Antiviral Polymers: A Review
Abstract
:1. Introduction
2. Natural Polymers
2.1. Polysaccharides
2.1.1. Carrageenan
2.1.2. Chitosan and Its Derivatives
2.1.3. Fucoidan
2.2. Nucleic Acid Polymers
3. Synthetic Polymers
3.1. Dendrimers
3.2. Sialyl-Based Polymers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CXCR4 | chemokine receptor type 4 |
| ERK | extracellular signal-regulated kinase |
| gB | glycoprotein B |
| H1N1 | novel influenza A virus |
| HBV | hepatitis B virus |
| HCoV-OC43 | human coronavirus OC43 |
| HCV | hepatitis C virus |
| HDV | hepatitis delta virus |
| HIV | human immunodeficiency virus |
| hMPV | human metapneumovirus |
| HPV | human papillomavirus |
| HRV | human rhinovirus |
| HSV-1 | herpes simplex virus Type 1 |
| HSV-2 | herpes simplex virus Type 2 |
| IAV | influenza A virus |
| IC50 | Half maximal inhibitory concentration |
| NAPs | nucleic acid polymers |
| PBMC | primary peripheral blood mononuclear cells |
| PCD | polyanionic carbosilane dendrimers |
| PG | 2,3-O-disulfated-1,1,4-poly-L-guluronic acid |
| RSV | respiratory virus |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
References
- Zhang, N.; Wang, L.; Deng, X.; Liang, R.; Su, M.; He, C.; Hu, L.; Su, Y.; Ren, J.; Yu, F. Recent advances in the detection of respiratory virus infection in humans. J. Med. Virol. 2020, 92, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Gannon, O.; Antonsson, A.; Bennett, I.; Saunders, N. Viral infections and breast cancer—A current perspective. Cancer Lett. 2018, 420, 182–189. [Google Scholar] [CrossRef]
- Feldmann, H.; Jones, S.; Klenk, H.D.; Schnittler, H.J. Ebola virus: From discovery to vaccine. Nat. Rev. Immunol. 2003, 3, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, N.; Yang, Z.-Y.; Nabel, G.J. Ebola virus pathogenesis: Implications for vaccines and therapies. J. Virol. 2003, 77, 9733–9737. [Google Scholar] [CrossRef] [Green Version]
- Colson, P.; La Scola, B.; Levasseur, A.; Caetano-Anolles, G.; Raoult, D. Mimivirus: Leading the way in the discovery of giant viruses of amoebae. Nat. Rev. Microbiol. 2017, 15, 243–254. [Google Scholar] [CrossRef]
- Villanueva, R.A.; Rouillé, Y.; Dubuisson, J. Interactions between virus proteins and host cell membranes during the viral life cycle. Int. Rev. Cytol. 2005, 245, 171–244. [Google Scholar] [PubMed]
- Choi, Y.; Bowman, J.W.; Jung, J.U. Autophagy during viral infection—a double-edged sword. Nat. Rev. Microbiol. 2018, 16, 341–354. [Google Scholar] [CrossRef]
- Ricci, S.; Francisci, D.; Longo, V.; Delfavero, A. Central nervous-system side-effects of antiviral drugs. Int. J. Clin. Pharmacol. Ther. Toxicol. 1988, 26, 400–408. [Google Scholar]
- Chaudhuri, S.; Symons, J.A.; Deval, J. Innovation and trends in the development and approval of antiviral medicines: 1987–2017 and beyond. Antiviral Res. 2018, 155, 76–88. [Google Scholar] [CrossRef]
- Zareifopoulos, N.; Lagadinou, M.; Karela, A.; Kyriakopoulou, O.; Velissaris, D. Neuropsychiatric effects of antiviral drugs. Cureus 2020, 12, e9536. [Google Scholar] [CrossRef]
- Chun, H.; Yeom, M.; Kim, H.-O.; Lim, J.-W.; Na, W.; Park, G.; Park, C.; Kang, A.; Yun, D.; Kim, J. Efficient antiviral co-delivery using polymersomes by controlling the surface density of cell-targeting groups for influenza A virus treatment. Polym. Chem. 2018, 9, 2116–2123. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Gong, L.D.; Yin, Y.; Zhang, L.; Sun, Q.M.; Feng, K.; Cui, Y.M.; Zhang, Q.; Zhang, X.H.; Deng, X.L.; et al. A gradient pH-sensitive polymer-based antiviral strategy via viroporin-induced membrane acidification. Adv. Mater. 2022, 2, e2109580. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.C.F.; Poletto, F.; Eberhardt, M.J.; Domingues, S.C.; De Sousa, F.B.; Tebaldi, M.L. Polymer-hybrid nanosystems for antiviral applications: Current advances. Biomed. Pharmacother. 2022, 146, 112249. [Google Scholar] [CrossRef]
- Chen, P.Y.; Lang, J.Y.; Zhou, Y.L.; Khlyustova, A.; Zhang, Z.Y.; Ma, X.J.; Liu, S.; Cheng, Y.F.; Yang, R. An imidazolium-based zwitterionic polymer for antiviral and antibacterial dual functional coatings. Sci. Adv. 2022, 8, eab18812. [Google Scholar] [CrossRef] [PubMed]
- Ordon, M.; Zdanowicz, M.; Nawrotek, P.; Stachurska, X.; Mizielinska, M. Polyethylene films containing plant extracts in the polymer matrix as antibacterial and antiviral materials. Int. J. Mol. Sci. 2021, 22, 13438. [Google Scholar] [CrossRef]
- Nasri, N.; Rusli, A.; Teramoto, N.; Jaafar, M.; Ishak, K.M.K.; Shafiq, M.D.; Hamid, Z.A.A. Past and current progress in the development of antiviral/antimicrobial polymer coating towards COVID-19 prevention: A Review. Polymers 2021, 13, 4234. [Google Scholar] [CrossRef]
- Li, J.H.; Wang, W.Z.; Jiang, R.; Guo, C.C. Antiviral electrospun polymer composites: Recent advances and opportunities for tackling COVID-19. Front. Mater. 2021, 8, 773205. [Google Scholar] [CrossRef]
- Leong, J.Y.; Shi, D.R.; Tan, J.P.K.; Yang, C.; Yang, S.C.; Wang, Y.M.; Ngow, Y.S.; Kng, J.; Balakrishnan, N.; Peng, S.Q.; et al. Potent antiviral and antimicrobial polymers as safe and effective disinfectants for the prevention of infections. Adv. Healthc. Mater. 2021, 2101898. [Google Scholar] [CrossRef]
- Kuroki, A.; Tay, J.; Lee, G.H.; Yang, Y.Y. Broad-Spectrum Antiviral Peptides and Polymers. Adv. Healthc. Mater. 2021, 10, e2101898. [Google Scholar] [CrossRef]
- Steinman, N.Y.; Hu, T.; Dombrovsky, A.; Reches, M.; Domb, A.J. Antiviral polymers based on N-halamine polyurea. Biomacromolecules 2021, 22, 4357–4364. [Google Scholar] [CrossRef]
- Yadavalli, T.; Mallick, S.; Patel, P.; Koganti, R.; Shukla, D.; Date, A.A. Pharmaceutically acceptable carboxylic acid-terminated polymers show activity and selectivity against HSV-1 and HSV-2 and synergy with antiviral drugs. ACS Infect. Dis. 2020, 6, 2926–2937. [Google Scholar] [CrossRef] [PubMed]
- Bianculli, R.H.; Mase, J.D.; Schulz, M.D. Antiviral polymers: Past approaches and future possibilities. Macromolecules 2020, 53, 9158–9186. [Google Scholar] [CrossRef]
- Soria-Martinez, L.; Bauer, S.; Giesler, M.; Schelhaas, S.; Materlik, J.; Janus, K.; Pierzyna, P.; Becker, M.; Snyder, N.L.; Hartmann, L. Prophylactic antiviral activity of sulfated glycomimetic oligomers and polymers. J. Am. Chem. Soc. 2020, 142, 5252–5265. [Google Scholar] [CrossRef] [PubMed]
- Roner, M.R.; Carraher, C.E.; Miller, L.; Mosca, F.; Slawek, P.; Haky, J.E.; Frank, J. Organotin polymers as antiviral agents including inhibition of Zika and Vaccinia viruses. J. Inorg. Organomet. Polym. Mater. 2020, 30, 684–694. [Google Scholar] [CrossRef]
- Gunther, S.C.; Maier, J.D.; Vetter, J.; Podvalnyy, N.; Khanzhin, N.; Hennet, T.; Stertz, S. Antiviral potential of 3′-sialyllactose- and 6′-sialyllactose-conjugated dendritic polymers against human and avian influenza viruses. Sci. Rep. 2020, 10, 768. [Google Scholar] [CrossRef] [Green Version]
- Fox, M.E.; Szoka, F.C.; Fréchet, J.M. Soluble polymer carriers for the treatment of cancer: The importance of molecular architecture. Acc. Chem. Res. 2009, 42, 1141–1151. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Canady, T.D.; Zhou, Z.; Tang, Y.; Price, D.N.; Bear, D.G.; Chi, E.Y.; Schanze, K.S.; Whitten, D.G. Cationic phenylene ethynylene polymers and oligomers exhibit efficient antiviral activity. ACS Appl. Mater. Interf. 2011, 3, 2209–2214. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural polymers for organ 3D bioprinting. Polymers 2018, 10, 1278. [Google Scholar] [CrossRef] [Green Version]
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Antiviral surfaces and coatings and their mechanisms of action. Commun. Mater. 2021, 2, 1–19. [Google Scholar] [CrossRef]
- Randazzo, W.; Fabra, M.J.; Falcó, I.; López-Rubio, A.; Sánche, G. Polymers and biopolymers with antiviral activity: Potential applications for improving food safety. Comp. Rev. Food Sci. Food Saf. 2018, 17, 754–768. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.-H.; Jin, M.-L.; Morris, G.A.; Zha, X.-Q.; Chen, H.-Q.; Yi, Y.; Li, J.-E.; Wang, Z.-J.; Gao, J.; Nie, S.-P. Advances on bioactive polysaccharides from medicinal plants. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. S1), S60–S84. [Google Scholar] [CrossRef] [PubMed]
- Dwek, R.A. Glycobiology: Toward understanding the function of sugars. Chem. Rev. 1996, 96, 683–720. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Shen, M.; Hong, Y.; Ye, H.; Huang, L.; Xie, J. Chemical modifications of polysaccharides and their anti-tumor activities. Carbohydr. Polym. 2020, 229, 115436. [Google Scholar] [CrossRef] [PubMed]
- Pawar, H.A.; Kamat, S.R.; Choudhary, P.D. An overview of natural polysaccharides as biological macromolecules: Their chemical modifications and pharmaceutical applications. Biol. Med. 2015, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.-L.; Zhang, W.-Z.; Ni, W.-X.; Shao, J.-W. Insight on structure-property relationships of carrageenan from marine red algal: A review. Carbohydr. Polym. 2021, 257, 117642. [Google Scholar] [CrossRef]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Obtaining chitin, chitosan and their melanin complexes from insects. Int. J. Biol. Macromol. 2021, 167, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Neves, N.M.; Reis, R.L.; Martins, A.; Silva, T.H. A review on fucoidan antitumor strategies: From a biological active agent to a structural component of fucoidan-based systems. Carbohydr. Polym. 2020, 239, 116131. [Google Scholar] [CrossRef]
- Darge, H.F.; Andrgie, A.T.; Tsai, H.-C.; Lai, J.-Y. Polysaccharide and polypeptide based injectable thermo-sensitive hydrogels for local biomedical applications. Int. J. Biol. Macromol. 2019, 133, 545–563. [Google Scholar] [CrossRef]
- Gupta, N.; Jangid, A.K.; Pooja, D.; Kulhari, H. Inulin: A novel and stretchy polysaccharide tool for biomedical and nutritional applications. Int. J. Biol. Macromol. 2019, 132, 852–863. [Google Scholar] [CrossRef]
- Shi, Q.M.; Wang, A.J.; Lu, Z.H.; Qin, C.J.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453–454, 1–9. [Google Scholar] [CrossRef]
- Lu, W.J.; Yang, Z.F.; Chen, J.; Wang, D.; Zhang, Y. Recent advances in antiviral activities and potential mechanisms of sulfated polysaccharides. Carbohydr. Polym. 2021, 272, 118526. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Cui, B.; Liu, X.W.; Wang, L.C.; Xian, Q.J.; Lu, Z.X.; Liu, S.T.; Cao, Y.G.; Zhao, Y.R. Virucidal activity and the antiviral mechanism of acidic polysaccharides against Enterovirus 71 infection in vitro. Microbiol. Immunol. 2020, 64, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Gao, L.; Wang, C.; Liu, B.; Jin, Y.; Xing, Z. Structural characterization and antiviral activity of a novel heteropolysaccharide isolated from Grifola frondosa against enterovirus 71. Carbohydr. Polym. 2016, 144, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Leibbrandt, A.; Meier, C.; König-Schuster, M.; Weinmüllner, R.; Kalthoff, D.; Pflugfelder, B.; Graf, P.; Frank-Gehrke, B.; Beer, M.; Fazekas, T. Iota-carrageenan is a potent inhibitor of influenza A virus infection. PLoS ONE 2010, 5, e14320. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.B.; Thompson, C.D.; Roberts, J.N.; Müller, M.; Lowy, D.R.; Schiller, J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006, 2, e69. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Luo, Q.; Li, S.; Li, C.; Liao, S.; Yang, X.; Zhou, R.; Zhu, Y.; Teng, L.; Chen, H. Antiviral activity against porcine epidemic diarrhea virus of Pogostemon cablin polysaccharide. J. Ethnopharmacol. 2020, 259, 113009. [Google Scholar] [CrossRef]
- Liang, Y.-Y.; Li, K.-W.; Niu, F.-J.; Li, Y.; Wei, H.-C.; Dai, Y.-L.; Wang, Y.-Y.; Zhou, C.-Z.; Wan, X.-H. Salvia plebeia R. Br. polysaccharides (SPP) against RSV (respiratory syncytial virus) infection: Antiviral effect and mechanisms of action. Biomed. Pharmacother. 2021, 141, 111843. [Google Scholar] [CrossRef]
- Wang, T.; Wang, X.; Zhuo, Y.; Si, C.; Yang, L.; Meng, L.; Zhu, B. Antiviral activity of a polysaccharide from Radix Isatidis (Isatis indigotica Fortune) against hepatitis B virus (HBV) in vitro via activation of JAK/STAT signal pathway. J. Ethnopharmacol. 2020, 257, 112782. [Google Scholar] [CrossRef]
- Chen, X.Y.; Han, W.W.; Wang, G.X.; Zhao, X. Application prospect of polysaccharides in the development of anti-novel coronavirus drugs and vaccines. Int. J. Biol. Macromol. 2020, 164, 331–343. [Google Scholar] [CrossRef]
- Yu, C.; Wei, K.; Liu, L.; Yang, S.; Hu, L.; Zhao, P.; Meng, X.; Shao, M.; Wang, C.; Zhu, L. Taishan Pinus massoniana pollen polysaccharide inhibits subgroup J avian leucosis virus infection by directly blocking virus infection and improving immunity. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Carbone, D.A.; Pellone, P.; Lubritto, C.; Ciniglia, C. Evaluation of microalgae antiviral activity and their bioactive compounds. Antibiotics 2021, 10, 746. [Google Scholar] [CrossRef] [PubMed]
- Sanniyasi, E.; Venkatasubramanian, G.; Anbalagan, M.M.; Raj, P.P.; Gopal, R.K. In vitro anti-HIV-1 activity of the bioactive compound extracted and purified from two different marine macroalgae (seaweeds) (Dictyota bartayesiana J.V.Lamouroux and Turbinaria decurrens Bory). Sci. Rep. 2019, 9, 12185. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Critchley, A.T. The COVID 19 novel coronavirus pandemic 2020: Seaweeds to the rescue? Why does substantial, supporting research about the antiviral properties of seaweed polysaccharides seem to go unrecognized by the pharmaceutical community in these desperate times? J. Appl. Phycol. 2020, 32, 1875–1877. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Parra-Saldivar, R.; Bilal, M.; Afroze, C.A.; Ahmed, M.N.; Iqbal, H.M.N.; Xu, J.L. Algae-derived bioactive molecules for the potential treatment of SARS-CoV-2. Molecules 2021, 26, 2134. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.; Jayaraman, G. Marine sulfated polysaccharides as potential antiviral drug candidates to treat corona virus disease (COVID-19). Carbohydr. Res. 2021, 505, 108326. [Google Scholar] [CrossRef]
- Wang, W.; Wu, J.; Zhang, X.; Hao, C.; Zhao, X.; Jiao, G.; Shan, X.; Tai, W.; Yu, G. Inhibition of influenza A virus infection by fucoidan targeting viral neuraminidase and cellular EGFR pathway. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, X.; Miao, Y.; Zhou, Y.; Shi, J.; Yan, M.; Chen, A. Studies on antiviral and immuno-regulation activity of low molecular weight fucoidan from Laminaria japonica. J. Ocean Univ. China 2018, 17, 705–711. [Google Scholar] [CrossRef]
- Kalitnik, A.A.; Byankina Barabanova, A.; Nagorskaya, V.; Reunov, A.; Glazunov, V.; Solov’eva, T.; Yermak, I. Low molecular weight derivatives of different carrageenan types and their antiviral activity. J. Appl. Phycol. 2013, 25, 65–72. [Google Scholar] [CrossRef]
- Aga, M.B.; Dar, A.H.; Nayik, G.A.; Panesar, P.S.; Allai, F.; Khan, S.A.; Shams, R.; Kennedy, J.F.; Altaf, A. Recent insights into carrageenan-based bio-nanocomposite polymers in food applications: A review. Int. J. Biol. Macromol. 2021, 192, 197–209. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef]
- He, X.; Fang, J.; Guo, Q.; Wang, M.; Li, Y.; Meng, Y.; Huang, L. Advances in antiviral polysaccharides derived from edible and medicinal plants and mushrooms. Carbohydr. Polym. 2020, 229, 115548. [Google Scholar] [CrossRef] [PubMed]
- Hans, N.; Malik, A.; Naik, S. Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: Mini review. Bioresour. Technol. Rep. 2020, 13, 100623. [Google Scholar] [CrossRef] [PubMed]
- Ana, P.; Nathalie, B.; Gilles, B.; Daniel, R.; Tomás, M.-S.; Yolanda, F.-P. Anti-Herpes simplex virus (HSV-1) activity and antioxidant capacity of carrageenan-rich enzymatic extracts from Solieria filiformis (Gigartinales, Rhodophyta). Int. J. Biol. Macromol. 2021, 168, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Talarico, L.B.; Damonte, E.B. Interference in dengue virus adsorption and uncoating by carrageenans. Virology 2007, 363, 473–485. [Google Scholar] [CrossRef] [Green Version]
- Kolender, A.A.; Pujol, C.A.; Damonte, E.B.; Cerezo, A.S.; Matulewicz, M. Sulfation of kappa-carrageenan and antiviral activity. An. Asoc. Quim. Argent. 1998, 86, 304–311. [Google Scholar]
- Baba, M.; Snoeck, R.; Pauwels, R.; De Clercq, E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 1988, 32, 1742–1745. [Google Scholar] [CrossRef] [Green Version]
- Morokutti-Kurz, M.; König-Schuster, M.; Koller, C.; Graf, C.; Graf, P.; Kirchoff, N.; Reutterer, B.; Seifert, J.-M.; Unger, H.; Grassauer, A. The intranasal application of Zanamivir and carrageenan is synergistically active against influenza A virus in the murine model. PLoS ONE 2015, 10, e0128794. [Google Scholar] [CrossRef] [Green Version]
- Morokutti-Kurz, M.; Fröba, M.; Graf, P.; Große, M.; Grassauer, A.; Auth, J.; Schubert, U.; Prieschl-Grassauer, E. Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro. PLoS ONE 2021, 16, e0237480. [Google Scholar] [CrossRef]
- Witvrouw, M.; De Clercq, E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Pharmacol. Vasc. Sys. 1997, 29, 497–511. [Google Scholar] [CrossRef]
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karmakar, P.; Mandal, P.; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure–activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar] [CrossRef]
- Piccini, L.E.; Carro, A.C.; Quintana, V.M.; Damonte, E.B. Antibody-independent and dependent infection of human myeloid cells with dengue virus is inhibited by carrageenan. Virus Res. 2020, 290, 198150. [Google Scholar] [CrossRef] [PubMed]
- Vissani, A.; Galdo Novo, S.; Ciancia, M.; Zabal, O.A.; Thiry, E.; Bratanich, A.C.; Barrandeguy, M.E. Effects of lambda-carrageenan on equid herpesvirus 3 in vitro. J. Equine Vet. Sci. 2016, 39, S61–S62. [Google Scholar] [CrossRef]
- Ciejka, J.; Botwina, P.; Nowakowska, M.; Szczubiałka, K.; Pyrc, K. Synthetic sulfonated derivatives of poly (allylamine hydrochloride) as inhibitors of human metapneumovirus. PLoS ONE 2019, 14, e0214646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenighofer, M.; Lion, T.; Bodenteich, A.; Prieschl-Grassauer, E.; Grassauer, A.; Unger, H.; Mueller, C.A.; Fazekas, T. Carrageenan nasal spray in virus confirmed common cold: Individual patient data analysis of two randomized controlled trials. Multidiscip. Respir. Med. 2014, 9, 1–12. [Google Scholar] [CrossRef]
- Morokutti-Kurz, M.; Graf, C.; Prieschl-Grassauer, E. Amylmetacresol/2, 4-dichlorobenzyl alcohol, hexylresorcinol, or carrageenan lozenges as active treatments for sore throat. Int. J. Gen. Med. 2017, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Buck, C.B.; Cheng, N.; Thompson, C.D.; Lowy, D.R.; Steven, A.C.; Schiller, J.T.; Trus, B.L. Arrangement of L2 within the papillomavirus capsid. J. Virol. 2008, 82, 5190–5197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, A.; Kleinbeck, K.; Mizenina, O.; Kizima, L.; Levendosky, K.; Jean-Pierre, N.; Villegas, G.; Ford, B.E.; Cooney, M.L.; Teleshova, N. In vitro and in vivo evaluation of two carrageenan-based formulations to prevent HPV acquisition. Antivir. Res. 2014, 108, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.; Shin, H.; Lee, M.K.; Kwon, O.S.; Shin, J.S.; Kim, Y.-i.; Kim, C.W.; Lee, H.-R.; Kim, M. Antiviral activity of lambda-carrageenan against influenza viruses and severe acute respiratory syndrome coronavirus 2. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Schütz, D.; Conzelmann, C.; Fois, G.; Groß, R.; Weil, T.; Wettstein, L.; Stenger, S.; Zelikin, A.; Hoffmann, T.K.; Frick, M. Carrageenan containing over-the-counter nasal and oral sprays inhibit SARS-CoV-2 infection of airway epithelial cultures. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L750–L756. [Google Scholar] [CrossRef]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Riaz Rajoka, M.S.; Mehwish, H.M.; Wu, Y.; Zhao, L.; Arfat, Y.; Majeed, K.; Anwaar, S. Chitin/chitosan derivatives and their interactions with microorganisms: A comprehensive review and future perspectives. Crit. Rev. Biotechnol. 2020, 40, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Shamshina, J.L.; Berton, P.; Rogers, R.D. Advances in functional chitin materials: A review. ACS Sustain. Chem. Eng. 2019, 7, 6444–6457. [Google Scholar] [CrossRef]
- Safarzadeh, M.; Sadeghi, S.; Azizi, M.; Rastegari-Pouyani, M.; Pouriran, R.; Hoseini, M.H.M. Chitin and chitosan as tools to combat COVID-19: A triple approach. Int. J. Biol. Macromol. 2021, 183, 235–244. [Google Scholar] [CrossRef]
- Jana, B.; Chatterjee, A.; Roy, D.; Ghorai, S.; Pan, D.; Pramanik, S.K.; Chakraborty, N.; Ganguly, J. Chitosan/benzyloxy-benzaldehyde modified ZnO nano template having optimized and distinct antiviral potency to human cytomegalovirus. Carbohydr. Polym. 2022, 278, 118965. [Google Scholar] [CrossRef] [PubMed]
- Davydova, V.; Nagorskaya, V.; Gorbach, V.; Kalitnik, A.; Reunov, A.; Solov’Eva, T.; Ermak, I. Chitosan antiviral activity: Dependence on structure and depolymerization method. Appl. Biochem. Microbiol. 2011, 47, 103–108. [Google Scholar] [CrossRef]
- Chirkov, S. The antiviral activity of chitosan. Appl. Biochem. Microbiol. 2002, 38, 1–8. [Google Scholar] [CrossRef]
- Sharma, N.; Modak, C.; Singh, P.K.; Kumar, R.; Khatri, D.; Singh, S.B. Underscoring the immense potential of chitosan in fighting a wide spectrum of viruses: A plausible molecule against SARS-CoV-2? Int. J. Biol. Macromol. 2021, 179, 33–44. [Google Scholar] [CrossRef]
- Nishimura, S.-I.; Kai, H.; Shinada, K.; Yoshida, T.; Tokura, S.; Kurita, K.; Nakashima, H.; Yamamoto, N.; Uryu, T. Regioselective syntheses of sulfated polysaccharides: Specific anti-HIV-1 activity of novel chitin sulfates. Carbohydr. Res. 1998, 306, 427–433. [Google Scholar] [CrossRef]
- Shibata, Y.; Foster, L.A.; Metzger, W.J.; Myrvik, Q.N. Alveolar macrophage priming by intravenous administration of chitin particles, polymers of N-acetyl-D-glucosamine, in mice. Infect. Immunity 1997, 65, 1734–1741. [Google Scholar] [CrossRef] [Green Version]
- Sosa, M.A.G.; Fazely, F.; Koch, J.A.; Vercellotti, S.V.; Ruprecht, R.M. N-Carboxymethylchitosan-N, O-sulfate as an anti-HIV-1 agent. Biochem. Biophys. Res. Commun. 1991, 174, 489–496. [Google Scholar] [CrossRef]
- Ishihara, C.; Yoshimatsu, K.; Tsuji, M.; Arikawa, J.; Saiki, I.; Tokura, S.; Azuma, I. Anti-viral activity of sulfated chitin derivatives against Friend murine leukaemia and herpes simplex type-1 viruses. Vaccine 1993, 11, 670–674. [Google Scholar] [CrossRef]
- Hassan, M.I.; Mohamed, A.F.; Taher, F.A.; Kamel, M.R. Antimicrobial activities of chitosan nanoparticles prepared from Lucilia cuprina maggots (Diptera: Calliphoridae). J. Egypt. Soc. Parasitol. 2016, 46, 563–570. [Google Scholar] [PubMed]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. Chitosan, alginate, hyaluronic acid, gums, and β-glucan as potent adjuvants and vaccine delivery systems for viral threats including SARS-CoV-2: A review. Int. J. Biol. Macromol. 2021, 182, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Loutfy, S.A.; Elberry, M.H.; Farroh, K.Y.; Mohamed, H.T.; Mohamed, A.A.; Mohamed, E.B.; Faraag, A.H.I.; Mousa, S.A. Antiviral activity of chitosan nanoparticles encapsulating curcumin against hepatitis C virus genotype 4a in human hepatoma cell lines. Int. J. Nanomed. 2020, 15, 2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Ensinas, A.; Verrier, B.; Primard, C.; Cuvillier, A.; Champier, G.; Paul, S.; Delair, T. Zinc-stabilized chitosan-chondroitin sulfate nanocomplexes for HIV-1 infection inhibition application. Mol. Pharmaceut. 2016, 13, 3279–3291. [Google Scholar] [CrossRef]
- Milewska, A.; Chi, Y.; Szczepanski, A.; Barreto-Duran, E.; Dabrowska, A.; Botwina, P.; Obloza, M.; Liu, K.; Liu, D.; Guo, X. HTCC as a polymeric inhibitor of SARS-CoV-2 and MERS-CoV. J. Virol. 2021, 95, e01622-20. [Google Scholar] [CrossRef]
- Pyrć, K.; Milewska, A.; Duran, E.B.; Botwina, P.; Lopes, R.; Arenas-Pinto, A.; Badr, M.; Mellor, R.; Kalber, T.L.; Fernandes-Reyes, D. SARS-CoV-2 inhibition in human airway epithelial cells using a mucoadhesive, amphiphilic chitosan that may serve as an anti-viral nasal spray. BioRxiv 2021, 11, 20012. [Google Scholar]
- Russo, E.; Gaglianone, N.; Baldassari, S.; Parodi, B.; Cafaggi, S.; Zibana, C.; Donalisio, M.; Cagno, V.; Lembo, D.; Caviglioli, G. Preparation, characterization and in vitro antiviral activity evaluation of foscarnet-chitosan nanoparticles. Colloids Surf. B Biointerf. 2014, 118, 117–125. [Google Scholar] [CrossRef]
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013, 8, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Sofy, A.R.; Hmed, A.A.; Abd El Haliem, N.F.; Zein, M.A.-E.; Elshaarawy, R.F. Polyphosphonium-oligochitosans decorated with nanosilver as new prospective inhibitors for common human enteric viruses. Carbohydr. Polym. 2019, 226, 115261. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, W.; Wang, W.; Zhang, X.; Zhao, X. The inhibitory effects and mechanisms of 3, 6-O-sulfated chitosan against human papillomavirus infection. Carbohydr. Polym. 2018, 198, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; Ulber, R. Fucoidans: Downstream processes and recent applications. Mar. Drugs 2020, 18, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Sun, J.; Su, X.; Yu, Q.; Yu, Q.; Zhang, P. A review about the development of fucoidan in antitumor activity: Progress and challenges. Carbohydr. Polym. 2016, 154, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.T.T.; Ly, B.M.; Van, T.T.T.; Van Quang, N.; Tu, H.C.; Zheng, Y.; Seguin-Devaux, C.; Mi, B.; Ai, U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr. Polym. 2015, 115, 122–128. [Google Scholar] [CrossRef]
- Cao, Y.-G.; Hao, Y.; Li, Z.-H.; Liu, S.-T.; Wang, L.-X. Antiviral activity of polysaccharide extract from Laminaria japonica against respiratory syncytial virus. Biomed. Pharmacother. 2016, 84, 1705–1710. [Google Scholar] [CrossRef]
- Trejo-Avila, L.M.; Elizondo-Gonzalez, R.; Rodriguez-Santillan, P.; Aguilar-Briseño, J.A.; Ricque-Marie, D.; Rodriguez-Padilla, C.; Cruz-Suarez, L.E. Innocuity and anti-Newcastle-virus-activity of Cladosiphon okamuranus fucoidan in chicken embryos. Poult. Sci. 2016, 95, 2795–2802. [Google Scholar] [CrossRef]
- Diaz-Resendiz, K.J.G.; Toledo-Ibarra, G.A.; Ruiz-Manzano, R.; Perez, D.A.G.; Covantes-Rosales, C.E.; Benitez-Trinidad, A.B.; Ramirez-Ibarra, K.M.; Escobedo, A.T.H.; Gonzalez-Navarro, I.; Ventura-Ramon, G.H.; et al. Ex vivo treatment with fucoidan of mononuclear cells from SARS-CoV-2 infected patients. Int. J. Environ. Health Res. 2021, 1–19. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, E.-A.; Ahn, G.; Jee, Y.; Jeon, Y.-J. Anti-inflammatory activity of a sulfated polysaccharide isolated from an enzymatic digest of brown seaweed Sargassum horneri in RAW 264.7 cells. Nutr. Res. Pract. 2017, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.L.; Mawad, D.; Dokos, S.; Koshy, P.; Martens, P.J.; Sorrell, C.C. Immunomodulatory properties of photopolymerizable fucoidan and carrageenans. Carbohydr. Polym. 2020, 230, 115691. [Google Scholar] [CrossRef]
- Rasin, A.B.; Silchenko, A.S.; Kusaykin, M.I.; Malyarenko, O.S.; Zueva, A.O.; Kalinovsky, A.I.; Airong, J.; Surits, V.V.; Ermakova, S.P. Enzymatic transformation and anti-tumor activity of Sargassum horneri fucoidan. Carbohydr. Polym. 2020, 246, 116635. [Google Scholar] [CrossRef]
- Dai, Y.-L.; Jiang, Y.-F.; Lee, H.G.; Jeon, Y.-J.; Kang, M.-C. Characterization and screening of anti-tumor activity of fucoidan from acid-processed hijiki (Hizikia fusiforme). Int. J. Biol. Macromol. 2019, 139, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Ponce, N.M.A.; Flores, M.L.; Pujol, C.A.; Becerra, M.B.; Navarro, D.A.; Córdoba, O.; Damonte, E.B.; Stortz, C.A. Fucoidans from the phaeophyta Scytosiphon lomentaria: Chemical analysis and antiviral activity of the galactofucan component. Carbohydr. Res. 2019, 478, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.L.; Li, Y.; Ni, L.Q.; Li, Y.X.; Cui, Y.S.; Jiang, S.L.; Xie, E.Y.; Du, J.; Deng, F.; Dong, C.X. Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydr. Polym. 2020, 229, 115487. [Google Scholar] [CrossRef]
- Li, Y.; Hu, H.; Zhou, Q.; Ao, Y.; Xiao, C.; Wan, J.; Wan, Y.; Xu, H.; Li, Z.; Yang, X. α-Amylase-and redox-responsive nanoparticles for tumor-targeted drug delivery. ACS Appl. Mater. Interf. 2017, 9, 19215–19230. [Google Scholar] [CrossRef] [PubMed]
- Elizondo-Gonzalez, R.; Cruz-Suarez, L.E.; Ricque-Marie, D.; Mendoza-Gamboa, E.; Rodriguez-Padilla, C.; Trejo-Avila, L.M. In vitro characterization of the antiviral activity of fucoidan from Cladosiphon okamuranus against Newcastle Disease Virus. Virol. J. 2012, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-B.; Hayashi, K.; Hashimoto, M.; Nakano, T.; Hayashi, T. Novel antiviral fucoidan from sporophyll of Undaria pinnatifida (Mekabu). Chem. Pharmaceut. Bull. 2004, 52, 1091–1094. [Google Scholar] [CrossRef] [Green Version]
- Krylova, N.V.; Ermakova, S.P.; Lavrov, V.F.; Leneva, I.A.; Kompanets, G.G.; Iunikhina, O.V.; Nosik, M.N.; Ebralidze, L.K.; Falynskova, I.N.; Silchenko, A.S. The comparative analysis of antiviral activity of native and modified fucoidans from brown algae Fucus evanescens in vitro and in vivo. Mar. Drugs 2020, 18, 224. [Google Scholar] [CrossRef] [Green Version]
- Vaillant, A. Nucleic acid polymers: Broad spectrum antiviral activity, antiviral mechanisms and optimization for the treatment of hepatitis B and hepatitis D infection. Antivir. Res. 2016, 133, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Vaillant, A.; Juteau, J.-M.; Lu, H.; Liu, S.; Lackman-Smith, C.; Ptak, R.; Jiang, S. Phosphorothioate oligonucleotides inhibit human immunodeficiency virus type 1 fusion by blocking gp41 core formation. Antimicrob. Agents Chemother. 2006, 50, 1393–1401. [Google Scholar] [CrossRef] [Green Version]
- Guzman, E.M.; Cheshenko, N.; Shende, V.; Keller, M.J.; Goyette, N.; Juteau, J.-M.; Boivin, G.; Vaillant, A.; Herold, B.C. Amphipathic DNA polymers are candidate vaginal microbicides and block herpes simplex virus binding, entry and viral gene expression. Antivir. Ther. 2007, 12, 1147. [Google Scholar] [CrossRef]
- Lee, A.M.; Rojek, J.M.; Gundersen, A.; Ströher, U.; Juteau, J.-M.; Vaillant, A.; Kunz, S. Inhibition of cellular entry of lymphocytic choriomeningitis virus by amphipathic DNA polymers. Virology 2008, 372, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, D.I.; Goyette, N.; Cardin, R.; Kern, E.R.; Boivin, G.; Ireland, J.; Juteau, J.-M.; Vaillant, A. Amphipathic DNA polymers exhibit antiherpetic activity in vitro and in vivo. Antimicrob. Agents Chemother. 2008, 52, 2727–2733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardin, R.D.; Bravo, F.J.; Sewell, A.P.; Cummins, J.; Flamand, L.; Juteau, J.-M.; Bernstein, D.I.; Vaillant, A. Amphipathic DNA polymers exhibit antiviral activity against systemic murine Cytomegalovirus infection. Virol. J. 2009, 6, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, A.; Kanda, T.; Beyene, A.; Saito, K.; Meyer, K.; Ray, R. Sulfated homologues of heparin inhibit hepatitis C virus entry into mammalian cells. J. Virol. 2007, 81, 3933–3941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, K.-S.; Jiang, J.; Cai, Z.; Luo, G. Human apolipoprotein e is required for infectivity and production of hepatitis C virus in cell culture. J. Virol. 2007, 81, 13783–13793. [Google Scholar] [CrossRef] [Green Version]
- Noordeen, F.; Vaillant, A.; Jilbert, A.R. Nucleic acid polymers inhibit duck hepatitis B virus infection in vitro. Antimicrob. Agents Chemother. 2013, 57, 5291–5298. [Google Scholar] [CrossRef] [Green Version]
- Al-Mahtab, M.; Bazinet, M.; Vaillant, A. Safety and efficacy of nucleic acid polymers in monotherapy and combined with immunotherapy in treatment-naive Bangladeshi patients with HBeAg+ chronic hepatitis B infection. PLoS ONE 2016, 11, e0156667. [Google Scholar] [CrossRef] [Green Version]
- Noordeen, F.; Scougall, C.A.; Grosse, A.; Qiao, Q.; Ajilian, B.B.; Reaiche-Miller, G.; Finnie, J.; Werner, M.; Broering, R.; Schlaak, J.F. Therapeutic antiviral effect of the nucleic acid polymer REP 2055 against persistent duck hepatitis B virus infection. PLoS ONE 2015, 10, e0140909. [Google Scholar] [CrossRef] [Green Version]
- Bazinet, M.; Pantea, V.; Placinta, G.; Moscalu, I.; Cebotarescu, V.; Cojuhari, L.; Jimbei, P.; Iarovoi, L.; Smesnoi, V.; Musteata, T.; et al. Safety and efficacy of 48 weeks REP 2139 or REP 2165, tenofovir disoproxil, and pegylated interferon alfa-2a in patients with chronic HBV infection naive to nucleos(t)ide therapy. Gastroenterology 2020, 158, 2180–2194. [Google Scholar] [CrossRef]
- Blanchet, M.; Sinnathamby, V.; Vaillant, A.; Labonté, P. Inhibition of HBsAg secretion by nucleic acid polymers in HepG2. 2.15 cells. Antivir. Res. 2019, 164, 97–105. [Google Scholar] [CrossRef]
- Blome, S.; Staubach, C.; Henke, J.; Carlson, J.; Beer, M. Classical swine fever—An updated review. Viruses 2017, 9, 86. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, H.; Demirer, G.S.; González-Grandío, E.; Fan, C.; Landry, M.P. Engineering DNA nanostructures for siRNA delivery in plants. Nat. Protoc. 2020, 15, 3064–3087. [Google Scholar] [CrossRef]
- Kleiboeker, S.B. Swine fever: Classical swine fever and African swine fever. Vet. Clin. Food Anim. Pract. 2002, 18, 431–451. [Google Scholar] [CrossRef]
- Dong, H.; Song, G.; Ma, D.; Wang, T.; Jing, S.; Yang, H.; Tao, Y.; Tang, Y.; Shi, Y.; Dai, Z. Improved antiviral activity of classical swine fever virus-targeted siRNA by tetrahedral framework nucleic acid-enhanced delivery. ACS Appl. Mater. Interf. 2021, 13, 29416–29423. [Google Scholar] [CrossRef]
- Woo, J.; Seo, H.; Na, Y.; Choi, S.; Kim, S.; Choi, W.I.; Park, M.H.; Sung, D. Facile synthesis and coating of aqueous antifouling polymers for inhibiting pathogenic bacterial adhesion on medical devices. Prog. Org. Coat. 2020, 147, 10. [Google Scholar] [CrossRef]
- Kouhi, M.; Prabhakaran, M.P.; Ramakrishna, S. Edible polymers: An insight into its application in food, biomedicine and cosmetics. Trends Food Sci. Technol. 2020, 103, 248–263. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Worley, S.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Vacas-Córdoba, E.; Maly, M.; De la Mata, F.J.; Gomez, R.; Pion, M.; Muñoz-Fernández, M.Á. Antiviral mechanism of polyanionic carbosilane dendrimers against HIV-1. Int. J. Nanomed. 2016, 11, 1281. [Google Scholar]
- Kim, Y.; Park, E.J.; Na, D.H. Recent progress in dendrimer-based nanomedicine development. Arch. Pharm. Res. 2018, 41, 571–582. [Google Scholar] [CrossRef]
- Caminade, A.M.; Turrin, C.O.; Laurent, R.; Rebout, C.; Majoral, J.P. Phosphorus dendritic architectures: Polyanionic and polycationic derivatives. Polym. Int. 2006, 55, 1155–1160. [Google Scholar] [CrossRef]
- Dias, A.P.; da Silva Santos, S.; da Silva, J.V.; Parise-Filho, R.; Ferreira, E.I.; El Seoud, O.; Giarolla, J. Dendrimers in the context of nanomedicine. Int. J. Pharmaceut. 2020, 573, 118814. [Google Scholar] [CrossRef] [PubMed]
- Witvrouw, M.; Fikkert, V.; Pluymers, W.; Matthews, B.; Mardel, K.; Schols, D.; Raff, J.; Debyser, Z.; De Clercq, E.; Holan, G. Polyanionic (i.e., polysulfonate) dendrimers can inhibit the replication of human immunodeficiency virus by interfering with both virus adsorption and later steps (reverse transcriptase/integrase) in the virus replicative cycle. Mol. Pharmacol. 2000, 58, 1100–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, D.; Stanberry, L.; Sacks, S.; Ayisi, N.; Gong, Y.; Ireland, J.; Mumper, R.; Holan, G.; Matthews, B.; McCarthy, T. Evaluations of unformulated and formulated dendrimer-based microbicide candidates in mouse and guinea pig models of genital herpes. Antimicrob. Agents Chemother. 2003, 47, 3784–3788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Y.; Matthews, B.; Cheung, D.; Tam, T.; Gadawski, I.; Leung, D.; Holan, G.; Raff, J.; Sacks, S. Evidence of dual sites of action of dendrimers: SPL-2999 inhibits both virus entry and late stages of herpes simplex virus replication. Antivir. Res. 2002, 55, 319–329. [Google Scholar] [CrossRef]
- Mumper, R.J.; Bell, M.A.; Worthen, D.R.; Cone, R.A.; Lewis, G.R.; Paull, J.R.; Moench, T.R. Formulating a sulfonated antiviral dendrimer in a vaginal microbicidal gel having dual mechanisms of action. Drug Dev. Ind. Pharm. 2009, 35, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Paull, J.R.A.; Heery, G.P.; Bobardt, M.D.; Castellarnau, A.; Luscombe, C.A.; Fairley, J.K.; Gallay, P.A. Virucidal and antiviral activity of astodrimer sodium against SARS-CoV-2 in vitro. Antivir. Res. 2021, 191, 105089. [Google Scholar] [CrossRef]
- Martínez-Gualda, B.; Sun, L.; Rivero-Buceta, E.; Flores, A.; Quesada, E.; Balzarini, J.; Noppen, S.; Liekens, S.; Schols, D.; Neyts, J. Structure-activity relationship studies on a Trp dendrimer with dual activities against HIV and enterovirus A71. Modifications on the amino acid. Antivir. Res. 2017, 139, 32–40. [Google Scholar] [CrossRef]
- Anderson, L.J.; Dormitzer, P.R.; Nokes, D.J.; Rappuoli, R.; Roca, A.; Graham, B.S. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine 2013, 31 (Suppl. S2), B209–B215. [Google Scholar] [CrossRef] [Green Version]
- Kozhikhova, K.V.; Shilovskiy, I.P.; Shatilov, A.A.; Timofeeva, A.V.; Turetskiy, E.A.; Vishniakova, L.I.; Nikolskii, A.A.; Barvinskaya, E.D.; Karthikeyan, S.; Smirnov, V.V. Linear and dendrimeric antiviral peptides: Design, chemical synthesis and activity against human respiratory syncytial virus. J. Mater. Chem. B 2020, 8, 2607–2617. [Google Scholar] [CrossRef]
- Ortega, M.A.; Guzman Merino, A.; Fraile-Martinez, O.; Recio-Ruiz, J.; Pekarek, L.; Guijarro, L.G.; Garcia-Honduvilla, N.; Alvarez-Mon, M.; Bujan, J.; Garcia-Gallego, S. Dendrimers and dendritic materials: From laboratory to medical practice in infectious diseases. Pharmaceutics 2020, 12, 874. [Google Scholar] [CrossRef]
- Sepulveda-Crespo, D.; Lorente, R.; Leal, M.; Gomez, R.; Francisco, J.; Jiménez, J.L.; Muñoz-Fernández, M.Á. Synergistic activity profile of carbosilane dendrimer G2-STE16 in combination with other dendrimers and antiretrovirals as topical anti-HIV-1 microbicide. Nanomedicine 2014, 10, 609–618. [Google Scholar] [CrossRef]
- Asaftei, S.; Huskens, D.; Schols, D. HIV-1 X4 activities of polycationic “viologen” based dendrimers by interaction with the chemokine receptor CXCR4: Study of structure–activity relationship. J. Med. Chem. 2012, 55, 10405–10413. [Google Scholar] [CrossRef] [PubMed]
- Relaño-Rodríguez, I.; Muñoz-Fernández, M.Á. Emergence of nanotechnology to fight HIV sexual transmission: The trip of G2-S16 polyanionic carbosilane dendrimer to possible pre-clinical trials. Int. J. Mol. Sci. 2020, 21, 9403. [Google Scholar] [CrossRef]
- Sepúlveda-Crespo, D.; Jiménez, J.L.; Gómez, R.; De La Mata, F.J.; Majano, P.L.; Muñoz-Fernández, M.Á.; Gastaminza, P. Polyanionic carbosilane dendrimers prevent hepatitis C virus infection in cell culture. Nanomedicine 2017, 13, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Chonco, L.; Pion, M.; Vacas, E.; Rasines, B.; Maly, M.; Serramia, M.; Lopez-Fernandez, L.; De la Mata, J.; Alvarez, S.; Gomez, R. Carbosilane dendrimer nanotechnology outlines of the broad HIV blocker profile. J. Contr. Rel. 2012, 161, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M.; Umemura, S.; Sugiyama, N.; Kuwano, N.; Koizumi, A.; Sawada, T.; Yanase, M.; Takaha, T.; Kadokawa, J.-I.; Usui, T. Synthesis of multivalent sialyllactosamine-carrying glyco-nanoparticles with high affinity to the human influenza virus hemagglutinin. Carbohydr. Polym. 2016, 153, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Yu, Y.; Song, J.-Q.; Jia, T.-W.; Liu, A.-Y.; Zhao, T.-F.; He, H.-J.; Yang, M.-B.; Zhang, W.-X.; Yang, Y. Amide-sialoside protein conjugates as neomucin bioshields prevent influenza virus infection. Carbohydr. Res. 2020, 495, 108088. [Google Scholar] [CrossRef]
- Stencel-Baerenwald, J.E.; Reiss, K.; Reiter, D.M.; Stehle, T.; Dermody, T.S. The sweet spot: Defining virus–sialic acid interactions. Nat. Rev. Microbiol. 2014, 12, 739–749. [Google Scholar] [CrossRef] [Green Version]
- Gulati, S.; Smith, D.F.; Cummings, R.D.; Couch, R.B.; Griesemer, S.B.; George, K.S.; Webster, R.G.; Air, G.M. Human H3N2 influenza viruses isolated from 1968 to 2012 show varying preference for receptor substructures with no apparent consequences for disease or spread. PLoS ONE 2013, 8, e66325. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Qin, H.-J.; Shi, X.-X.; Song, J.-Q.; Zhou, J.-P.; Yu, P.; Fan, Z.-C.; Zhong, M.; Yang, Y. Thiosialoside-decorated polymers use a two-step mechanism to inhibit both early and late stages of influenza virus infection. Eur. J. Med. Chem. 2020, 199, 112357. [Google Scholar] [CrossRef]
- Nagao, M.; Fujiwara, Y.; Matsubara, T.; Hoshino, Y.; Sato, T.; Miura, Y. Design of glycopolymers carrying sialyl oligosaccharides for controlling the interaction with the influenza virus. Biomacromolecules 2017, 18, 4385–4392. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Matsubara, T.; Hoshino, Y.; Sato, T.; Miura, Y. Topological design of star glycopolymers for controlling the interaction with the influenza virus. Bioconj. Chem. 2019, 30, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Puryear, W.B.; Seifried, B.M.; Dong, X.; Runstadler, J.A.; Ribbeck, K.; Olsen, B.D. Antiviral agents from multivalent presentation of sialyl oligosaccharides on brush polymers. ACS Macro Lett. 2016, 5, 413–418. [Google Scholar] [CrossRef]
- Matrosovich, M.N.; Gambaryan, A.S.; Teneberg, S.; Piskarev, V.E.; Yamnikova, S.S.; Lvov, D.K.; Robertson, J.S.; Karlsson, K.A. Avian influenza a viruses differ from human viruses by recognition of sialyloligosaccharides and gangliosides and by a higher conservation of the HA receptor-binding site. Virology 1997, 233, 224–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
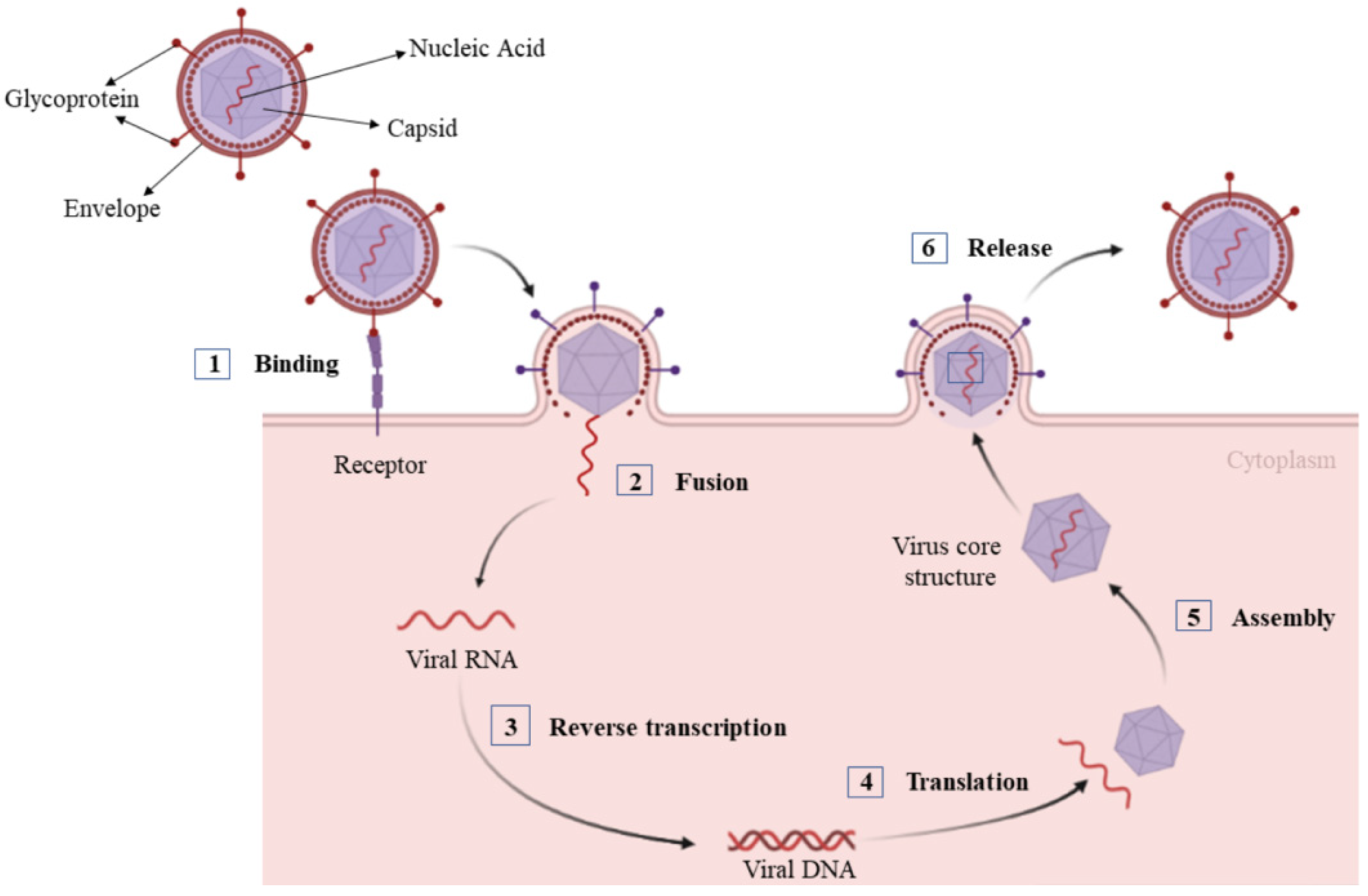

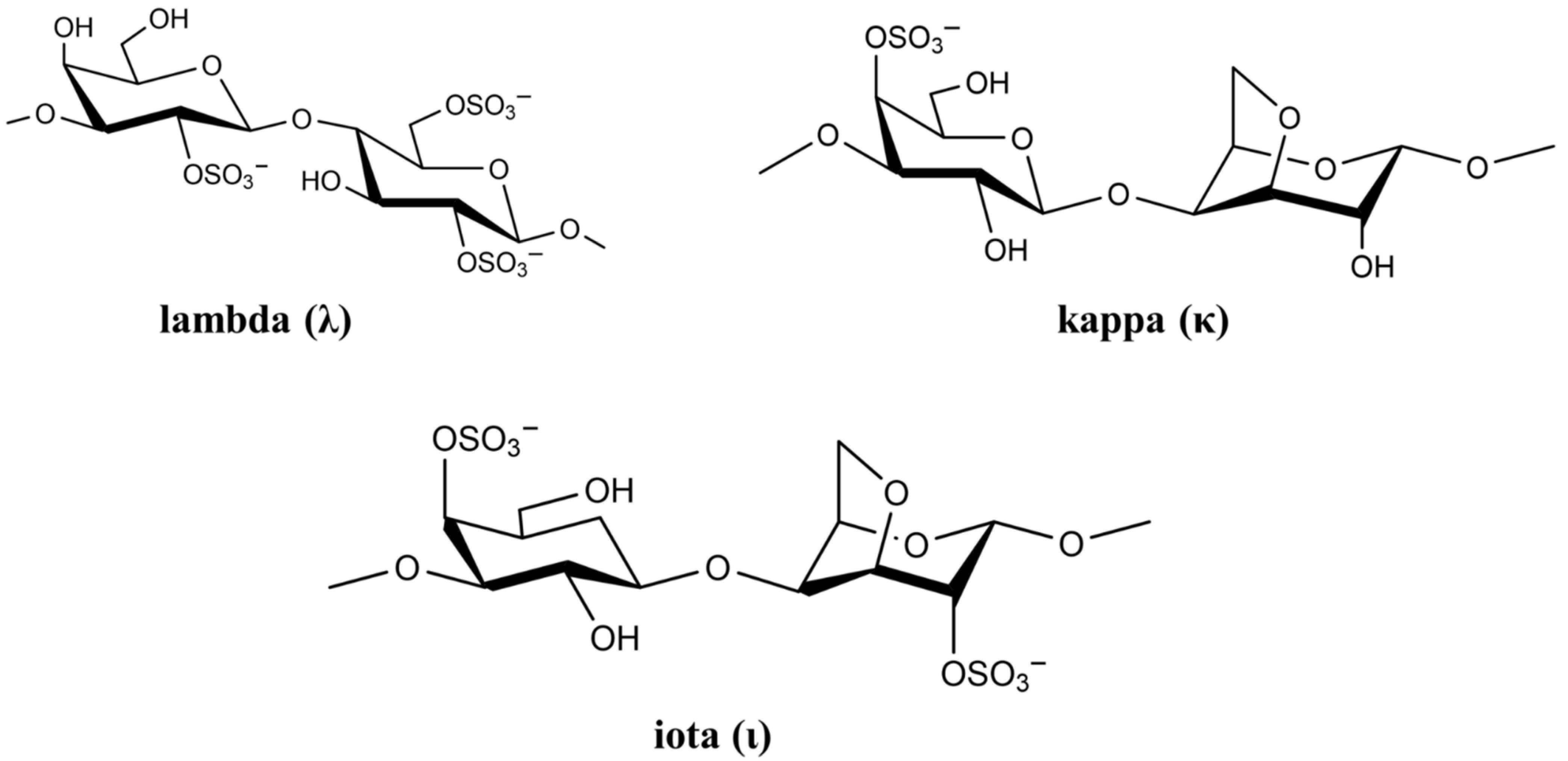








| Polysaccharide | Origin | Occurrence/Function | Molecular Characteristics | Ref. |
|---|---|---|---|---|
| Carrageenan | Algae | Structural polysaccharides of marine red algae | Heteropolysaccharide, linear, anionic | [35] |
| Chitin/chitosan | Animal | Chitin: structural polysaccharide from exoskeleton of insects and shells of crustaceans Chitosan: derivative of chitin prepared by deacetylation | Chitin: homopolysaccharide, linear, neutral Chitosan: heteropolysaccharide, linear, cationic | [36] |
| Fucoidan | Algae | Structure and composition dependent on species with diverse structures | Heteropolysaccharide, anionic, linear. | [37] |
| Type | Virus | Genome | Cell Line | IC50/EC50 (µg/mL) | Ref. |
|---|---|---|---|---|---|
| κ | DENV-1 | RNA | Vero | >50 (EC50) | [64] |
| κ | DENV-2 | RNA | Vero | 1.8 (EC50) | [64] |
| κ | HSV-1 | DNA | Vero | 1.9 (EC50) | [65] |
| κ | HSV-2 | DNA | Vero | 1.6 (EC50) | [65] |
| κ | HIV-1 | RNA | MT-4 | 12 (IC50) | [66] |
| ι | DENV-1 | RNA | Vero | 41 (EC50) | [64] |
| ι | DENV-2 | RNA | Vero | 0.40 (EC50) | [64] |
| ι | H1N1 | RNA | MDCK | 0.39 (IC50) | [67] |
| ι | SARS-CoV-2 | RNA | Vero | 0.05 (IC50) | [68] |
| λ | HIV-1 | RNA | MT-4 | 1.9 (IC50) | [66] |
| λ | H1N1 | RNA | MDCK | 0.04 (IC50) | [44] |
| λ | VSV | RNA | HeLa | 4.0 (IC50) | [66] |
| Chitosan | Virus | Cell Line | Concentration | Ref. |
|---|---|---|---|---|
| Zinc stabilized chitosan | HIV-1 | Human peripheral blood mononuclear | 0.1–0.2% w/v chitosan in acetic acid | [95] |
| Curcumin-loaded chitosan nanocomposite | Hepatitis C | human hepatoma | 20 µg/mL curcumin in 4% chitosan composite | [94] |
| Chitosan with medium molecular weight | AD169 strain of HCMV | Human embryonic lung fibroblasts | 9–14 mg Fostcanet antiviral drug in 4.5 mg chitosan | [98] |
| Silver-loaded chitosan | H1N1 Influenza A | Madin-Darby canine kidney | Ag NPs in 10 mg/mL chitosan | [99] |
| Nanosilver-loaded polyquaternary phosphonium oligochitosan | Hepatitis A | Vero | 100 µL/mL silver NPs | [100] |
| 3,6-O-sulfated chitosan | HPV | 293FT, Hela, HaCaT | 2.4, 4.7, 4.2 µg/mL chitosan | [101] |
| HTCC | SARS-CoV2 | human airway epithelium (HAE) | 12.5 µg/mL HTCC | [96] |
| GCPQ | SARS-CoV2 | Vero E6 and A549 | 10 µg/mL GCPQ | [97] |
| Fucoidan | Virus | Cell Line | IC50 (μg/mL) | Ref. |
|---|---|---|---|---|
| Cladosiphon okamuranus | Newcastle Disease (NDV) | Vero | 0.75 | [115] |
| Scytosiphon lomentaria | HSV-1, HSV-2 | Vero | 1.12–1.22 | [112] |
| Sargassum henslowianum | HSV-1, HSV-2 | Vero | 0.48–0.89 | [113] |
| Sporophyll of Undaria pinnatifida (Mekabu) | Influenza A | MDCK | 15 | [116] |
| Brown Algae: Fucus evanescens | HSV-1, HSV-2, HIV-1 | MT-4 | 25–80 | [117] |
| NAP | Virus | Cell Line | Mechanism of Action | Ref. |
|---|---|---|---|---|
| Phosphorothioate Oligonucleotides | HIV-1 | H9 | Inhibiting viral entry to the host | [119] |
| amphipathic DNA polymer (40-nucleotide polycytidine) | HSV | Human cervical epithelial (CaSki) | Reduced viral and protein expression | [120] |
| Amphipathic DNA polymers (REP 9, REP 2015 and REP 9C) | Animal | NIH 3T3 | Reduced viral replication | [123] |
| REP 2006 | HBV | DHBV | Reduced viral entry to the host | [126] |
| REP 2055 | HBV | DHBV | blocking release of DHBsAg from the infected hepatocytes | [128] |
| Dendrimer Type | Name | Virus | Cell Line | Mechanism of Action | Ref. |
|---|---|---|---|---|---|
| Polyanionic | Phenyldicarboxylic acid (BRI6195), Naphthyldisulfonic acid (BRI2923) | HIV-1 | MT-4 | Inhibit viral binding and replication | [142] |
| Polyanionic | SPL-2999 | HSV-1, HSV-2 | Vero | Inhibit late stages of viral replication | [144] |
| Polyanionic | carbosilane dendrimer G2-STE16 | HIV-1 | TZM.bl | Inhibit viral entry | [151] |
| Polyanionic | G2-S16 | HIV | PBMC | Inhibit viral replication by blocking gp120/CD4/CCR5 interaction | [155] |
| Polyanionic | G3-S16 and G2-NF16 | HIV-1 | HeLa P4.R5MAGI, TZM.bl | Inhibit viral replication by blocking gp120/CD4/CCR5 interaction | [138] |
| Polycationic | Viologen | HIV-1 | MT-4 cells | Block viral entry | [152] |
| Sialyl-Based Polymers | Virus | Cell Line | Mechanism of Action | Ref. |
|---|---|---|---|---|
| Amide-sialoside protein conjugates | IAV | H1N1, H3N2, H9N2 | Binding to the virus and inhibiting replication | [157] |
| Thiosialoside-modified poly (methyl vinyl ether-alt-maleic anhydride) | IAV | MDCK | inhibiting both early attachment and late release during viral infection | [160] |
| silyl oligosaccharides modified glycopolymers | IAV | H1N1 | Binding with hemagglutinin on viral surface | [161] |
| Bioinspired brush polymers containing α-2,6-linked sialic acid | IAV | H1N1 | binding hemagglutination on viral surface | [163] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbari, A.; Bigham, A.; Rahimkhoei, V.; Sharifi, S.; Jabbari, E. Antiviral Polymers: A Review. Polymers 2022, 14, 1634. https://doi.org/10.3390/polym14091634
Akbari A, Bigham A, Rahimkhoei V, Sharifi S, Jabbari E. Antiviral Polymers: A Review. Polymers. 2022; 14(9):1634. https://doi.org/10.3390/polym14091634
Chicago/Turabian StyleAkbari, Ali, Ashkan Bigham, Vahid Rahimkhoei, Sina Sharifi, and Esmaiel Jabbari. 2022. "Antiviral Polymers: A Review" Polymers 14, no. 9: 1634. https://doi.org/10.3390/polym14091634
APA StyleAkbari, A., Bigham, A., Rahimkhoei, V., Sharifi, S., & Jabbari, E. (2022). Antiviral Polymers: A Review. Polymers, 14(9), 1634. https://doi.org/10.3390/polym14091634








