Polymeric Coatings and Antimicrobial Peptides as Efficient Systems for Treating Implantable Medical Devices Associated-Infections
Abstract
:1. Introduction
2. Insight into the Biofilm Formation and Resistance
3. Methods for Obtaining Polymeric Coatings
4. Drug Delivery from Polymeric Coatings
Drug-Eluting Implantable Devices
5. Antimicrobial Peptides
5.1. Short Overview on Their Structural Features
5.2. Sources
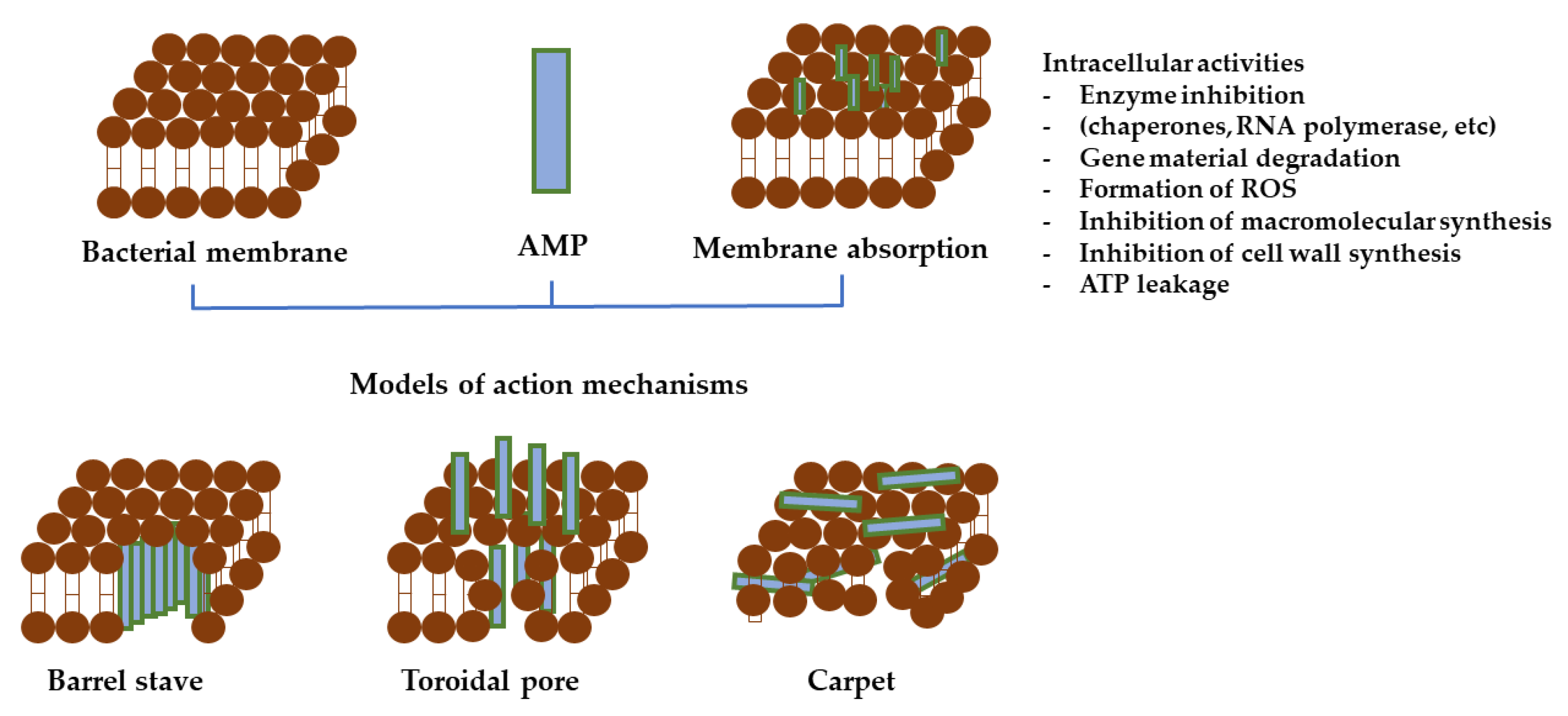
5.3. Insights into AMPs Antimicrobial Action Mechanism
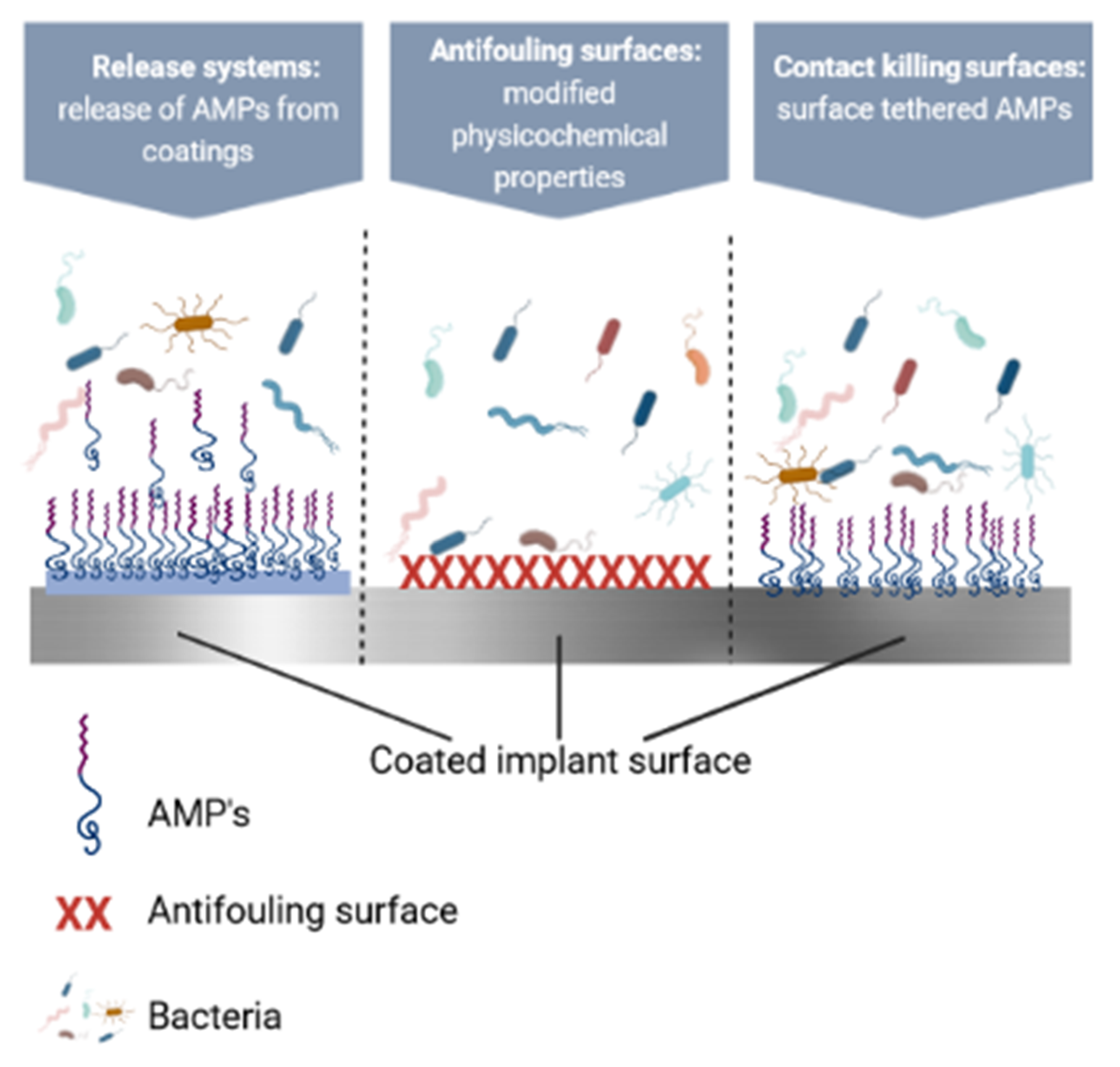
6. AMPs and/in Polymeric Coatings against Infections
6.1. Contact-Killing Surfaces
6.2. Antifouling Surfaces
6.3. Polymeric Coatings as Release Systems for AMPs
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilkins, L.J.; Monga, M.; Miller, A.W. Defining dysbiosis for a cluster of chronic diseases. Sci. Rep. 2019, 9, 12918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.J.; Bell, C.M.; Matelski, J.J.; Detsky, A.S.; Cram, P. Payments by US pharmaceutical and medical device manufacturers to US medical journal editors: Retrospective observational study. BMJ 2017, 359, j4619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.A.; Neuzil, P.; Dukkipati, S.R.; Reddy, V.Y. Leadless cardiac pacemakers: Back to the future. J. Am. Coll. Cardiol. 2015, 66, 1179–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruben, R.B.; Fernandes, P.R.; Folgado, J. On the optimal shape of hip implants. J. Biomech. 2012, 45, 239–246. [Google Scholar] [CrossRef]
- Franco, P.; de Marco, I. Contact lenses as ophthalmic drug delivery systems: A review. Polymers 2021, 13, 1102. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Kanamori, H.; Gergen, M.F.; Knelson, L.P.; Sickbert-Bennett, E.E.; Chen, L.F.; Anderson, D.J.; Sexton, D.J.; Weber, D.J.; CDC Prevention Epicenters Program. Enhanced disinfection leads to reduction of microbial contamination and a decrease in patient colonization and infection. Infect. Control Hosp. Epidemiol. 2018, 39, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Caldara, M.; Belgiovine, C.; Secchi, E.; Rusconi, R. Environmental, Microbiological, and Immunological Features of Bacterial Biofilms Associated with Implanted Medical Devices. Clin. Microbiol. Rev. 2022, 35, e00221-20. [Google Scholar] [CrossRef]
- Muñoz, M.; Acheson, A.G.; Bisbe, E.; Butcher, A.; Gómez-Ramírez, S.; Khalafallah, A.; Kehlet, H.; Kietaibl, S.; Liumbruno, G.M.; Meybohm, P.; et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia 2018, 73, 1418–1431. [Google Scholar] [CrossRef] [Green Version]
- Leahy, M.F.; Hofmann, A.; Towler, S.; Trentino, K.M.; Burrows, S.A.; Swain, S.G.; Hamdorf, J.; Gallagher, T.; Koay, A.; Geelhoed, G.C.; et al. Improved outcomes and reduced costs associated with a health-system–wide patient blood management program: A retrospective observational study in four major adult tertiary-care hospitals. Transfusion 2017, 57, 1347–1358. [Google Scholar] [CrossRef]
- Frieri, K.K.M.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Namivandi-Zangeneh, R.; Wong, E.H.; Boyer, C. Synthetic antimicrobial polymers in combination therapy: Tackling antibiotic resistance. ACS Infect. Dis. 2021, 7, 215–253. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, D.C.; Meza-Rodriguez, S.M. Development of antimicrobial resistance: Future challenges. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 383–408. [Google Scholar]
- Yu, L.; Li, K.; Zhang, J.; Jin, H.; Saleem, A.; Song, Q.; Jia, Q.; Li, P. Antimicrobial Peptides and Macromolecules for Combating Microbial Infections: From Agents to Interfaces. ACS Appl. Bio Mater. 2022, 5, 366–393. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (U.S.). Antibiotic Resistance Threats in the United States, 2019; Centers for Disease Control and Prevention (U.S.): Atlanta, GA, USA, 2019. [Google Scholar] [CrossRef] [Green Version]
- Jamar, F.; Buscombe, J.; Chiti, A.; Christian, P.E.; Delbeke, D.; Donohoe, K.J.; Israel, O.; Martin-Comin, J.; Signore, A. EANM/SNMMI guideline for 18F-FDG use in inflammation and infection. J. Nucl. Med. 2013, 54, 647–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masters, E.A.; Ricciardi, B.F.; Bentley, K.L.; Moriarty, T.F.; Schwarz, E.M.; Muthukrishnan, G. Skeletal infections: Microbial pathogenesis, immunity and clinical management. Nat. Rev. Microbiol. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Khan, A.U. Global economic impact of antibiotic resistance: A review. J. Glob. Antimicrob. Resist. 2019, 19, 313–316. [Google Scholar] [CrossRef]
- Makvandi, P.; Wang, C.; Zare, E.N.; Borzacchiello, A.; Niu, L.; Tay, F.R. Metal-based nanomaterials in biomedical applications: Antimicrobial activity and cytotoxicity aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- Nikfarjam, N.; Ghomi, M.; Agarwal, T.; Hassanpour, M.; Sharifi, E.; Khorsandi, D.; Ali Khan, M.; Rossi, F.; Rossetti, A.; Zare, E.; et al. Antimicrobial ionic liquid-based materials for biomedical applications. Adv. Funct. Mater. 2021, 31, 2104148. [Google Scholar] [CrossRef]
- Mitra, D.; Kang, E.-T.; Neoh, K.G. Antimicrobial copper-based materials and coatings: Potential multifaceted biomedical applications. ACS Appl. Mater. Interfaces 2019, 12, 21159–21182. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Y.; Wu, Y.; Wei, T.; Lu, K.; Li, L.; Lin, Y.; Wu, Y.; Huang, C.; Zhang, Y.; et al. Universal Antifouling and Photothermal Antibacterial Surfaces Based on Multifunctional Metal–Phenolic Networks for Prevention of Biofilm Formation. ACS Appl. Mater. Interfaces 2021, 13, 48403–48413. [Google Scholar] [CrossRef]
- Soria-Castro, M.; la Rosa-García, D.; Quintana, P.; Gómez-Cornelio, S.; Sierra-Fernandez, A.; Gómez-Ortíz, N. Broad spectrum antimicrobial activity of Ca (Zn (OH) 3) 2· 2H2O and ZnO nanoparticles synthesized by the sol–gel method. J. Sol-Gel Sci. Technol. 2019, 89, 284–294. [Google Scholar] [CrossRef]
- la Rosa-García, D.; Fuentes, A.F.; Gómez-Cornelio, S.; Zagada-Domínguez, U.; Quintana, P. Structural characterization of antifungal CaZn2 (OH) 6· 2H2O nanoparticles obtained via mechanochemical processing. J. Mater. Sci. 2018, 53, 13758–13768. [Google Scholar] [CrossRef]
- Ong, G.; Kasi, R.; Subramaniam, R. A review on plant extracts as natural additives in coating applications. Prog. Org. Coat. 2021, 151, 106091. [Google Scholar] [CrossRef]
- Vaz, J.M.; Pezzoli, D.; Chevallier, P.; Campelo, C.S.; Candiani, G.; Mantovani, D. Antibacterial coatings based on chitosan for pharmaceutical and biomedical applications. Curr. Pharm. Des. 2018, 24, 866–885. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Tian, L.; Bing, W.; Zhao, J.; Ren, L. Bioinspired marine antifouling coatings: Status, prospects, and future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Nguyen, T.K.N.; Dierre, B.; Grasset, F.; Dumait, N.; Cordier, S.; Lemoine, P.; Renaud, A.; Fudouzi, H.; Ohashi, N.; Uchikoshi, T. Electrophoretic coating of octahedral molybdenum metal clusters for UV/NIR light screening. Coatings 2017, 7, 114. [Google Scholar] [CrossRef] [Green Version]
- Floroian, L.; Samoila, C.; Badea, M.; Munteanu, D.; Ristoscu, C.; Sima, F.; Negut, I.; Chifiriuc, M.C.; Mihailescu, I.N. Stainless steel surface biofunctionalization with PMMA-bioglass coatings: Compositional, electrochemical corrosion studies and microbiological assay. J. Mater. Sci Mater. Med. 2015, 26, 195. [Google Scholar] [CrossRef]
- Chen, L.; Song, X.; Xing, F.; Wang, Y.; Wang, Y.; He, Z.; Sun, L. A Review on Antimicrobial Coatings for Biomaterial Implants and Medical Devices. J. Biomed. Nanotechnol. 2020, 16, 789–809. [Google Scholar] [CrossRef]
- He, J.; Renard, E.; Lord, P.; Cohen, D.; Gu, B.; Wang, X.; Yenduri, G.; Burgess, D.J. Strategies for extended lifetime of implantable intraperitoneal insulin catheters. J. Control. Release 2022, 341, 487–497. [Google Scholar] [CrossRef]
- Mitra, A.K.; Cholkar, K.; Mandal, A. Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; William, Andrew: Norwich, NY, USA, 2017. [Google Scholar]
- Levien, M.; Farka, Z.; Pastucha, M.; Skládal, P.; Nasri, Z.; Weltmann, K.-D.; Fricke, K. Functional plasma-polymerized hydrogel coatings for electrochemical biosensing. Appl. Surf. Sci. 2022, 584, 152511. [Google Scholar] [CrossRef]
- Gherasim, O.; Grumezescu, A.; Grumezescu, V.; Negut, I.; Dumitrescu, M.; Stan, M.; Nica, I.; Holban, A.; Socol, G.; Andronescu, E. Bioactive Coatings Based on Hydroxyapatite, Kanamycin, and Growth Factor for Biofilm Modulation. Antibiotics 2021, 10, 160. [Google Scholar] [CrossRef]
- Gherasim, O.; Grumezescu, A.M.; Grumezescu, V.; Andronescu, E.; Negut, I.; Bîrcă, A.C.; Gălățeanu, B.; Hudiță, A. Bioactive Coatings Loaded with Osteogenic Protein for Metallic Implants. Polymers 2021, 13, 4303. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Fischer, J.T.; Daniels, R. Development of probiotic orodispersible tablets using mucoadhesive polymers for buccal mucoadhesion. Drug Dev. Ind. Pharm. 2020, 46, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Wajs, E.; Nielsen, T.T.; Larsen, K.L.; Fragoso, A. Preparation of stimuli-responsive nano-sized capsules based on cyclodextrin polymers with redox or light switching properties. Nano Res. 2016, 9, 2070–2078. [Google Scholar] [CrossRef]
- Chaudhary, V.; Sharma, S. Suspension polymerization technique: Parameters affecting polymer properties and application in oxidation reactions. J. Polym. Res. 2019, 26, 102. [Google Scholar] [CrossRef]
- Amaral, A.J.; Pasparakis, G. Stimuli responsive self-healing polymers: Gels, elastomers and membranes. Polym. Chem. 2017, 8, 6464–6484. [Google Scholar] [CrossRef] [Green Version]
- Nutan, B.; Chandel, A.K.S.; Biswas, A.; Kumar, A.; Yadav, A.; Maiti, P.; Jewrajka, S.K. Gold Nanoparticle Promoted Formation and Biological Properties of Injectable Hydrogels. Biomacromolecules 2020, 21, 3782–3794. [Google Scholar] [CrossRef]
- Jamakandi, V.G.; Mulla, J.S.; Vinay, B.L.; Shivakumar, H.N. Formulation, characterization, and evaluation of matrix-type transdermal patches of a model antihypertensive drug. Asian J. Pharm. (AJP) 2014, 3, 59. [Google Scholar] [CrossRef]
- Abdelkader, H.; Fathalla, Z.; Seyfoddin, A.; Farahani, M.; Thrimawithana, T.; Allahham, A.; Alani, A.W.; Al-Kinani, A.A.; Alany, R.G. Polymeric long-acting drug delivery systems (LADDS) for treatment of chronic diseases: Inserts, patches, wafers, and implants. Adv. Drug Deliv. Rev. 2021, 177, 113957. [Google Scholar] [CrossRef]
- Bao, Y.; Paunović, N.; Leroux, J.-C. Challenges and Opportunities in 3D Printing of Biodegradable Medical Devices by Emerging Photopolymerization Techniques. Adv. Funct. Mater. 2022, 32, 2109864. [Google Scholar] [CrossRef]
- Ghosh, S.; Mukherjee, S.; Patra, D.; Haldar, J. Polymeric Biomaterials for Prevention and Therapeutic Intervention of Microbial Infections. Biomacromolecules 2022, 23, 592–608. [Google Scholar] [CrossRef]
- Gong, H.; Hajizadeh, S.; Liu, W.; Ye, L. Imprinted Polymer Beads Loaded with Silver Nanoparticles for Antibacterial Applications. ACS Appl. Bio Mater. 2021, 4, 2829–2838. [Google Scholar] [CrossRef] [PubMed]
- Nutan, B.; Chandel, A.K.S.; Jewrajka, S.K. Synthesis and Multi-Responsive Self-Assembly of Cationic Poly(caprolactone)–Poly(ethylene glycol) Multiblock Copolymers. Chem. A Eur. J. 2017, 23, 8166–8170. [Google Scholar] [CrossRef] [PubMed]
- Saidin, S.; Jumat, M.A.; Amin, N.A.A.M.; Al-Hammadi, A.S.S. Organic and inorganic antibacterial approaches in combating bacterial infection for biomedical application. Mater. Sci. Eng. C 2021, 118, 111382. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, L. Nanomaterials arising amid antibiotic resistance. Nat. Rev. Microbiol. 2021, 19, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Deng, S.; Lu, Z.; She, Y.; Xie, J.; Cong, Z.; Zhang, W.; Liu, R. Using In Vivo Assessment on Host Defense Peptide Mimicking Polymer-Modified Surfaces for Combating Implant Infections. ACS Appl. Bio Mater. 2021, 4, 3811–3829. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhang, C.; Qin, X.; Shi, L.; Zhao, M. Identification and function of penaeidin 3 and penaeidin 5 in Fenneropenaeus merguiensis. Fish Shellfish Immunol. 2019, 89, 623–631. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial peptides: Diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Thapa, R.K.; Diep, D.B.; Tønnesen, H.H. Topical antimicrobial peptide formulations for wound healing: Current developments and future prospects. Acta Biomater. 2020, 103, 52–67. [Google Scholar] [CrossRef]
- Atriwal, T.; Azeem, K.; Husain, F.M.; Hussain, A.; Khan, M.N.; Alajmi, M.F.; Abid, M. Mechanistic Understanding of Candida albicans Biofilm Formation and Approaches for Its Inhibition. Front. Microbiol. 2022, 12, 932. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Reen, F.J.; Gutiérrez-Barranquero, J.A.; Parages, M.L.; O´Gara, F. Coumarin: A novel player in microbial quorum sensing and biofilm formation inhibition. Appl. Microbiol. Biotechnol. 2018, 102, 2063–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lianou, A.; Nychas, G.-J.E.; Koutsoumanis, K.P. Strain variability in biofilm formation: A food safety and quality perspective. Food Res. Int. 2020, 137, 109424. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.C.; Mergulhão, F.J. A selection of platforms to evaluate surface adhesion and biofilm formation in controlled hydrodynamic conditions. Microorganisms 2021, 9, 1993. [Google Scholar] [CrossRef] [PubMed]
- Afonso, T.B.; Simões, L.C.; Lima, N. Occurrence of filamentous fungi in drinking water: Their role on fungal-bacterial biofilm formation. Res. Microbiol. 2021, 172, 103791. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, A.; Gupta, A.; Rani, K.; Singh, T. Monitoring Gene Expression in Sessile Forms of Microbial Biofilm: Polymerase Chain Reaction (PCR) and Real-Time Polymerase Chain Reaction (RT-PCR). In Analytical Methodologies for Biofilm Research; Springer: Berlin/Heidelberg, Germany, 2021; pp. 317–343. [Google Scholar]
- Nag, M.; Lahiri, D.; Sarkar, T.; Ghosh, S.; Dey, A.; Edinur, H.A.; Pati, S.; Ray, R.R. Microbial fabrication of nanomaterial and its role in disintegration of exopolymeric matrices of biofilm. Front. Chem. 2021, 9, 690590. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Soto, I.; McTiernan, C.; Gonzalez-Gomez, M.; Ross, A.; Gupta, K.; Suuronen, E.J.; Mah, T.-F.; Griffith, M.; Alarcon, E.I. Mimicking biofilm formation and development: Recent progress in in vitro and in vivo biofilm models. Iscience 2021, 24, 102443. [Google Scholar] [CrossRef]
- Mirzaei, R.; Ranjbar, R. Hijacking host components for bacterial biofilm formation: An advanced mechanism. Int. Immunopharmacol. 2022, 103, 108471. [Google Scholar] [CrossRef]
- Krsmanovic, M.; Biswas, D.; Ali, H.; Kumar, A.; Ghosh, R.; Dickerson, A.K. Hydrodynamics and surface properties influence biofilm proliferation. Adv. Colloid Interface Sci. 2021, 288, 102336. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.W.; Phillips, K.S.; Gu, H.; Kazemzadeh-Narbat, M.; Ren, D. How microbes read the map: Effects of implant topography on bacterial adhesion and biofilm formation. Biomaterials 2021, 268, 120595. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Manoil, D. Microbial Community-Driven Etiopathogenesis of Peri-Implantitis. J. Dent. Res. 2021, 100, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, X.; Li, H.; Gelinsky, M.; Gu, Z. Tailoring Materials for Modulation of Macrophage Fate. Adv. Mater. 2021, 33, 2004172. [Google Scholar] [CrossRef] [PubMed]
- Abaricia, J.O.; Farzad, N.; Heath, T.J.; Simmons, J.; Morandini, L.; Olivares-Navarrete, R. Control of Innate Immune Response by Biomaterial Surface Topography, Energy and Stiffness. Acta Biomater. 2021, 133, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Bogut, A.; Magryś, A. The road to success of coagulase-negative staphylococci: Clinical significance of small colony variants and their pathogenic role in persistent infections. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2249–2270. [Google Scholar] [CrossRef]
- Priddy-Arrington, T.R.; Ward, M.S.; Edwards, R.E.; Caldorera-Moore, M.E. Proactive biomaterials for chronic wound management and treatment. Curr. Opin. Biomed. Eng. 2021, 20, 100327. [Google Scholar] [CrossRef]
- Ramesh, M.; Rangappa, S.M.; Parameswaranpillai, J.; Siengchin, S. Introduction to biodegradable polymers. In Biodegradable Polymers, Blends and Composites; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–18. [Google Scholar]
- Cristache, C.M.; Totu, E.E. 3D Printing-Processed Polymers for Dental Applications. In Reactive and Functional Polymers; Springer: Berlin/Heidelberg, Germany, 2021; Volume 3, pp. 141–164. [Google Scholar]
- Toh, H.W.; Toong, D.W.Y.; Ng, J.C.K.; Ow, V.; Lu, S.; Tan, L.P.; Wong, P.E.H.; Venkatraman, S.; Huang, Y.; Ang, H.Y. Polymer blends and polymer composites for cardiovascular implants. Eur. Polym. J. 2021, 146, 110249. [Google Scholar] [CrossRef]
- Sharma, S.; Sudhakara, P.; Singh, J.; Ilyas, R.A.; Asyraf, M.R.M.; Razman, M.R. Critical review of biodegradable and bioactive polymer composites for bone tissue engineering and drug delivery applications. Polymers 2021, 13, 2623. [Google Scholar] [CrossRef]
- Biswal, T. Biopolymers for tissue engineering applications: A review. Mater. Today Proc. 2021, 41, 397–402. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Dana, H.R. Poly lactic acid (PLA) polymers: From properties to biomedical applications. Int. J. Polym. Mater. Polym. Biomater. 2021, 1–14. [Google Scholar] [CrossRef]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-Based Composites for Various Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, P. Effect of Modifications in Poly (Lactide-co-Glycolide)(PLGA) on Drug Release and Degradation Characteristics: A Mini Review. Curr. Drug Deliv. 2021, 18, 1378–13901. [Google Scholar] [CrossRef] [PubMed]
- Perez-Puyana, V.; Wieringa, P.; Yuste, Y.; de la Portilla, F.; Guererro, A.; Romero, A.; Moroni, L. Fabrication of hybrid scaffolds obtained from combinations of PCL with gelatin or collagen via electrospinning for skeletal muscle tissue engineering. J. Biomed. Mater. Res. Part A 2021, 109, 1600–1612. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-Y.; Cui, L.Y.; Zeng, R.C.; Li, S.Q.; Chen, X.B.; Zheng, Y.; Kannan, M.B. Advances in functionalized polymer coatings on biodegradable magnesium alloys–A review. Acta Biomater. 2018, 79, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. Polyurethane nanocomposite coatings: State of the art and perspectives. Polym. Int. 2018, 67, 1470–1477. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Chong, E.W.N.; Owolabi, F.A.T.; Asniza, M.; Tye, Y.Y.; Rizal, S.; Fazita, M.R.N.; Haafiz, M.K.M.; Nurmiati, Z.; Paridah, M.T. Enhancement of basic properties of polysaccharide-based composites with organic and inorganic fillers: A review. J. Appl. Polym. Sci. 2019, 136, 47251. [Google Scholar] [CrossRef] [Green Version]
- Bhong, S.Y.; More, N.; Choppadandi, M.; Kapusetti, G. Review on carbon nanomaterials as typical candidates for orthopaedic coatings. SN Appl. Sci. 2019, 1, 76. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh-Osgouei, M.; Li, Y.; Wen, C. A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications. Bioact. Mater. 2019, 4, 22–36. [Google Scholar] [CrossRef]
- Fu, M.; Liang, Y.; Lv, X.; Li, C.; Yang, Y.Y.; Yuan, P.; Ding, X. Recent advances in hydrogel-based anti-infective coatings. J. Mater. Sci. Technol. 2021, 85, 169–183. [Google Scholar] [CrossRef]
- Peranidze, K.K.; Safronova, T.; Kil’Deeva, N.; Chernogortseva, M.; Selezneva, I.; Shatalova, T.; Rau, J. Biocompatible composite films and fibers based on Poly (Vinyl alcohol) and powders of calcium salts. Smart Mater. Med. 2021, 2, 292–301. [Google Scholar] [CrossRef]
- Abegunde, O.O.; Akinlabi, E.T.; Oladijo, O.P.; Akinlabi, S.; Ude, A.U. Overview of thin film deposition techniques. AIMS Mater. Sci. 2019, 6, 174–199. [Google Scholar] [CrossRef]
- Li, P.H.; Chu, P.K. Thin film deposition technologies and processing of biomaterials. In Thin Film Coatings for Biomaterials and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2016; pp. 3–28. [Google Scholar]
- Stiff-Roberts, A.D.; Ge, W. Organic/hybrid thin films deposited by matrix-assisted pulsed laser evaporation (MAPLE). Appl. Phys. Rev. 2017, 4, 041303. [Google Scholar] [CrossRef]
- Serra, P.; Piqué, A. Laser-Induced Forward Transfer: Fundamentals and Applications. Adv. Mater. Technol. 2019, 4, 1800099. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, O.N.; Caseli, L.; Ariga, K. The Past and the Future of Langmuir and Langmuir–Blodgett Films. Chem. Rev. 2022, 122, 6459–6513. [Google Scholar] [CrossRef]
- Moreira, J.; Vale, A.C.; Alves, N.M. Spin-Coated Freestanding Films for Biomedical Applications. J. Mater. Chem. B 2021, 9, 3778–3799. [Google Scholar] [CrossRef]
- Naebea, M.; Haquea, A.N.M.A.; Haji, A. Plasma-Assisted Antimicrobial Finishing of Textiles: A Review. Engineering 2021, in press. Available online: https://www.sciencedirect.com/science/article/pii/S2095809921001430 (accessed on 21 February 2022).
- Wang, Q.; Xue, Q.; Chen, T.; Li, J.; Liu, Y.; Shan, X.; Liu, F.; Jia, J. Recent advances in electrochemical sensors for antibiotics and their applications. Chin. Chem. Lett. 2021, 32, 609–619. [Google Scholar] [CrossRef]
- Sandhu, H.S.; Phull, G.S.; Saini, M.S.; Singh, J.I.P.; Gulati, P. A Review: Bio-compatible Thermal Spray Coating on Bio-implant. In Recent Trends in Engineering Design; Springer: Singapore, 2021; pp. 71–77. [Google Scholar] [CrossRef]
- Vega-Hernández, M.; Cano-Díaz, G.; Vivaldo-Lima, E.; Rosas-Aburto, A.; Hernández-Luna, M.; Martinez, A.; Palacios-Alquisira, J.; Mohammadi, Y.; Penlidis, A. A Review on the Synthesis, Characterization, and Modeling of Polymer Grafting. Processes 2021, 9, 375. [Google Scholar] [CrossRef]
- Chisini, L.A.; Paganotto, G.F.D.R.; Guergolette, R.; Conde, M.C.M.; Alcázar, J.C.B.; de Carvalho, R.V.; Piva, E.; Carreño, N.L.V. Hydroxyapatite Synthesis and Covering of Titanium Surfaces by Dip-Coating Method. Braz. Arch. Biol. Technol. 2021, e21200344. [Google Scholar] [CrossRef]
- Hadzhieva, Z.; Boccaccini, A.R. Recent developments in electrophoretic deposition (EPD) of antibacterial coatings for biomedical applications—A review. Curr. Opin. Biomed. Eng. 2022, 21, 100367. [Google Scholar] [CrossRef]
- Le Low, J.; Kao, P.H.-N.; Tambyah, P.A.; Koh, G.L.E.; Ling, H.; Kline, K.A.; Cheow, W.S.; Leong, S.S.J. Development of a polymer-based antimicrobial coating for efficacious urinary catheter protection. Biotechnol. Notes 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Tiyyagura, H.R.; Rudolf, R.; Gorgieva, S.; Fuchs-Godec, R.; Boyapati, V.R.; Mantravadi, K.M.; Kokol, V. The chitosan coating and processing effect on the physiological corrosion behaviour of porous magnesium monoliths. Prog. Org. Coat. 2016, 99, 147–156. [Google Scholar] [CrossRef]
- Fu, Y.; Dudley, E.G. Antimicrobial-coated films as food packaging: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3404–3437. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Han, Y.; Liu, D.; Chen, S.; Qie, J.; Qu, J.; Lin, Q. Centrifugally concentric ring-patterned drug-loaded polymeric coating as an intraocular lens surface modification for efficient prevention of posterior capsular opacification. Acta Biomater. 2021, 138, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Sukthavorn, K.; Nootsuwan, N.; Wuttisarn, R.; Jongrungruangchok, S.; Veranitisagul, C.; Laobuthee, A. Golden Glittering Biocomposite Fibers from Poly(lactic acid) and Nanosilver-Coated Titanium Dioxide with Unique Properties; Antimicrobial, Photocatalytic, and Ion-Sensing Properties. ACS Omega 2021, 6, 16307–16315. [Google Scholar] [CrossRef]
- Van, T.T.T.; Makkar, P.; Farwa, U.; Lee, B.-T. Development of a novel polycaprolactone based composite membrane for periodontal regeneration using spin coating technique. J. Biomater. Sci. Polym. Ed. 2021, 33, 783–800. [Google Scholar] [CrossRef]
- Wu, P.; Chen, D.; Yang, H.; Lai, C.; Xuan, C.; Chen, Y.; Shi, X. Antibacterial peptide-modified collagen nanosheet for infected wound repair. Smart Mater. Med. 2021, 2, 172–181. [Google Scholar] [CrossRef]
- Poli, R.; Colleoni, C.; Calvimontes, A.; Polášková, H.; Dutschk, V.; Rosace, G. Innovative sol–gel route in neutral hydroalcoholic condition to obtain antibacterial cotton finishing by zinc precursor. J. Sol-Gel Sci. Technol. 2014, 74, 151–160. [Google Scholar] [CrossRef]
- Raknam, P.; Balekar, N.; Teanpaisan, R.; Amnuaikit, T. Thermoresponsive sol–gel containing probiotic’s cell free supernatant for dental caries prophylaxis. J. Oral Microbiol. 2022, 14, 2012390. [Google Scholar] [CrossRef]
- Miroiu, F.M.; Stefan, N.; Visan, A.I.; Nita, C.; Luculescu, C.R.; Rasoga, O.; Socol, M.; Zgura, I.; Cristescu, R.; Craciun, D.; et al. Composite biodegradable biopolymer coatings of silk fibroin—Poly(3-hydroxybutyric-acid-co-3-hydroxyvaleric-acid) for biomedical applications. Appl. Surf. Sci. 2015, 355, 1123–1131. [Google Scholar] [CrossRef]
- Laser-Induced Forward Transfer (LIFT) Based Bioprinting of the Collagen I with Retina Photoreceptor Cells—ProQuest. Available online: https://www.proquest.com/openview/5594a0684301d43899bcbb6d4662ccc1/1?pq-origsite=gscholar&cbl=18750&diss=y (accessed on 21 February 2022).
- Yusupov, V.; Churbanov, S.; Churbanova, E.; Bardakova, K.; Antoshin, A.; Evlashin, S.; Timashev, P.; Minaev, N. Laser-induced Forward Transfer Hydrogel Printing: A Defined Route for Highly Controlled Process. Int. J. Bioprint. 2020, 6, 271. [Google Scholar] [CrossRef]
- Grumezescu, V.; Negut, I.; Grumezescu, A.M.; Ficai, A.; Dorcioman, G.; Socol, G.; Iordache, F.; Truşcă, R.; Vasile, B.S.; Holban, A.M. MAPLE fabricated coatings based on magnetite nanoparticles embedded into biopolymeric spheres resistant to microbial colonization. Appl. Surf. Sci. 2018, 448, 230–236. [Google Scholar] [CrossRef]
- Caciandone, M.; Niculescu, A.-G.; Roșu, A.R.; Grumezescu, V.; Negut, I.; Holban, A.M.; Oprea, O.; Vasile, B.; Bîrcă, A.C.; Grumezescu, A.M.; et al. PEG-Functionalized Magnetite Nanoparticles for Modulation of Microbial Biofilms on Voice Prosthesis. Antibiotics 2021, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Spirescu, V.A.; Niculescu, A.-G.; Slave, S.; Bîrcă, A.C.; Dorcioman, G.; Grumezescu, V.; Holban, A.M.; Oprea, O.-C.; Vasile, B.; Grumezescu, A.M.; et al. Anti-Biofilm Coatings Based on Chitosan and Lysozyme Functionalized Magnetite Nanoparticles. Antibiotics 2021, 10, 1269. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Chen, Q.; Ren, H.; Wu, X.; Liu, X.; Lu, T. Electrophoretic deposition of dexamethasone-loaded gelatin nanospheres/chitosan coating and its dual function in anti-inflammation and osteogenesis. Colloids Surfaces B Biointerfaces 2018, 169, 249–256. [Google Scholar] [CrossRef]
- Jia, Z.; Ma, C.; Zhang, H. PLGA Coatings and PLGA Drug-Loading Coatings for Cardiac Stent Samples: Degradation Characteristics and Blood Compatibility. Coatings 2021, 11, 1427. [Google Scholar] [CrossRef]
- Chen, R.; Cai, X.; Ma, K.; Zhou, Y.; Wang, Y.; Jiang, T. The fabrication of double-layered chitosan/gelatin/genipin nanosphere coating for sequential and controlled release of therapeutic proteins. Biofabrication 2017, 9, 025028. [Google Scholar] [CrossRef]
- Chintada, V.B.; Gurugubelli, S.; Uppada, S. Review on Materials and Method Used to Develop Antimicrobial Coatings in Medical and Food Processing Industry. In Recent Advances in Manufacturing, Automation, Design and Energy Technologies; Springer: Singapore, 2022; pp. 57–63. [Google Scholar] [CrossRef]
- Huo, L.; Wei, Y.; Zhang, H.; Wang, Y.; Deng, B.; Wang, Y.; Jin, L. Preparation and properties of triethyl citrate plasticized chitosan-based membranes for efficient release of curcumin. J. Appl. Polym. Sci. 2022, 139, 51908. [Google Scholar] [CrossRef]
- Kim, S.; Foulandian, P.; Afinjuomo, F.; Song, Y.; Youssef, S.; Vaidya, S.; Garg, S. Effect of plasticizers on drug-in-adhesive patches containing 5-fluorouracil. Int. J. Pharm. 2021, 611, 121316. [Google Scholar] [CrossRef]
- Lestido-Cardama, A.; Vázquez-Loureiro, P.; Sendón, R.; Bustos, J.; Santillana, M.I.; Losada, P.P.; de Quirós, A.R.B. Characterization of Polyester Coatings Intended for Food Contact by Different Analytical Techniques and Migration Testing by LC-MSn. Polymers 2022, 14, 487. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, L.; Mao, X.; Shao, Y.; Cao, M.; Zhang, L.; Liang, X. Pullulan-based nanocomposite films with enhanced hydrophobicity and antibacterial performances. Polym. Bull. 2022, 1–17. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, W.; Chen, H.; Liu, J.; Zhang, W. Novel application method of talcum powder to prevent sticking tendency and modify release of esomeprazole magnesium enteric-coated pellets. Pharm. Dev. Technol. 2016, 21, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Bodratti, A.M.; Alexandridis, P. Amphiphilic block copolymers in drug delivery: Advances in formulation structure and performance. Expert Opin. Drug Deliv. 2018, 15, 1085–1104. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.S.; Shimizu, A.; Hasegawa, K.; Ito, T. Advancement of Biomaterial-Based Postoperative Adhesion Barriers. Macromol. Biosci. 2021, 21, 2000395. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.C. Chapter 27—Coating of pharmaceutical dosage forms. In Remington, 23rd ed.; Adejare, A., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 551–564. [Google Scholar] [CrossRef]
- Miyazawa, T.; Itaya, M.; Burdeos, G.C.; Nakagawa, K.; Miyazawa, T. A Critical Review of the Use of Surfactant-Coated Nanoparticles in Nanomedicine and Food Nanotechnology. Int. J. Nanomed. 2021, 16, 3937. [Google Scholar] [CrossRef]
- Saikh, M.A.A. Aqueous Film Coating the Current Trend. J. Drug Deliv. Ther. 2021, 11, 212–224. [Google Scholar] [CrossRef]
- Fu, M.; Blechar, J.A.; Sauer, A.; Al-Gousous, J.; Langguth, P. In Vitro Evaluation of Enteric-Coated HPMC Capsules—Effect of Formulation Factors on Product Performance. Pharmaceutics 2020, 12, 696. [Google Scholar] [CrossRef]
- Ballesteros-Mártinez, L.; Pérez-Cervera, C.; Andrade-Pizarro, R. Effect of glycerol and sorbitol concentrations on mechanical, optical, and barrier properties of sweet potato starch film. NFS J. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Dastpak, A.; Hannula, P.-M.; Lundström, M.; Wilson, B.P. A sustainable two-layer lignin-anodized composite coating for the corrosion protection of high-strength low-alloy steel. Prog. Org. Coat. 2020, 148, 105866. [Google Scholar] [CrossRef]
- Bagheri, F.; Radi, M.; Amiri, S. Drying conditions highly influence the characteristics of glycerol-plasticized alginate films. Food Hydrocoll. 2018, 90, 162–171. [Google Scholar] [CrossRef]
- Singh, A.; Radhakrishnan, S.; Vijayalakshmi, R.; Talawar, M.B.; Kumar, A.; Kumar, D. Screening of polymer-plasticizer systems for propellant binder applications: An experimental and simulation approach. Energetic Mater. 2019, 37, 365–377. [Google Scholar] [CrossRef]
- Aliotta, L.; Canesi, I.; Lazzeri, A. Study on the preferential distribution of acetyl tributyl citrate in poly(lactic) acid-poly(butylene adipate-co-terephthalate) blends. Polym. Test. 2021, 98, 107163. [Google Scholar] [CrossRef]
- Yu, D.-G.; Li, J.-J.; Williams, G.R.; Zhao, M. Electrospun amorphous solid dispersions of poorly water-soluble drugs: A review. J. Control. Release 2018, 292, 91–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özakar, R.S.; Özakar, E. Current Overview of Oral Thin Films. Turk. J. Pharm. Sci. 2021, 18, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Ketema, A.; Worku, A. Review on Intermolecular Forces between Dyes Used for Polyester Dyeing and Polyester Fiber. J. Chem. 2020, 2020, e6628404. [Google Scholar] [CrossRef]
- Weichold, O. Introduction to Polymer Chemistry. In Encyclopedia of Glass Science, Technology, History, and Culture; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 1043–1055. [Google Scholar] [CrossRef]
- Haywood, A.; Glass, B.D. Pharmaceutical Excipients—Where Do We Begin? Aust. Prescr. 2011, 34, pp. 112–114. [CrossRef]
- Felton, L.A.; Porter, S.C. An update on pharmaceutical film coating for drug delivery. Expert Opin. Drug Deliv. 2013, 10, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Tabasum, S.; Younas, M.; Zaeem, M.A.; Majeed, I.; Majeed, M.; Noreen, A.; Iqbal, M.N.; Zia, K.M. A review on blending of corn starch with natural and synthetic polymers, and inorganic nanoparticles with mathematical modeling. Int. J. Biol. Macromol. 2018, 122, 969–996. [Google Scholar] [CrossRef] [PubMed]
- Nimkulrat, S.; Suchiva, K.; Phinyocheep, P.; Puttipipatkhachorn, S. Influence of selected surfactants on the tackiness of acrylic polymer films. Int. J. Pharm. 2004, 287, 27–37. [Google Scholar] [CrossRef]
- Canu, I.G.; Fraize-Frontier, S.; Michel, C.; Charles, S. Weight of epidemiological evidence for titanium dioxide risk assessment: Current state and further needs. J. Expo Sci. Environ. Epidemiol. 2019, 30, 430–435. [Google Scholar] [CrossRef]
- Pérez-Ibarbia, L.; Majdanski, T.; Schubert, S.; Windhab, N.; Schubert, U.S. Safety and regulatory review of dyes commonly used as excipients in pharmaceutical and nutraceutical applications. Eur. J. Pharm. Sci. 2016, 93, 264–273. [Google Scholar] [CrossRef]
- Felton, L.A.; McGinity, J.W. Influence of Insoluble Excipients on Film Coating Systems. Drug Dev. Ind. Pharm. 2002, 28, 225–243. [Google Scholar] [CrossRef]
- Gaur, P.K.; Mishra, S.; Gautam, R.; Singh, A.P.; Yasir, M. Film Coating Technology: Past, Present and Future. Pharm. Sci. Pharmacol. 2014, 1, 57–67. [Google Scholar] [CrossRef]
- Shenoy, A.V. Rheology of Filled Polymer Systems; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Varma, M.V.S.; Kaushal, A.M.; Garg, A.; Garg, S. Factors affecting mechanism and kinetics of drug release from matrix-based oral controlled drug delivery systems. Am. J. Drug Deliv. 2004, 2, 43–57. [Google Scholar] [CrossRef]
- Tagami, T.; Nagata, N.; Hayashi, N.; Ogawa, E.; Fukushige, K.; Sakai, N.; Ozeki, T. Defined drug release from 3D-printed composite tablets consisting of drug-loaded polyvinylalcohol and a water-soluble or water-insoluble polymer filler. Int. J. Pharm. 2018, 543, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.M.; Brijesh, R. A review on: Sustained release technology. Int. J. Ther. Appl. 2012, 8, 18–23. [Google Scholar]
- Ding, Y.; Li, W.; Zhang, F.; Liu, Z.; Ezazi, N.Z.; Liu, D.; Santos, H.A. Electrospun Fibrous Architectures for Drug Delivery, Tissue Engineering and Cancer Therapy. Adv. Funct. Mater. 2019, 29, 1802852. [Google Scholar] [CrossRef]
- Choi, H.; Schulte, A.; Müller, M.; Park, M.; Jo, S.; Schönherr, H. Drug Release from Thermo-Responsive Polymer Brush Coatings to Control Bacterial Colonization and Biofilm Growth on Titanium Implants. Adv. Healthc. Mater. 2021, 10, 2100069. [Google Scholar] [CrossRef]
- Józó, M.; Simon, N.; Yi, L.; Móczó, J.; Pukánszky, B. Improved Release of a Drug with Poor Water Solubility by Using Electrospun Water-Soluble Polymers as Carriers. Pharmaceutics 2021, 14, 34. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, P.; Zhang, X.; Wu, D. Emerging porous organic polymers for biomedical applications. Chem. Soc. Rev. 2022, 51, 1377–1414. [Google Scholar] [CrossRef]
- Bruschi, M.L. Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
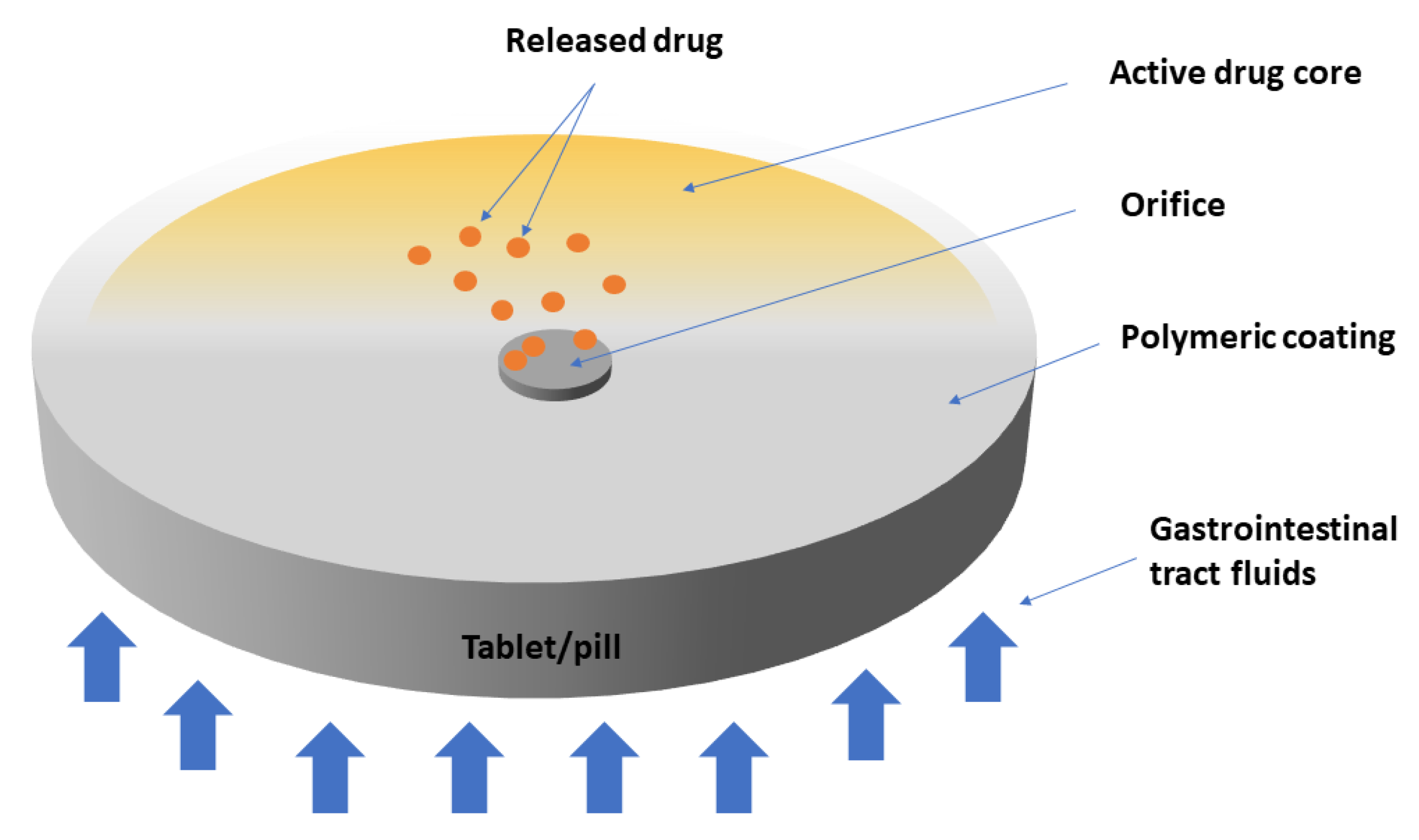
- Patel, J.; Parikh, S.; Patel, S. Comprehensive review on osmotic drug delivery system. World J. Pharm. Res. 2021, 10, 29. [Google Scholar]
- Ogueri, K.S.; Shamblin, S.L. Osmotic-controlled release oral tablets: Technology and functional insights. Trends Biotechnol. 2021, 40, 606–619. [Google Scholar] [CrossRef]
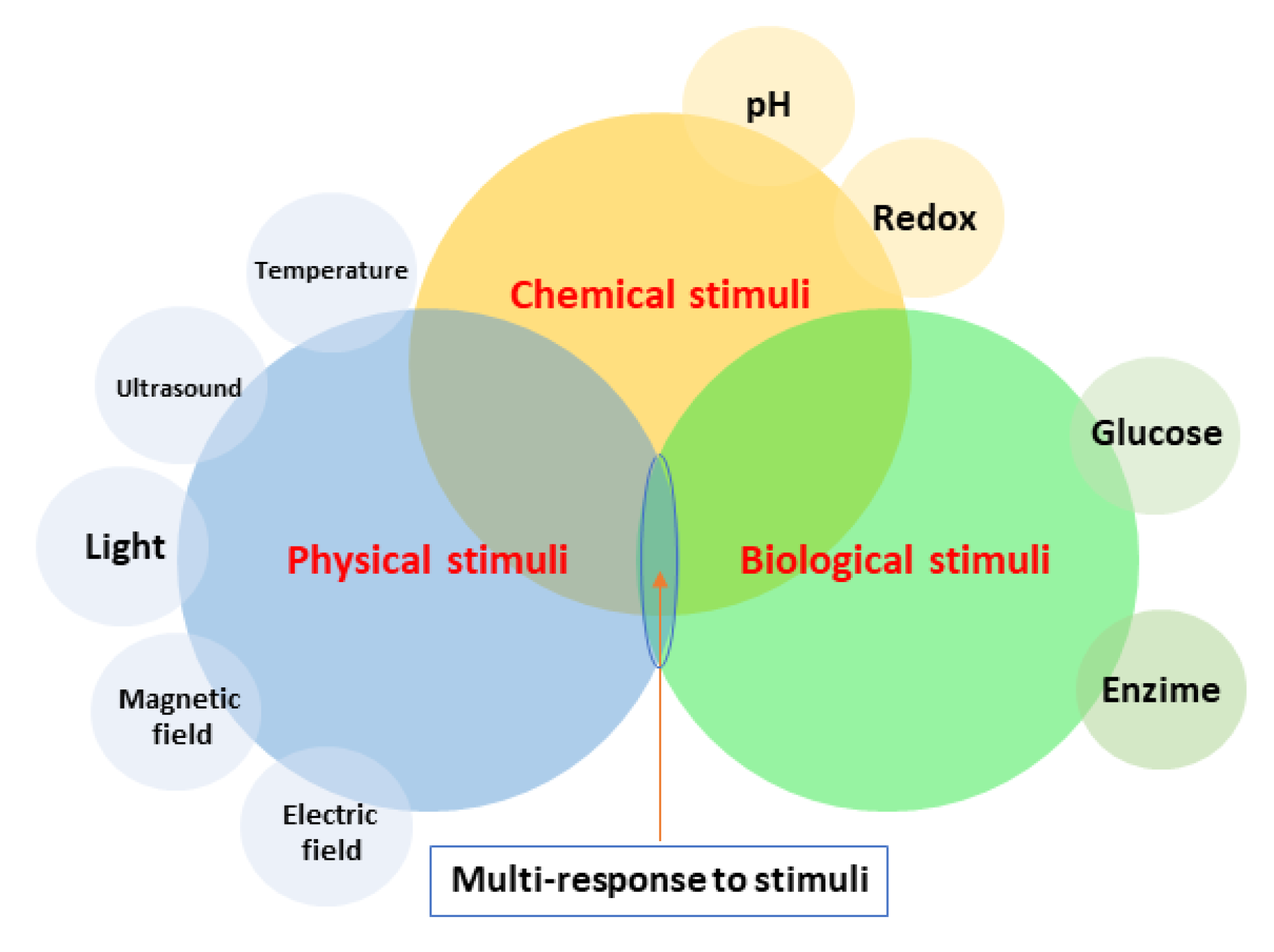
- James, H.P.; John, R.; Alex, A.; Anoop, K.R. Smart polymers for the controlled delivery of drugs—A concise overview. Acta Pharm. Sin. B 2014, 4, 120–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spósito, L.; Fortunato, G.C.; de Camargo, B.A.F.; Ramos, M.A.D.S.; de Souza, M.P.C.; Meneguin, A.B.; Bauab, T.M.; Chorilli, M. Exploiting drug delivery systems for oral route in the peptic ulcer disease treatment. J. Drug Target 2021, 29, 1029–1047. [Google Scholar] [CrossRef] [PubMed]
- Khuroo, T.; Mohamed, E.M.; Dharani, S.; Afrooz, H.; Ali, S.F.B.; Cook, P.; Khan, M.A.; Rahman, Z. Coating characterization by hyperspectroscopy and predictive dissolution models of tablets coated with blends of cellulose acetate and cellulose acetate phthalate. APS PharmSciTech 2021, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.K.; Sahoo, R.N.; Mallick, S.; Mohapatra, R. Enteric Dissolution Enhancement of Engineered Gastro Resistant Omeprazole Tablets using Hydroxypropyl Methylcellulose Acetate Succinate. Indian J. Pharm. Educ. Res. 2021, 55, 677–684. [Google Scholar] [CrossRef]
- Katona, M.T.; Kakuk, M.; Szabó, R.; Tonka-Nagy, P.; Takács-Novák, K.; Borbás, E. Towards a Better Understanding of the Post-Gastric Behavior of Enteric-Coated Formulations. Pharm Res. 2022, 39, 201–211. [Google Scholar] [CrossRef]
- Turanlı, Y.; Acartürk, F. Preparation and characterization of colon-targeted pH/Time-dependent nanoparticles using anionic and cationic polymethacrylate polymers. Eur. J. Pharm. Sci. 2022, 171, 106122. [Google Scholar] [CrossRef]
- Chandel, A.K.S.; Bera, A.; Nutan, B.; Jewrajka, S.K. Reactive compatibilizer mediated precise synthesis and application of stimuli responsive polysaccharides-polycaprolactone amphiphilic co-network gels. Polymer 2016, 99, 470–479. [Google Scholar] [CrossRef]
- Deirram, N.; Zhang, C.; Kermaniyan, S.S.; Johnston, A.P.R.; Such, G.K. pH-Responsive Polymer Nanoparticles for Drug Delivery. Macromol. Rapid Commun. 2019, 40, 1800917. [Google Scholar] [CrossRef] [Green Version]
- Chandel, A.K.S.; Kannan, D.; Nutan, B.; Singh, S.; Jewrajka, S.K. Dually crosslinked injectable hydrogels of poly(ethylene glycol) and poly[(2-dimethylamino)ethyl methacrylate]-b-poly(N-isopropyl acrylamide) as a wound healing promoter. J. Mater. Chem. B 2017, 5, 4955–4965. [Google Scholar] [CrossRef]
- Shevtsova, T.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Donchak, V.; Harhay, K.; Korolko, S.; Budkowski, A.; Stetsyshyn, Y. Temperature-responsive hybrid nanomaterials based on modified halloysite nanotubes uploaded with silver nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 641, 128525. [Google Scholar] [CrossRef]
- Fan, B.; Cui, N.; Xu, Z.; Chen, K.; Yin, P.; Yue, K.; Tang, W. Thermoresponsive and Self-Healing Hydrogel Based on Chitosan Derivatives and Polyoxometalate as an Antibacterial Coating. Biomacromolecules 2022, 23, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Z.; Zhuo, R.-X.; Cui, J.-Z.; Zhang, J.-T. A novel thermo-responsive drug delivery system with positive controlled release. Int. J. Pharm. 2002, 235, 43–50. [Google Scholar] [CrossRef]
- Bae, Y.H.; Okano, T.; Kim, S.W. ‘On–Off’ Thermocontrol of Solute Transport. I. Temperature Dependence of Swelling of N-Isopropylacrylamide Networks Modified with Hydrophobic Components in Water. Pharm. Res. 1991, 8, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Cinar, H.; Fetahaj, Z.; Cinar, S.; Vernon, R.M.; Chan, H.S.; Winter, R.H.A. Temperature, Hydrostatic Pressure, and Osmolyte Effects on Liquid–Liquid Phase Separation in Protein Condensates: Physical Chemistry and Biological Implications. Chem. A Eur. J. 2019, 25, 13049–13069. [Google Scholar] [CrossRef]
- Pérez-Köhler, B.; Pascual, G.; Benito-Martínez, S.; Bellón, J.M.; Eglin, D.; Guillaume, O. Thermo-Responsive Antimicrobial Hydrogel for the In-Situ Coating of Mesh Materials for Hernia Repair. Polymers 2020, 12, 1245. [Google Scholar] [CrossRef]
- Ter Boo, G.-J.; Arens, D.; Metsemakers, W.-J.; Zeiter, S.; Richards, R.; Grijpma, D.W.; Eglin, D.; Moriarty, T.F. Injectable gentamicin-loaded thermo-responsive hyaluronic acid derivative prevents infection in a rabbit model. Acta Biomater. 2016, 43, 185–194. [Google Scholar] [CrossRef]
- AO Research Institute Davos. Local application of a gentamicin-loaded thermo-responsive hydrogel allows for fracture healing upon clearance of a high Staphylococcus aureus load in a rabbit model. Eur. Cells Mater. 2018, 35, 151–164. [Google Scholar] [CrossRef]
- Hu, J.; Li, H.-Y.; Williams, G.R.; Yang, H.-H.; Tao, L.; Zhu, L.-M. Electrospun Poly(N-isopropylacrylamide)/Ethyl Cellulose Nanofibers as Thermoresponsive Drug Delivery Systems. J. Pharm. Sci. 2016, 105, 1104–1112. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, Y.; Wang, Q.; Liu, T.; Sun, J.; Zhang, R. Remote Light-Responsive Nanocarriers for Controlled Drug Delivery: Advances and Perspectives. Small 2019, 15, 1903060. [Google Scholar] [CrossRef]
- Cho, H.J.; Chung, M.; Shim, M.S. Engineered photo-responsive materials for near-infrared-triggered drug delivery. J. Ind. Eng. Chem. 2015, 31, 15–25. [Google Scholar] [CrossRef]
- Cazin, I.; Rossegger, E.; de la Cruz, G.G.; Griesser, T.; Schlögl, S. Recent Advances in Functional Polymers Containing Coumarin Chromophores. J. Ind. Eng. Chem. 2015, 31, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Marín-Caba, L.; Bodelón, G.; Negrín-Montecelo, Y.; Correa-Duarte, M.A. Sunlight-Sensitive Plasmonic Nanostructured Composites as Photocatalytic Coating with Antibacterial Properties. Adv. Funct. Mater. 2021, 31, 2105807. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, J.; Stachurski, Z.H.; Holl, M.M.B.; Xiao, P. Visible-Light-Sensitive Triazine-Coated Silica Nanoparticles: A Dual Role Approach to Polymer Nanocomposite Materials with Enhanced Properties. ACS Appl. Mater. Interfaces 2021, 13, 46033–46042. [Google Scholar] [CrossRef] [PubMed]
- Marturano, V.; Abate, F.; Ambrogi, V.; Califano, V.; Cerruti, P.; Pepe, G.; Vicari, L.; Ausanio, G. Smart Coatings Prepared via MAPLE Deposition of Polymer Nanocapsules for Light-Induced Release. Molecules 2021, 26, 2736. [Google Scholar] [CrossRef] [PubMed]
- Makhlouf, A.S.H.; Perez, A.; Guerrero, E. Chapter 13—Recent trends in smart polymeric coatings in biomedicine and drug delivery applications. In Advances in Smart Coatings and Thin Films for Future Industrial and Biomedical Engineering Applications; Makhlouf, A.S.H., Abu-Thabit, N.Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 359–381. [Google Scholar] [CrossRef]
- Girija, A.R. 12—Medical Applications of Polymer/Functionalized Nanoparticle Systems. In Polymer Composites with Functionalized Nanoparticles; Pielichowski, K., Majka, T.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 381–404. [Google Scholar] [CrossRef]
- Rodríguez, A.M.O.; Martínez, C.J.P.; Castro, T.d.; Ortega, M.M.C.; Félix, D.E.R.; García, J.R. Nanocomposite hydrogel of poly(vinyl alcohol) and biocatalytically synthesized polypyrrole as potential system for controlled release of metoprolol. Polym. Bull. 2020, 77, 1217–1232. [Google Scholar] [CrossRef]
- Purkait, M.K.; Sinha, M.K.; Mondal, P.; Singh, R. Chapter 6—Electric Field-Responsive Membranes. In Interface Science and Technology 25; Purkait, M.K., Sinha, M.K., Mondal, P., Singh, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 173–191. [Google Scholar] [CrossRef]
- Ge, J.; Neofytou, E.; Cahill, T.J.; Beygui, R.E.; Zare, R.N. Drug Release from Electric-Field-Responsive Nanoparticles. ACS Nano 2012, 6, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized ‘smart’ drug release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef]
- Zhao, Y.; Tavares, A.C.; Gauthier, M.A. Nano-engineered electro-responsive drug delivery systems. J. Mater. Chem. B 2016, 4, 3019–3030. [Google Scholar] [CrossRef]
- Qu, J.; Liang, Y.; Shi, M.; Guo, B.; Gao, Y.; Yin, Z. Biocompatible conductive hydrogels based on dextran and aniline trimer as electro-responsive drug delivery system for localized drug release. Int. J. Biol. Macromol. 2019, 140, 255–264. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, H.; Deng, H.; Xiao, L.; Qin, C.; Du, Y.; Shi, X. A study of chitosan hydrogel with embedded mesoporous silica nanoparticles loaded by ibuprofen as a dual stimuli-responsive drug release system for surface coating of titanium implants. Colloids Surf. B Biointerfaces 2014, 123, 657–663. [Google Scholar] [CrossRef]
- Saravanakumar, G.; Kim, W.J. Stimuli-Responsive Polymeric Nanocarriers as Promising Drug and Gene Delivery Systems. In Intracellular Delivery II: Fundamentals and Applications; Prokop, A., Iwasaki, Y., Harada, A., Eds.; Springer: Cham, Switzerland, 2014; pp. 55–91. [Google Scholar] [CrossRef]
- Wu, P.; Jia, Y.; Qu, F.; Sun, Y.; Wang, P.; Zhang, K.; Xu, C.; Liu, Q.; Wang, X. Ultrasound-Responsive Polymeric Micelles for Sonoporation-Assisted Site-Specific Therapeutic Action. ACS Appl. Mater. Interfaces 2017, 9, 25706–25716. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.; Harris, M.A.; LeVine, D.; Ghimire, M.; Jennings, J.A.; Morshed, B.I.; Haggard, W.O.; Bumgardner, J.D.; Mishra, S.R.; Fujiwara, T. Magnetic stimulus responsive vancomycin drug delivery system based on chitosan microbeads embedded with magnetic nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, J.; Xie, X.; Li, X. Development of photo-magnetic drug delivery system by facile-designed dual stimuli-responsive modified biopolymeric chitosan capped nano-vesicle to improve efficiency in the anesthetic effect and its biological investigations. J. Photochem. Photobiol. B Biol. 2019, 202, 111716. [Google Scholar] [CrossRef] [PubMed]
- Silva-Freitas, E.L.; Pontes, T.R.F.; Araújo-Neto, R.P.; Damasceno, H.M.; Silva, K.L.; Carvalho, J.F.; Medeiros, A.C.; Silva, R.B.; Silva, A.K.A.; Morales, M.A.; et al. Design of Magnetic Polymeric Particles as a Stimulus-Responsive System for Gastric Antimicrobial Therapy. AAPS PharmSciTech 2016, 18, 2026–2036. [Google Scholar] [CrossRef]
- Wu, P.; Grainger, D.W. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials 2006, 27, 2450–2467. [Google Scholar] [CrossRef]
- Alt, V. Antimicrobial coated implants in trauma and orthopaedics–A clinical review and risk-benefit analysis. Injury 2017, 48, 599–607. [Google Scholar] [CrossRef]
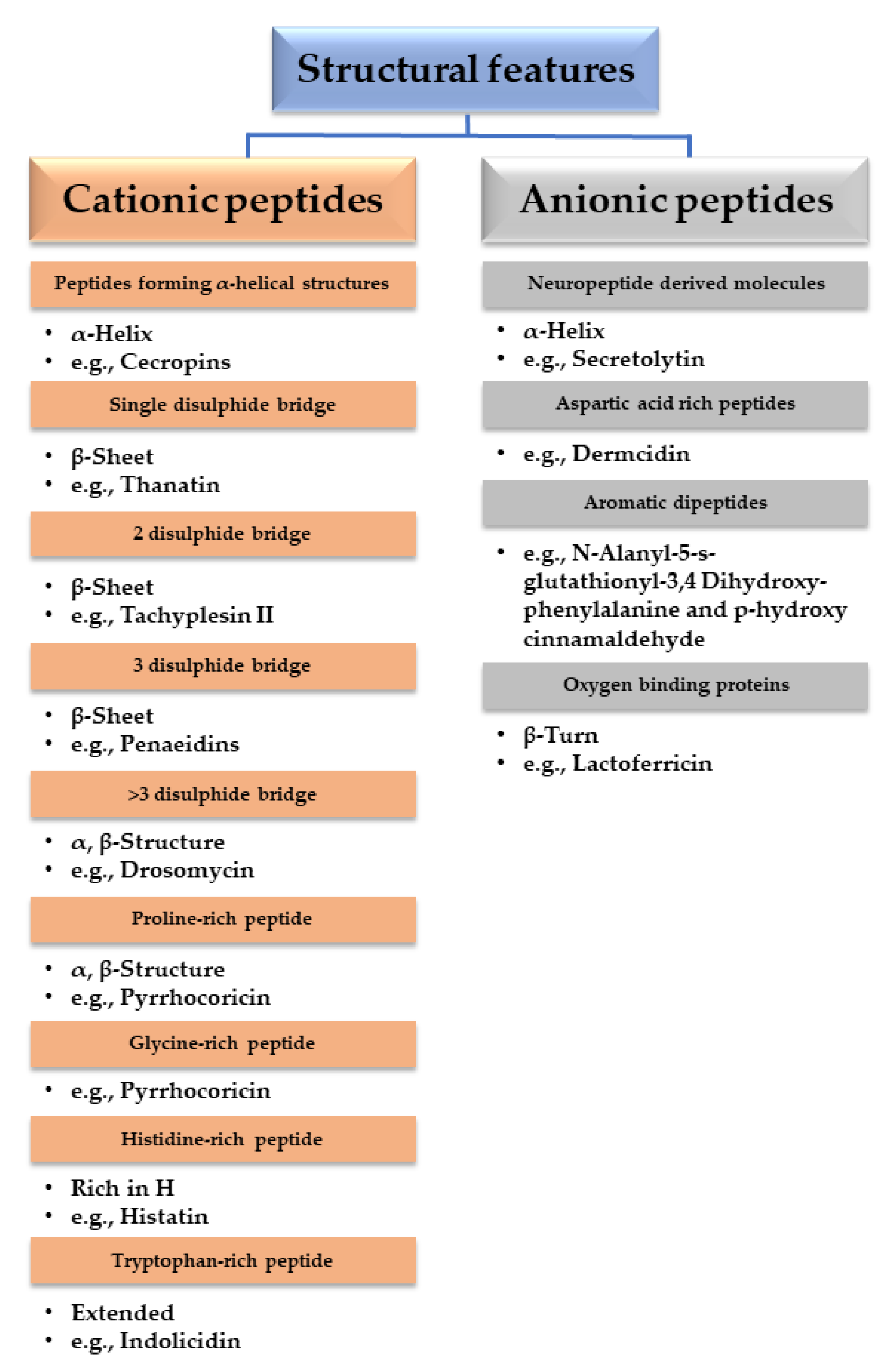
- Bulet, P.; Hetru, C.; Dimarcq, J.-L.; Hoffmann, D. Antimicrobial peptides in insects; structure and function. Dev. Comp. Immunol. 1999, 23, 329–344. [Google Scholar] [CrossRef]
- Mishra, B.; Reiling, S.; Zarena, D.; Wang, G. Host defense antimicrobial peptides as antibiotics: Design and application strategies. Curr. Opin. Chem. Biol. 2017, 38, 87–96. [Google Scholar] [CrossRef]
- Nayab, S.; Aslam, M.A.; Rahman, S.U.; Sindhu, Z.U.D.; Sajid, S.; Zafar, N.; Razaq, M.; Kanwar, R. A Review of Antimicrobial Peptides: Its Function, Mode of Action and Therapeutic Potential. Int. J. Pept. Res. Ther. 2022, 28, 46. [Google Scholar] [CrossRef]
- Prasad, A.K.; Tiwari, C.; Ray, S.; Holden, S.; Armstrong, D.A.; Rosengren, K.J.; Rodger, A.; Panwar, A.S.; Martin, L.L. Secondary Structure Transitions for a Family of Amyloidogenic, Antimicrobial Uperin 3 Peptides in Contact with Sodium Dodecyl Sulfate. ChemPlusChem 2022, 87, e202100408. [Google Scholar] [CrossRef]
- Yi, H.-Y.; Chowdhury, M.; Huang, Y.-D.; Yu, X.-Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grünewald, J.; Marahiel, M.A. Chemoenzymatic and Template-Directed Synthesis of Bioactive Macrocyclic Peptides. Microbiol. Mol. Biol. Rev. 2006, 70, 121–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papagianni, M. Ribosomally synthesized peptides with antimicrobial properties: Biosynthesis, structure, function, and applications. Biotechnol. Adv. 2003, 21, 465–499. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Sahl, H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef]
- Wang, G. Antimicrobial Peptides: Discovery, Design and Novel Therapeutic Strategies, 2nd ed.; CABI: Boston, MA, USA, 2017. [Google Scholar]
- Graf, M.; Mardirossian, M.; Nguyen, F.; Seefeldt, A.C.; Guichard, G.; Scocchi, M.; Innis, C.A.; Wilson, D.N. Proline-rich antimicrobial peptides targeting protein synthesis. Nat. Prod. Rep. 2017, 34, 702–711. [Google Scholar] [CrossRef]
- Harris, F.; Dennison, S.R.; Phoenix, D.A. Anionic Antimicrobial Peptides from Eukaryotic Organisms. Curr. Protein Pept. Sci. 2009, 10, 585–606. [Google Scholar] [CrossRef]
- Castillo-Juárez, I.; Blancas-Luciano, B.E.; García-Contreras, R.; Fernández-Presas, A.M. Antimicrobial peptides properties beyond growth inhibition and bacterial killing. PeerJ 2022, 10, e12667. [Google Scholar] [CrossRef]
- Ryu, M.; Park, J.; Yeom, J.-H.; Joo, M.; Lee, K. Rediscovery of antimicrobial peptides as therapeutic agents. J. Microbiol. 2021, 59, 113–123. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, P.; Niu, Z.-W. A perspective on general direction and challenges facing antimicrobial peptides. Chin. Chem. Lett. 2017, 28, 703–708. [Google Scholar] [CrossRef]
- Marchini, G.; Lindow, S.; Brismar, H.; Stabi, B.; Berggren, V.; Ulfgren, A.-K.; Lonne-Rahm, S.; Agerberth, B.; Gudmundsson, G. The newborn infant is protected by an innate antimicrobial barrier: Peptide antibiotics are present in the skin and vernix caseosa. Br. J. Dermatol. 2002, 147, 1127–1134. [Google Scholar] [CrossRef]
- Gschwandtner, M.; Zhong, S.; Tschachler, A.; Mlitz, V.; Karner, S.; Elbe-Bürger, A.; Mildner, M. Fetal Human Keratinocytes Produce Large Amounts of Antimicrobial Peptides: Involvement of Histone-Methylation Processes. J. Investig. Dermatol. 2014, 134, 2192–2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittersheim, M.; Cordes, J.; Meyer-Hoffert, U.; Harder, J.; Hedderich, J.; Gläser, R. Differential expression and in vivo secretion of the antimicrobial peptides psoriasin (S100A7), RNase 7, human beta-defensin-2 and -3 in healthy human skin. Exp. Dermatol. 2013, 22, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Gläser, R.; Meyer-Hoffert, U.; Harder, J.; Cordes, J.; Wittersheim, M.; Kobliakova, J.; Fölster-Holst, R.; Proksch, E.; Schröder, J.-M.; Schwarz, T. The Antimicrobial Protein Psoriasin (S100A7) Is Upregulated in Atopic Dermatitis and after Experimental Skin Barrier Disruption. J. Investig. Dermatol. 2009, 129, 641–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDermott, A.M. Antimicrobial compounds in tears. Exp. Eye Res. 2013, 117, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Underwood, M.; Bakaletz, L. Innate Immunity and the Role of Defensins in Otitis Media. Curr. Allergy Asthma Rep. 2011, 11, 499–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, J.; Nishimura, M.; Yamazaki, M.; Yoshida, K.; Kurashige, Y.; Saitoh, M.; Abiko, Y. Expression profile of drosomycin-like defensin in oral epithelium and oral carcinoma cell lines. Arch. Oral Biol. 2013, 58, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Human Antimicrobial Peptides and Proteins. Pharmaceuticals 2014, 7, 545–594. [Google Scholar] [CrossRef] [Green Version]
- Nelsen, D.R.; Nisani, Z.; Cooper, A.M.; Fox, G.A.; Gren, E.C.K.; Corbit, A.G.; Hayes, W.K. Poisons, toxungens, and venoms: Redefining and classifying toxic biological secretions and the organisms that employ them. Biol. Rev. 2014, 89, 450–465. [Google Scholar] [CrossRef]
- König, E.; Bininda-Emonds, O.R.P.; Shaw, C. The diversity and evolution of anuran skin peptides. Peptides 2015, 63, 96–117. [Google Scholar] [CrossRef]
- Conlon, B.P.; Nakayasu, E.S.; Fleck, L.E.; LaFleur, M.D.; Isabella, V.M.; Coleman, K.; Leonard, S.N.; Smith, R.D.; Adkins, J.N.; Lewis, K. Activated ClpP Kills Persisters and Eradicates a Chronic Biofilm Infection. Nature 2013, 503, 365–370. [Google Scholar] [CrossRef] [Green Version]
- Bellotto, O.; Semeraro, S.; Bandiera, A.; Tramer, F.; Pavan, N.; Marchesan, S. Polymer Conjugates of Antimicrobial Peptides (AMPs) with d-Amino Acids (d-aa): State of the Art and Future Opportunities. Pharmaceutics 2022, 14, 446. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, Y.; Shimizu, K.; Kitahashi, Y.; Ohyama, A.; Kawamura, I.; Kawano, R. Electrophysiological Analysis of Membrane Disruption by Bombinin and Its Isomer Using the Lipid Bilayer System. ACS Appl. Bio Mater. 2019, 2, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Makarova, O.; Rodríguez-Rojas, A.; Eravci, M.; Weise, C.; Dobson, A.; Johnston, P.; Rolff, J. Antimicrobial defence and persistent infection in insects revisited. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilcinskas, A. Evolutionary plasticity of insect immunity. J. Insect Physiol. 2013, 59, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Gerardo, N.M.; Altincicek, B.; Anselme, C.; Atamian, H.; Barribeau, S.M.; De Vos, M.; Duncan, E.J.; Evans, J.D.; Gabaldón, T.; Ghanim, M.; et al. Immunity and other defenses in pea aphids, Acyrthosiphon pisum. Genome Biol. 2010, 11, R21. [Google Scholar] [CrossRef] [PubMed]
- Steiner, H.; Hultmark, D.; Engström, Å.; Bennich, H.; Boman, H.G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 1981, 292, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Misof, B.; Liu, S.; Meusemann, K.; Peters, R.S.; Donath, A.; Mayer, C.; Frandsen, P.B.; Ware, J.; Flouri, T.; Beutel, R.G.; et al. Phylogenomics resolves the timing and pattern of insect evolution. Science 2014, 346, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Aponte, C.A.; Silva-Sanchez, J.; Quintero-Hernández, V.; Rodriguez-Romero, A.; Balderas, C.; Possani, L.D.; Gurrola, G.B. Vejovine, a new antibiotic from the scorpion venom of Vaejovis mexicanus. Toxicon 2011, 57, 84–92. [Google Scholar] [CrossRef]
- Bontems, F.; Roumestand, C.; Gilquin, B.; Ménez, A.; Toma, F. Refined Structure of Charybdotoxin: Common Motifs in Scorpion Toxins and Insect Defensins. Science 1991, 254, 1521–1523. [Google Scholar] [CrossRef]
- Montesinos, E. Antimicrobial peptides and plant disease control. FEMS Microbiol. Lett. 2007, 270, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tam, J.P.; Wang, S.; Wong, K.H.; Tan, W.L. Antimicrobial Peptides from Plants. Pharmaceuticals 2015, 8, 711–757. [Google Scholar] [CrossRef] [PubMed]
- Naimah, A.K.; Al-Manhel, A.J.A.; Al-Shawi, M.J. Isolation, Purification and Characterization of Antimicrobial Peptides Produced from Saccharomyces boulardii. Int. J. Pept. Res. Ther. 2017, 24, 455–461. [Google Scholar] [CrossRef]
- Hadinegoro, S.R.; Arredondo-Garcia, J.L.; Capeding, M.R.; Deseda, C.; Chotpitayasunondh, T.; Dietze, R.; Ismail, H.H.M.; Reynales, H.; Limkittikul, K.; Rivera-Medina, D.M.; et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N. Engl. J. Med. 2015, 373, 1195–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora, C.; Tittensor, D.P.; Adl, S.; Simpson, A.G.B.; Worm, B. How Many Species Are There on Earth and in the Ocean? PLoS Biol. 2011, 9, e1001127. [Google Scholar] [CrossRef] [Green Version]
- Holo, H.; Faye, T.; Brede, D.A.; Nilsen, T.; Ødegård, I.; Langsrud, T.; Brendehaug, J.; Nes, I.F. Bacteriocins of propionic acid bacteria. Le Lait 2002, 82, 59–68. [Google Scholar] [CrossRef]
- Kaunietis, A.; Buivydas, A.; Čitavičius, D.J.; Kuipers, O.P. Heterologous biosynthesis and characterization of a glycocin from a thermophilic bacterium. Nat. Commun. 2019, 10, 1115. [Google Scholar] [CrossRef]
- Lima, P.G.; Oliveira, J.T.A.; Amaral, J.L.; Freitas, C.D.T.; Souza, P.F.N. Synthetic antimicrobial peptides: Characteristics, design, and potential as alternative molecules to overcome microbial resistance. Life Sci. 2021, 278, 119647. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [Green Version]
- Waghu, F.H.; Idicula-Thomas, S. Collection of antimicrobial peptides database and its derivatives: Applications and beyond. Protein Sci. 2020, 29, 36–42. [Google Scholar] [CrossRef]
- Wu, H.; Huang, J.; Wang, W.; Ge, K.; Li, G.; Zhong, J.; Huang, Q. LAMP2: A major update of the database linking antimicrobial peptides. Database 2020, 2020, baaa061. [Google Scholar] [CrossRef]
- Bazzaz, B.S.F.; Seyedi, S.; Goki, N.H.; Khameneh, B. Human Antimicrobial Peptides: Spectrum, Mode of Action and Resistance Mechanisms. Int. J. Pept. Res. Ther. 2020, 27, 801–816. [Google Scholar] [CrossRef]
- Joseph, J.; Abirami, B.; Manigundan, K.; Gopikrishnan, V.; Radhakrishnan, M. Chapter 9—Antimicrobial peptides from Actinobacteria: Current status and future prospects. In Microbial and Natural Macromolecules; Das, S., Dash, H.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 205–231. [Google Scholar] [CrossRef]
- Li, J.; Hu, S.; Jian, W.; Xie, C.; Yang, X. Plant antimicrobial peptides: Structures, functions, and applications. Bot. Stud. 2021, 62, 5. [Google Scholar] [CrossRef] [PubMed]
- Manniello, M.D.; Moretta, A.; Salvia, R.; Scieuzo, C.; Lucchetti, D.; Vogel, H.; Sgambato, A.; Falabella, P. Insect antimicrobial peptides: Potential weapons to counteract the antibiotic resistance. Cell. Mol. Life Sci. 2021, 78, 4259–4282. [Google Scholar] [CrossRef] [PubMed]
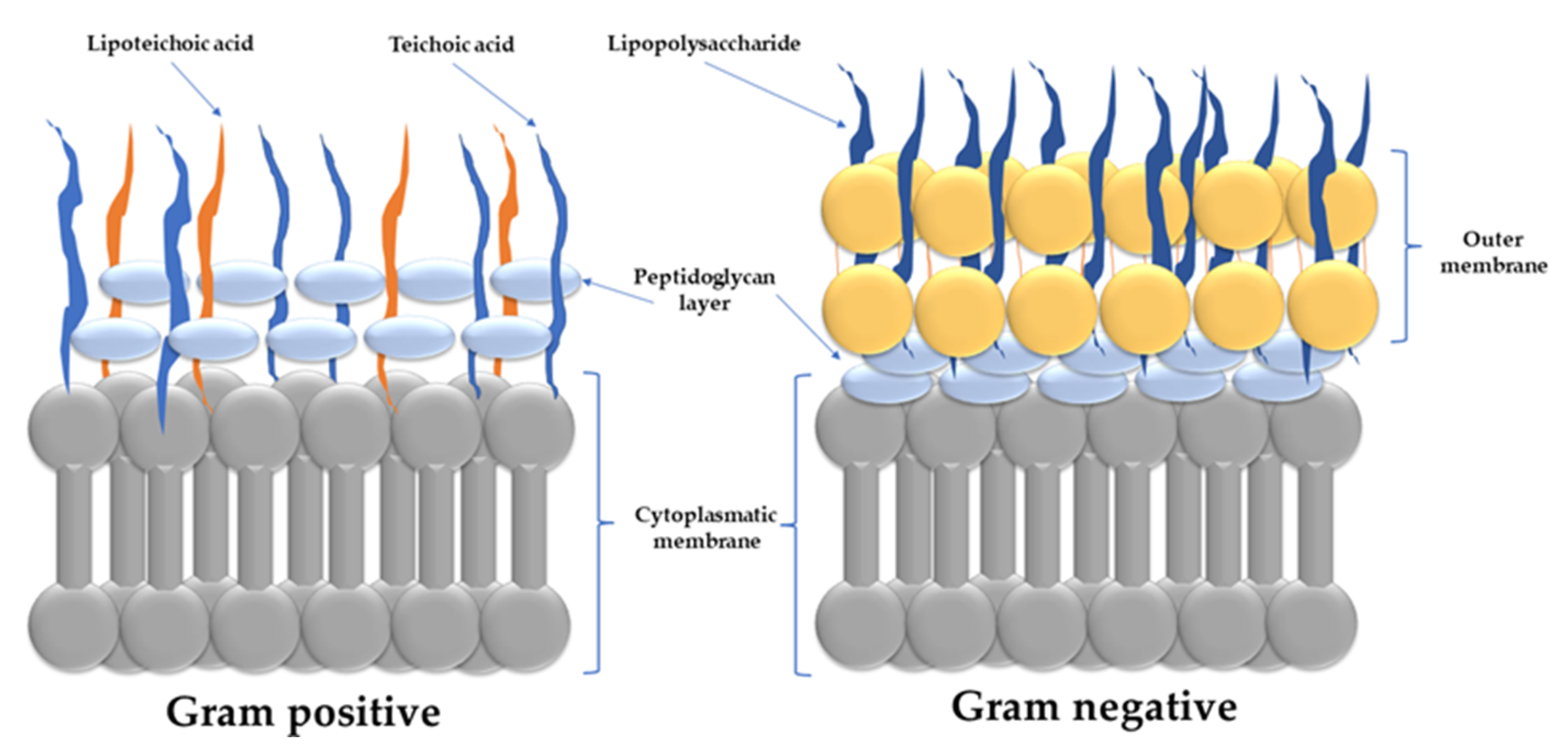
- Vollmer, W.; Blanot, D.; De Pedro, M.A. Peptidoglycan Structure and Architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Chen, Y.; Song, Z.; Tan, Z.; Cheng, J. Recent advances in design of antimicrobial peptides and polypeptides toward clinical translation. Adv. Drug Deliv. Rev. 2021, 170, 261–280. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Briers, Y. Lysins breaking down the walls of Gram-negative bacteria, no longer a no-go. Curr. Opin. Biotechnol. 2020, 68, 15–22. [Google Scholar] [CrossRef]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Håkansson, J.; Hansen, P.R.; Svenson, J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar] [CrossRef]
- Randall, C.P.; Mariner, K.R.; Chopra, I.; O’Neill, A.J. The Target of Daptomycin Is Absent from Escherichia coli and Other Gram-Negative Pathogens. Antimicrob. Agents Chemother. 2013, 57, 637–639. [Google Scholar] [CrossRef] [Green Version]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Genet. 2015, 13, 605–619. [Google Scholar] [CrossRef] [Green Version]
- Malanovic, N.; Lohner, K. Gram-positive bacterial cell envelopes: The impact on the activity of antimicrobial peptides. Biochim. Et Biophys. Acta (BBA) Biomembr. 2015, 1858, 936–946. [Google Scholar] [CrossRef] [Green Version]
- Shafee, T.M.A.; Lay, F.T.; Phan, T.K.; Anderson, M.A.; Hulett, M.D. Convergent evolution of defensin sequence, structure and function. Cell. Mol. Life Sci. 2017, 74, 663–682. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Forsman, J.; Woodward, C.E. Molecular Simulations of Melittin-Induced Membrane Pores. J. Phys. Chem. B 2017, 121, 10209–10214. [Google Scholar] [CrossRef] [PubMed]
- Xhindoli, D.; Pacor, S.; Benincasa, M.; Scocchi, M.; Gennaro, R.; Tossi, A. The human cathelicidin LL-37—A pore-forming antibacterial peptide and host-cell modulator. Biochim. Biophys. Acta (BBA) Biomembr. 2016, 1858, 546–566. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.H.; Ishibashi, N.; Inoue, T.; Himeno, K.; Masuda, Y.; Sawa, N.; Zendo, T.; Wilaipun, P.; Leelawatcharamas, V.; Nakayama, J.; et al. Functional Analysis of Genes Involved in the Biosynthesis of Enterocin NKR-5-3B, a Novel Circular Bacteriocin. J. Bacteriol. 2016, 198, 291–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belmadani, A.; Semlali, A.; Rouabhia, M. Dermaseptin-S1 decreases Candida albicans growth, biofilm formation and the expression of hyphal wall protein 1 and aspartic protease genes. J. Appl. Microbiol. 2018, 125, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Sani, M.-A.; Separovic, F. How Membrane-Active Peptides Get into Lipid Membranes. Acc. Chem. Res. 2016, 49, 1130–1138. [Google Scholar] [CrossRef]
- Rocha, E.D.; Ferreira, M.R.S.; Neto, E.D.S.; Barbosa, E.J.; Löbenberg, R.; Lourenço, F.R.; Bou-Chacra, N. Enhanced In Vitro Antimicrobial Activity of Polymyxin B–Coated Nanostructured Lipid Carrier Containing Dexamethasone Acetate. J. Pharm. Innov. 2020, 16, 125–135. [Google Scholar] [CrossRef]
- Olaitan, A.O.; Morand, S.; Rolain, J.-M. Emergence of colistin-resistant bacteria in humans without colistin usage: A new worry and cause for vigilance. Int. J. Antimicrob. Agents 2016, 47, 1–3. [Google Scholar] [CrossRef]
- Li, J.; Koh, J.-J.; Liu, S.; Lakshminarayanan, R.; Verma, C.S.; Beuerman, R.W. Membrane Active Antimicrobial Peptides: Translating Mechanistic Insights to Design. Front. Neurosci. 2017, 11, 73. [Google Scholar] [CrossRef] [Green Version]
- Patocka, J.; Nepovimova, E.; Klimova, B.; Wu, Q.; Kuca, K. Antimicrobial Peptides: Amphibian Host Defense Peptides. Curr. Med. Chem. 2019, 26, 5924–5946. [Google Scholar] [CrossRef]
- Upert, G.; Luther, A.; Obrecht, D.; Ermert, P. Emerging peptide antibiotics with therapeutic potential. Med. Drug Discov. 2020, 9, 100078. [Google Scholar] [CrossRef] [PubMed]
- Büyükkiraz, M.E.; Kesmen, Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. J. Appl. Microbiol. 2022, 132, 1573–1596. [Google Scholar] [CrossRef] [PubMed]
- Pushpanathan, M.; Gunasekaran, P.; Rajendhran, J. Antimicrobial Peptides: Versatile Biological Properties. Int. J. Pept. 2013, 2013, e675391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batoni, G.; Maisetta, G.; Esin, S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta (BBA) Biomembr. 2016, 1858, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Järvå, M.; Lay, F.T.; Phan, T.K.; Humble, C.; Poon, I.K.; Bleackley, M.R.; Anderson, M.A.; Hulett, M.D.; Kvansakul, M. X-ray Structure of a Carpet-Like Antimicrobial Defensin–Phospholipid Membrane Disruption Complex. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Ongey, E.L.; Pflugmacher, S.; Neubauer, P. Bioinspired Designs, Molecular Premise and Tools for Evaluating the Ecological Importance of Antimicrobial Peptides. Pharmaceuticals 2018, 11, 68. [Google Scholar] [CrossRef] [Green Version]
- Costa, B.; Martínez-de-Tejada, G.; Gomes, P.A.C.; Martins, M.C.L.; Costa, F. Antimicrobial Peptides in the Battle against Orthopedic Implant-Related Infections: A Review. Pharmaceutics 2021, 13, 1918. [Google Scholar] [CrossRef]
- DeFlorio, W.; Liu, S.; White, A.R.; Taylor, T.M.; Cisneros-Zevallos, L.; Min, Y.; Scholar, E.M.A. Recent developments in antimicrobial and antifouling coatings to reduce or prevent contamination and cross-contamination of food contact surfaces by bacteria. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3093–3134. [Google Scholar] [CrossRef]
- Sun, H.; Hong, Y.; Xi, Y.; Zou, Y.; Gao, J.; Du, J. Synthesis, Self-Assembly, and Biomedical Applications of Antimicrobial Peptide–Polymer Conjugates. Biomacromolecules 2018, 19, 1701–1720. [Google Scholar] [CrossRef]
- Andrade, C.A. Chemical immobilization of antimicrobial peptides on biomaterial surfaces. Front Biosci. 2016, 8, 129–142. [Google Scholar] [CrossRef] [Green Version]
- Costa, F.; Carvalho, I.F.; Montelaro, R.C.; Gomes, P.; Martins, M.C.L. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011, 7, 1431–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleophas, R.T.C.; Riool, M.; van Ufford, H.C.Q.; Zaat, S.A.J.; Kruijtzer, J.A.W.; Liskamp, R.M.J. Convenient Preparation of Bactericidal Hydrogels by Covalent Attachment of Stabilized Antimicrobial Peptides Using Thiolene Click Chemistry. ACS Macro Lett. 2014, 3, 477–480. [Google Scholar] [CrossRef]
- Gao, G.; Lange, D.; Hilpert, K.; Kindrachuk, J.; Zou, Y.; Cheng, J.T.J.; Kazemzadeh-Narbat, M.; Yu, K.; Wang, R.; Straus, S.K.; et al. The biocompatibility and biofilm resistance of implant coatings based on hydrophilic polymer brushes conjugated with antimicrobial peptides. Biomaterials 2011, 32, 3899–3909. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Yu, K.; Kindrachuk, J.; Brooks, D.E.; Hancock, R.E.W.; Kizhakkedathu, J.N. Antibacterial Surfaces Based on Polymer Brushes: Investigation on the Influence of Brush Properties on Antimicrobial Peptide Immobilization and Antimicrobial Activity. Biomacromolecules 2011, 12, 3715–3727. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Ganesan, K.; Simionescu, D.T.; Vyavahare, N.R. Novel porous aortic elastin and collagen scaffolds for tissue engineering. Biomaterials 2004, 25, 5227–5237. [Google Scholar] [CrossRef]
- De Zoysa, G.H.; Sarojini, V. Feasibility Study Exploring the Potential of Novel Battacin Lipopeptides as Antimicrobial Coatings. ACS Appl. Mater. Interfaces 2017, 9, 1373–1383. [Google Scholar] [CrossRef]
- Nie, B.; Ao, H.; Long, T.; Zhou, J.; Tang, T.; Yue, B. Immobilizing bacitracin on titanium for prophylaxis of infections and for improving osteoinductivity: An in vivo study. Colloids Surfaces B Biointerfaces 2017, 150, 183–191. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.C.; Liu, S.; Wu, R.F.; Aparicio, C.; Wu, J.Y. In vivo osseointegration of dental implants with an antimicrobial peptide coating. J. Mater. Sci. Mater. Med. 2017, 28, 76. [Google Scholar] [CrossRef]
- Masurier, N.; Tissot, J.-B.; Boukhriss, D.; Jebors, S.; Pinese, C.; Verdié, P.; Amblard, M.; Mehdi, A.; Martinez, J.; Humblot, V.; et al. Site-specific grafting on titanium surfaces with hybrid temporin antibacterial peptides. J. Mater. Chem. B 2018, 6, 1782–1790. [Google Scholar] [CrossRef]
- Majhi, S.; Mishra, A. Exploring potential of glass surface immobilized short antimicrobial peptide (AMP) as antibacterial coatings. Mater. Today Proc. 2021, 49, 1367–1377. [Google Scholar] [CrossRef]
- Hoyos-Nogués, M.; Velasco, F.; Ginebra, M.-P.; Manero, J.M.; Gil, F.J.; Mas-Moruno, C. Regenerating Bone via Multifunctional Coatings: The Blending of Cell Integration and Bacterial Inhibition Properties on the Surface of Biomaterials. ACS Appl. Mater. Interfaces 2017, 9, 21618–21630. [Google Scholar] [CrossRef] [PubMed]
- Nilebäck, L.; Hedin, J.; Widhe, M.; Floderus, L.S.; Krona, A.; Bysell, H.; Hedhammar, M. Self-Assembly of Recombinant Silk as a Strategy for Chemical-Free Formation of Bioactive Coatings: A Real-Time Study. Biomacromolecules 2017, 18, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Yuan, C.; Xiao, J.; He, X.; Bai, X. A biofilm resistance surface yielded by grafting of antimicrobial peptides on stainless steel surface. Surf. Interface Anal. 2018, 50, 516–521. [Google Scholar] [CrossRef]
- Parreira, P.; Monteiro, C.; Graça, V.; Gomes, J.; Maia, S.; Gomes, P.; Gonçalves, I.; Martins, M.C.L. Surface Grafted MSI-78A Antimicrobial Peptide has High Potential for Gastric Infection Management. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Xiao, M.; Jasensky, J.; Gerszberg, J.; Chen, J.; Tian, J.; Lin, T.; Lu, T.; Lahann, J.; Chen, Z. Chemically Immobilized Antimicrobial Peptide on Polymer and Self-Assembled Monolayer Substrates. Langmuir 2018, 34, 12889–12896. [Google Scholar] [CrossRef]
- Onaizi, S.A.; Leong, S.S.J. Tethering antimicrobial peptides: Current status and potential challenges. Biotechnol. Adv. 2011, 29, 67–74. [Google Scholar] [CrossRef]
- Dutta, D.; Kumar, N.; Willcox, M.D.P. Antimicrobial activity of four cationic peptides immobilised to poly-hydroxyethylmethacrylate. Biofouling 2016, 32, 429–438. [Google Scholar] [CrossRef]
- Boelens, J.J.; Dankert, J.; Murk, J.L.; Weening, J.V.; van der Poll, T.; Dingemans, K.P.; Koole, L.; Laman, J.D.; Zaat, S.A.J. Biomaterial-Associated Persistence of Staphylococcus Epidermidis in Pericatheter Macrophages. J. Infect. Dis. 2000, 181, 1337–1349. [Google Scholar] [CrossRef] [Green Version]
- Busscher, H.J.; Van Der Mei, H.C.; Subbiahdoss, G.; Jutte, P.C.; van den Dungen, J.J.A.M.; Zaat, S.A.J.; Schultz, M.J.; Grainger, D.W. Biomaterial-Associated Infection: Locating the Finish Line in the Race for the Surface. Sci. Transl. Med. 2012, 4, 153rv10. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Lo, J.C.Y.; Mei, Y.; Haney, E.F.; Siren, E.; Kalathottukaren, M.T.; Hancock, R.E.; Lange, D.; Kizhakkedathu, J.N. Toward Infection-Resistant Surfaces: Achieving High Antimicrobial Peptide Potency by Modulating the Functionality of Polymer Brush and Peptide. ACS Appl. Mater. Interfaces 2015, 7, 28591–28605. [Google Scholar] [CrossRef]
- Muszanska, A.K.; Rochford, E.T.J.; Gruszka, A.; Bastian, A.A.; Busscher, H.J.; Norde, W.; Van Der Mei, H.C.; Herrmann, A. Antiadhesive Polymer Brush Coating Functionalized with Antimicrobial and RGD Peptides to Reduce Biofilm Formation and Enhance Tissue Integration. Biomacromolecules 2014, 15, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.; Costa, F.; Pirttilä, A.M.; Tejesvi, M.V.; Martins, M.C.L. Prevention of urinary catheter-associated infections by coating antimicrobial peptides from crowberry endophytes. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-Y.; Zhao, Y.-Q.; Zhang, Y.; Wang, A.; Ding, X.; Li, Y.; Duan, S.; Ding, X.; Xu, F.-J. Antimicrobial Peptide-Conjugated Hierarchical Antifouling Polymer Brushes for Functionalized Catheter Surfaces. Biomacromolecules 2019, 20, 4171–4179. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Yu, K.; Manero, J.M.; Gil, F.J.; Kizhakkedathu, J.N.; Rodriguez, D. Antibacterial Properties of hLf1–11 Peptide onto Titanium Surfaces: A Comparison Study Between Silanization and Surface Initiated Polymerization. Biomacromolecules 2015, 16, 483–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta, S.; Ibañez-Fonseca, A.; Aparicio, C.; Rodríguez-Cabello, J.C. Antibiofilm coatings based on protein-engineered polymers and antimicrobial peptides for preventing implant-associated infections. Biomater. Sci. 2020, 8, 2866–2877. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.; Xue, Y.; Ding, T.; Zhu, S.; Mao, M.; Zhang, L.; Han, Y. Polymer brush grafted antimicrobial peptide on hydroxyapatite nanorods for highly effective antibacterial performance. Chem. Eng. J. 2021, 423, 130133. [Google Scholar] [CrossRef]
- Bhalani, D.V.; Bera, A.; Chandel, A.K.S.; Kumar, S.B.; Jewrajka, S.K. Multifunctionalization of Poly(vinylidene fluoride)/Reactive Copolymer Blend Membranes for Broad Spectrum Applications. ACS Appl. Mater. Interfaces 2017, 9, 3102–3112. [Google Scholar] [CrossRef]
- Pal, S.; Mondal, R.; Chandel, A.K.S.; Chatterjee, U. Composite Anion Exchange Membranes with Antibacterial Properties for Desalination and Fluoride Ion Removal. CS ES&T Water 2021, 1, 2206–2216. [Google Scholar] [CrossRef]
- Bera, A.; Trivedi, J.S.; Kumar, S.B.; Chandel, A.K.S.; Haldar, S.; Jewrajka, S.K. Anti-organic fouling and anti-biofouling poly(piperazineamide) thin film nanocomposite membranes for low pressure removal of heavy metal ions. Hazard. Mater. 2018, 343, 86–97. [Google Scholar] [CrossRef]
- Ramburrun, P.; Pringle, N.A.; Dube, A.; Adam, R.Z.; D’Souza, S.; Aucamp, M. Recent Advances in the Development of Antimicrobial and Antifouling Biocompatible Materials for Dental Applications. Materials 2021, 14, 3167. [Google Scholar] [CrossRef]
- Shahid, A.; Aslam, B.; Muzammil, S.; Aslam, N.; Shahid, M.; Almatroudi, A.; Allemailem, K.S.; Saqalein, M.; Nisar, M.A.; Rasool, M.H.; et al. The prospects of antimicrobial coated medical implants. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211040304. [Google Scholar] [CrossRef] [PubMed]
- Mateescu, M.; Baixe, S.; Garnier, T.; Jierry, L.; Ball, V.; Haikel, Y.; Metz-Boutigue, M.H.; Nardin, M.; Schaaf, P.; Etienne, O.; et al. Antibacterial Peptide-Based Gel for Prevention of Medical Implanted-Device Infection. PLoS ONE 2015, 10, e0145143. [Google Scholar]
- Li, T.; Wang, N.; Chen, S.; Lu, R.; Li, H.; Zhang, Z. Antibacterial activity and cytocompatibility of an implant coating consisting of TiO2 nanotubes combined with a GL13K antimicrobial peptide. Int. J. Nanomed. 2017, 12, 2995–3007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riool, M.; De Breij, A.; De Boer, L.; Kwakman, P.H.S.; Cordfunke, R.A.; Cohen, O.; Malanovic, N.; Emanuel, N.; Lohner, K.; Drijfhout, J.W.; et al. Controlled Release of LL-37-Derived Synthetic Antimicrobial and Anti-Biofilm Peptides SAAP-145 and SAAP-276 Prevents Experimental Biomaterial-Associated Staphylococcus aureus Infection. Adv. Funct. Mater. 2017, 27, 1606623. [Google Scholar] [CrossRef]
- De Breij, A.; Riool, M.; Kwakman, P.; de Boer, L.; Cordfunke, R.; Drijfhout, J.; Cohen, O.; Emanuel, N.; Zaat, S.; Nibbering, P.; et al. Prevention of Staphylococcus aureus biomaterial-associated infections using a polymer-lipid coating containing the antimicrobial peptide OP-145. J. Control. Release 2016, 222, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zarghami, V.; Ghorbani, M.; Bagheri, K.P.; Shokrgozar, M.A. Melittin antimicrobial peptide thin layer on bone implant chitosan-antibiotic coatings and their bactericidal properties. Mater. Chem. Phys. 2021, 263, 124432. [Google Scholar] [CrossRef]
- Chakraborty, I.; Bodurtha, K.J.; Heeder, N.J.; Godfrin, M.P.; Tripathi, A.; Hurt, R.H.; Shukla, A.; Bose, A. Massive Electrical Conductivity Enhancement of Multilayer Graphene/Polystyrene Composites Using a Nonconductive Filler. ACS Appl. Mater. Interfaces 2014, 6, 16472–16475. [Google Scholar] [CrossRef]
- Lim, K.; Saravanan, R.; Chong, K.K.L.; Goh, S.H.M.; Chua, R.R.Y.; Tambyah, P.A.; Chang, M.W.; Kline, K.; Leong, S.S.J. Anhydrous polymer-based coating with sustainable controlled release functionality for facile, efficacious impregnation, and delivery of antimicrobial peptides. Biotechnol. Bioeng. 2018, 115, 2000–2012. [Google Scholar] [CrossRef]
- Lin, E.M.J.; Lay, C.L.; Subramanian, G.S.; Tan, W.S.; Leong, S.S.J.; Moh, L.C.H.; Lim, K. Control Release Coating for Urinary Catheters with Enhanced Released Profile for Sustained Antimicrobial Protection. ACS Appl. Mater. Interfaces 2021, 13, 59263–59274. [Google Scholar] [CrossRef]
- Miao, Q.; Sun, J.-L.; Huang, F.; Wang, J.; Wang, P.; Zheng, Y.-F.; Wang, F.; Ma, C.-F. Antibacterial Peptide HHC-36 Sustained-Release Coating Promotes Antibacterial Property of Percutaneous Implant. Front. Bioeng. Biotechnol. 2021, 9, 735889. [Google Scholar] [CrossRef]
- Yu, L.; Dou, S.; Ma, J.; Gong, Q.; Zhang, M.; Zhang, X.; Li, M.; Zhang, W. An Antimicrobial Peptide-Loaded Chitosan/Polyethylene Oxide Nanofibrous Membrane Fabricated by Electrospinning Technology. Front. Mater. 2021, 8, 70. [Google Scholar] [CrossRef]
- Divakarla, S.K.; Das, T.; Chatterjee, C.; Ionescu, M.; Pastuovic, Z.; Jang, J.-H.; Al-Khoury, H.; Loppnow, H.; Yamaguchi, S.; Groth, T.; et al. Antimicrobial and Anti-inflammatory Gallium–Defensin Surface Coatings for Implantable Devices. ACS Appl. Mater. Interfaces 2022, 14, 9685–9696. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Y.; Zuo, E.; Chai, S.; Ren, X.; Fei, T.; Ma, G.; Wang, X.; Liu, H. A Novel Antibacterial Titanium Modification with a Sustained Release of Pac-525. Nanomaterials 2021, 11, 3306. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Yue, K.; Kazemzadeh-Narbat, M.; Liu, Y.; Khalilpour, A.; Li, B.; Zhang, Y.S.; Annabi, N.; Khademhosseini, A. Mussel-Inspired Multifunctional Hydrogel Coating for Prevention of Infections and Enhanced Osteogenesis. ACS Appl. Mater. Interfaces 2017, 9, 11428–11439. [Google Scholar] [CrossRef] [Green Version]
- Department of Medical Microbiology, Center for Infection and Immunity Amsterdam (CINIMA), Academic Medical Center, University of Amsterdam. A chlorhexidine-releasing epoxy-based coating on titanium implants prevents Staphylococcus aureus experimental biomaterial-associated infection. ECM 2017, 33, 143–157. [Google Scholar] [CrossRef]
- Ye, J.; Liu, E.; Yu, Z.; Pei, X.; Chen, S.; Zhang, P.; Shin, M.-C.; Gong, J.; He, H.; Yang, V.C. CPP-Assisted Intracellular Drug Delivery, What Is Next? Int. J. Mol. Sci. 2016, 17, 1892. [Google Scholar] [CrossRef] [Green Version]

| Method | Advantages | Disadvantages | Polymer | Ref. |
|---|---|---|---|---|
| Dip-coating |
|
| PCL | [99,100,101] |
| Chitosan | ||||
| Dextran | ||||
| Spin coating |
|
| PLGA/PCL composite | [102,103,104,105] |
| PCL | ||||
| Collagen | ||||
| Sol-gel |
|
| 3-glycidoxypropyltrimethoxysilane | [106,107] |
| Poloxamer 407-gellan gum-sodium alginate-xyloglucan | ||||
| LIFT |
|
| Silk fibroin–Poly (3-hydroxybutyric-acid-co-3-hydroxyvaleric-acid) | [108,109,110] |
| Collagen | ||||
| Hyaluronic acid sodium salt-methylcellulose—sodium alginate | ||||
| MAPLE |
|
| PLGA-Fe3O4 | [111,112,113] |
| PEG-Fe3O4 | ||||
| Chitosan and Lysozyme | ||||
| Electrostatic deposition |
|
| PLGA | [114,115] |
| Chitosan | ||||
| Layer-by-layer (LbL) adsorption technique |
|
| Gelatin | [116] |
| Chitosan | ||||
| Sputtering |
|
| [117] |
| Additives | Role | Additive | Ref. |
|---|---|---|---|
| Plasticizer |
| Triethyl and tributyl citrates Diethyl phthalate Dibutyl sebacate | [118,119,120,121] |
| Anti-adherents |
| Talc Glyceryl monostearate | [122,123,124] |
| Pigments |
| Aluminum lakes Iron oxides TiO2 | [125] |
| Surfactants |
| Polysorbate 80 Sorbitan monooleate Sodium dodecyl sulfate | [126] |
| Matrix | Eluted Drug | Commercial Name/Company | Application |
|---|---|---|---|
| Sulbactam/Cefoperazone | Sulperazone® (Pfiser, New York, NY, USA) | Orthopedics | |
| Sulbactam/Ampicillin | Duocid® (Pfiser, New York, NY, USA) | ||
| PMMA | Tobramycin | Simplex® (Stryker, Kalamazoo, MI, USA) | |
| Gentamicin sulphate | Palacos® (Zimmer biomet, Warsaw, IN, USA) | ||
| CMW® (DePuy, Raynham, MA, USA) | |||
| Septopal® (Zimmer biomet, Warsaw, IN, USA) | |||
| Triamcinolone acetonide | Relieva Stratus® (Acclarent, CA, USA) | Breathing system | |
| PLGA | Mometasone furoate | Propel® (Intersect Ent, CA, USA) | |
| Silicone | Paclitaxel | Exhale® (Broncus, San Jose, CA, USA) | |
| PEVA blend with PBMA | Sirolimus | Cypher® (Johnson & Johnson/Cordis, New Brunswick, NJ, USA) | Stenting procedures |
| Poly(styrene-b-isobutylene-b-styrene) (SIBS) | Paclitaxel | Taxus® (Boston Scientific, Marlborough, MA, USA) | |
| PLLA | Everolimus | Absorb®/(Abbott Vascular, Chicago, IL, USA) | |
| PLA | Champion® (Guidant, Indianapolis, IN, USA) | ||
| PLGA | Synergy® (Boston Scientific, Marlborough, MA, USA) | ||
| Microporous stainless steel | Rapamycin | Yukon® (Traslumina, Hechingen, Germany) | |
| Olefinic block copolymer | Triclosan | Triumph® (Boston Scientific, Marlborough, MA, USA) | |
| Micro-structured abluminal surface | Biolimus A9 | BioFreedom® (Biosensors International, Singapore) | |
| Polifeprosan 20 | Carmustine | Gliadel® (Eisai, Tokyo, Japan) | Brain disorders |
| Liposome | Cytarabine | DepoCyt® (Sigma-Tau, Gaithersburg, MD, USA) | |
| Polyurethane foam | Ag | Contreet® (Coloplast, Humlebaek, Denmark) | Wound management |
| Nylon fibers | Silvercel® (Acelity, San Antonio, TX, USA) | ||
| Polyester | Acticoat® (Smith & Nephew, London, UK) | ||
| Collagen | Gentamicin | Collatamp® (Eusa Pharma, Hemel Hempstead, UK) | |
| Septocoll® (Zimmer biomet, Warsaw, IN, USA) |
| AMPs | Coating Type | Surface | Antimicrobial Activity | Ref. |
|---|---|---|---|---|
| Cateslytin | Hydrogel | Ti | Surface activity against P. gingivalis in vitro | [304] |
| GL13K | TiO2 nanotubes | Ti | Prevented the growth of Fusobacterium nucleatum and P. gingivalis in vitro | [305] |
| SAAP-145, SAAP-276 | PLEX | Ti | Reduction of S. aureus implant and tissue colonization in a subcutaneous mouse implant infection model | [306] |
| OP-145 | PLEX | Ti | Reduction of S. aureus in a rabbit humerus intramedullary nail infection model | [307] |
| Melittin | chitosan\vancomycin and oxacillin antibiotic | Etched Ti | Activity against MRSA and VRSA bacteria in vitro | [308] |
| HHC36 | TiO2 nanotubes | Ti | Surface activity against S. aureus, S. epidermidis, E. coli, and P. aeruginosa in vitro | [309] |
| PCL—dual layer | Silicone urinary catheters | Reduction of E. coli, S. aureus and P. aeruginosa in vitro and in vivo assessment using an experimental mouse wounding model | [310] | |
| PEG-PCL | Retarded E. coli in vitro | [311] | ||
| PDLLA-PLGA | TiO2 nanotubes | Significant antibacterial activity against the proliferation of S. aureus in vitro and biocompatible and antibacterial in vivo on Male C57BL/6J mice | [312] | |
| NP10 | CS—PEO nanofiber membranes | Activity against E. coli and S. aureus in vitro | [313] | |
| hBD-1 | Gallium + AMP | PLA | Activity against A. baumanii in vitro | [314] |
| Pac-525 | PLGA | Ti | Surface activity against S. aureus and E. coli in vitro | [315] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negut, I.; Bita, B.; Groza, A. Polymeric Coatings and Antimicrobial Peptides as Efficient Systems for Treating Implantable Medical Devices Associated-Infections. Polymers 2022, 14, 1611. https://doi.org/10.3390/polym14081611
Negut I, Bita B, Groza A. Polymeric Coatings and Antimicrobial Peptides as Efficient Systems for Treating Implantable Medical Devices Associated-Infections. Polymers. 2022; 14(8):1611. https://doi.org/10.3390/polym14081611
Chicago/Turabian StyleNegut, Irina, Bogdan Bita, and Andreea Groza. 2022. "Polymeric Coatings and Antimicrobial Peptides as Efficient Systems for Treating Implantable Medical Devices Associated-Infections" Polymers 14, no. 8: 1611. https://doi.org/10.3390/polym14081611
APA StyleNegut, I., Bita, B., & Groza, A. (2022). Polymeric Coatings and Antimicrobial Peptides as Efficient Systems for Treating Implantable Medical Devices Associated-Infections. Polymers, 14(8), 1611. https://doi.org/10.3390/polym14081611







