Preparing Biomass Carbon Fiber Derived from Waste Rabbit Hair as a Carrier of TiO2 for Photocatalytic Degradation of Methylene Blue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
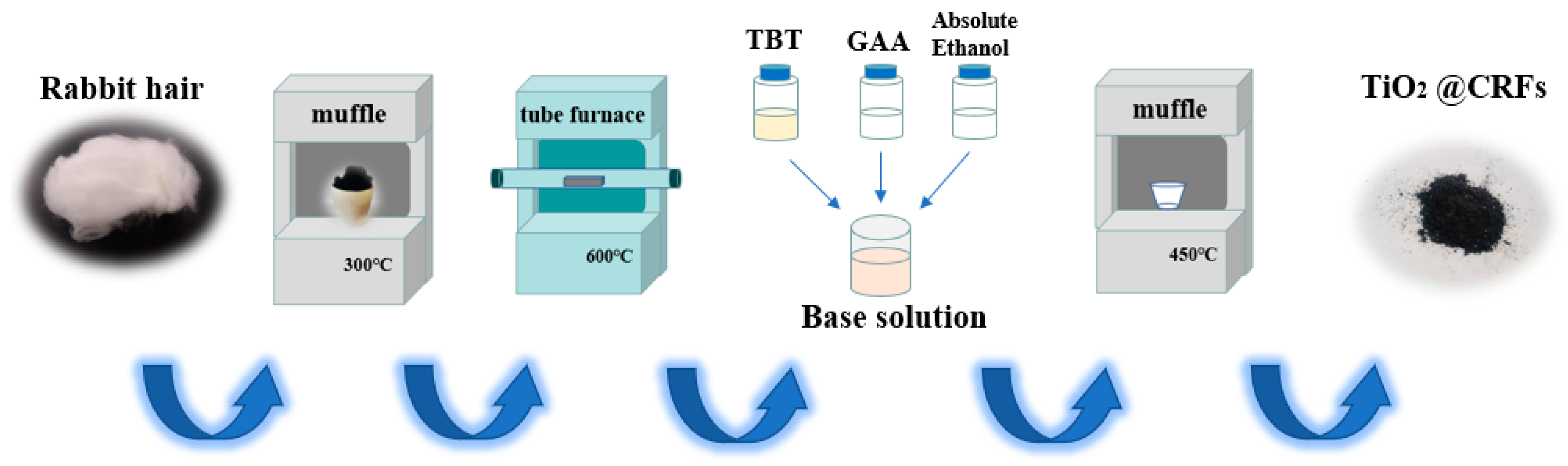
2.2. Preparation of CRFs Carbon Carrier
2.3. Fabrication of TiO2/Carbonized Rabbit Hair Fibers Composites (TiO2/CRFs)
2.4. Characterization
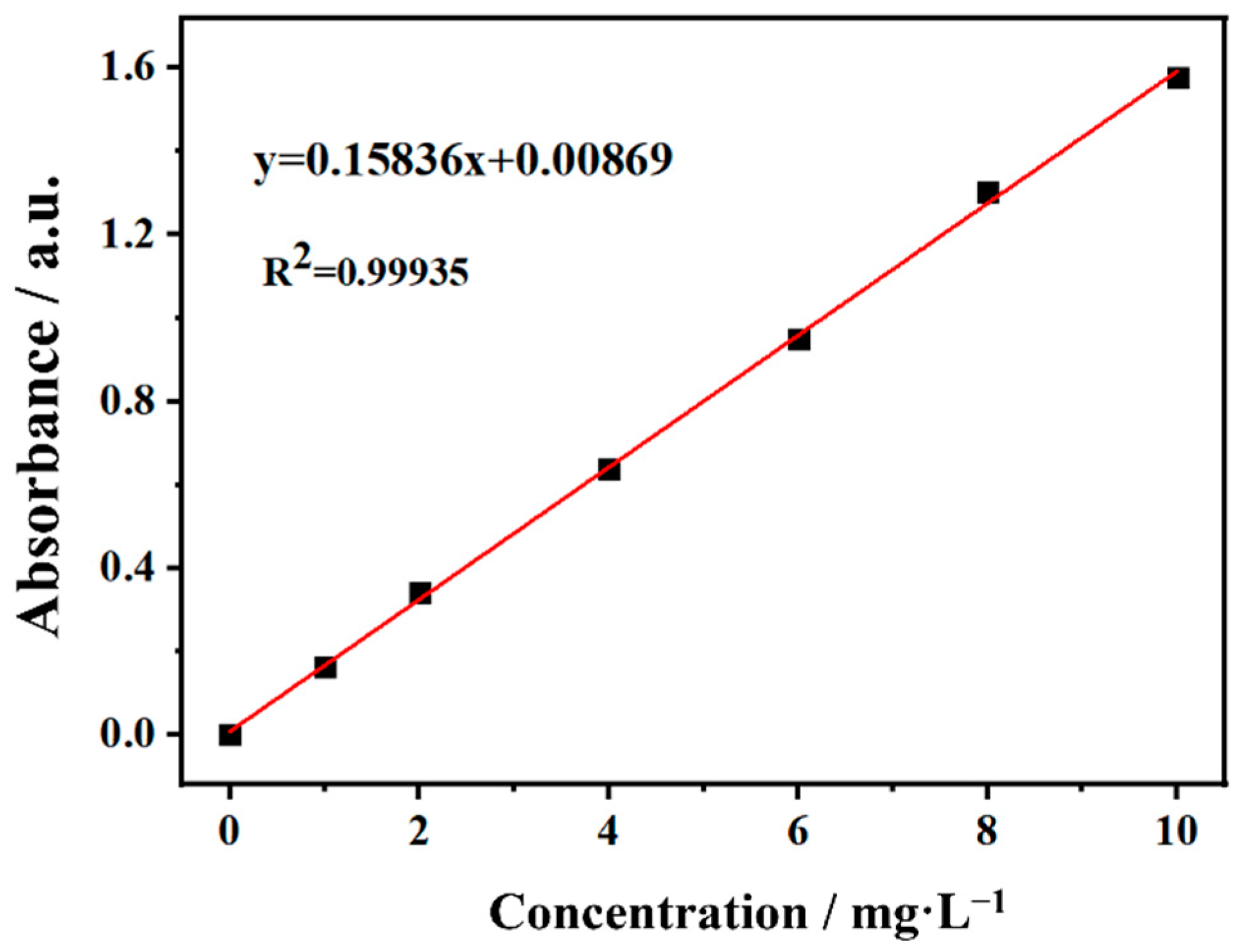
2.5. Evaluation of Photocatalytic Activity
3. Results
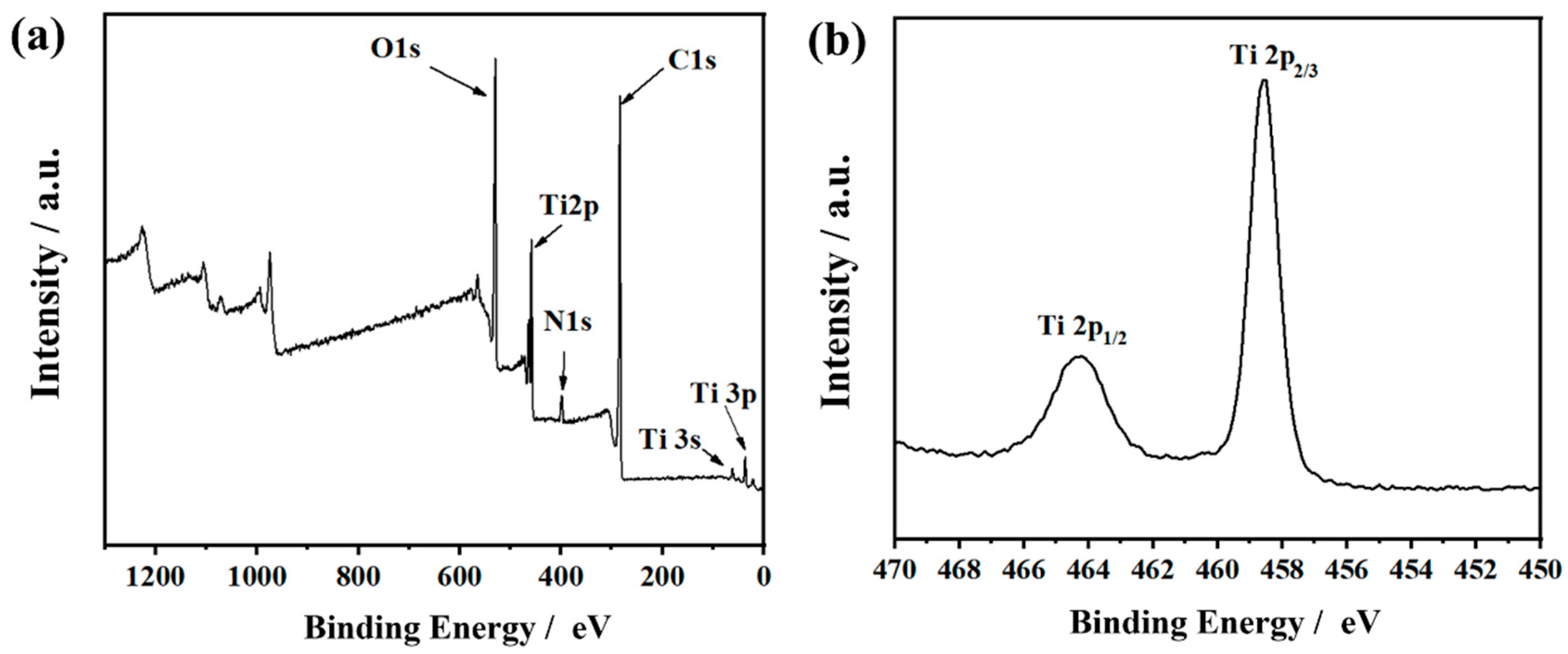
3.1. Morphologies and Chemical Properties
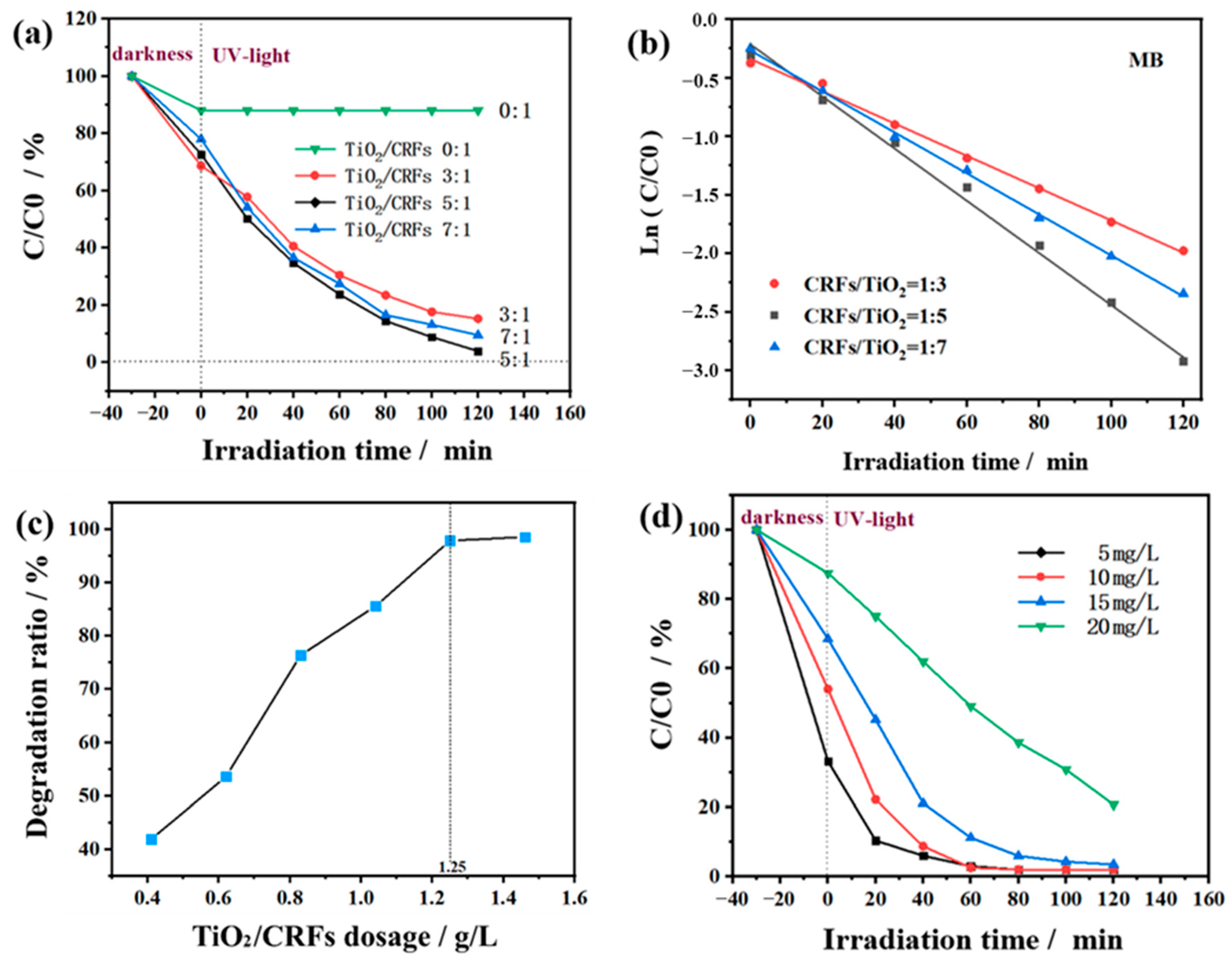
3.2. Photocatalytic Activity Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jain, P.; Kumar, A.; Verma, N.; Gupta, R.K. In-situ synthesis of TiO2 nanoparticles in ACF: Photocatalytic degradation under continuous flow. Sol. Energy 2019, 189, 35–44. [Google Scholar] [CrossRef]
- Bahrudin, N.N.; Nawi, M.A.; Nawawi, W.I. Photocatalytic enhancement of immobilized TiO2-polyaniline bilayer (TiO2-PBL) system for decolorization of methyl orange dye. Mater. Res. Bull. 2018, 106, 388–395. [Google Scholar] [CrossRef]
- Hu, H.X.; Pang, B.Y.; Zhu, Y.F.; Fu, Y.Q. Preparation of titanium dioxide immobilized on carbon fibers annealed in steam ambient and their photocatalytic properties. Text. Res. J. 2017, 87, 2233–2241. [Google Scholar] [CrossRef]
- Seabold, J.A.; Choi, K.-S. Effect of a Cobalt-Based Oxygen Evolution Catalyst on the Stability and the Selectivity of Photo-Oxidation Reactions of a WO3Photoanode. Chem. Mater. 2011, 23, 1105–1112. [Google Scholar] [CrossRef]
- Choudhary, S.; Hasina, D.; Saini, M.; Ranjan, M.; Mohapatra, S. Facile synthesis, morphological, structural, photocatalytic and optical properties of ZnFe2O4-ZnO hybrid nanostructures. J. Alloy. Compd. 2022, 895, 162723. [Google Scholar] [CrossRef]
- Murata, N.; Suzuki, T.; Kobayashi, M.; Togoh, F.; Asakura, K. Characterization of Pt-doped SnO2 catalyst for a high-performance micro gas sensor. Phys. Chem. Chem. Phys. 2013, 15, 17938–17946. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.P.; Guan, Z.C.; Shi, H.Y.; Wang, X.; Jin, P.A.; Song, G.L.; Du, R.G. Ag/SnO2/TiO2 nanotube composite film used in photocathodic protection for stainless steel. J. Photochem. Photobiol. A-Chem. 2021, 417, 113353. [Google Scholar] [CrossRef]
- Chen, P. A promising strategy to fabricate the Cu/BiVO4 photocatalysts and their enhanced visible-light-driven photocatalytic activities. J. Mater. Sci. Mater. Electron. 2015, 27, 2394–2403. [Google Scholar] [CrossRef]
- Lv, Y.-R.; Liu, C.-J.; He, R.-K.; Li, X.; Xu, Y.-H. BiVO4/TiO2 heterojunction with enhanced photocatalytic activities and photoelectochemistry performances under visible light illumination. Mater. Res. Bull. 2019, 117, 35–40. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.; Xu, H.; Sun, S.; Shang, M. Bi2O3 Hierarchical Nanostructures: Controllable Synthesis, Growth Mechanism, and their Application in Photocatalysis. Chem. A Eur. J. 2009, 15, 1776–1782. [Google Scholar] [CrossRef]
- Zou, H.; Song, M.; Yi, F.; Bian, L.; Liu, P.; Zhang, S. Simulated-sunlight-activated photocatalysis of Methyl Orange using carbon and lanthanum co-doped Bi2O3–TiO2 composite. J. Alloy. Compd. 2016, 680, 54–59. [Google Scholar] [CrossRef]
- Ding, P.R.; Ji, H.D.; Li, P.S.; Liu, Q.M.; Wu, Y.Y.; Guo, M.; Zhou, Z.A.; Gao, S.; Xu, W.L.; Liu, W.; et al. Visible-light degradation of antibiotics catalyzed by titania/zirconia/graphitic carbon nitride ternary nanocomposites: A combined experimental and theoretical study. Appl. Catal. B Environ. 2022, 300, 120633. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.; Luan, Q.; Duan, R.; Guan, R.; Cao, X.; Hu, X. Photocatalytic Properties Dependent on the Interfacial Defects of Intergrains within TiO2 Mesocrystals. Chemistry 2018, 24, 17105–17116. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Meng, Z.; Trisha, G.; Oh, W.C. Hydrothermal Synthesis of Porous Ag2S Sensitized TiO2 Catalysts and Their Photocatalytic Activities in the Visible Light Range. Chin. J. Catal. 2012, 33, 254–260. [Google Scholar] [CrossRef]
- Nagaveni, K.; Sivalingam, G.; Hegde, M.S.; Madras, G. Solar photocatalytic degradation of dyes: High activity of combustion synthesized nano TiO2. Appl. Catal. B: Environ. 2004, 48, 83–93. [Google Scholar] [CrossRef]
- Maeda, K. Photocatalytic properties of rutile TiO2 powder for overall water splitting. Catal. Sci. Technol. 2014, 4, 1949–1953. [Google Scholar] [CrossRef]
- Turkten, N.; Bekbolet, M. Photocatalytic performance of titanium dioxide and zinc oxide binary system on degradation of humic matter. J. Photochem. Photobiol. A-Chem. 2021, 417, 113281. [Google Scholar] [CrossRef]
- Inagaki, M.; Kojin, F.; Tryba, B.; Toyoda, M. Carbon-coated anatase: The role of the carbon layer for photocatalytic performance. Carbon 2005, 43, 1652–1659. [Google Scholar] [CrossRef]
- Tryba, B.; Morawski, A.W.; Tsumura, T.; Toyoda, M.; Inagaki, M. Hybridization of adsorptivity with photocatalytic activity—carbon-coated anatase. J. Photochem. Photobiol. A Chem. 2004, 167, 127–135. [Google Scholar] [CrossRef]
- Tsumura, T.; Kojitani, N.; Izumi, I.; Iwashita, N.; Toyoda, M.; Inagaki, M. Carbon coating of anatase-type TiO2 and photoactivity. J. Mater. Chem. 2002, 12, 1391–1396. [Google Scholar] [CrossRef]
- Wang, B.; Karthikeyan, R.; Lu, X.Y.; Xuan, J.; Leung, M.K. High photocatalytic activity of immobilized TiO2 nanorods on carbonized cotton fibers. J. Hazard. Mater. 2013, 263 Pt 2, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Xu, F.F.; Xiong, J.Y.; Tian, F.; Ding, J.; Stadler, F.J.; Chen, R. Enhanced adsorption and photocatalysis capability of generally synthesized TiO2-carbon materials hybrids. Adv. Powder Technol. 2016, 27, 1949–1962. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Fu, C.-C.; Juang, R.-S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Orooji, Y.; Tanhaei, B.; Ayati, A.; Tabrizi, S.H.; Alizadeh, M.; Bamoharram, F.F.; Karimi, F.; Salmanpour, S.; Rouhi, J.; Afshar, S.; et al. Heterogeneous UV-Switchable Au nanoparticles decorated tungstophosphoric acid/TiO2 for efficient photocatalytic degradation process. Chemosphere 2021, 281, 130795. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Jin, Y.; Zhu, J.; Li, H. Highly active TiO2−x−yNxFy visible photocatalyst prepared under supercritical conditions in NH4F/EtOH fluid. Appl. Catal. B Environ. 2009, 89, 543–550. [Google Scholar] [CrossRef]
- Powell, M.J.; Palgrave, R.G.; Dunnill, C.W.; Parkin, I.P. A fast and effective method for N-doping TiO2 by post treatment with liquid ammonia: Visible light photocatalysis. Thin Solid Film. 2014, 562, 223–228. [Google Scholar] [CrossRef]
- Zhang, X.L.; Yuan, N.; Li, Y.; Han, L.J.; Wang, Q.B. Fabrication of new MIL-53(Fe)@TiO2 visible-light responsive adsorptive photocatalysts for efficient elimination of tetracycline. Chem. Eng. J. 2022, 428, 131077. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, Z.; Shi, H.; Xiao, T.; Yan, Z. Preparation of highly visible-light active N-doped TiO2 photocatalyst. J. Mater. Chem. 2010, 20, 5301–5309. [Google Scholar] [CrossRef]
- Wang, X.Q.; Shi, Z.M.; Zhao, Q.L.; Yun, Y. Study on the Structure and Properties of Biofunctional Keratin from Rabbit Hair. Materials 2021, 14, 379. [Google Scholar] [CrossRef]
- Kiatkittipong, K.; Lim, J.W.; Cheng, C.K.; Kiatkittipong, W.; Assabumrungrat, S. Simultaneous Enhancement of Photocatalytic Bactericidal Activity and Strength Properties of Acrylonitrile-Butadiene-Styrene Plastic Via a Facile Preparation with Silane/TiO2. Polymers 2020, 12, 917. [Google Scholar] [CrossRef]
- Eom, Y.; Son, S.M.; Kim, Y.E.; Lee, J.E.; Hwang, S.H.; Chae, H.G. Structure evolution mechanism of highly ordered graphite during carbonization of cellulose nanocrystals. Carbon 2019, 150, 142–152. [Google Scholar] [CrossRef]
- Ju, J.; Lv, Y.; An, X.; Liu, W.; Li, Z.; Kang, W.; Cheng, B. The stereoscopic honeycomb-like porous carbon nanofibers as a carrier of TiO2 nanoparticles for high-performance Li-ion capacitor. J. Alloy. Compd. 2019, 791, 1248–1256. [Google Scholar] [CrossRef]
- Katal, R.; Masudy-Panah, S.; Tanhaei, M.; Farahani, M.; Hu, J.Y. A review on the synthesis of the various types of anatase TiO2 facets and their applications for photocatalysis. Chem. Eng. J. 2020, 384, 123384. [Google Scholar] [CrossRef]
- Zuliani, J.E.; Tong, S.; Kirk, D.W.; Jia, C.Q. Isolating the effect of pore size distribution on electrochemical double-layer capacitance using activated fluid coke. J. Power Sources 2015, 300, 190–198. [Google Scholar] [CrossRef]
- Zou, X.; Yang, Y.; Chen, H.; Shi, X.-L.; Song, S.; Chen, Z.-G. Hierarchical meso/macro-porous TiO2/graphitic carbon nitride nanofibers with enhanced hydrogen evolution. Mater. Des. 2021, 202, 109542. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Zhu, J.; Kou, Z.; Hu, P.; Liu, L.; Li, S.; Mu, S.; Huang, Y. Defect and pyridinic nitrogen engineering of carbon-based metal-free nanomaterial toward oxygen reduction. Nano Energy 2018, 52, 307–314. [Google Scholar] [CrossRef]
- Wu, Y.; Zang, Y.; Xu, L.; Wang, J.; Jia, H.; Miao, F. Synthesis of functional conjugated microporous polymer/TiO2 nanocomposites and the mechanism of the photocatalytic degradation of organic pollutants. J. Mater. Sci. 2021, 56, 7936–7950. [Google Scholar] [CrossRef]
- Valadez-Renteria, E.; Oliva, J.; Rodriguez-Gonzalez, V. Photocatalytic materials immobilized on recycled supports and their role in the degradation of water contaminants: A timely review. Sci. Total Environ. 2022, 807, 150820. [Google Scholar] [CrossRef]
- Wenqi, X.; Yang, J.; Yongsheng, B.; Jun, L.; Zhizhen, W.; Changsheng, B.; Haitao, C.; Ming, C. Synergy mechanism for TiO2/activated carbon composite material: Photocatalytic degradation ofmethylene blue solution. Can. J. Chem. Eng. 2020, 100, 276–290. [Google Scholar] [CrossRef]
- Camera-Roda, G.; Augugliaro, V.; Cardillo, A.G.; Loddo, V.; Palmisano, L.; Parrino, F.; Santarelli, F. A reaction engineering approach to kinetic analysis of photocatalytic reactions in slurry systems. Catalysis Today 2016, 259, 87–96. [Google Scholar] [CrossRef]
- Beltran, F.J.; Aguinaco, A.; Garcia-Araya, J.F. Kinetic modelling of TOC removal in the photocatalytic ozonation of diclofenac aqueous solutions. Appl. Catal. B Environ. 2010, 100, 289–298. [Google Scholar] [CrossRef]
- Aoudjit, L.; Salazar, H.; Zioui, D.; Sebti, A.; Martins, P.M.; Lanceros-Mendez, S. Reusable Ag@TiO2-Based Photocatalytic Nanocomposite Membranes for Solar Degradation of Contaminants of Emerging Concern. Polymers 2021, 13, 3718. [Google Scholar] [CrossRef] [PubMed]
- Alvaro, M.; Carbonell, E.; Fornes, V.; Garcia, H. Enhanced photocatalytic activity of zeolite-encapsulated TiO2 clusters by complexation with organic additives and N-doping. Chemphyschem 2006, 7, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Li, X.; Ni, T.; Lin, F.; Li, G. Carbonaceous–TiO2 materials: Unique morphologies for photocatalytic applications. J. Mater. Sci. 2019, 55, 2725–2740. [Google Scholar] [CrossRef]
- Ling, L.; Wang, C.; Ni, M.L.; Shang, C. Enhanced photocatalytic activity of TiO2/single-walled carbon nanotube (SWCNT) composites under UV-A irradiation. Sep. Purif. Technol. 2016, 169, 273–278. [Google Scholar] [CrossRef]
- Yun, J.; Kim, H.I.; Lee, Y.S. A hybrid gas-sensing material based on porous carbon fibers and a TiO2 photocatalyst. J. Mater. Sci. 2013, 48, 8320–8328. [Google Scholar] [CrossRef]
- Vital-Grappin, A.D.; Ariza-Tarazona, M.C.; Luna-Hernandez, V.M.; Villarreal-Chiu, J.F.; Hernandez-Lopez, J.M.; Siligardi, C.; Cedillo-Gonzalez, E.I. The Role of the Reactive Species Involved in the Photocatalytic Degradation of HDPE Microplastics Using C,N-TiO2 Powders. Polymers 2021, 13, 999. [Google Scholar] [CrossRef]
- Xu, Z.W.; Li, X.H.; Wang, W.; Shi, J.; Teng, K.Y.; Qian, X.M.; Shan, M.J.; Li, C.Y.; Yang, C.Y.; Liu, L.S. Microstructure and photocatalytic activity of electrospun carbon nanofibers decorated by TiO2 nanoparticles from hydrothermal reaction/blended spinning. Ceram. Int. 2016, 42, 15012–15022. [Google Scholar] [CrossRef]
- Lee, K.; Yoon, H.; Ahn, C.; Park, J.; Jeon, S. Strategies to improve the photocatalytic activity of TiO2: 3D nanostructuring and heterostructuring with graphitic carbon nanomaterials. Nanoscale 2019, 11, 7025–7040. [Google Scholar] [CrossRef]
- Wang, B.; Liu, B.; Ji, X.X.; Ma, M.G. Synthesis, Characterization, and Photocatalytic Properties of Bamboo Charcoal/TiO2 Composites Using Four Sizes Powder. Materials 2018, 11, 670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huy, T.H.; Phat, B.D.; Kang, F.; Wang, Y.F.; Liu, S.H.; Thi, C.M.; You, S.J.; Chang, G.M.; Viet, P.V. SnO2/TiO2 nanotube heterojunction: The first investigation of NO degradation by visible light-driven photocatalysis. Chemosphere 2019, 215, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Lisowski, P.; Colmenares, J.C.; Masek, O.; Lomot, D.; Chernyayeva, O.; Lisovytskiy, D. Novel biomass-derived hybrid TiO2/carbon material using tar-derived secondary char to improve TiO2 bonding to carbon matrix. J. Anal. Appl. Pyrolysis 2018, 131, 35–41. [Google Scholar] [CrossRef]
- Ramasamy, K.; Dhavamani, S.; Natesan, G.; Sengodan, K.; Sengottayan, S.N.; Tiwari, M.; Vikram, S.S.; Perumal, V. A potential role of green engineered TiO2 nanocatalyst towards enhanced photocatalytic and biomedical applications. Environ. Sci. Pollut. Res. 2021, 28, 41207–41223. [Google Scholar] [CrossRef]
- Lin, Z.H.; Huang, J.G. A hierarchical H3PW12O40/TiO2 nanocomposite with cellulose as scaffold for photocatalytic degradation of organic pollutants. Sep. Purif. Technol. 2021, 264, 118427. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Y.; Ding, Y.; Zheng, J. High-performance antibacterial film via synergistic effect between uniformly dispersed TiO2 nanoparticles and multifunctional quaternary ammonium cationic ligand. Prog. Org. Coat. 2021, 157, 106322. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, W.; Liu, X.; Tang, T.; Xiong, J. Fabrication of Titanium Dioxide/Carbon Fiber (TiO2CF) Composites for Removal of Methylene Blue (MB) from Aqueous Solution with Enhanced Photocatalytic Activity. J. Chem. 2021, 2021, 9986158. [Google Scholar] [CrossRef]


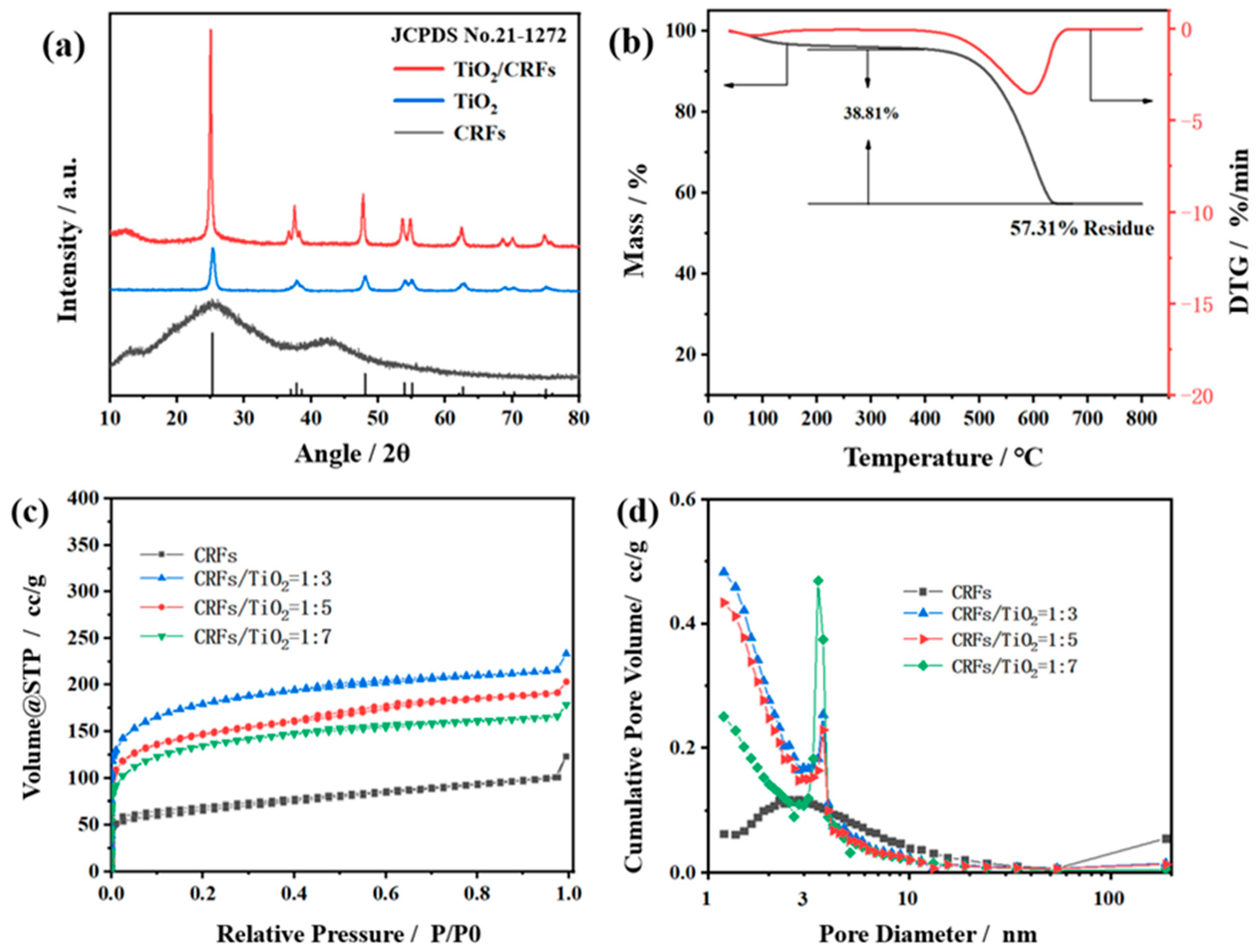



| Sample | Specific Surface Area (m2 g−1) | Pore Volume (cc g−1) |
|---|---|---|
| CRFs | 237.828 | 0.1027 |
| TiO2:CRFs = 3:1 | 488.942 | 0.2099 |
| TiO2:CRFs = 5:1 | 345.757 | 0.1627 |
| TiO2:CRFs = 7:1 | 324.588 | 0.1580 |
| Material | Synthesis Method | MB (mg L−1) | Catalyst Loading (g L−1) | Light Source | Highest Degradation | Cyclic Degradation | Ref. |
|---|---|---|---|---|---|---|---|
| TiO2 | — | 20 | 0.4 | UV light | 60 min, 85.45% | 4 cycles, 62.49% | [40] |
| Bamboo biochar/TiO2 | Calcination method | 12.8 | 0.2 | UV light | 60 min, 95% | — | [51] |
| Visible light | 60 min, 97% | 4 cycles, 75% | |||||
| SnO2/TiO2 | Hydrothermal techniques | 20 | 0.5 | Visible light | 50 min, 90% | 4 cycles, 87% | [52] |
| Lignin biochar/TiO2 | Microwave-hydrothermal and calcination method | 12.8 | 0.2 | UV light | 25 min, 93% | — | [53] |
| CMP/TiO2 | Sonogashira–Hagihara coupling reaction | 8.6 | 0.28 | Visible light | 60 min, 96.8% | 5 cycles, 93.1% | [38] |
| Biomolecules wrapped TiO2 | Microwave irradiation method | 10 | 0.2 | Visible light | 6 h, 90.6% | — | [54] |
| Hierarchical H3PW12O40/TiO2 | Impregnation and layer-by-layer methods | 10 | 0.25 | UV light | 5 min, 95% | 5 cycles, 65% 6 cycles after calcination, 96% | [55] |
| P(MMA-co-BA-coMTC)/TiO2 | Suspension polymerization | 6 | 20 | UV light | 270 min, 99.66% | 20 cycles, 98.7% | [56] |
| TiO2/AC | Sol–gel method | 20 | 0.4 | UV light | 60 min, 99.43% | 4 cycles, 88.06% | [40] |
| B-TiO2/CF | Hydrothermal method | 10 | 0.75 | UV light | 10 min, 76% | 5 cycles, slight decrease | [57] |
| Visible light | 10 min, 69% | ||||||
| TiO2/CRFs | Carbonization, impregnation and calcination method | 10 | 1.25 | UV light | 60 min, 97.9% | — | This study |
| Visible light | 80 min, 98.1% | 5 cycles, 90.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, C.; Chen, J.; Wang, S.; Ju, J.; Kang, W. Preparing Biomass Carbon Fiber Derived from Waste Rabbit Hair as a Carrier of TiO2 for Photocatalytic Degradation of Methylene Blue. Polymers 2022, 14, 1593. https://doi.org/10.3390/polym14081593
Chen Y, Wang C, Chen J, Wang S, Ju J, Kang W. Preparing Biomass Carbon Fiber Derived from Waste Rabbit Hair as a Carrier of TiO2 for Photocatalytic Degradation of Methylene Blue. Polymers. 2022; 14(8):1593. https://doi.org/10.3390/polym14081593
Chicago/Turabian StyleChen, Yanfei, Chunyan Wang, Junyan Chen, Shuaishuai Wang, Jingge Ju, and Weimin Kang. 2022. "Preparing Biomass Carbon Fiber Derived from Waste Rabbit Hair as a Carrier of TiO2 for Photocatalytic Degradation of Methylene Blue" Polymers 14, no. 8: 1593. https://doi.org/10.3390/polym14081593
APA StyleChen, Y., Wang, C., Chen, J., Wang, S., Ju, J., & Kang, W. (2022). Preparing Biomass Carbon Fiber Derived from Waste Rabbit Hair as a Carrier of TiO2 for Photocatalytic Degradation of Methylene Blue. Polymers, 14(8), 1593. https://doi.org/10.3390/polym14081593






