Bioabsorption of Subcutaneous Nanofibrous Scaffolds Influences the Engraftment and Function of Neonatal Porcine Islets
Abstract
1. Introduction
2. Materials and Methods
2.1. PCL and PLGA Fibrous Scaffold Fabrication
2.2. NPI Isolation, Transplantation and Metabolic Follow-Up
2.2.1. NPI Isolation
2.2.2. Scaffold Implantation, Transplantation and Metabolic Follow-Up
2.3. Characterization of Grafts
2.4. Statistical Analysis
3. Results
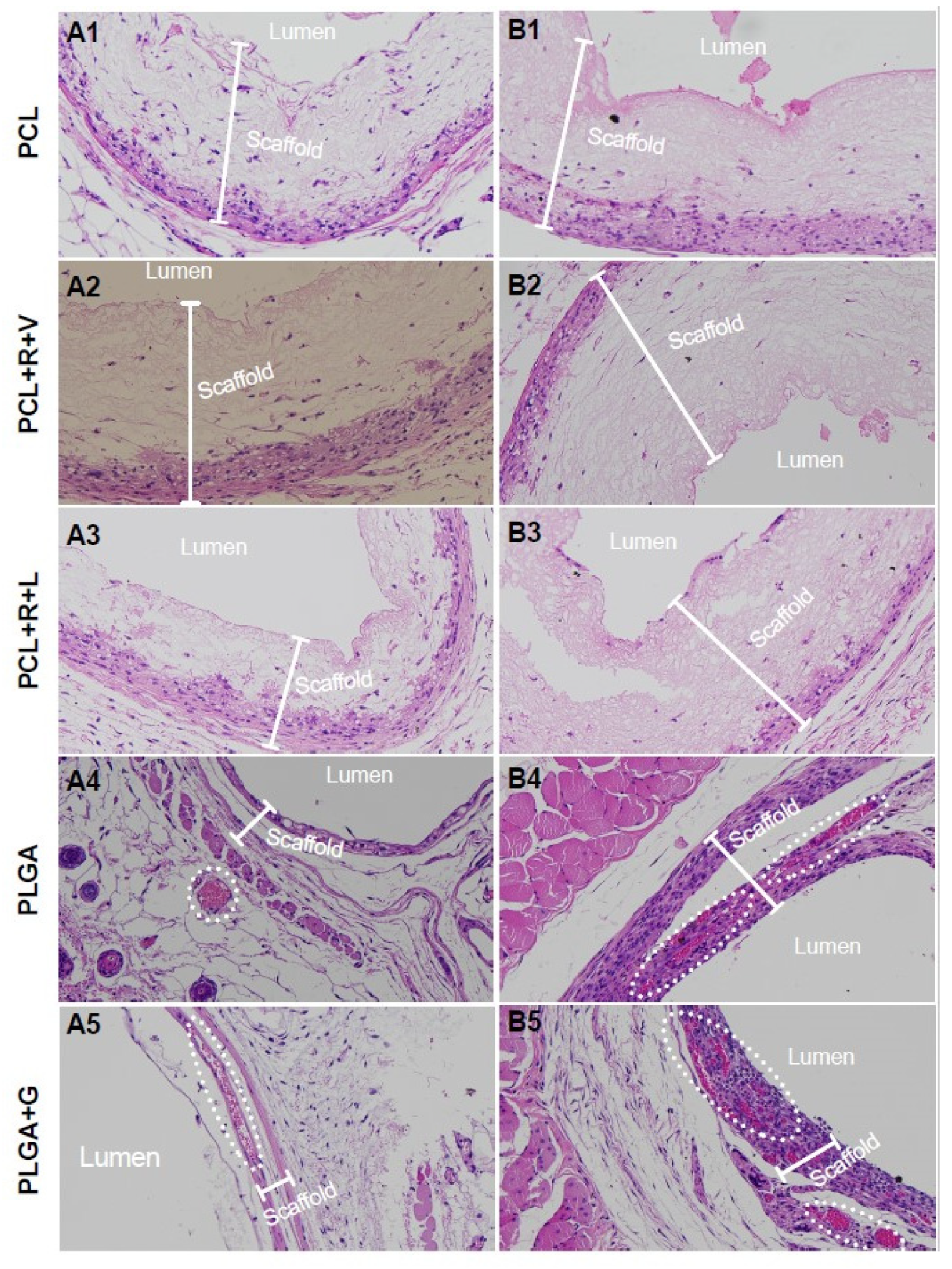
3.1. Scaffold Bioabsorption and Biocompatibility
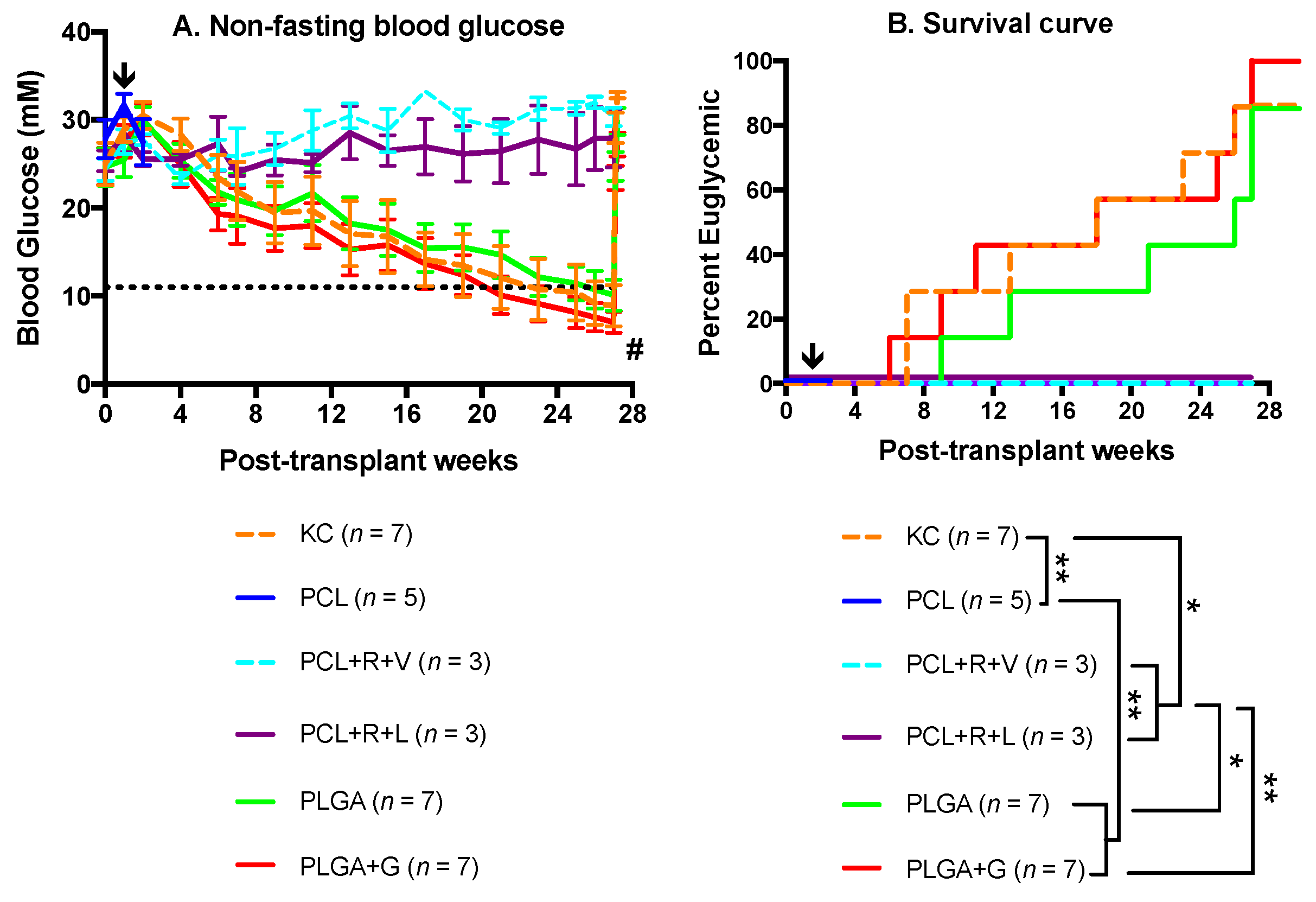
3.2. Metabolic Follow-Up of Transplant Recipients
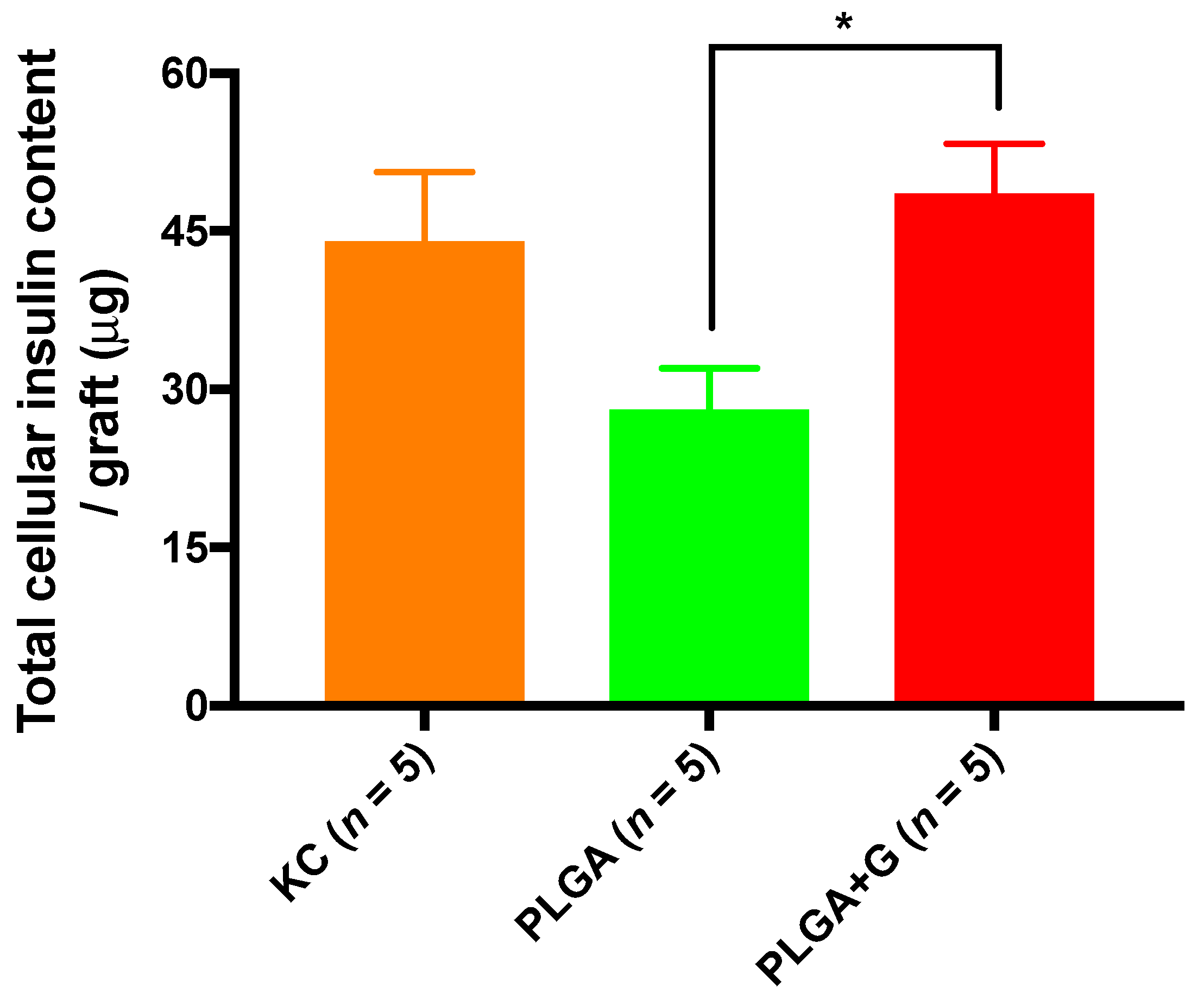
3.3. Graft Morphological Characterization and Cellular Insulin Content of Grafts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hering, B.J.; Clarke, W.R.; Bridges, N.D.; Eggerman, T.L.; Alejandro, R.; Bellin, M.D.; Chaloner, K.; Czarniecki, C.W.; Goldstein, J.S.; Hunsicker, L.G.; et al. Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes Care 2016, 39, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- McCall, M.; Shapiro, A.M. Update on islet transplantation. Cold Spring Harb. Perspect. Med. 2012, 2, a007823. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.A.; Paty, B.W.; Senior, P.A.; Bigam, D.; Alfadhli, E.; Kneteman, N.M.; Lakey, J.R.; Shapiro, A.M. Five-year follow-up after clinical islet transplantation. Diabetes 2005, 54, 2060–2069. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef]
- Shapiro, A.M.; Pokrywczynska, M.; Ricordi, C. Clinical pancreatic islet transplantation. Nat. Rev. Endocrinol. 2017, 13, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Krickhahn, M.; Buhler, C.; Meyer, T.; Thiede, A.; Ulrichs, K. The morphology of islets within the porcine donor pancreas determines the isolation result: Successful isolation of pancreatic islets can now be achieved from young market pigs. Cell Transplant. 2002, 11, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Kin, T.; Korbutt, G.S. Delayed functional maturation of neonatal porcine islets in recipients under strict glycemic control. Xenotransplantation 2007, 14, 333–338. [Google Scholar] [CrossRef]
- Korbutt, G.S.; Elliott, J.F.; Ao, Z.; Smith, D.K.; Warnock, G.L.; Rajotte, R.V. Large scale isolation, growth, and function of porcine neonatal islet cells. J. Clin. Investig. 1996, 97, 2119–2129. [Google Scholar] [CrossRef]
- Vizzardelli, C.; Molano, R.D.; Pileggi, A.; Berney, T.; Cattan, P.; Fenjves, S.; Peel, A.; Fraker, C.; Ricordi, C.; Inverardi, L. Neonatal porcine pancreatic cell clusters as a potential source for transplantation in humans: Characterization of proliferation, apoptosis, xenoantigen expression and gene delivery with recombinant AAV. Xenotransplantation 2002, 9, 14–24. [Google Scholar] [CrossRef]
- Kemter, E.; Wolf, E. Recent progress in porcine islet isolation, culture and engraftment strategies for xenotransplantation. Curr. Opin. Organ Transplant. 2018, 23, 633–641. [Google Scholar] [CrossRef]
- Barra, J.M.; Kozlovskaya, V.; Kepple, J.D.; Seeberger, K.L.; Kuppan, P.; Hunter, C.S.; Korbutt, G.S.; Kharlampieva, E.; Tse, H.M. Xenotransplantation of tannic acid-encapsulated neonatal porcine islets decreases proinflammatory innate immune responses. Xenotransplantation 2021, 28, e12706. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.S.; Yamada, K.; Okumi, M.; Weiner, J.; O’Malley, P.E.; Tseng, Y.L.; Dor, F.J.; Cooper, D.K.; Saidman, S.L.; Griesemer, A.; et al. Allosensitization does not increase the risk of xenoreactivity to alpha1,3-galactosyltransferase gene-knockout miniature swine in patients on transplantation waiting lists. Transplantation 2006, 82, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Hassouna, T.; Seeberger, K.L.; Salama, B.; Korbutt, G.S. Functional Maturation and In Vitro Differentiation of Neonatal Porcine Islet Grafts. Transplantation 2018, 102, e413–e423. [Google Scholar] [CrossRef] [PubMed]
- Kin, T.; Korbutt, G.S.; Kobayashi, T.; Dufour, J.M.; Rajotte, R.V. Reversal of diabetes in pancreatectomized pigs after transplantation of neonatal porcine islets. Diabetes 2005, 54, 1032–1039. [Google Scholar] [CrossRef][Green Version]
- Cardona, K.; Korbutt, G.S.; Milas, Z.; Lyon, J.; Cano, J.; Jiang, W.; Bello-Laborn, H.; Hacquoil, B.; Strobert, E.; Gangappa, S.; et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat. Med. 2006, 12, 304–306. [Google Scholar] [CrossRef]
- Thompson, P.; Cardona, K.; Russell, M.; Badell, I.R.; Shaffer, V.; Korbutt, G.; Rayat, G.R.; Cano, J.; Song, M.; Jiang, W.; et al. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. Am. J. Transplant. 2011, 11, 947–957. [Google Scholar] [CrossRef]
- Boneva, R.S.; Folks, T.M.; Chapman, L.E. Infectious disease issues in xenotransplantation. Clin. Microbiol. Rev. 2001, 14, 584–640. [Google Scholar] [CrossRef]
- Matsumoto, S.; Abalovich, A.; Wechsler, C.; Wynyard, S.; Elliott, R.B. Clinical Benefit of Islet Xenotransplantation for the Treatment of Type 1 Diabetes. EBioMedicine 2016, 12, 255–262. [Google Scholar] [CrossRef]
- Morozov, V.A.; Wynyard, S.; Matsumoto, S.; Abalovich, A.; Denner, J.; Elliott, R. No PERV transmission during a clinical trial of pig islet cell transplantation. Virus Res. 2017, 227, 34–40. [Google Scholar] [CrossRef]
- Matsumoto, S.; Wynyard, S.; Giovannangelo, M.; Hemdev, S.L.; Abalovich, A.; Carulla, M.E.; Wechsler, C.J. Long-term follow-up for the microbiological safety of clinical microencapsulated neonatal porcine islet transplantation. Xenotransplantation 2020, 27, e12631. [Google Scholar] [CrossRef]
- Porrett, P.M.; Orandi, B.J.; Kumar, V.; Houp, J.; Anderson, D.; Killian, A.C.; Hauptfeld-Dolejsek, V.; Martin, D.E.; Macedon, S.; Budd, N.; et al. First clinical-grade porcine kidney xenotransplant using a human decedent model. Am. J. Transplant. 2022. [Google Scholar] [CrossRef] [PubMed]
- Burki, T. Pig-heart transplantation surgeons look to the next steps. Lancet 2022, 399, 347. [Google Scholar] [CrossRef]
- Bhargava, R.; Senior, P.A.; Ackerman, T.E.; Ryan, E.A.; Paty, B.W.; Lakey, J.R.; Shapiro, A.M. Prevalence of hepatic steatosis after islet transplantation and its relation to graft function. Diabetes 2004, 53, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Markmann, J.F.; Rosen, M.; Siegelman, E.S.; Soulen, M.C.; Deng, S.P.; Barker, C.F.; Naji, A. Magnetic resonance-defined periportal steatosis following intraportal islet transplantation—A functional footprint of islet graft survival? Diabetes 2003, 52, 1591–1594. [Google Scholar] [CrossRef] [PubMed]
- Korsgren, O.; Lundgren, T.; Felldin, M.; Foss, A.; Isaksson, B.; Permert, J.; Persson, N.H.; Rafael, E.; Ryden, M.; Salmela, K.; et al. Optimising islet engraftment is critical for successful clinical islet transplantation. Diabetologia 2008, 51, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.; Goto, M.; Sekiguchi, S.; Fujimori, K.; Ushiyama, A.; Satomi, S. Assessment for revascularization of transplanted pancreatic islets at subcutaneous site in mice with a highly sensitive imaging system. Transplant. Proc. 2011, 43, 3239–3240. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Ohashi, K.; Utoh, R.; Shimizu, H.; Ise, K.; Suzuki, H.; Yamato, M.; Okano, T.; Gotoh, M. Reversal of diabetes by the creation of neo-islet tissues into a subcutaneous site using islet cell sheets. Transplantation 2011, 92, 1231–1236. [Google Scholar] [CrossRef]
- Sakata, N.; Aoki, T.; Yoshimatsu, G.; Tsuchiya, H.; Hata, T.; Katayose, Y.; Egawa, S.; Unno, M. Strategy for clinical setting in intramuscular and subcutaneous islet transplantation. Diabetes Metab Res. Rev. 2014, 30, 1–10. [Google Scholar] [CrossRef]
- Burke, J.A.; Zhang, X.M.; Bobbala, S.; Frey, M.A.; Fuentes, C.B.; Haddad, H.F.; Allen, S.D.; Richardson, R.A.K.; Ameer, G.A.; Scott, E.A. Subcutaneous nanotherapy repurposes the immunosuppressive mechanism of rapamycin to enhance allogeneic islet graft viability. Nat. Nanotechnol. 2022, 1–12. [Google Scholar] [CrossRef]
- Hou, H.Y.; Fu, S.H.; Liu, C.H.; Chen, J.P.; Hsu, B.R.S. The graft survival protection of subcutaneous allogeneic islets with hydrogel grafting and encapsulated by CTLA4Ig and IL1ra. Polym. J. 2014, 46, 136–144. [Google Scholar] [CrossRef]
- Pathak, S.; Regmi, S.; Gupta, B.; Poudel, B.K.; Pham, T.T.; Yong, C.S.; Kim, J.O.; Kim, J.R.; Park, M.H.; Bae, Y.K.; et al. Single synchronous delivery of FK506-loaded polymeric microspheres with pancreatic islets for the successful treatment of streptozocin-induced diabetes in mice. Drug Deliv. 2017, 24, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Simeonovic, C.J.; Dhall, D.P.; Wilson, J.D.; Lafferty, K.J. A comparative study of transplant sites for endocrine tissue transplantation in the pig. Aust. J. Exp. Biol. Med. Sci. 1986, 64 Pt 1, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.Z.; Ma, J.X.; Bei, J.Z.; Wang, S.G. Surface Modification of Aliphatic Polyester to Enhance Biocompatibility. Front. Bioeng. Biotechnol. 2019, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.Y.; Huang, J.H.; Zhu, T.H.; Lu, J.X.; Liu, J.L.; Li, X.L.; Yan, X.Y.; Liu, F. Liraglutide-loaded PLGA/gelatin electrospun nanofibrous mats promote angiogenesis to accelerate diabetic wound healing via the modulation of miR-29b-3p. Biomater. Sci. 2020, 8, 4225–4238. [Google Scholar] [CrossRef] [PubMed]
- Carmagnola, I.; Chiono, V.; Ruocco, G.; Scalzone, A.; Gentile, P.; Taddei, P.; Ciardelli, G. PLGA Membranes Functionalized with Gelatin through Biomimetic Mussel-Inspired Strategy. Nanomaterials 2020, 10, 2184. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.; Kuppan, P.; Raj, S.; Salama, B.; Korbutt, G.S.; Montemagno, C.D. Developing Hybrid Polymer Scaffolds Using Peptide Modified Biopolymers for Cell Implantation. ACS Biomater. Sci. Eng. 2017, 3, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Salama, B.F.; Seeberger, K.L.; Korbutt, G.S. Fibrin supports subcutaneous neonatal porcine islet transplantation without the need for pre-vascularization. Xenotransplantation 2020, 27, e12575. [Google Scholar] [CrossRef]
- Kuppan, P.; Seeberger, K.; Kelly, S.; Rosko, M.; Adesida, A.; Pepper, A.R.; Korbutt, G.S. Co-transplantation of human adipose-derived mesenchymal stem cells with neonatal porcine islets within a prevascularized subcutaneous space augments the xenograft function. Xenotransplantation 2020, 27, e12581. [Google Scholar] [CrossRef]
- Pepper, A.R.; Gala-Lopez, B.; Pawlick, R.; Merani, S.; Kin, T.; Shapiro, A.M. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat. Biotechnol. 2015, 33, 518–523. [Google Scholar] [CrossRef]
- Yu, M.; Agarwal, D.; Korutla, L.; May, C.L.; Wang, W.; Griffith, N.N.; Hering, B.J.; Kaestner, K.H.; Velazquez, O.C.; Markmann, J.F.; et al. Islet transplantation in the subcutaneous space achieves long-term euglycaemia in preclinical models of type 1 diabetes. Nat. Metab. 2020, 2, 1013–1020. [Google Scholar] [CrossRef]
- Pathak, S.; Regmi, S.; Gupta, B.; Pham, T.T.; Yong, C.S.; Kim, J.O.; Yook, S.; Kim, J.R.; Park, M.H.; Bae, Y.K.; et al. Engineered islet cell clusters transplanted into subcutaneous space are superior to pancreatic islets in diabetes. FASEB J. 2017, 31, 5111–5121. [Google Scholar] [CrossRef] [PubMed]
- Perez-Basterrechea, M.; Esteban, M.M.; Vega, J.A.; Obaya, A.J. Tissue-engineering approaches in pancreatic islet transplantation. Biotechnol. Bioeng. 2018, 115, 3009–3029. [Google Scholar] [CrossRef] [PubMed]
- Salg, G.A.; Giese, N.A.; Schenk, M.; Huttner, F.J.; Felix, K.; Probst, P.; Diener, M.K.; Hackert, T.; Kenngott, H.G. The emerging field of pancreatic tissue engineering: A systematic review and evidence map of scaffold materials and scaffolding techniques for insulin-secreting cells. J. Tissue Eng. 2019, 10, 2041731419884708. [Google Scholar] [CrossRef] [PubMed]
- Smink, A.M.; de Haan, B.J.; Lakey, J.R.T.; de Vos, P. Polymer scaffolds for pancreatic islet transplantation—Progress and challenges. Am. J. Transplant. 2018, 18, 2113–2119. [Google Scholar] [CrossRef]
- Lu, J.M.; Wang, X.; Marin-Muller, C.; Wang, H.; Lin, P.H.; Yao, Q.; Chen, C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev. Mol. Diagn. 2009, 9, 325–341. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, C.Y.; Hollister, S.J. The interaction between bone marrow stromal cells and RGD-modified three-dimensional porous polycaprolactone scaffolds. Biomaterials 2009, 30, 4063–4069. [Google Scholar] [CrossRef]
- Norouzi, M.; Shabani, I.; Ahvaz, H.H.; Soleimani, M. PLGA/gelatin hybrid nanofibrous scaffolds encapsulating EGF for skin regeneration. J. Biomed. Mater. Res. A 2015, 103, 2225–2235. [Google Scholar] [CrossRef]
- Rickel, A.P.; Deng, X.; Engebretson, D.; Hong, Z. Electrospun nanofiber scaffold for vascular tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 129, 112373. [Google Scholar] [CrossRef]
- Minardi, S.; Guo, M.; Zhang, X.; Luo, X. An elastin-based vasculogenic scaffold promotes marginal islet mass engraftment and function at an extrahepatic site. J. Immunol. Regen. Med. 2019, 3, 1–12. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Sakata, N.; Yoshimatsu, G.; Fukase, M.; Aoki, T.; Ishida, M.; Katayose, Y.; Egawa, S.; Unno, M. Extracellular Matrix and Growth Factors Improve the Efficacy of Intramuscular Islet Transplantation. PLoS ONE 2015, 10, e0140910. [Google Scholar] [CrossRef] [PubMed]
- Yap, W.T.; Salvay, D.M.; Silliman, M.A.; Zhang, X.; Bannon, Z.G.; Kaufman, D.B.; Lowe, W.L., Jr.; Shea, L.D. Collagen IV-modified scaffolds improve islet survival and function and reduce time to euglycemia. Tissue Eng. Part A 2013, 19, 2361–2372. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Kim, J.H.; Choi, E.S.; Kim, E.; Choi, S.K.; Jeon, W.B. RGD-containing elastin-like polypeptide improves islet transplantation outcomes in diabetic mice. Acta Biomater. 2019, 94, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Salvay, D.M.; Rives, C.B.; Zhang, X.; Chen, F.; Kaufman, D.B.; Lowe, W.L., Jr.; Shea, L.D. Extracellular matrix protein-coated scaffolds promote the reversal of diabetes after extrahepatic islet transplantation. Transplantation 2008, 85, 1456–1464. [Google Scholar] [CrossRef]
- Stendahl, J.C.; Kaufman, D.B.; Stupp, S.I. Extracellular matrix in pancreatic islets: Relevance to scaffold design and transplantation. Cell Transplant. 2009, 18, 1–12. [Google Scholar] [CrossRef]
- Smink, A.M.; de Vos, P. Therapeutic Strategies for Modulating the Extracellular Matrix to Improve Pancreatic Islet Function and Survival After Transplantation. Curr. Diab. Rep. 2018, 18, 39. [Google Scholar] [CrossRef]
- Coindre, V.F.; Kinney, S.M.; Sefton, M.V. Methacrylic acid copolymer coating of polypropylene mesh chamber improves subcutaneous islet engraftment. Biomaterials 2020, 259, 120324. [Google Scholar] [CrossRef]
- Yang, S.Y.; Yang, K.C.; Sumi, S. Prevascularization-free Primary Subcutaneous Transplantation of Xenogeneic Islets Coencapsulated with Hepatocyte Growth Factor. Transplant. Direct 2020, 6, e620. [Google Scholar] [CrossRef]
- Pileggi, A.; Molano, R.D.; Ricordi, C.; Zahr, E.; Collins, J.; Valdes, R.; Inverardi, L. Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation 2006, 81, 1318–1324. [Google Scholar] [CrossRef]
- Bitar, K.N.; Zakhem, E. Design strategies of biodegradable scaffolds for tissue regeneration. Biomed. Eng. Comput. Biol. 2014, 6, 13–20. [Google Scholar] [CrossRef]
- Bolgen, N.; Menceloglu, Y.Z.; Acatay, K.; Vargel, I.; Piskin, E. In vitro and in vivo degradation of non-woven materials made of poly(epsilon-caprolactone) nanofibers prepared by electrospinning under different conditions. J. Biomater. Sci. Polym. Ed. 2005, 16, 1537–1555. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Mei, L.; Song, C.; Cui, X.; Wang, P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 2006, 27, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Kuppan, P.; Kelly, S.; Polishevska, K.; Hojanepesov, O.; Seeberger, K.; Korbutt, G.S.; Pepper, A.R. Co-localized immune protection using dexamethasone-eluting micelles in a murine islet allograft model. Am. J. Transplant. 2020, 20, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuppan, P.; Kelly, S.; Seeberger, K.; Castro, C.; Rosko, M.; Pepper, A.R.; Korbutt, G.S. Bioabsorption of Subcutaneous Nanofibrous Scaffolds Influences the Engraftment and Function of Neonatal Porcine Islets. Polymers 2022, 14, 1120. https://doi.org/10.3390/polym14061120
Kuppan P, Kelly S, Seeberger K, Castro C, Rosko M, Pepper AR, Korbutt GS. Bioabsorption of Subcutaneous Nanofibrous Scaffolds Influences the Engraftment and Function of Neonatal Porcine Islets. Polymers. 2022; 14(6):1120. https://doi.org/10.3390/polym14061120
Chicago/Turabian StyleKuppan, Purushothaman, Sandra Kelly, Karen Seeberger, Chelsea Castro, Mandy Rosko, Andrew R. Pepper, and Gregory S. Korbutt. 2022. "Bioabsorption of Subcutaneous Nanofibrous Scaffolds Influences the Engraftment and Function of Neonatal Porcine Islets" Polymers 14, no. 6: 1120. https://doi.org/10.3390/polym14061120
APA StyleKuppan, P., Kelly, S., Seeberger, K., Castro, C., Rosko, M., Pepper, A. R., & Korbutt, G. S. (2022). Bioabsorption of Subcutaneous Nanofibrous Scaffolds Influences the Engraftment and Function of Neonatal Porcine Islets. Polymers, 14(6), 1120. https://doi.org/10.3390/polym14061120





