Impact of Starch Coating Embedded with Silver Nanoparticles on Strawberry Storage Time
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Starch-Silver Nanoparticles (St-AgNPs)
2.2.2. Characterization Methods
Visual Examination
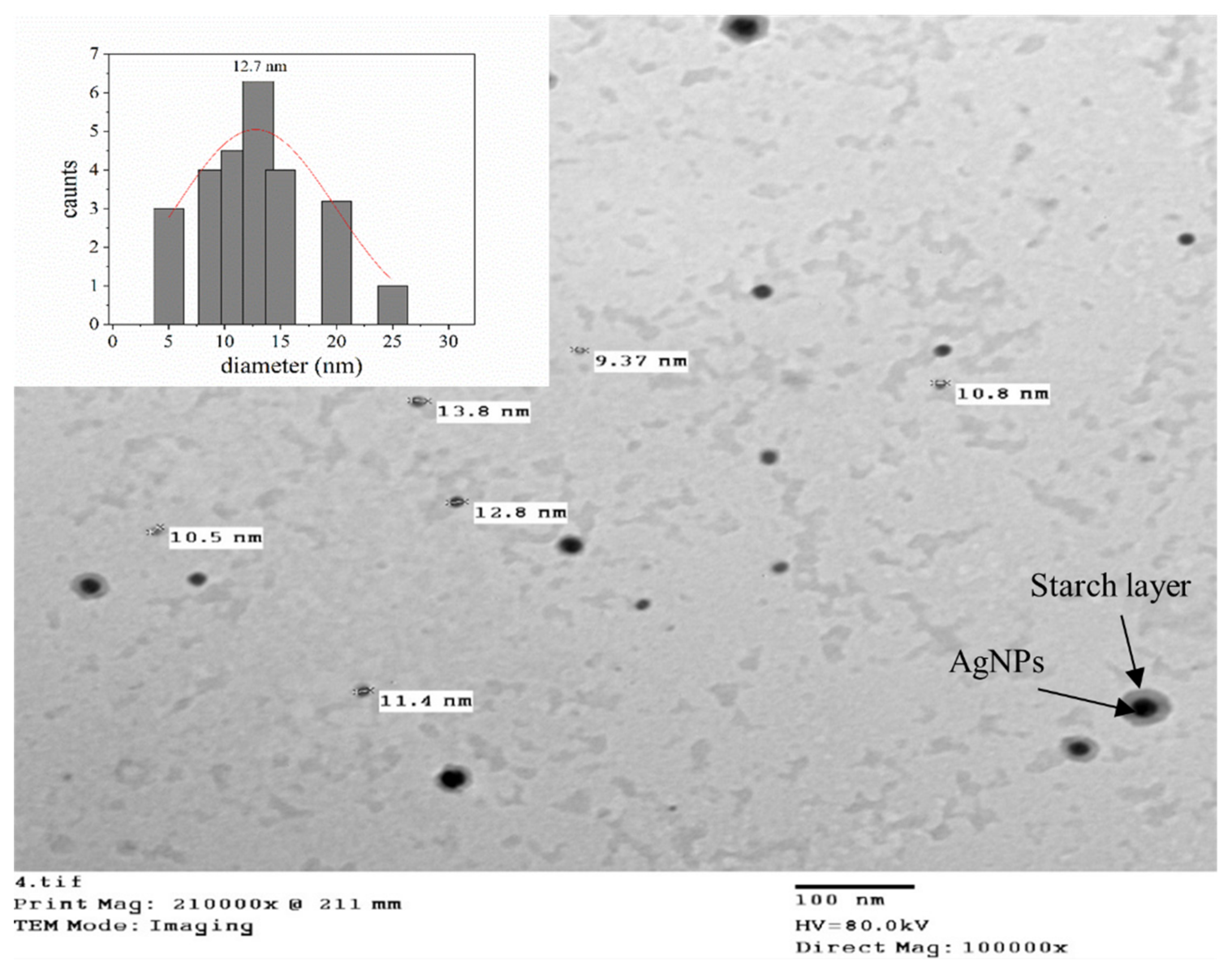
Transmission Electron Microscopic (TEM)
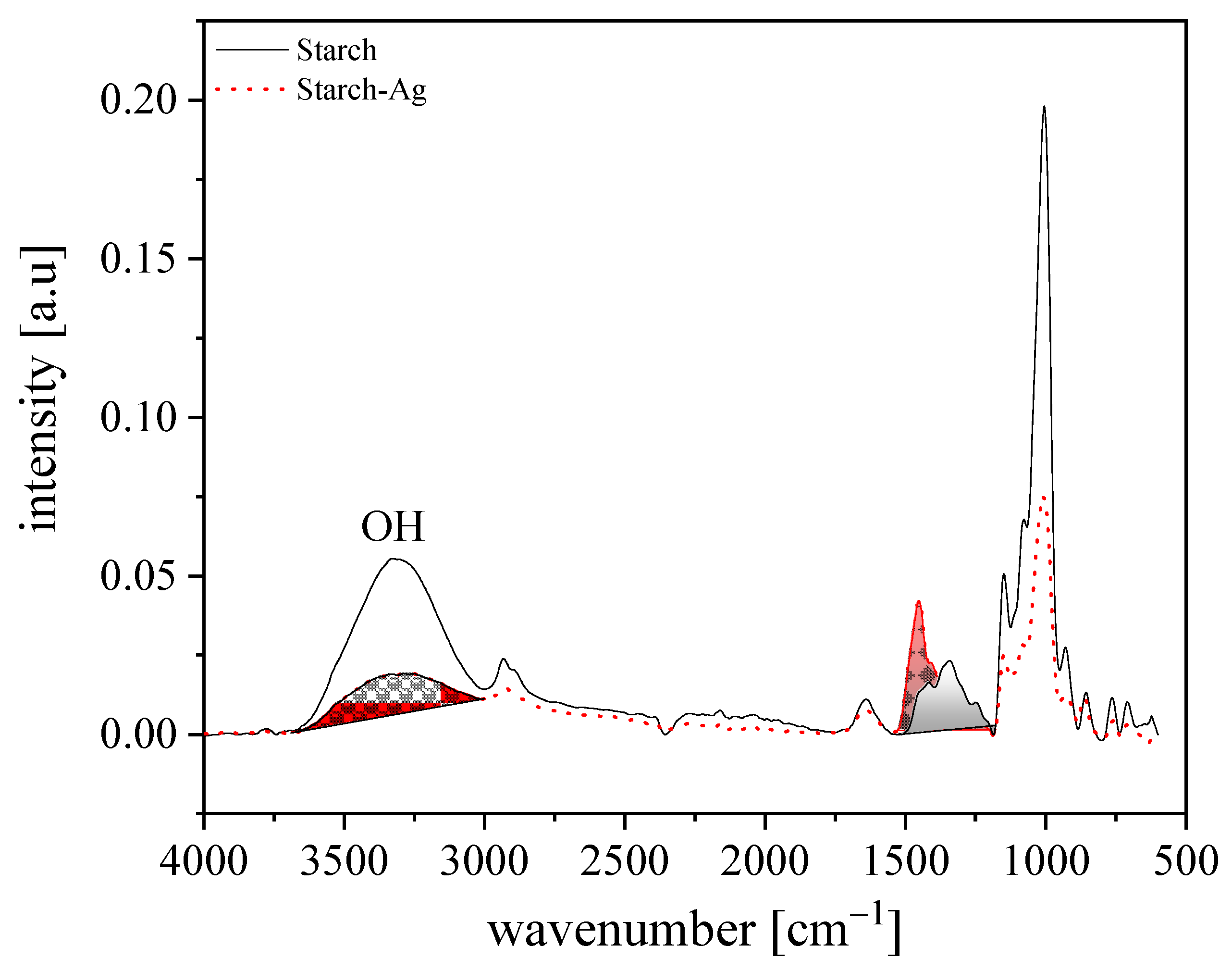
Fourier Transform Infrared (FTIR)
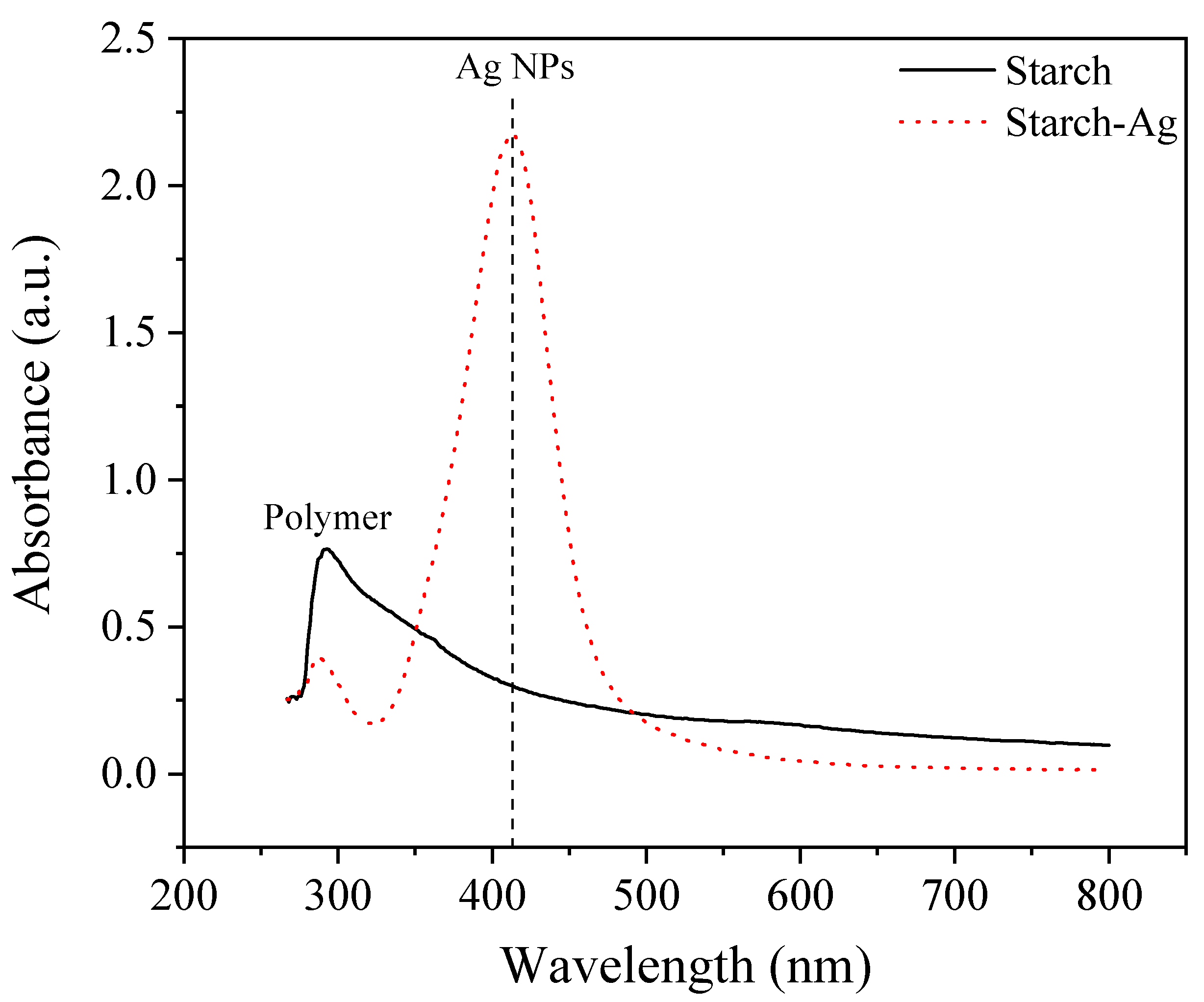
UV-Visible Analysis
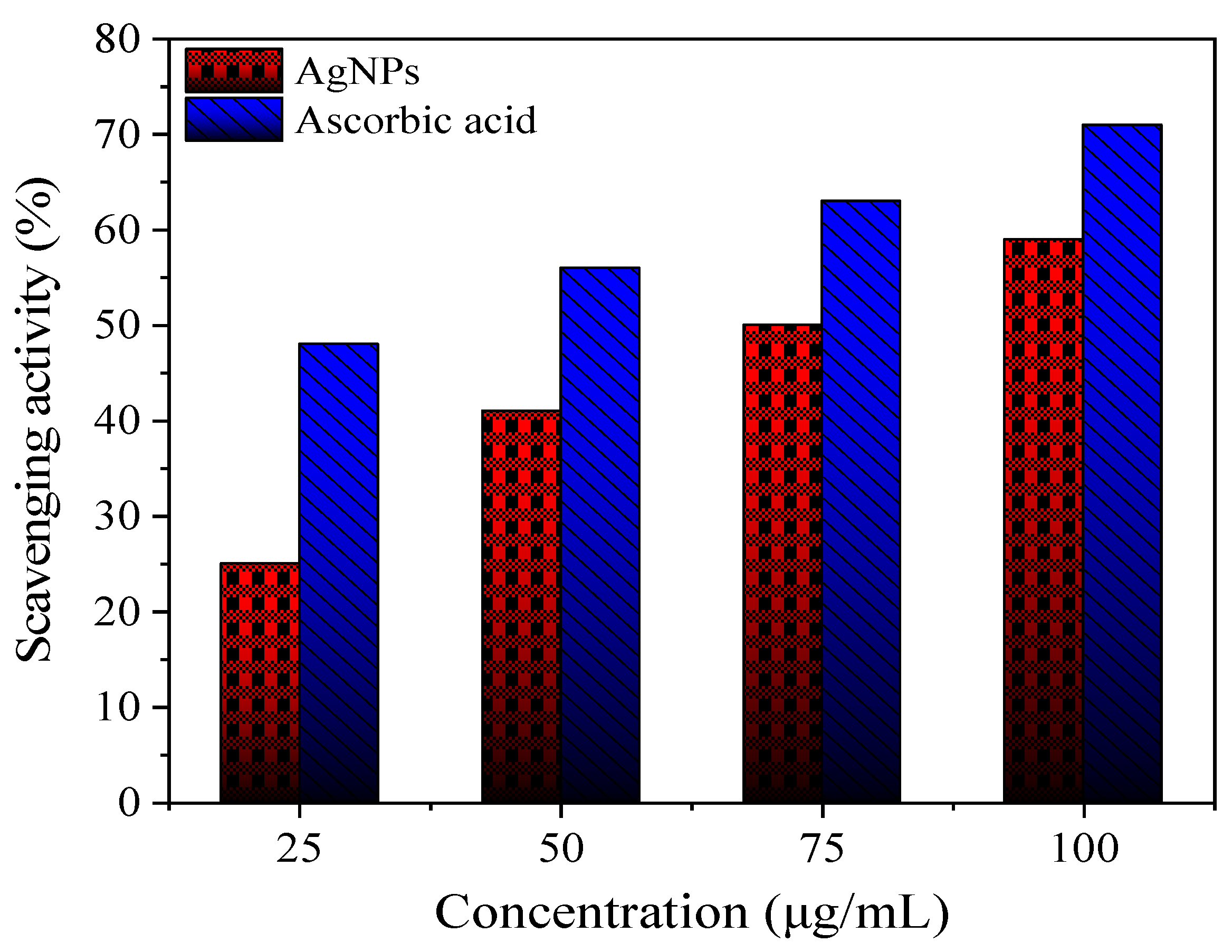
Antioxidant Activity
2.2.3. Starch-AgNPs Application as Active Coatings in the Preservation of Strawberries
2.2.4. Quality Criteria of Strawberry Fruit
Loss of Weight
Inspection of Visual Decay (%)
Soluble Solids Content (%)
Total Titratable Acidity (%)
Anthocyanin Content
Induced Coupled Plasma Optical Emission Spectrometry
Microbial Counts
Strawberry Shelf-Life
Analytical Statistics
3. Results
3.1. Characterization Results
3.1.1. Nanoparticle Visual Examination
3.1.2. Morphology and Microstructure
3.1.3. Chemical Composition of Nanocomposite
3.1.4. Investigation of St-AgNPs Formation Using UV-Visible Spectroscopy
3.1.5. Antioxidant Activity of AgNPs
3.2. Quality Criteria of Strawberry Fruit
3.2.1. Weight Loss
3.2.2. Visual Decay
3.2.3. Total Soluble Solid Content
3.2.4. Total Acidity Content
3.2.5. Content of Anthocyanin
3.2.6. Impact of the Washing Process on Silver Concentration
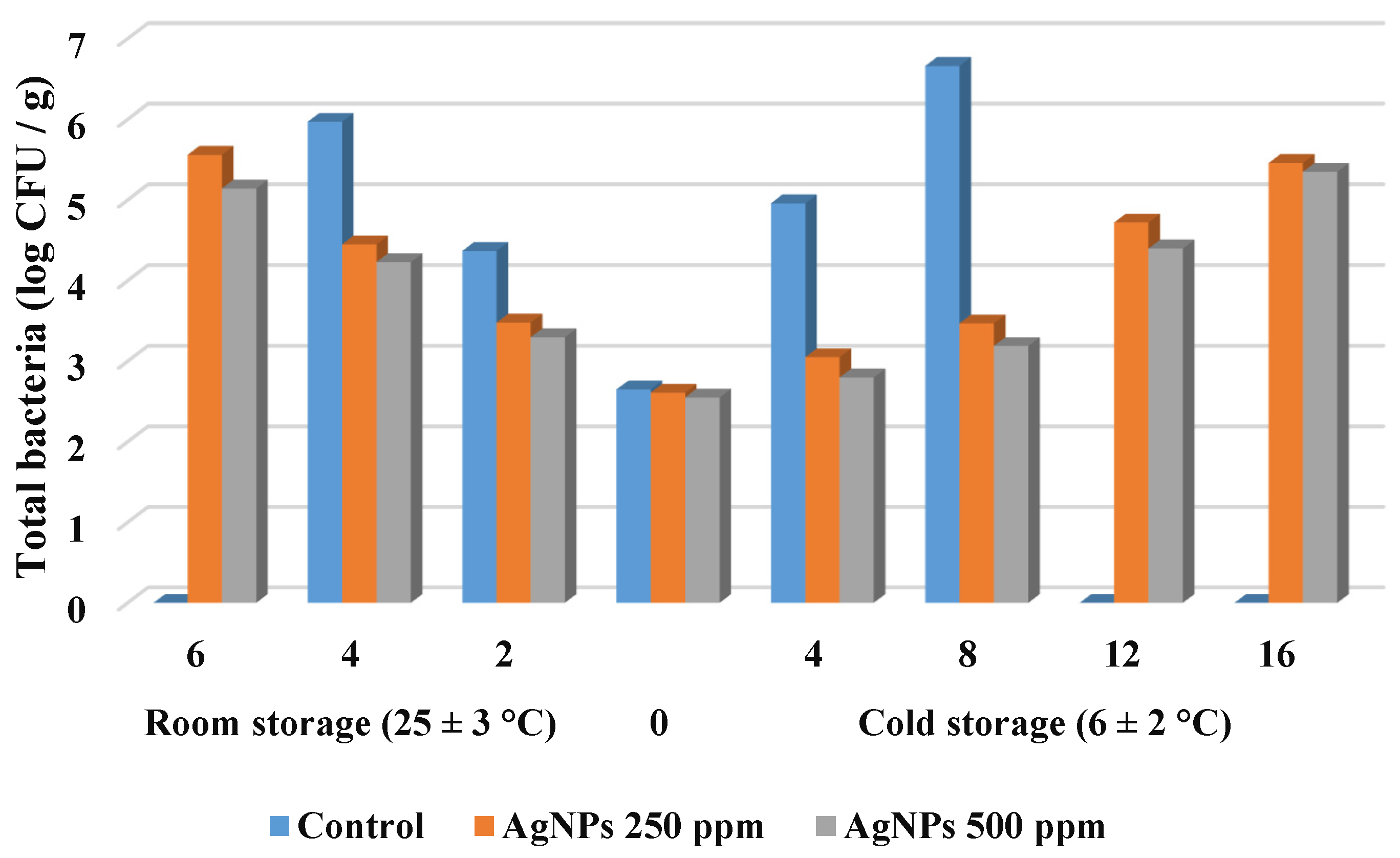
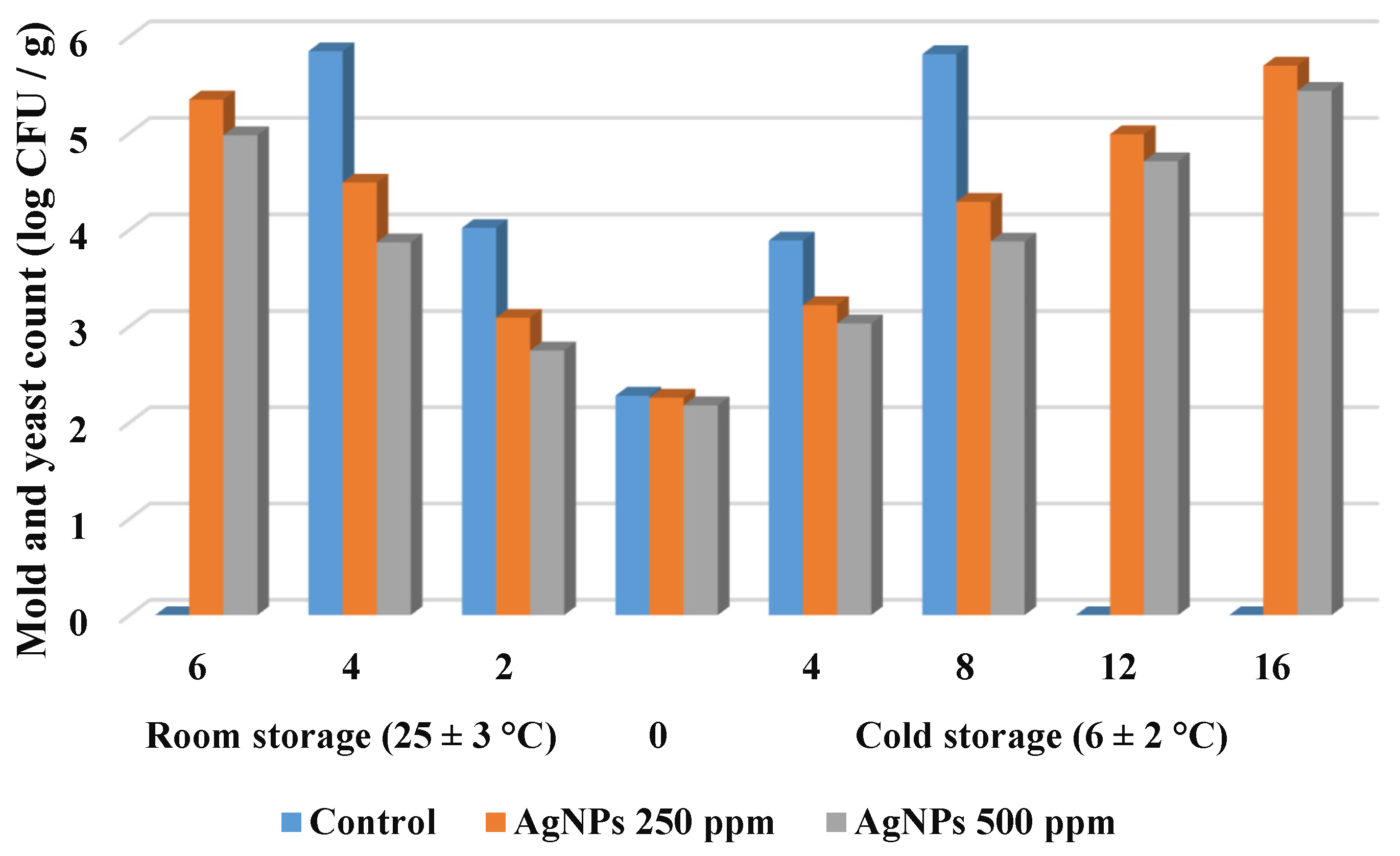
3.2.7. Changes in Microbial Load for Strawberry Fruit during Storage
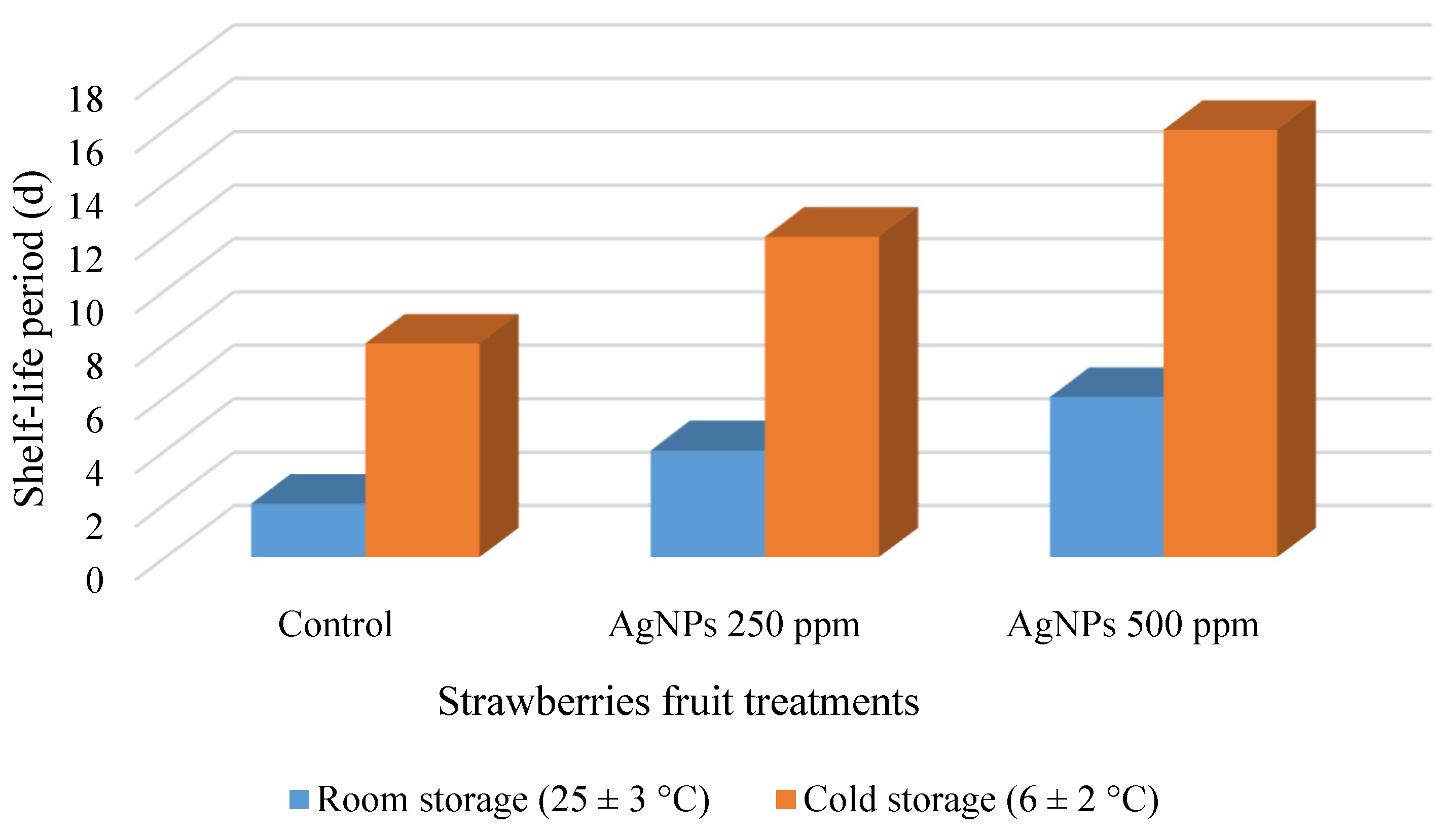
3.2.8. Shelf-Life Periods for Strawberry Fruit
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, J.; Yang, M.; Son, D.; Park, S.; Cho, S.I. Estimation of Tomato Bruising by Mechanical Impact Force Using Multivariate Analysis. HortScience 2018, 53, 1352–1359. [Google Scholar] [CrossRef]
- Zhang, H.; Li, K.; Zhang, X.; Dong, C.; Ji, H.; Ke, R.; Ban, Z.; Hu, Y.; Lin, S.; Chen, C. Effects of ozone treatment on the antioxidant capacity of postharvest strawberry. RSC Adv. 2020, 10, 38142–38157. [Google Scholar] [CrossRef]
- Khodaei, D.; Hamidi-Esfahani, Z. Influence of bioactive edible coatings loaded with Lactobacillus plantarum on physicochemical properties of fresh strawberries. Postharvest Biol. Technol. 2019, 156, 110944. [Google Scholar] [CrossRef]
- Watson, T.T.; Noling, J.W.; Desaeger, J.A. Fluopyram as a rescue nematicide for managing sting nematode (Belonolaimus longicaudatus) on commercial strawberry in Florida. Crop. Prot. 2020, 132, 105108. [Google Scholar] [CrossRef]
- Pinzon, M.I.; Sanchez, L.T.; Garcia, O.R.; Gutierrez, R.; Luna, J.C.; Villa, C.C. Increasing shelf life of strawberries (Fragaria ssp) by using a banana starch-chitosan-Aloe vera gel composite edible coating. Int. J. Food Sci. Technol. 2020, 55, 92–98. [Google Scholar] [CrossRef]
- Majid, I.; Ahmad Nayik, G.; Mohammad Dar, S.; Nanda, V. Novel food packaging technologies: Innovations and future prospective. J. Saudi Soc. Agric. Sci. 2018, 17, 454–462. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Zou, P.; Xiong, H.; Tang, H. Effect of nano-SiO2 on the performance of starch/polyvinyl alcohol blend films. Carbohydr. Polym. 2008, 72, 521–526. [Google Scholar] [CrossRef]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Borowski, G.; Szczypiński, M.; Klepka, T.; Thakur, V.K. Recent progress in biodegradable polymers and nanocomposite-based packaging materials for sustainable environment. Int. J. Polym. Anal. Charact. 2018, 23, 383–395. [Google Scholar] [CrossRef]
- Sam, S.T.; Nuradibah, M.A.; Chin, K.M.; Hani, N. Current Application and Challenges on Packaging Industry Based on Natural Polymer Blending. Nat. Polym. Ind. Tech. Appl. 2016, 163–184. [Google Scholar] [CrossRef]
- Xing, Y.; Li, W.; Wang, Q.; Li, X.; Xu, Q.; Guo, X.Y.H. Antimicrobial nanoparticles incorporated in edible coatings and films for the preservation of fruit and vegetables. Molecules 2019, 24, 1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbagh, F.; Kiarostami, K.; Mahmoudi Khatir, N.; Rezania, S.; Muhamad, I.I. Green synthesis of Mg0. 99 Zn0. 01O nanoparticles for the fabrication of κ-Carrageenan/NaCMC hydrogel in order to deliver catechin. Polymers 2020, 12, 861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbagh, F.; Kiarostami, K.; Khatir, N.M.; Rezania, S.; Muhamad, I.I.; Hosseini, F. Effect of zinc content on structural, functional, morphological, and thermal proper-ties of kappa-carrageenan/NaCMC nanocomposites. Polym. Test. 2021, 93, 106922. [Google Scholar] [CrossRef]
- Onitsuka, S.; Hamada, T.; Okamura, H. Preparation of antimicrobial gold and silver nanoparticles from tea leaf extracts. Colloids Surf. B Biointerfaces 2019, 173, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, A.; Agudo Jácome, L.; Schönhals, A. Effect of Silver Nanoparticles on the Dielectric Properties and the. Homogeneity of Plasma Poly(acrylic acid) Thin Films. J. Phys. Chem. C 2020, 124, 22817–22826. [Google Scholar] [CrossRef]
- Monge, M.; Moreno-Arribas, M.V. Applications of Nanotechnology in Wine Production and Quality and Safety Control; Moreno-Arribas, M.V., Suáldea, B.B., Eds.; Springer: Cham, Switzerland, 2016; pp. 51–69. [Google Scholar]
- Commission Regulation (EU) No 1129/2011 of 11 November 2011 Amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011R1129 (accessed on 11 November 2011).
- European Food Safety Authority Scientific opinion on the re-evaluation of silver (E 174) as food additive. EFSA J. 2016, 14, 4364–4428.
- Azlin-Hasim, S.; Cruz-Romero, M.C.; Morris, M.A.; Cummins, E.; Kerry, J.P. Effects of a combination of antimicrobial silver low density polyethylene nanocomposite films and modified atmosphere packaging on the shelf life of chicken breast fillets. Food Packag. Shelf Life 2015, 4, 26–35. [Google Scholar] [CrossRef]
- García-Ruiz, A.; Crespo, J.; López-de-Luzuriaga, J.M.; Olmos, M.E.; Monge, M.; Rodríguez-Álfaro, M.P.; Martín-Álvarez, P.J.; Bartolome, B.; Moreno-Arribas, M.V. Novel biocompatible silver nanoparticles for controlling the growth of lactic acid bacteria and acetic acid bacteria in wines. Food Control 2015, 50, 613–619. [Google Scholar] [CrossRef]
- Gil-Sánchez, I.; Monge, M.; Miralles, B.; Armentia, G.; Cueva, C.; Crespo, J.; López de Luzuriaga, J.M.; Olmos, M.E.; Bartolomé, B.; González de Llano, D.; et al. Some new findings on the potential use of biocompatible silver nanoparticles in winemaking. Innov. Food Sci. Emerg. Technol. 2019, 51, 64–72. [Google Scholar] [CrossRef]
- Gaillet, S.; Rouanet, J.M. Silver nanoparticles: Their potential toxic effects after oral exposure and underlying mechanisms—A review. Food Chem. Toxicol. 2015, 77, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Bakhy, E.A.; Zidan, N.S.; Aboul-Anean, H.E.D. The effect of nano materials on edible coating and films’ improvement. Int. J. Pharm. Res. Allied Sci 2018, 7, 20–41. [Google Scholar]
- Wasiu Oluwatofarati, S.; Akeem Olayemi, R.; Rahman, A. Effects of silver bio-nanoparticle treatment on the wet preservation, technological, and chemical qualities of meat. Food Qual. Saf. 2018, 2, 159–164. [Google Scholar] [CrossRef]
- Ortiz-Duarte, G.; Pérez-Cabrera, L.E.; Artés-Hernández, F.; Martínez-Hernández, G.B. Ag-chitosan nanocomposites in edible coatings affect the quality of fresh-cut melon. Postharvest Biol. Technol. 2019, 147, 174–184. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Hu, X.; Chelliah, R.; Oh, D.H.; Kathiresan, K.; Wang, M.H. Biogenic silver nanoparticles-polyvinylpyrrolidone based glycerosomes coating to expand the shelf life of fresh-cut bell pepper (Capsicum annuum L. var. grossum (L.) Sendt). Postharvest Biol. Technol. 2020, 160, 111039. [Google Scholar] [CrossRef]
- Shahat, M.S.; Ibrahim, M.I.; Osheba, A.S.; Taha, I.M. Preparation and characterization of silver nanoparticles and their use for improving the quality of apricot fruit. Al-Azhar J. Agric. Res. 2020, 45, 38–43. [Google Scholar] [CrossRef]
- El-Rafie, M.H.; Ahmed, H.B.; Zahran, M.K. Facile Precursor for Synthesis of Silver Nanoparticles Using Alkali Treated Maize Starch. Int. Scholarly Res. Not. 2014, 2014, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, S.; Jacob, G.; Gupta, B. Investigation of the herbal synthesis of silver nanoparticles using Cinnamon zeylanicum extract. Emergent Mater. 2019, 2, 113–122. [Google Scholar] [CrossRef]
- Sundararajan, B.; Mahendran, G.; Thamaraiselvi, R.; Ranjitha Kumari, B.D. Biological activities of synthesized silver nanoparticles from Cardiospermum halicacabum L. Bull. Mater. Sci. 2016, 39, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Zahid, N.; Manickam, S.; Siddiqui, Y.; Alderson, P.G.; Maqbool, M. Effectiveness of submicron chitosan dispersions in controlling anthracnose and maintaining quality of dragon fruit. Postharvest Biol. Technol. 2013, 86, 147–153. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Huynh Nguyen, T.T.; Tran Pham, B.T.; Van Tran, T.; Bach, L.G.; Bui Thi, P.Q.; Ha Thuc, C.N. Development of poly (vinyl alcohol)/agar/maltodextrin coating containing silver nanoparticles for banana (Musa acuminate) preservation. Food Packag. Shelf Life 2021, 29, 100740. [Google Scholar] [CrossRef]
- Li, J.; Fu, Y.; Yan, J.; Song, H.; Jiang, W. Forced Air Precooling Enhanced Storage Quality by Activating the Antioxidant System of Mango Fruits. J. Food Qual. 2019, 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- AOAC International. Official Methods of Analysis of the Association Official Methods of Analysis of the Association of Official Analytical. Chemists International, 20th ed.; AOAC International: Rockville, MD, USA, 2016; p. 3172. [Google Scholar]
- Hajji, S.; Younes, I.; Affes, S.; Boufi, S.; Nasri, M. Optimization of the formulation of chitosan edible coatings supplemented with carotenoproteins and their use for extending strawberries postharvest life. Food Hydrocoll. 2018, 83, 375–392. [Google Scholar] [CrossRef]
- Holzwarth, M.; Korhummel, S.; Carle, R.; Kammerer, D.R. Impact of enzymatic mash maceration and storage on anthocyanin and color retention of pasteurized strawberry purées. Eur. Food Res. Technol. 2012, 234, 207–222. [Google Scholar] [CrossRef]
- Vargas, M.; Albors, A.; Chiralt, A.; González-Martínez, C. Quality of cold-stored strawberries as affected by chitosan–oleic acid edible coatings. Postharvest Biol. Technol. 2006, 41, 164–171. [Google Scholar] [CrossRef]
- American Public Health Association, American Water Works Association, and W.E.F. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- American Public Health. Association Compendium of Methods for the Microbiological Examination of Foods. Available online: https://ajph.aphapublications.org/doi/abs/10.2105/MBEF.0222 (accessed on 1 August 2015).
- Mondal, M.F. Production and Storage of Fruit (in Google). Available online: https://scholar.google.com.eg/scholar?hl=ar&as_sdt=0%2C5&q=Mondal%2C+MF%2C+“Production+and+storage+of+fruit+%28in+Bangla%29.”+Published+by+Mrs.+Afia+Mondal.+BAU+Campus%2C+Mymsningh%2C+2202%2C+312+%282000%29&btnG= (accessed on 28 February 2022).
- CoStat Program; Version 6.311; CoHort Software: Monterey, CA, USA, 2005.
- Mano, J.F.; Koniarova, D.; Reis, R.L. Thermal properties of thermoplastic starch/synthetic polymer blends with potential biomedical applicability. J. Mater. Sci. Mater. Med. 2003, 14, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.B.; Francis, A.P.; Devasena, T. Chitosan–starch nanocomposite particles as a drug carrier for the delivery of bis-desmethoxy curcumin analog. Carbohydr. Polym. 2014, 114, 170–178. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A.; Pathak, R.N. Sonochemical synthesis of silver nanoparticles using starch: A comparison. Bioinorg. Chem. Appl. 2014, 2014, 8. [Google Scholar] [CrossRef]
- Fahmy, A.; Friedrich, J.; Poncin-Epaillard, F.; Debarnot, D. Plasma polymerized allyl alcohol/O2 thin films embedded with silver nanoparticles. Thin Solid Films 2016, 616, 339–347. [Google Scholar] [CrossRef]
- Cheviron, P.; Gouanvé, F.; Espuche, E. Green synthesis of colloid silver nanoparticles and resulting biodegradable starch/silver nanocomposites. Carbohydr. Polym. 2014, 108, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, A.; El-Zomrawy, A.; Saeed, A.M.; Sayed, A.Z.; El-Arab, M.A.E.; Shehata, H.A.; Friedrich, J. One-step synthesis of silver nanoparticles embedded with polyethylene glycol as thin films. J. Adhes. Sci. Technol. 2016, 31, 1422–1440. [Google Scholar] [CrossRef]
- Jung, J.; Kasi, G.; Seo, J. Development of functional antimicrobial papers using chitosan/starch-silver nanoparticles. Int. J. Biol. Macromol. 2018, 112, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Vigneshwaran, N.; Nachane, R.P.; Balasubramanya, R.H.; Varadarajan, P.V. A novel one-pot ‘green’ synthesis of stable silver nanoparticles using soluble starch. Carbohydr. Res. 2006, 341, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Wu, R.; Strik, B.C.; Zhao, Y. Effect of edible coatings on the quality of fresh blueberries (Duke and Elliott) under commercial storage conditions. Postharvest Biol. Technol. 2011, 59, 71–79. [Google Scholar] [CrossRef]
- Svagan, A.J.; Hedenqvist, M.S.; Berglund, L. Reduced water vapour sorption in cellulose nanocomposites with starch matrix. Compos. Sci. Technol. 2009, 69, 500–506. [Google Scholar] [CrossRef]
- Khodaei, D.; Hamidi-Esfahani, Z.; Rahmati, E. Effect of edible coatings on the shelf-life of fresh strawberries: A comparative study using TOPSIS-Shannon entropy method. NFS J. 2021, 23, 17–23. [Google Scholar] [CrossRef]
- Nguyen, V.T.B.; Nguyen, D.H.H.; Nguyen, H.V.H. Combination effects of calcium chloride and nano-chitosan on the postharvest quality of strawberry (Fragaria x ananassa Duch.). Postharvest Biol. Technol. 2020, 162, 111103. [Google Scholar] [CrossRef]
- Rasouli, M.; Koushesh Saba, M.; Ramezanian, A. Inhibitory effect of salicylic acid and Aloe vera gel edible coating on microbial load and chilling injury of orange fruit. Sci. Hortic. (Amst.) 2019, 247, 27–34. [Google Scholar] [CrossRef]
- Riaz, A.; Aadil, R.M.; Amoussa, A.M.O.; Bashari, M.; Abid, M.; Hashim, M.M. Application of chitosan-based apple peel polyphenols edible coating on the preservation of strawberry (Fragaria ananassa cv Hongyan) fruit. J. Food Process. Preserv. 2021, 45, e15018. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I.; El-Nouby, M.A.M.; Ismail, R.I.A.; Taktak, N.E.M. Strawberry Shelf Life, Composition, and Enzymes Activity in Response to Edible Chitosan Coatings. Int. J. Fruit Sci. 2016, 17, 117–136. [Google Scholar] [CrossRef]
- Guidelines for Drinking-Water Quality, 4th ed., Incorporating the 1st Addendum. Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 24 April 2017).
- Rani, Z.T.; Hugo, A.; Hugo, C.J.; Vimiso, P.; Muchenje, V. Effect of post-slaughter handling during distribution on. microbiological quality and safety of meat in the formal and informal sectors of South Africa: A review. S. Afr. J. Anim. Sci. 2017, 47, 255–267. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Li, W.; Zhu, B.; Chen, H.; Chi, H.; Li, L.; Qin, Y.; Xue, J. The Quality Evaluation of Postharvest Strawberries Stored in Nano-Ag Packages at Refrigeration Temperature. Polymers 2018, 10, 894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahbazi, Y.; Shavisi, N.; Karami, N. Development of edible bioactive coating based on mucilages for increasing the shelf life of strawberries. J. Food Meas. Charact. 2021, 15, 394–405. [Google Scholar] [CrossRef]
- Shahbazi, Y. Application of carboxymethyl cellulose and chitosan coatings containing Mentha spicata essential oil in fresh strawberries. Int. J. Biol. Macromol. 2018, 112, 264–272. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Jahani, M. Effect of potassium permanganate, 1-Methylcyclopropene and modified atmosphere packaging on postharvest losses and quality of fresh apricot cv. Shahroudi. J. Hortic. Postharvest Res. 2019, 2, 39–48. [Google Scholar] [CrossRef]

| Operating Conditions | Silver Ions |
|---|---|
| Wavelength (nm) | 328.1 |
| Intensity | 82675 |
| RF plasma torches power (kW) | 1.2 |
| Nebulizer flow (L/min) | 0.7 |
| Plasma flow (L/min) | 12 |
| Viewing mode | Axial |
| Background correction | Fitted |
| Correlation coefficient | 0.98 |
| Calibration error (%) | 10 |
| Calibration fit | Rational |
| Limit of quantification (ug/dl) | 5 |
| Treatment | Storage Period (d) at 25 ± 3 °C | Storage Period (d) at 6 ± 2 °C | ||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 4 | 8 | 12 | 16 | ||
| Control | 5.80 Ca | 11.50 Ba | 28.00 Aa | 2.35 Da | 4.35 Ca | 8.35 Ba | 13.65 Aa | |
| AgNPs | 250 mg·L−1 | 4.15 Cb | 7.45 Bb | 16.89 Ab | 2.21 Da | 2.77 Cb | 3.40 Bb | 5.65 Ab |
| 500 mg·L−1 | 4. 25 Cb | 7.35 Bb | 15.75 Ac | 2.08 Da | 2.65 Cb | 3.28 Bb | 4.44 Ac | |
| Treatments | Storage Period (d) at 25 ± 3 °C | Storage Period (d) at 6 ± 2 °C | ||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 4 | 8 | 12 | 16 | ||
| Control | 10.0 | 80.0 | 100.0 | 15.0 | 45.0 | 100.0 | ND | |
| AgNPs | 250 mg·L−1 | ND | 20.0 | 60.0 | ND | ND | 20.0 | 50.0 |
| 500 mg·L−1 | ND | ND | 40.0 | ND | ND | 10.0 | 30.0 | |
| Treatments | Storage Period (d) at 25 ± 3 °C | Storage Period (d) at 6 ± 2 °C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 0 | 4 | 8 | 12 | 16 | ||
| Control | 5.70 Ba | 6.58 Aa | ND | ND | 5.70 Aa | 5.87 Aa | 5.65 Aa | ND | ND | |
| AgNPs | 250 mg·L−1 | 5.70 Ba | 6.04 Ab | 6.20 Aa | 6.18 Aa | 5.70 Ba | 5.84 Ba | 5.95 ABa | 6.22 Aa | 6.21 Aa |
| 500 mg·L−1 | 5.65 Ba | 5.93A Bb | 6.10 Aa | 6.20 Aa | 5.65 Ca | 5.72 BCa | 5.80 ABCa | 6.02 Aa | 6.08 Aa | |
| Treatments | Storage Period (d) at 25 ± 3 °C | Storage Period (d) at 6 ± 2 °C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 0 | 4 | 8 | 12 | 16 | ||
| Control | 0.48 Aa | 0.37 Ba | ND | ND | 0.48 Aa | 0.41 ABa | 0.35 Ba | ND | ND | |
| AgNPs | 250 mg·L−1 | 0. 47 Aa | 0.42 Aa | 0.38 Aa | 0.32 Aa | 0.47 Aa | 0.45 Aa | 0.42 Aa | 0.40 Aa | 0.37 Aa |
| 500 mg·L−1 | 0.46 Aa | 0.42 Aa | 0.39 Aa | 0.34 Aa | 0.46 Aa | 0.47 Aa | 0.43 Aa | 0.41 Aa | 0.38 Aa | |
| Treatments | Storage Period (d) at 25 ± 3 °C | Storage Period (d) at 6 ± 2 °C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 0 | 4 | 8 | 12 | 16 | ||
| Control | 325.2 Ba | 527.6 Aa | ND | ND | 325.2 Ca | 446.8 Aa | 383.6 Bb | ND | ND | |
| AgNPs | 250 mg·L−1 | 322.5 Da | 475.0 Cb | 572.5 Ba | 629.0 Aa | 322.5 Da | 371.5 Cb | 414.5 Ba | 485.0 Aa | 467.0 Aa |
| 500 mg·L−1 | 324.4 Da | 471.6 Cb | 562.0 Ba | 606.0 Ab | 324.4 Da | 363.5 Cb | 409.5 Ba | 471.5 Aa | 465.6 Aa | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, I.M.; Zaghlool, A.; Nasr, A.; Nagib, A.; El Azab, I.H.; Mersal, G.A.M.; Ibrahim, M.M.; Fahmy, A. Impact of Starch Coating Embedded with Silver Nanoparticles on Strawberry Storage Time. Polymers 2022, 14, 1439. https://doi.org/10.3390/polym14071439
Taha IM, Zaghlool A, Nasr A, Nagib A, El Azab IH, Mersal GAM, Ibrahim MM, Fahmy A. Impact of Starch Coating Embedded with Silver Nanoparticles on Strawberry Storage Time. Polymers. 2022; 14(7):1439. https://doi.org/10.3390/polym14071439
Chicago/Turabian StyleTaha, Ibrahim M., Ayman Zaghlool, Ali Nasr, Ashraf Nagib, Islam H. El Azab, Gaber A. M. Mersal, Mohamed M. Ibrahim, and Alaa Fahmy. 2022. "Impact of Starch Coating Embedded with Silver Nanoparticles on Strawberry Storage Time" Polymers 14, no. 7: 1439. https://doi.org/10.3390/polym14071439
APA StyleTaha, I. M., Zaghlool, A., Nasr, A., Nagib, A., El Azab, I. H., Mersal, G. A. M., Ibrahim, M. M., & Fahmy, A. (2022). Impact of Starch Coating Embedded with Silver Nanoparticles on Strawberry Storage Time. Polymers, 14(7), 1439. https://doi.org/10.3390/polym14071439











