Review on the Relationship between Nano Modifications of Geopolymer Concrete and Their Structural Characteristics
Abstract
:1. Introduction
1.1. Nanofabrication Technique
1.2. Production of Nanomaterials
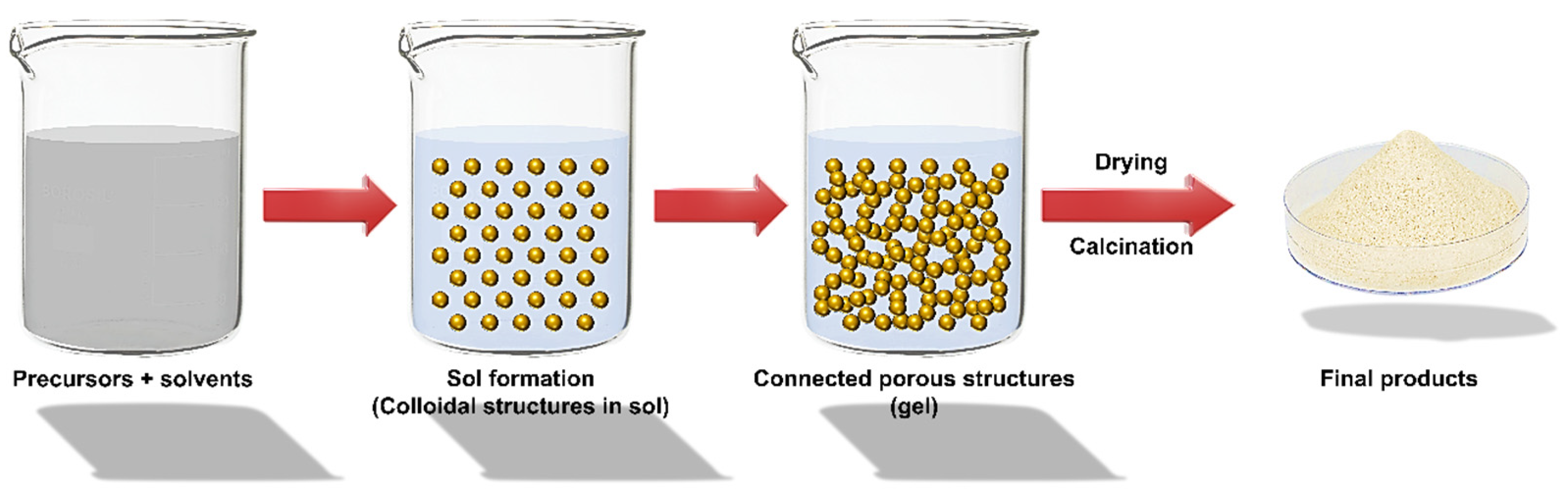
Sol–Gel Technique
1.3. Structure Interaction of Nanomaterials in GPC
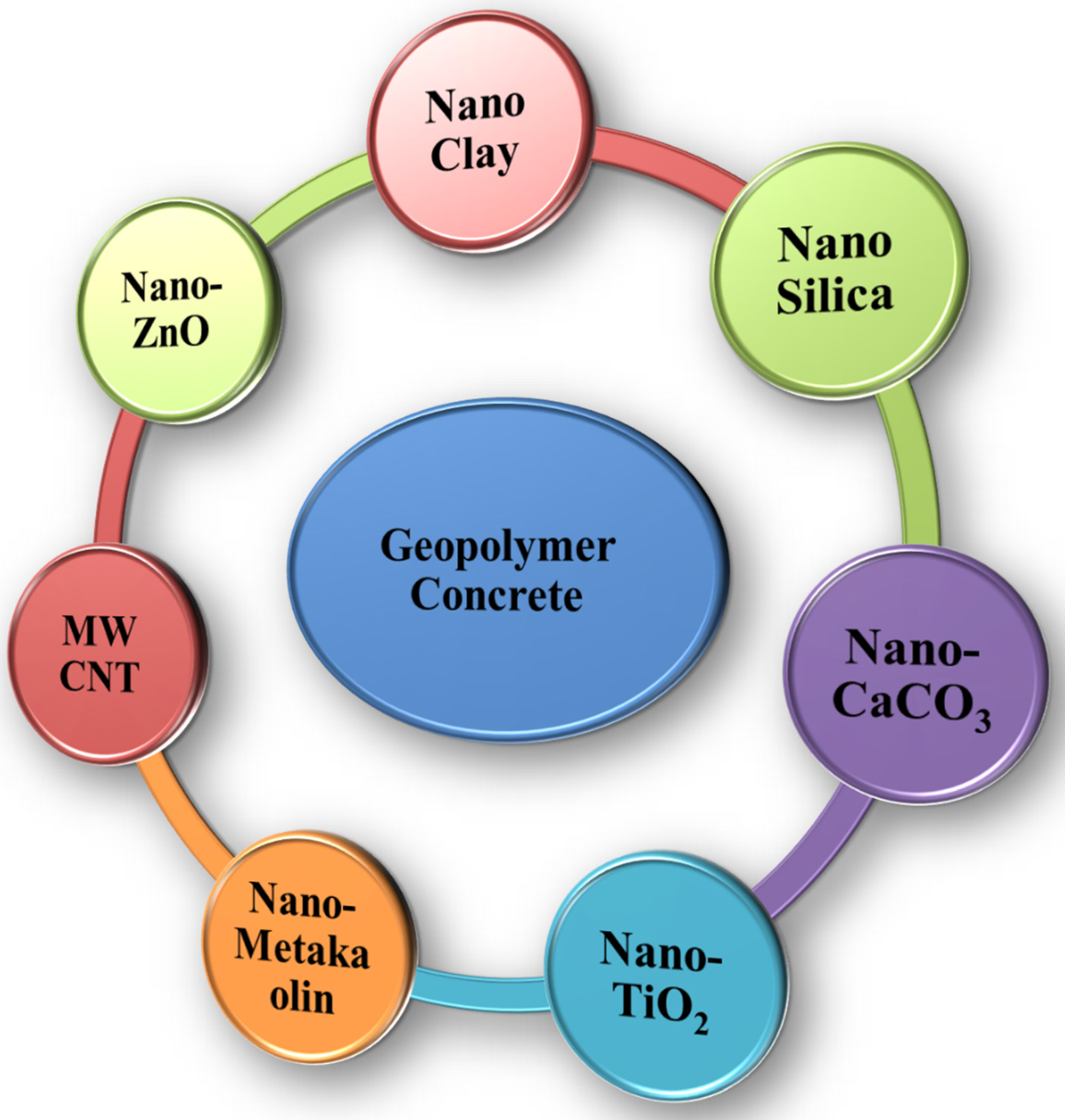
2. Types of Nanomaterials Used in GPC
2.1. Nanomaterials Used as Binders in GPC
2.1.1. Nano Silica (NS)
2.1.2. Nano-TiO2 (NT)
2.1.3. Nano Metakaolin (NM)
2.1.4. Carbon Nanotubes (CNT)
2.1.5. Multi-Walled Carbon Nanotubes (MVCNT)
2.1.6. Nano Clay (NC)
2.1.7. Nano-CaCO3 (NCC)
2.1.8. Nano-ZnO (NZ)
2.2. Geopolymerization Process
2.2.1. Effect of Alkaline to Binder Ratio in Strength Development
2.2.2. Influences of the Ratio of Sodium Silicate to Sodium Hydroxide (SS/SH)
3. Influences of Nanomaterials on Fresh and Hardened Properties of GPC
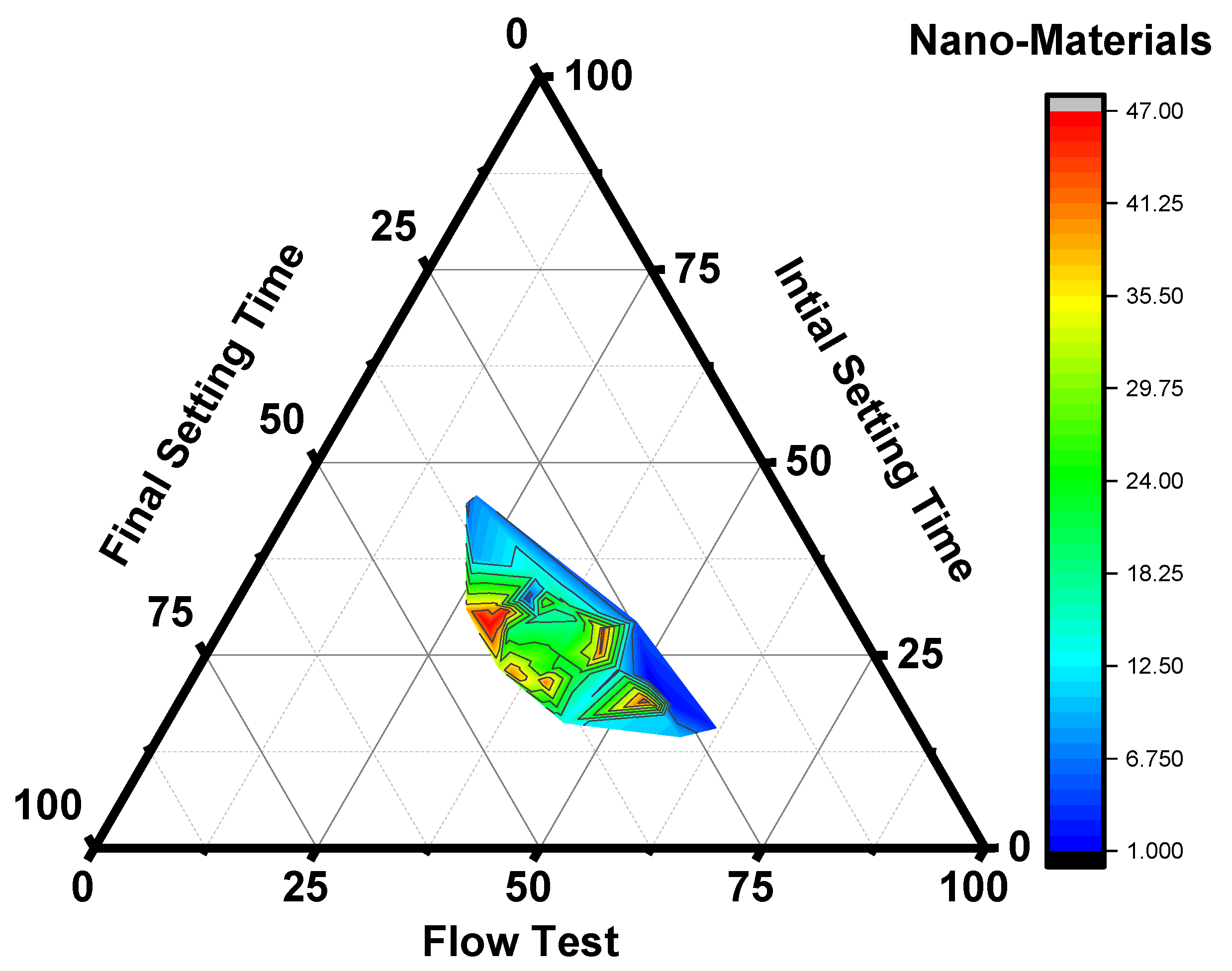
3.1. Relationship between Setting Time and Workability of Mortar Using Nanomaterials
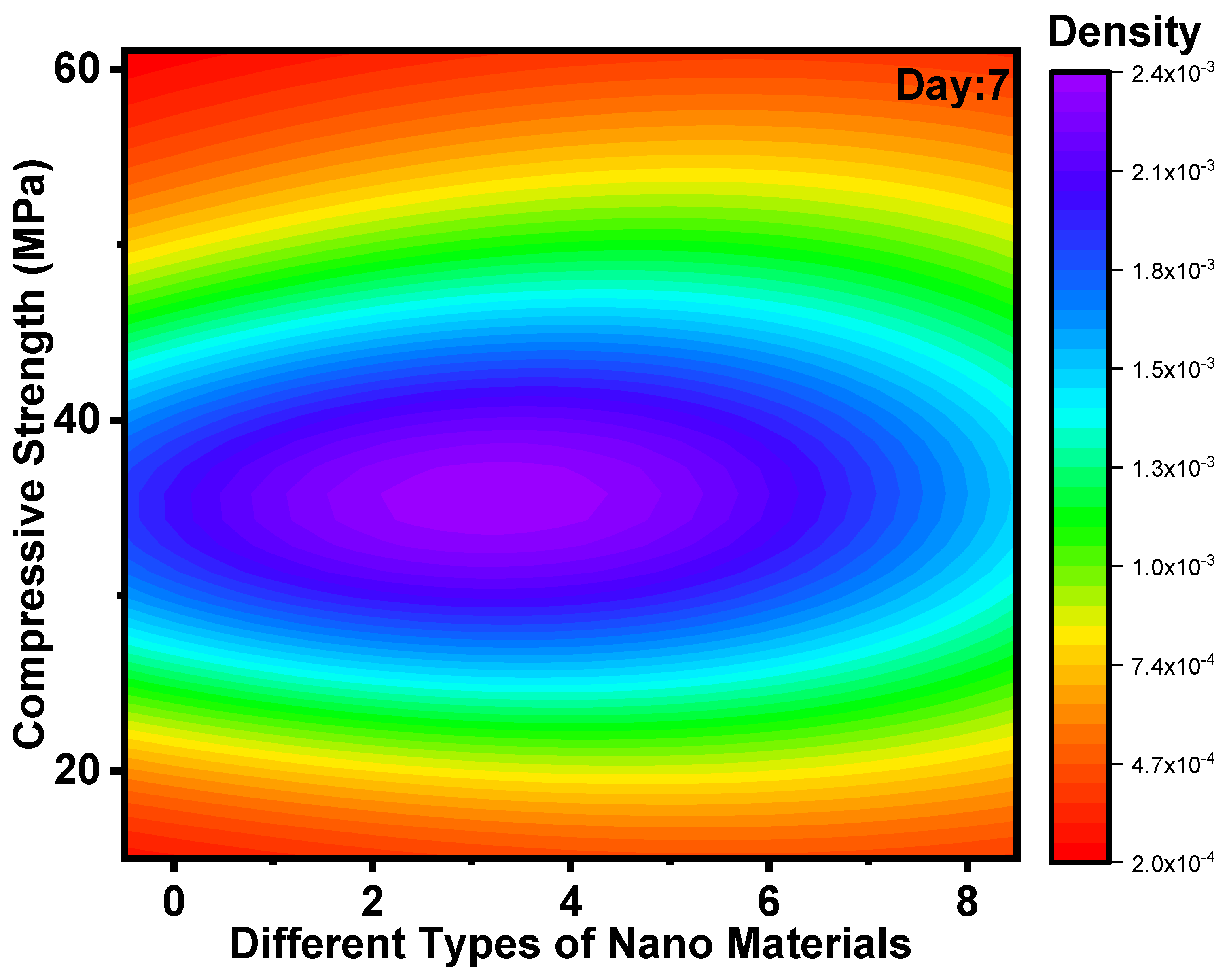
3.2. Impact of Nanomaterials on Development of CS
3.3. Impact of Nanomaterials on STS and FS
3.4. Relationship between Bulk Density (BD) and Water Absorption (WA) of Nanomaterials
3.5. Microstructure Ansysis of Nanomaterial-Based GPC
3.5.1. Scanning Electron Microscope (SEM)
3.5.2. X-ray Diffraction (XRD)
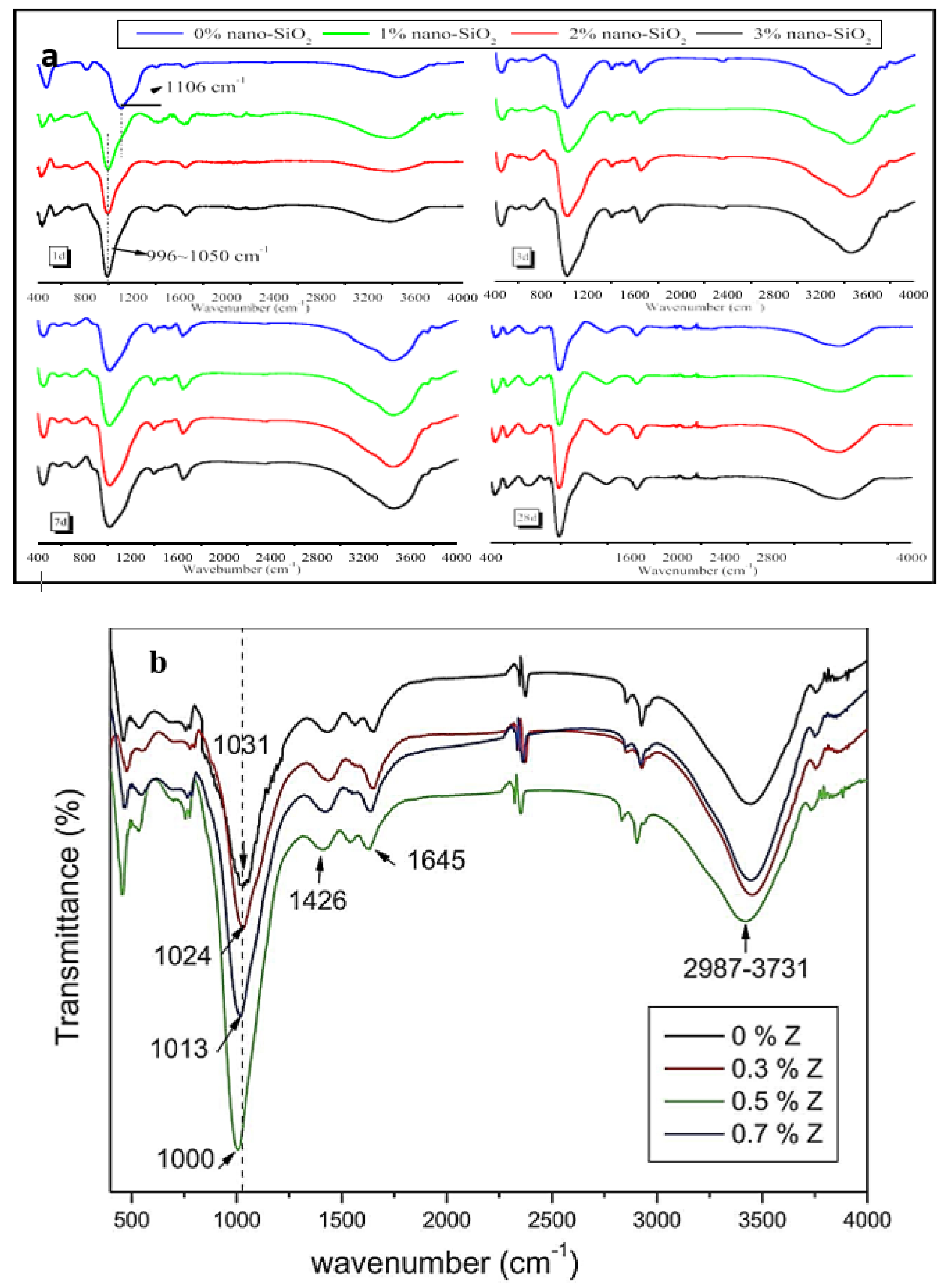
3.5.3. Fourier Transform Infrared Spectroscopy (FTIR)
4. Effect of Nanomaterials on Health Issues
5. Practical Application of Nanomaterials
6. Conclusions
- The inclusion of nanoparticles into geopolymers considerably lowers the geopolymers setting time. Nanomaterials are particularly useful in making up for the limitations of ambient-cured GPC.
- Incorporating nanomaterials such as nano silica, nano alumina, and nano clay into the GPC composite morphology significantly refines the structural stability of the GPC composite, improving its durability. The various products in GPC composites emerging from the incorporation of highly reactive nanoparticles also contribute significantly to geopolymer durability by sealing the holes and cracks in its matrix.
- The microstructure of geopolymer-containing nanoparticles was more compact and uniform. Nanomaterials in geopolymers enhance the polymerization rate, according to SEM, XRD, and FTIR analysis. Moreover, these characterization tests show that adding NS, CNT, NT, and NCC to GP does not form new phases.
- Nanoscale materials provide new possibilities in a wide range of sectors, with applications that are diverse and expanding all the time. As a result, numerous sectors have embraced the benefits that nanoparticles may give, propelling industry-specific products forward. This may be seen in a wide range of applications, including building with nanoparticle-enriched recycled materials that have similar mechanical qualities to new materials, medicine delivery, and antibacterial capabilities in clothes.
- The nanomaterials demonstrated remarkable reactivity; they can be used as superfine pozzolanic materials in GPC composites to improve the mechanical properties of geopolymers by attaining a higher degree of hydration. High nanomaterial dosage in GPC results in a lower polymerization process level due to insufficient dissolving and possible material agglomerate, resulting in lower structural performances of GPC.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, C.; Alengaram, U.J.; Wong, L.S.; Mo, K.H.; Jumaat, M.Z.; Ramesh, S. A review on microstructural study and compressive strength of geopolymer mortar, paste and concrete. Constr. Build. Mater. 2018, 186, 550–576. [Google Scholar] [CrossRef]
- Cheng, T.-W.; Lo, K.-W.; Lin, K.-L.; Lan, J.-Y. Study on the effects Nano-SiO2 and spent catalyst ratios on characteristics of metakaolin-based geopolymers. Environ. Prog. Sustain. Energy 2019, 38, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Das, P.; Beulah, M.; Arjun, H.R.; Ignatius, G. Utilization of Iron Ore Tailings for the Production of Fly Ash—GGBS-Based Geopolymer Bricks. J. Adv. Manuf. Syst. 2017, 16, 275–290. [Google Scholar] [CrossRef] [Green Version]
- Davidovits, J. Geopolymers Based on Natural and Synthetic Metakaolin a Critical Review. In Proceedings of the 41st International Conference on Advanced Ceramics and Composites; John and Wiley and Sons: Hoboken, NJ, USA, 2018; pp. 201–214. [Google Scholar] [CrossRef]
- Polymers/Free Full-Text/Evaluation of the Effect of Granite Waste Powder by Varying the Molarity of Activator on the Mechanical Properties of Ground Granulated Blast-Furnace Slag-Based Geopolymer Concrete. Available online: https://www.mdpi.com/2073-4360/14/2/306 (accessed on 16 March 2022).
- Ravitheja, A.; Kumar, N.L.N.K. A study on the effect of nano clay and GGBS on the strength properties of fly ash based geopolymers. Mater. Today Proc. 2019, 19, 273–276. [Google Scholar] [CrossRef]
- Abdullah, M.M.A.B.; Kamarudin, H.; Abdulkareem, O.A.K.A.; Ghazali, C.M.R.; Rafiza, A.R.; Norazian, M.N. Optimization of Alkaline Activator/Fly ASH Ratio on the Compressive Strength of Manufacturing Fly ASH-BASED Geopolymer. Appl. Mech. Mater. 2012, 110, 734–739. [Google Scholar] [CrossRef]
- Alouani, M.E.L.; Alehyen, S.; Achouri, M.E.L.; Hajjaji, A.; Ennawaoui, C.; Taibi, M. Influence of the Nature and Rate of Alkaline Activator on the Physicochemical Properties of Fly Ash-Based Geopolymers. Adv. Civ. Eng. 2020, 2020, e8880906. [Google Scholar] [CrossRef]
- Xu, S.; Xie, N.; Cheng, X.; Huang, S.; Feng, L.; Hou, P.; Zhu, Y. Environmental resistance of cement concrete modified with low dosage nano particles. Constr. Build. Mater. 2018, 164, 535–553. [Google Scholar] [CrossRef]
- Huseien, G.F.; Hamzah, H.K.; Sam, A.R.M.; Khalid, N.H.A.; Shah, K.W.; Deogrescu, D.P.; Mirza, J. Alkali-activated mortars blended with glass bottle waste nano powder: Environmental benefit and sustainability. J. Clean. Prod. 2020, 243, 118636. [Google Scholar] [CrossRef]
- Faheem, M.T.M.; Abdullah, M.M.a.; Hussin, K.; Binhussain, M.; Ghazali, C.M.R.; Izzat, A.M. Application of Clay-Based Geopolymer in Brick Production: A Review. Adv. Mater. Res. 2013, 626, 878–882. [Google Scholar] [CrossRef]
- Rao, A.K.; Kumar, D.R. Comparative study on the behaviour of GPC using silica fume and fly ash with GGBS exposed to elevated temperature and ambient curing conditions. Mater. Today Proc. 2020, 27, 1833–1837. [Google Scholar] [CrossRef]
- Luo, Z.; Li, W.; Wang, K.; Castel, A.; Shah, S.P. Comparison on the properties of ITZs in fly ash-based geopolymer and Portland cement concretes with equivalent flowability. Cem. Concr. Res. 2021, 143, 106392. [Google Scholar] [CrossRef]
- Jayarajan, G.; Arivalagan, S. An experimental studies of geopolymer concrete incorporated with fly-ash & GGBS. Mater. Today Proc. 2021, 45, 6915–6920. [Google Scholar] [CrossRef]
- Verma, M.; Dev, N. Sodium hydroxide effect on the mechanical properties of flyash-slag based geopolymer concrete. Struct. Concr. 2021, 22, E368–E379. [Google Scholar] [CrossRef]
- Saini, G.; Vattipalli, U. Assessing properties of alkali activated GGBS based self-compacting geopolymer concrete using nano-silica. Case Stud. Constr. Mater. 2020, 12, e00352. [Google Scholar] [CrossRef]
- Huseien, G.F.; Ismail, M.; Khalid, N.H.A.; Hussin, M.W.; Mirza, J. Compressive strength and microstructure of assorted wastes incorporated geopolymer mortars: Effect of solution molarity. Alex. Eng. J. 2018, 57, 3375–3386. [Google Scholar] [CrossRef]
- Samadi, M.; Huseien, G.F.; Lim, N.H.A.S.; Mohammadhosseini, H.; Alyousef, R.; Mirza, J.; Rahman, A.B.A. Enhanced performance of nano-palm oil ash-based green mortar against sulphate environment. J. Build. Eng. 2020, 32, 101640. [Google Scholar] [CrossRef]
- Assaedi, H.; Alomayri, T.; Kaze, C.R.; Jindal, B.B.; Subaer, S.; Shaikh, F.; Alraddadi, S. Characterization and properties of geopolymer nanocomposites with different contents of nano-CaCO3. Constr. Build. Mater. 2020, 252, 119137. [Google Scholar] [CrossRef]
- Durak, U.; Karahan, O.; Uzal, B.; İlkentapar, S.; Atiş, C.D. Influence of nano SiO2 and nano CaCO3 particles on strength, workability, and microstructural properties of fly ash-based geopolymer. Struct. Concr. 2021, 22, E352–E367. [Google Scholar] [CrossRef]
- Khater, H.M.; el Gawaad, H.A.A. Characterization of alkali activated geopolymer mortar doped with MWCNT. Constr. Build. Mater. 2016, 102, 329–337. [Google Scholar] [CrossRef]
- Deb, P.S.; Sarker, P.K.; Barbhuiya, S. Sorptivity and acid resistance of ambient-cured geopolymer mortars containing nano-silica. Cem. Concr. Compos. 2016, 72, 235–245. [Google Scholar] [CrossRef] [Green Version]
- AlKhatib, A.; Maslehuddin, M.; Al-Dulaijan, S.U. Development of high performance concrete using industrial waste materials and nano-silica. J. Mater. Res. Technol. 2020, 9, 6696–6711. [Google Scholar] [CrossRef]
- Zidi, Z.; Ltifi, M.; Zafar, I. Synthesis and attributes of nano-SiO2 local metakaolin based-geopolymer. J. Build. Eng. 2021, 33, 101586. [Google Scholar] [CrossRef]
- Al-Majidi, M.H.; Lampropoulos, A.; Cundy, A.; Meikle, S. Development of geopolymer mortar under ambient temperature for in situ applications. Constr. Build. Mater. 2016, 120, 198–211. [Google Scholar] [CrossRef] [Green Version]
- Adeyanju, E.; Okeke, C.A.; Akinwumi, I.; Busari, A. Subgrade Stabilization using Rice Husk Ash-based Geopolymer (GRHA) and Cement Kiln Dust (CKD). Case Stud. Constr. Mater. 2020, 13, e00388. [Google Scholar] [CrossRef]
- Karthik, A.; Sudalaimani, K.; Vijayakumar, C.T. Durability study on coal fly ash-blast furnace slag geopolymer concretes with bio-additives. Ceram. Int. 2017, 43, 11935–11943. [Google Scholar] [CrossRef]
- Azad, N.M.; Samarakoon, S.M. Utilization of Industrial By-Products/Waste to Manufacture Geopolymer Cement/Concrete. Sustainability 2021, 13, 873. [Google Scholar] [CrossRef]
- Si, A.; Pal, K.; Kralj, S.; El-Sayyad, G.S.; de Souza, F.G.; Narayanan, T. Sustainable preparation of gold nanoparticles via green chemistry approach for biogenic applications. Mater. Today Chem. 2020, 17, 100327. [Google Scholar] [CrossRef]
- Moghaddam, S.C.; Madandoust, R.; Jamshidi, M.; Nikbin, I.M. Mechanical properties of fly ash-based geopolymer concrete with crumb rubber and steel fiber under ambient and sulfuric acid conditions. Constr. Build. Mater. 2021, 281, 122571. [Google Scholar] [CrossRef]
- Huynh, T.-P.; Hwang, C.-L.; Lin, K.-L.; Ngo, S.-H. Effect of residual rice husk ash on mechanical-microstructural properties and thermal conductivity of sodium-hydroxide-activated bricks. Environ. Prog. Sustain. Energy 2018, 37, 1647–1656. [Google Scholar] [CrossRef]
- Lee, B.; Kim, G.; Kim, R.; Cho, B.; Lee, S.; Chon, C.-M. Strength development properties of geopolymer paste and mortar with respect to amorphous Si/Al ratio of fly ash. Constr. Build. Mater. 2017, 151, 512–519. [Google Scholar] [CrossRef]
- Sun, K.; Peng, X.; Wang, S.; Zeng, L.; Ran, P.; Ji, G. Effect of nano-SiO2 on the efflorescence of an alkali-activated metakaolin mortar. Constr. Build. Mater. 2020, 253, 118952. [Google Scholar] [CrossRef]
- Amran, Y.H.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean production and properties of geopolymer concrete; A review. J. Clean. Prod. 2020, 251, 119679. [Google Scholar] [CrossRef]
- Amran, M.; Debbarma, S.; Ozbakkaloglu, T. Fly ash-based eco-friendly geopolymer concrete: A critical review of the long-term durability properties. Constr. Build. Mater. 2021, 270, 121857. [Google Scholar] [CrossRef]
- Hassan, A.; Arif, M.; Shariq, M. Use of geopolymer concrete for a cleaner and sustainable environment—A review of mechanical properties and microstructure. J. Clean. Prod. 2019, 223, 704–728. [Google Scholar] [CrossRef]
- Spitaleri, L.; Nicotra, G.; Zimbone, M.; Contino, A.; Maccarrone, G.; Alberti, A.; Gulino, A. Fast and Efficient Sun Light Photocatalytic Activity of Au_ZnO Core–Shell Nanoparticles Prepared by a One-Pot Synthesis. ACS Omega 2019, 4, 15061–15066. [Google Scholar] [CrossRef] [Green Version]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Meesala, C.R.; Verma, N.K.; Kumar, S. Critical review on fly-ash based geopolymer concrete. Struct. Concr. 2020, 21, 1013–1028. [Google Scholar] [CrossRef]
- Muraleedharan, M.; Nadir, Y. Factors affecting the mechanical properties and microstructure of geopolymers from red mud and granite waste powder: A review. Ceram. Int. 2021, 47, 13257–13279. [Google Scholar] [CrossRef]
- Zimbone, M.; Cacciato, G.; Spitaleri, L.; Egdell, R.G.; Grimaldi, M.G.; Gulino, A. Sb-Doped Titanium Oxide: A Rationale for Its Photocatalytic Activity for Environmental Remediation. ACS Omega 2018, 3, 11270–11277. [Google Scholar] [CrossRef] [Green Version]
- Sustainable Preparation of Metal Nanoparticles: Methods and Applications RSC Books; Royal Society of Chemistry: London, UK, 2012. [CrossRef]
- Shafeek, A.M.; Khedr, M.H.; El-Dek, S.I.; Shehata, N. Influence of ZnO nanoparticle ratio and size on mechanical properties and whiteness of White Portland Cement. Appl. Nanosci. 2020, 10, 3603–3615. [Google Scholar] [CrossRef]
- Contino, A.; Maccarrone, G.; Spitaleri, L.; Torrisi, L.; Nicotra, G.; Gulino, A. One Pot Synthesis of Au_ZnO Core-Shell Nanoparticles Using a Zn Complex Acting as ZnO Precursor, Capping and Reducing Agent During the Formation of Au NPs. Eur. J. Inorg. Chem. 2018, 2018, 4678–4683. [Google Scholar] [CrossRef]
- Assaedi, H.; Shaikh, F.U.A.; Low, I.M. Influence of mixing methods of nano silica on the microstructural and mechanical properties of flax fabric reinforced geopolymer composites. Constr. Build. Mater. 2016, 123, 541–552. [Google Scholar] [CrossRef]
- Abhishek, H.S.; Prashant, S.; Kamath, M.V.; Kumar, M. Fresh mechanical and durability properties of alkali-activated fy ash-slag concrete: A review, Innovative Infrastructure Solutions. Innov. Infrastruct. Solut. 2022, 7, 1–14. [Google Scholar] [CrossRef]
- Abdullah, M.M.a.; Tahir, M.F.M.; Hussin, K.; Binhussain, M.; Ekaputri, J.J. Effect of Microwave Curing to the Compressive Strength of Fly Ash Based Geopolymer Mortar. Mater. Sci. Forum. 2016, 841, 193–199. [Google Scholar] [CrossRef]
- Sofi, M.; van Deventer, J.S.J.; Mendis, P.A.; Lukey, G.C. Engineering properties of inorganic polymer concretes (IPCs). Cem. Concr. Res. 2007, 37, 251–257. [Google Scholar] [CrossRef]
- Adak, D.; Sarkar, M.; Mandal, S. Effect of nano-silica on strength and durability of fly ash based geopolymer mortar. Constr. Build. Mater. 2014, 70, 453–459. [Google Scholar] [CrossRef]
- Norhasri, M.S.M.; Hamidah, M.S.; Fadzil, A.M. Applications of using nano material in concrete: A review. Constr. Build. Mater. 2017, 133, 91–97. [Google Scholar] [CrossRef]
- Singh, N.B.; Saxena, S.K.; Kumar, M. Effect of nanomaterials on the properties of geopolymer mortars and concrete. Mater. Today Proc. 2018, 5, 9035–9040. [Google Scholar] [CrossRef]
- Ekmen, Ş.; Mermerdaş, K.; Algın, Z. Effect of oxide composition and ingredient proportions on the rheological and mechanical properties of geopolymer mortar incorporating pumice aggregate. J. Build. Eng. 2021, 34, 101893. [Google Scholar] [CrossRef]
- Akono, A.-T. Effect of nano-TiO2 on C-S-H phase distribution within Portland cement paste. J. Mater. Sci. 2020, 55, 11106–11119. [Google Scholar] [CrossRef]
- Dehkordi, B.A.; Nilforoushan, M.R.; Talebian, N.; Tayebi, M. A comparative study on the self-cleaning behavior and antibacterial activity of Portland cement by addition of TiO2 and ZnO nanoparticles. Mater. Res. Express 2021, 8, 035403. [Google Scholar] [CrossRef]
- Moya, J.S.; Cabal, B.; Sanz, J.; Torrecillas, R. Metakaolin-Nanosilver as Biocide Agent in Geopolymer. In Developments in Strategic Materials and Computational Design III; John and Wiley and Sons: Hoboken, NJ, USA, 2012; pp. 1–11. [Google Scholar] [CrossRef]
- Ding, Y.-C.; Cheng, T.-W.; Dai, Y.-S. Application of geopolymer paste for concrete repair. Struct. Concr. 2017, 18, 561–570. [Google Scholar] [CrossRef]
- Gao, X.; Yuan, B.; Yu, Q.L.; Brouwers, H.J.H. Characterization and application of municipal solid waste incineration (MSWI) bottom ash and waste granite powder in alkali activated slag. J. Clean. Prod. 2017, 164, 410–419. [Google Scholar] [CrossRef] [Green Version]
- Liew, Y.-M.; Heah, C.-Y.; Mustafa, A.B.M.; Kamarudin, H. Structure and properties of clay-based geopolymer cements: A review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- M Ziada, Y Tammam, S¸ Erdem and R A González Lezcano, Investigation of the Mechanical, Microstructure and 3D Fractal Analysis of Nanocalcite-Modified Environmentally Friendly and Sustainable Cementitious Composites. Buildings 2022, 12, 36. [CrossRef]
- Singh, S.; Aswath, M.U.; Ranganath, R.V. Effect of mechanical activation of red mud on the strength of geopolymer binder. Constr. Build. Mater. 2018, 177, 91–101. [Google Scholar] [CrossRef]
- Shilar, A.F.; Ganachari, S.V.; Patil, V.B. Investigation of the effect of granite waste powder as a binder for different molarity of geopolymer concrete on fresh and mechanical properties. Mater. Lett. 2022, 309, 131302. [Google Scholar] [CrossRef]
- Pavithra, P.; Reddy, M.S.; Dinakar, P.; Rao, B.H.; Satpathy, B.K.; Mohanty, A.N. Effect of the Na2SiO3/NaOH Ratio and NaOH Molarity on the Synthesis of Fly Ash-Based Geopolymer Mortar. Geo-Chicago 2016, 2016, 336–344. [Google Scholar] [CrossRef]
- Kotop, M.A.; El-Feky, M.S.; Alharbi, Y.R.; Abadel, A.A.; Binyahya, A.S. Engineering properties of geopolymer concrete incorporating hybrid nano-materials. Ain Shams Eng. J. 2021, 12, 3641–3647. [Google Scholar] [CrossRef]
- Cao, Y.-F.; Tao, Z.; Pan, Z.; Wuhrer, R. Effect of calcium aluminate cement on geopolymer concrete cured at ambient temperature. Constr. Build. Mater. 2018, 191, 242–252. [Google Scholar] [CrossRef]
- Nuaklong, P.; Wongsa, A.; Boonserm, K.; Ngohpok, C.; Jongvivatsakul, P.; Sata, V.; Sukontasukkul, P.; Chindaprasirt, P. Enhancement of mechanical properties of fly ash geopolymer containing fine recycled concrete aggregate with micro carbon fiber. J. Build. Eng. 2021, 41, 102403. [Google Scholar] [CrossRef]
- Bernal, S.A.; de Gutiérrez, R.M.; Pedraza, A.L.; Provis, J.L.; Rodriguez, E.D.; Delvasto, S. Effect of binder content on the performance of alkali-activated slag concretes. Cem. Concr. Res. 2011, 41, 1–8. [Google Scholar] [CrossRef]
- Chen, K.; Wu, D.; Chen, H.; Zhang, G.; Yao, R.; Pan, C.; Zhang, Z. Development of low-calcium fly ash-based geopolymer mortar using nanosilica and hybrid fibers. Ceram. Int. 2021, 47, 21791–21806. [Google Scholar] [CrossRef]
- Anshul, A.; Moinuddin, A.A.; Azad, A.M.; Khera, P.; Dehariya, K.; Bherwani, H.; Gupta, A.; Kumar, S. Morphologically designed micro porous zeolite-geopolymers as cool coating materials. J. Hazard. Mater. 2020, 398, 123022. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K.; Rangan, V.B. Early Age Properties of Low-calcium Fly Ash Geopolymer Concrete Suitable for Ambient Curing. Procedia Eng. 2015, 125, 601–607. [Google Scholar] [CrossRef] [Green Version]
- Hamzah, H.K.; Joudah, Z.H.; Georgescu, D.P.; Khalid, N.H.A.; Huseien, G.F. Laboratory evaluation of alkali-activated mortars modified with nanosilica from glass bottle wastes. Mater. Today Proc. 2021, 46, 2098–2104. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, K.; Wang, J.; Guo, J.; Ling, Y. Macroscopic and microscopic analyses on mechanical performance of metakaolin/fly ash based geopolymer mortar. J. Clean. Prod. 2021, 294, 126193. [Google Scholar] [CrossRef]
- Cai, J.; Pan, J.; Han, J.; Lin, Y.; Sheng, Z. Impact behaviours of engineered geopolymer composite exposed to elevated temperatures. Constr. Build. Mater. 2021, 312, 125421. [Google Scholar] [CrossRef]
- Seifan, M.; Mendoza, S.; Berenjian, A. Mechanical properties and durability performance of fly ash based mortar containing nano-and micro-silica additives. Constr. Build. Mater. 2020, 252, 119121. [Google Scholar] [CrossRef]
- Prakasam, G.; Murthy, A.R.; Reheman, M.S. Mechanical, durability and fracture properties of nano-modified FA/GGBS geopolymer mortar. Mag. Concr. Res. 2020, 72, 207–216. [Google Scholar] [CrossRef]
- Elyamany, H.E.; Elmoaty, A.E.M.A.; Elshaboury, A.M. Setting time and 7-day strength of geopolymer mortar with various binders. Constr. Build. Mater. 2018, 187, 974–983. [Google Scholar] [CrossRef]
- Investigation of Microstructure and Thermomechanical Properties of Nano-TiO2 Admixed Geopolymer for Thermal Resistance Applications, Springerprofessional.De. Available online: https://www.springerprofessional.de/en/investigation-of-microstructure-and-thermomechanical-properties-/19108492 (accessed on 22 October 2021).
- Lloyd, R.R.; Provis, J.L.; van Deventer, J.S.J. Pore solution composition and alkali diffusion in inorganic polymer cement. Cem. Concr. Res. 2010, 40, 1386–1392. [Google Scholar] [CrossRef]
- Al-Hedad, A.S.A.; Farhan, N.A.; Zhang, M.; Sheikh, M.N.; Hadi, M.N.S. Effect of geogrid reinforcement on the drying shrinkage and thermal expansion of geopolymer concrete. Struct. Concr. 2020, 21, 1029–1039. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, J.; Kaur, M. Microstructure and strength development of fly ash-based geopolymer mortar: Role of nano-metakaolin. Constr. Build. Mater. 2018, 190, 672–679. [Google Scholar] [CrossRef]
- Lu and Zhong J Infrastruct Preserv Resil Carbon-based nanomaterials engineered cement composites: A review. J. Infrastruct. Preserv. Resil. 2022, 3, 22. [CrossRef]
- Li, Z.; Fei, M.-E.; Huyan, C.; Shi, X. Nano-engineered, Fly Ash-Based Geopolymer Composites: An Overview. Resour. Conserv. Recycl. 2021, 168, 105334. [Google Scholar] [CrossRef]
- Luo, Z.; Li, W.; Gan, Y.; He, X.; Castel, A.; Sheng, D. Nanoindentation on micromechanical properties and microstructure of geopolymer with nano-SiO2 and nano-TiO2. Cem. Concr. Compos. 2021, 117, 103883. [Google Scholar] [CrossRef]
- Revathi, T.; Jeyalakshmi, R.; Rajamane, N.P. Study on the role of n-SiO2 incorporation in thermo-mechanical and microstructural properties of ambient cured FA-GGBS geopolymer matrix. Appl. Surf. Sci. 2018, 449, 322–331. [Google Scholar] [CrossRef]
- Luan, C.; Shi, X.; Zhang, K.; Utashev, N.; Yang, F.; Dai, J.; Wang, Q. A mix design method of fly ash geopolymer concrete based on factors analysis. Constr. Build. Mater. 2021, 272, 121612. [Google Scholar] [CrossRef]
- Hassan, A.; Arif, M.; Shariq, M. Age-dependent compressive strength and elastic modulus of fly ash-based geopolymer concrete. Struct. Concr. 2022, 23, 473–487. [Google Scholar] [CrossRef]
- Nagaraj, V.K.; Babu, D.L.V. Assessing the performance of molarity and alkaline activator ratio on engineering properties of self-compacting alkaline activated concrete at ambient temperature. J. Build. Eng. 2018, 20, 137–155. [Google Scholar] [CrossRef]
- Haruna, S.; Mohammed, B.S.; Wahab, M.M.A.; Kankia, M.U.; Amran, M.; Gora, A.M. Long-Term Strength Development of Fly Ash-Based One-Part Alkali-Activated Binders. Materials 2021, 14, 4160. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.K.; Kumar, D.R. Effect of various alkaline binder ratio on geopolymer concrete under ambient curing condition. Mater. Today Proc. 2020, 27, 1768–1773. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. Sulfuric acid resistance of fly ash based geopolymer concrete. Constr. Build. Mater. 2017, 146, 136–143. [Google Scholar] [CrossRef]
- Use of Heat-Treated Water Treatment Residuals in Fly Ash-Based Geopolymers-Guo-2010-Journal of the American Ceramic Society—Wiley Online Library. Available online: https://ceramics.onlinelibrary.wiley.com/doi/10.1111/j.1551-2916.2009.03331.x (accessed on 25 May 2021).
- Mendes, B.; Andrade, I.K.; de Carvalho, J.M.; Pedroti, L.; Júnior, A.d. Assessment of mechanical and microstructural properties of geopolymers produced from metakaolin, silica fume, and red mud. Int. J. Appl. Ceram. Technol. 2021, 18, 262–274. [Google Scholar] [CrossRef]
- Albidah, A.; Alqarni, A.S.; Abbas, H.; Almusallam, T.; Al-Salloum, Y. Behavior of Metakaolin-Based geopolymer concrete at ambient and elevated temperatures. Constr. Build. Mater. 2022, 317, 125910. [Google Scholar] [CrossRef]
- Matalkah, F.; Ababneh, A.; Aqel, R. Efflorescence Control in Calcined Kaolin-Based Geopolymer Using Silica Fume and OPC. J. Mater. Civ. Eng. 2021, 33, 04021119. [Google Scholar] [CrossRef]
- Padmakar, M.; Barhmaiah, B.; Priyanka, M.L. Characteristic compressive strength of a geo polymer concrete. Mater. Today Proc. 2021, 37, 2219–2222. [Google Scholar] [CrossRef]
- Das, S.K.; Mishra, J.; Singh, S.K.; Mustakim, S.M.; Patel, A.; Das, S.K.; Behera, U. Characterization and utilization of rice husk ash (RHA) in fly ash—Blast furnace slag based geopolymer concrete for sustainable future. Mater. Today Proc. 2020, 33, 5162–5167. [Google Scholar] [CrossRef]
- Zidi, Z.; Ltifi, M.; Ayadi, Z.B.; Mir, L.E.; Nóvoa, X.R. Effect of nano-ZnO on mechanical and thermal properties of geopolymer. J. Asian Ceram. Soc. 2020, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- De Azevedo, A.R.G.; Marvila, M.T.; de Oliveira, L.B.; Ferreira, W.M.; Colorado, H.; Teixeira, S.R.; Vieira, C.M.F. Circular economy and durability in geopolymers ceramics pieces obtained from glass polishing waste. Int. J. Appl. Ceram. Technol. 2021, 18, 1891–1900. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. Utilization of cement kiln dust (CKD) to enhance mine tailings-based geopolymer bricks. Constr. Build. Mater. 2013, 40, 1002–1011. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Elmoaty, A.E.M.A.; Salem, H.A. Effect of water addition, plasticizer and alkaline solution constitution on fly ash based geopolymer concrete performance. Constr. Build. Mater. 2016, 121, 694–703. [Google Scholar] [CrossRef]
- Assi, L.N.; Deaver, E.E.; Ziehl, P. Effect of source and particle size distribution on the mechanical and microstructural properties of fly Ash-Based geopolymer concrete. Constr. Build. Mater. 2018, 167, 372–380. [Google Scholar] [CrossRef]
- Thormark, C. A low energy building in a life cycle—its embodied energy, energy need for operation and recycling potential. Build. Environ. 2002, 37, 429–435. [Google Scholar] [CrossRef]
- Azari, R.; Abbasabadi, N. Embodied energy of buildings: A review of data, methods, challenges, and research trends. Energy Build. 2018, 168, 225–235. [Google Scholar] [CrossRef]

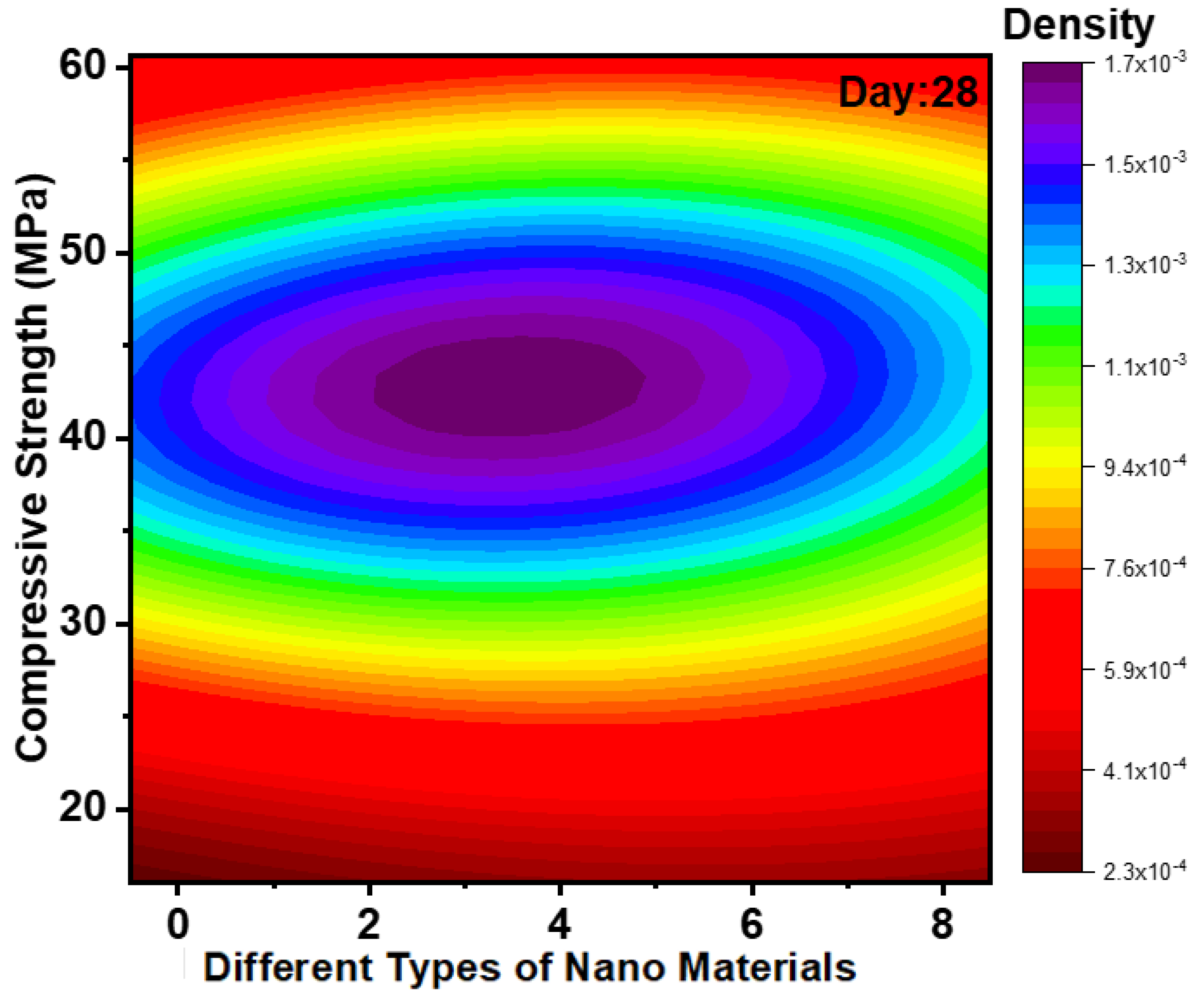


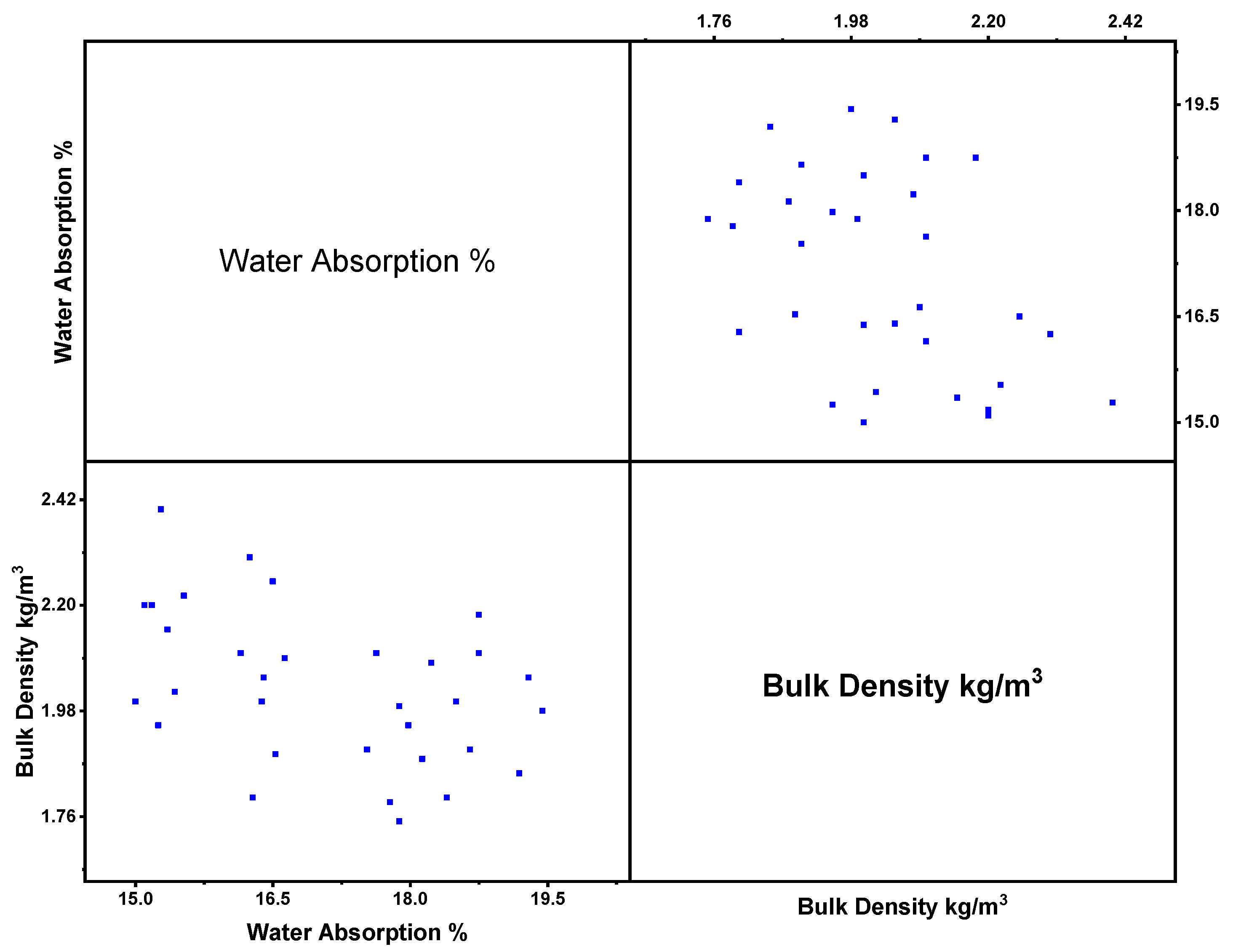
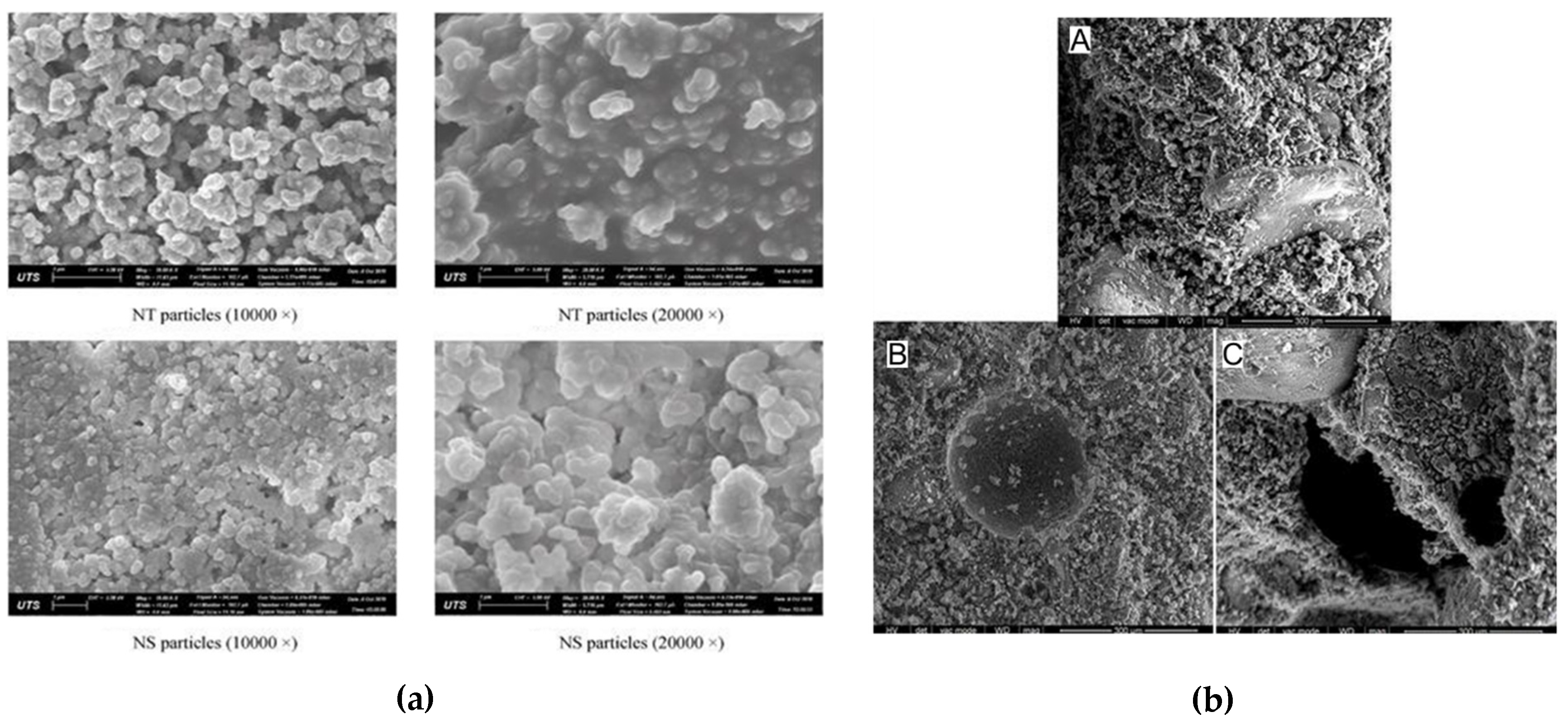
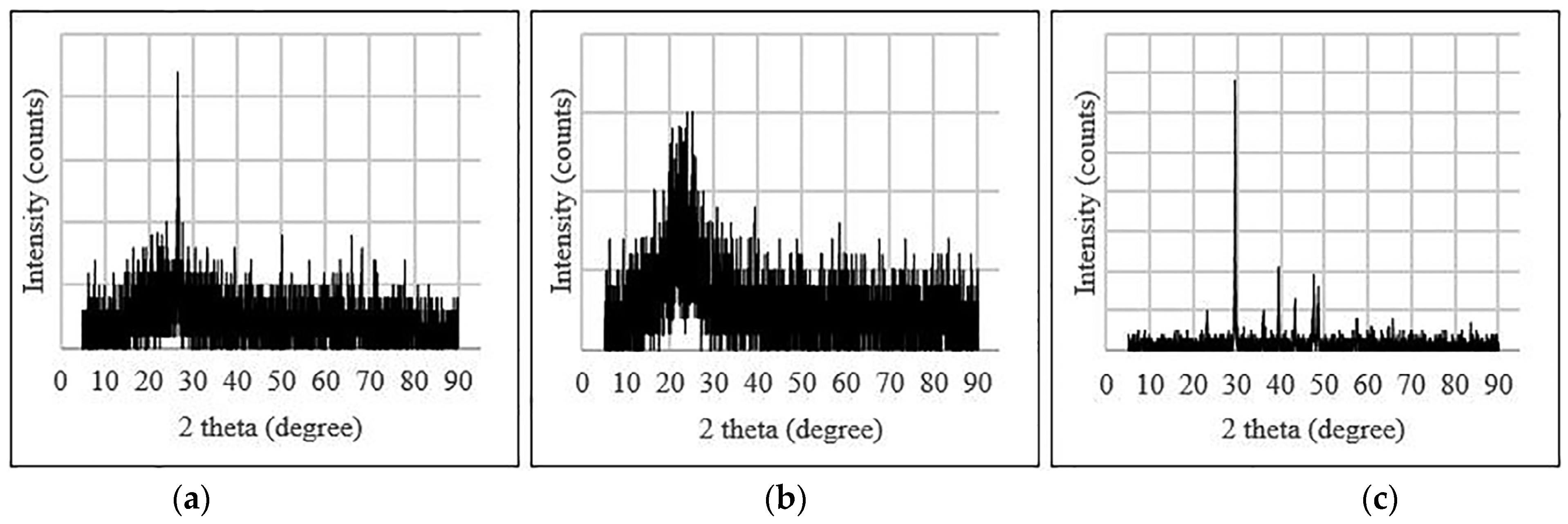

| Binder | Size | % of Variation | Max. CS/STS/FS/WA | Tests | Molarity | Code | References |
|---|---|---|---|---|---|---|---|
| Kaoline + Silica fume | 50 mm3 | 5, 10, 15 | 10%–46 MPa | CS, EDS, XRD | - | ASTM C109 | [45] |
| Metakaolin + NS | 40 mm3 | 1, 2, 3 | 2%–28 MPa | Efflorescence, CS, WA, FTIR, SEM, EDX, XRD | - | ASTM C109 | [4] |
| FA + NS + Micro Silica | - | 5, 10, 15 | 5%–15.2 MPa | Flow test, CS, WA, FTIR, XRD | - | ASTM C109, Flow test-ASTM1437-16WA-ASTM, C1403-15 | [46] |
| FA + Pumice (Sand) | 50 mm3-CS, 40 × 40 × 160 mm-FS | 35 | 14 M–29 MPa-CS 14 M–4 MPa-FS | Flow tests, CS, FS, Ultrasonic pulse velocity, Rheology | 10, 12, 14 | CS-ASTM C109 Flow Test-ASTM C 1437, FS-ASTM C348-18 UPV-ASTM C597-16 | [42] |
| FA + NS + NCC | 40 × 40 × 160, mm-FS | 1, 2, 3 | 12 M–69.7 MPa-CS 12 M–10.7 MPa-FS | CS, FS, FESEM, Ultrasonic pulse velocities | 8, 10, 12 | FS-TS EN 1015–11, Flow test-TSEN 1015–3 | [8] |
| Metakaolin + NZ | Dia. of 30 mm and ht. of 60 mm-CS | 0.3, 0.5, 0.7 | 0.5%–38 MPa | CS, SEM, XRD, FTIR, RXF, MIP, thermogravimetric, Density and WA | 10 | WA-ASTM C140, CS-ASTM D1633-00 | [47] |
| FA + GGBS + NS | 70.6 mm3-CS, | 0, 1, 2 | 2%–70.20 MPa-CS 2%–5.22 MPa-STS, 2%–5.57 MPa-FS | CS, STS, FS, WA, RCPT, Water Sorptivity | 3 | CS-ASTM (2001) C 109, STS-ASTM (2001) C496, FS-ASTM (2001) C 78 | [48] |
| FA + GGBS + Glass bottles waste Nano powder | 50 mm3 CS, 40 × 40 × 160, mm-FS, 150(L), 75(D) mm-STS | 5, 10, 15, 20 | 5%–65 MPa-CS, 5%–6.8 MPa-FS, 5%–4.7 MPa-STS, 20%–10.2%-WA | Flow test, CS, STS, FS, TGA, FTIR, SEM, XRD, WA | 2 | FS-ASTM C78, STS-ASTM C496/C496M-11, CS-ASTM C109/109M | [41] |
| FA + NS + NT | 50 mm3-CS | 2% | 35.8 MPa and 33.7 MPa for NS + NT | CS, XRD, SEM, TGA, Hydration heat test | - | CS-ASTM C109 | [49] |
| FA + GGBS + NS | 75(D) × 150 (H) mm-CS | 0, 0.5, 1, 1.5, 2.0, 2.5, 3.0 | 2.5%–53.2 MPa-CS | Flow test, CS, ATR-FTIR, FESEM, TGA | 10 | Flow tests-ASTM C1437 | [26] |
| FA + OGPC + GGBS + NS | 50 mm3-CS | 0, 0.5, 1, 1.5, 2.0, 2.5, 3.0 | 3%–56 MPa | Flow, CS, SEM, EDX, XRD | 8 | CS-ASTM C109, C1437 | [27] |
| FA + NT | 40 mm3-CS | 1, 3, 5 | 5%–22% higher than reference sample | Workability, CS, SEM, XRD | 10 | Workability-ASTM C230 | [50] |
| Metakaolin + NS | - | 0, 1, 2, 3 | 1%–15.8 MPa-FS | FS, MAS NMR, TG/DTA | - | - | [28] |
| Metakaolin + NS | 70.6 mm3-CS | 0, 2, 4, 6, 8, 10 | 4%–52.77 MPa-CS | CS, SEM | 14 | - | [31] |
| Air cooled slag + Water cooled slag + MVCNT | 25 mm3-CS | 0.1, 0.2, 0.3 | 0.1%–20.5 MPa-CS | SEM, XRD, FTIR | 6 | - | [7] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Khan, T.M.Y.; Almakayeel, N.M.; Alghamdi, S. Review on the Relationship between Nano Modifications of Geopolymer Concrete and Their Structural Characteristics. Polymers 2022, 14, 1421. https://doi.org/10.3390/polym14071421
Shilar FA, Ganachari SV, Patil VB, Khan TMY, Almakayeel NM, Alghamdi S. Review on the Relationship between Nano Modifications of Geopolymer Concrete and Their Structural Characteristics. Polymers. 2022; 14(7):1421. https://doi.org/10.3390/polym14071421
Chicago/Turabian StyleShilar, Fatheali A., Sharanabasava V. Ganachari, Veerabhadragouda B. Patil, T. M. Yunus Khan, Naif Mana Almakayeel, and Saleh Alghamdi. 2022. "Review on the Relationship between Nano Modifications of Geopolymer Concrete and Their Structural Characteristics" Polymers 14, no. 7: 1421. https://doi.org/10.3390/polym14071421
APA StyleShilar, F. A., Ganachari, S. V., Patil, V. B., Khan, T. M. Y., Almakayeel, N. M., & Alghamdi, S. (2022). Review on the Relationship between Nano Modifications of Geopolymer Concrete and Their Structural Characteristics. Polymers, 14(7), 1421. https://doi.org/10.3390/polym14071421










