospun nanofilm by the incorporation of lysozyme. RSC Adv. 2017, 7, 1572–1580. [Google Scholar] [CrossRef]
- Rafiq, M.; Hussain, T.; Abid, S.; Nazir, A.; Masood, R. Development of sodium alginate/PVA antibacterial nanofibers by the incorporation of essential oils. Mater. Res. Express 2018, 5, 035007. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Vicente, A.A.; Reis, M.A.M.; Torres-Giner, S.; Lagaron, J.M. Antimicrobial and antioxidant performance of various essential oils and natural extracts and their incorporation into biowaste derived poly(3-hydroxybutyrate-co-3-hydroxyvalerate) layers made from electrospun ultrathin fibers. Nanomaterials 2019, 9, 144. [Google Scholar] [CrossRef]
- Hasanpour Ardekani-Zadeh, A.; Hosseini, S.F. Electrospun essential oil-doped chitosan/poly(ε-caprolactone) hybrid nanofibrous mats for antimicrobial food biopackaging exploits. Carbohydr. Polym. 2019, 223, 115108. [Google Scholar] [CrossRef]
- Unalan, I.; Slavik, B.; Buettner, A.; Goldmann, W.H.; Frank, G.; Boccaccini, A.R. Physical and Antibacterial Properties of Peppermint Essential Oil Loaded Poly (ε-caprolactone) (PCL) Electrospun Fiber Mats for Wound Healing. Front. Bioeng. Biotechnol. 2019, 7, 1–11. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, Y.; Lan, X.; Huang, D.; Luo, T.; Ji, J.; Mafang, Z.; Miao, X.; Wang, H.; Wang, W. Electrospun Gelatin Nanofibers Encapsulated with Peppermint and Chamomile Essential Oils as Potential Edible Packaging. J. Agric. Food Chem. 2019, 67, 2227–2234. [Google Scholar] [CrossRef]
- Sahal, G.; Nasseri, B.; Ebrahimi, A.; Bilkay, I.S. Electrospun essential oil-polycaprolactone nanofibers as antibiofilm surfaces against clinical Candida tropicalis isolates. Biotechnol. Lett. 2019, 41, 511–522. [Google Scholar] [CrossRef]
- Liu, J.X.; Dong, W.H.; Mou, X.J.; Liu, G.S.; Huang, X.W.; Yan, X.; Zhou, C.F.; Jiang, S.; Long, Y.Z. In Situ Electrospun Zein/Thyme Essential Oil-Based Membranes as an Effective Antibacterial Wound Dressing. ACS Appl. Bio Mater. 2020, 3, 302–307. [Google Scholar] [CrossRef]
- Lin, L.; Liao, X.; Cui, H. Cold plasma treated thyme essential oil/silk fibroin nanofibers against Salmonella Typhimurium in poultry meat. Food Packag. Shelf Life 2019, 21, 100337. [Google Scholar] [CrossRef]
- Dias Antunes, M.; da Silva Dannenberg, G.; Fiorentini, Â.M.; Pinto, V.Z.; Lim, L.T.; da Rosa Zavareze, E.; Dias, A.R.G. Antimicrobial electrospun ultrafine fibers from zein containing eucalyptus essential oil/cyclodextrin inclusion complex. Int. J. Biol. Macromol. 2017, 104, 874–882. [Google Scholar] [CrossRef]
- Da Silva, F.T.; da Cunha, K.F.; Fonseca, L.M.; Antunes, M.D.; El Halal, S.L.M.; Fiorentini, Â.M.; da Zavareze, E.R.; Dias, A.R.G. Action of ginger essential oil (Zingiber officinale) encapsulated in proteins ultrafine fibers on the antimicrobial control in situ. Int. J. Biol. Macromol. 2018, 118, 107–115. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Lin, L. Plasma-treated poly(ethylene oxide) nanofibers containing tea tree oil/beta-cyclodextrin inclusion complex for antibacterial packaging. Carbohydr. Polym. 2018, 179, 360–369. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, C.; Kusmartseva, O.; Thomas, N.L.; Mele, E. Electrospinning of polylactic acid fibres containing tea tree and manuka oil. React. Funct. Polym. 2017, 117, 106–111. [Google Scholar] [CrossRef]
- Wang, P.; Mele, E. Effect of antibacterial plant extracts on the morphology of electrospun poly(lactic acid) fibres. Materials 2018, 11, 923. [Google Scholar] [CrossRef]
- Mele, E. Electrospinning of essential oils. Polymers 2020, 12, 908. [Google Scholar] [CrossRef]
- D’agostino, M.; Tesse, N.; Frippiat, J.P.; Machouart, M.; Debourgogne, A. Essential oils and their natural active compounds presenting antifungal properties. Molecules 2019, 24, 3713. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S.Y. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Huang, D.F.; Xu, J.G.; Liu, J.X.; Zhang, H.; Hu, Q.P. Chemical constituents, antibacterial activity and mechanism of action of the essential oil from Cinnamomum cassia bark against four food-related bacteria. Microbiology 2014, 83, 357–365. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; qin Shi, Y.; hua Pan, X.; hua Lu, Y.; Cao, P. Antibacterial effects of cinnamon (Cinnamomum zeylanicum) bark essential oil on Porphyromonas gingivalis. Microb. Pathog. 2018, 116, 26–32. [Google Scholar] [CrossRef]
- Goñi, P.; López, P.; Sánchez, C.; Gómez-Lus, R.; Becerril, R.; Nerín, C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 2009, 116, 982–989. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.H.; Feng, K.; Liu, F.J.; Lou, W.Y.; Li, N.; Zong, M.H.; Wu, H. Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/β-cyclodextrin inclusion complex for antimicrobial packaging. Food Chem. 2016, 196, 996–1004. [Google Scholar] [CrossRef]
- Lin, L.; Dai, Y.; Cui, H. Antibacterial poly(ethylene oxide) electrospun nanofibers containing cinnamon essential oil/beta-cyclodextrin proteoliposomes. Carbohydr. Polym. 2017, 178, 131–140. [Google Scholar] [CrossRef]
- Liakos, I.; Rizzello, L.; Hajiali, H.; Brunetti, V.; Carzino, R.; Pompa, P.P.; Athanassiou, A.; Mele, E. Fibrous wound dressings encapsulating essential oils as natural antimicrobial agents. J. Mater. Chem. B 2015, 3, 1583–1589. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial mechanism of oregano essential oil. Ind. Crops Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- Işcan, G.; Kirimer, N.; Kürkcüoǧlu, M.; Başer, K.H.C.; Demirci, F. Antimicrobial screening of Mentha piperita essential oils. J. Agric. Food Chem. 2002, 50, 3943–3946. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.K.; Malik, A. Antimicrobial potential and chemical composition of Mentha piperita oil in liquid and vapour phase against food spoiling microorganisms. Food Control 2011, 22, 1707–1714. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mani, M.P.; Khudzari, A.Z.M. Electrospun combination of peppermint oil and copper sulphate with conducive physico-chemical properties for wound dressing applications. Polymers 2019, 11, 586. [Google Scholar] [CrossRef] [PubMed]
- Suganya Bharathi, B.; Stalin, T. Cerium oxide and peppermint oil loaded polyethylene oxide/graphene oxide electrospun nanofibrous mats as antibacterial wound dressings. Mater. Today Commun. 2019, 21, 100664. [Google Scholar] [CrossRef]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Ben Kahla-Nakbi, A.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The Chemical Composition and Biological Activity of Clove Essential Oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A Short Review. Phytother. Res 2007, 21, 501–506. [Google Scholar] [CrossRef]
- Moon, S.E.; Kim, H.Y.; Cha, J.D. Synergistic effect between clove oil and its major compounds and antibiotics against oral bacteria. Arch. Oral Biol. 2011, 56, 907–916. [Google Scholar] [CrossRef]
- Unalan, I.; Endlein, S.J.; Slavik, B.; Buettner, A.; Goldmann, W.H.; Detsch, R.; Boccaccini, A.R. Evaluation of electrospun poly(ε-caprolactone)/gelatin nanofiber mats containing clove essential oil for antibacterial wound dressing. Pharmaceutics 2019, 11, 570. [Google Scholar] [CrossRef]
- Rota, M.C.; Herrera, A.; Martínez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Çallıoğlu, F.C.; Güler, H.K.; Çetin, E.S. Emulsion electrospinning of bicomponent poly (vinyl pyrrolidone)/gelatin nanofibers with thyme essential oil Emulsion electrospinning of bicomponent poly (vinyl pyrrolidone)/gelatin nano fi bers with thyme essential oil. Mater. Res. Express 2019, 6, 125013. [Google Scholar] [CrossRef]
- Berechet, M.D.; Gaidau, C.; Miletic, A.; Pilic, B.; Râpă, M.; Stanca, M.; Ditu, L.-M.; Constantinescu, R.; Lazea-Stoyanova, A. Bioactive Properties of Nanofibres Based on Concentrated Collagen Hydrolysate Loaded with Thyme and Oregano Essential Oils. Materials 2020, 13, 1618. [Google Scholar] [CrossRef]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological activities of lavender essential oil. Phyther. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Gille, E.; Trifan, A.; Luca, V.S.; Miron, A. Essential oils of Lavandula genus: A systematic review of their chemistry. Phytochem. Rev. 2017, 16, 761–799. [Google Scholar] [CrossRef]
- Electrospun, A.; Nanofibers, P.; Khunová, V.; Kováčová, M.; Olejniková, P.; Ondreáš, F.; Špitalský, Z.; Ghosal, K.; Berkeš, D. Antibacterial Electrospun Polycaprolactone Nanofibers Reinforced by Halloysite Nanotubes for Tissue Engineering. Polymers 2022, 14, 746. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Yakub, G.; Toncheva, A.; Manolova, N.; Rashkov, I.; Danchev, D.; Kussovski, V. Electrospun polylactide-based materials for curcumin release: Photostability, antimicrobial activity, and anticoagulant effect. J. Appl. Polym. Sci. 2016, 133, 1–11. [Google Scholar] [CrossRef]
- Sridhar, R.; Ravanan, S.; Venugopal, J.R.; Sundarrajan, S.; Pliszka, D.; Sivasubramanian, S.; Gunasekaran, P.; Prabhakaran, M.; Madhaiyan, K.; Sahayaraj, A.; et al. Curcumin-and natural extract-loaded nanofibres for potential treatment of lung and breast cancer: In vitro efficacy evaluation. J. Biomater. Sci. Polym. Ed. 2014, 25, 985–998. [Google Scholar] [CrossRef]
- Wang, C.; Ma, C.; Wu, Z.; Liang, H.; Yan, P.; Song, J.; Ma, N.; Zhao, Q. Enhanced Bioavailability and Anticancer Effect of Curcumin-Loaded Electrospun Nanofiber: In Vitro and In Vivo Study. Nanoscale Res. Lett. 2015, 10, 1–10. [Google Scholar] [CrossRef]
- Trung, H.; Bui, H.T.; Chung, O.H.; Park, J.S. Fabrication of Electrospun Antibacterial Curcumin-loaded Zein Nanofibers. Polymer 2014, 38, 744–751. [Google Scholar] [CrossRef]
- Fallah, M.; Bahrami, S.H.; Ranjbar-Mohammadi, M. Fabrication and characterization of PCL/gelatin/curcumin nanofibers and their antibacterial properties. J. Ind. Text. 2016, 46, 562–577. [Google Scholar] [CrossRef]
- Deng, L.; Kang, X.; Liu, Y.; Feng, F.; Zhang, H. Effects of surfactants on the formation of gelatin nanofibres for controlled release of curcumin. Food Chem. 2017, 231, 70–77. [Google Scholar] [CrossRef]
- Han, J.; Chen, T.X.; Branford-White, C.J.; Zhu, L.M. Electrospun shikonin-loaded PCL/PTMC composite fiber mats with potential biomedical applications. Int. J. Pharm. 2009, 382, 215–221. [Google Scholar] [CrossRef]
- Wolfgang, M.C.; Martin Dozois, C.; Schmelcher, M.; Zurich, E.; Mahlapuu margitmahlapuu, M.; Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Kang, S.J.; Park, S.J.; Mishig-Ochir, T.; Lee, B.J. Antimicrobial peptides: Therapeutic potentials. Expert Rev. Anti. Infect. Ther. 2014, 12, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Song, D.W.; Kim, S.H.; Kim, H.H.; Lee, K.H.; Ki, C.S.; Park, Y.H. Multi-biofunction of antimicrobial peptide-immobilized silk fibroin nanofiber membrane: Implications for wound healing. Acta Biomater. 2016, 39, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Suchánek, J.; Henke, P.; Mosinger, J.; Zelinger, Z.; Kubát, P. Effect of temperature on photophysical properties of polymeric nanofiber materials with porphyrin photosensitizers. J. Phys. Chem. B 2014, 118, 6167–6174. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.S.; Oh, K.W.; Kim, S.H. Antimicrobial activity of organic photosensitizers embedded in electrospun nylon 6 nanofibers. Polym. Int. 2012, 61, 1519–1524. [Google Scholar] [CrossRef]



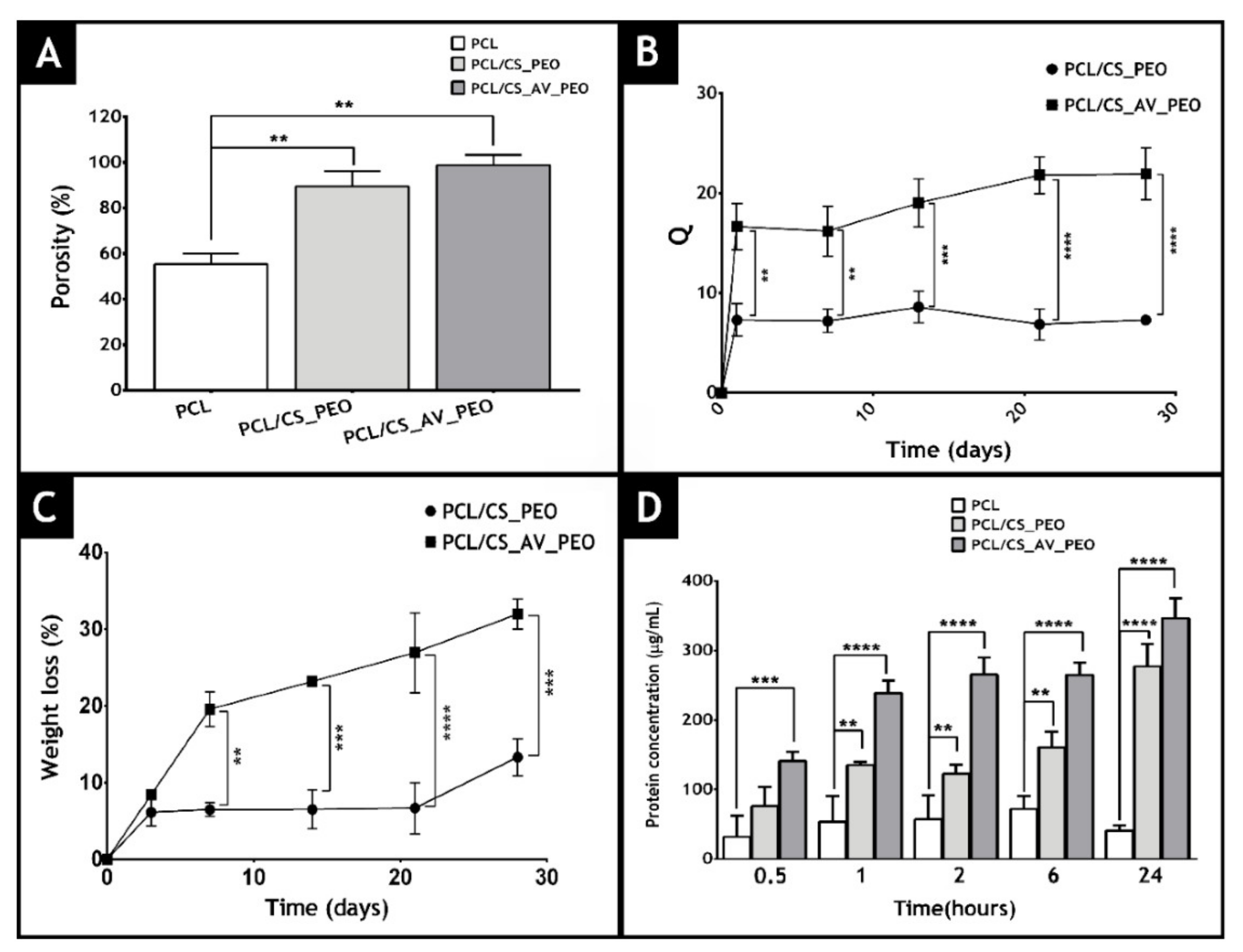

| Fabrication Method | Advantages | Disadvantages |
|---|---|---|
| Drawing |
|
|
| Template synthesis |
|
|
| Phase separation |
|
|
| Self-assembly |
|
|
| Electrospinning |
|
|
| Fabrication Method | Structure | Refs. |
|---|---|---|
| Drawing |
| [1,6] |
| Template synthesis |
| [7,8] |
| Phase separation |
| [4] |
| Self-assembly |
| [3,9] |
| Electrospinning |
| [10,11] |
| Electrospun Material | Agent | Microorganism | Refs. |
|---|---|---|---|
| Poly(vinyl alcohol-co-vinyl acetate)/octadecyl amine-montmorillonite | AgNPs | C. albicans, C. tropicalis, C. glabrata, C. keyfr, C. krusei, S. aureus, E. coli | [120] |
| Ethylene vinyl alcohol copolymer | AgNPs | L. monocytogenes, S. enterica | [121] |
| Polystyrene (PS) | AgNPs | S. xylosus | [122] |
| Polyvinyl alcohol (PVA)/silk fibroin (SF) | AgNPs | E. coli, S. aureus | [123] |
| Ascorbyl palmitate/poly (e-caprolactone) (PCL) | AgNPs | S. aureus | [124] |
| Poly(butylenes succinate)(PBS) | AgNPs | S. aureus, E. coli | [125] |
| Polyacrylonitrile | AgNPs | S. aureus, E. coli, M. albicans | [110] |
| Polycaprolactone | AgNPs | S. aureus, E. coli, C. albicans | [126] |
| Poly (acrylonitrile-co-methyl methacrylate | AgNPs | P. aeruginosa, S. aureus, E.coli, Acinetobacter sp, K. pneumoniae, Micrococcus sp, S. epidermidis, Candida sp. | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maliszewska, I.; Czapka, T. Electrospun Polymer Nanofibers with Antimicrobial Activity. Polymers 2022, 14, 1661. https://doi.org/10.3390/polym14091661
Maliszewska I, Czapka T. Electrospun Polymer Nanofibers with Antimicrobial Activity. Polymers. 2022; 14(9):1661. https://doi.org/10.3390/polym14091661
Chicago/Turabian StyleMaliszewska, Irena, and Tomasz Czapka. 2022. "Electrospun Polymer Nanofibers with Antimicrobial Activity" Polymers 14, no. 9: 1661. https://doi.org/10.3390/polym14091661
APA StyleMaliszewska, I., & Czapka, T. (2022). Electrospun Polymer Nanofibers with Antimicrobial Activity. Polymers, 14(9), 1661. https://doi.org/10.3390/polym14091661








