Threonine-Based Stimuli-Responsive Nanoparticles with Aggregation-Induced Emission-Type Fixed Cores for Detection of Amines in Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Threonine-Containing Amphiphilic Block Copolymer
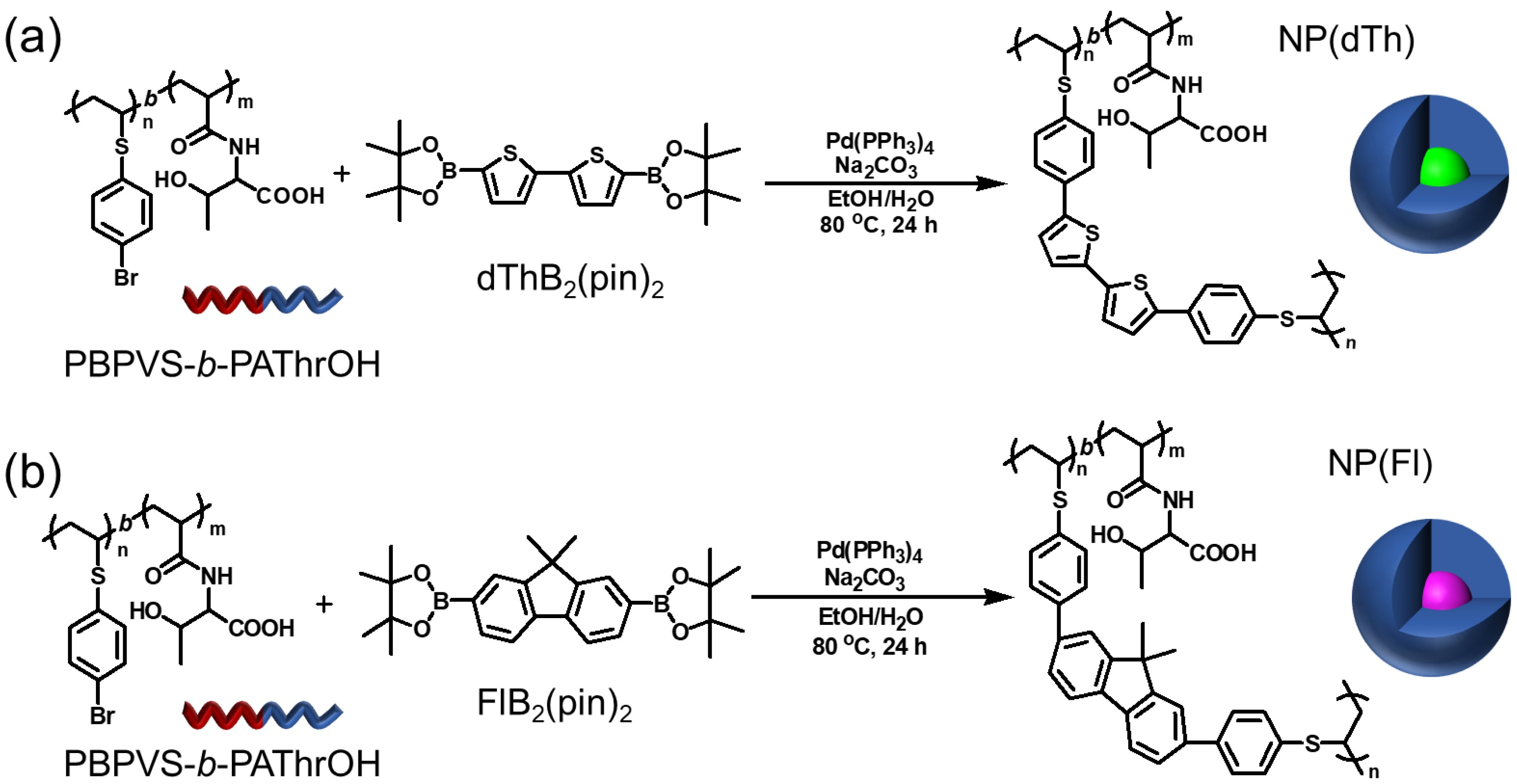
2.3. Synthesis of Fluorescent NPs with a Threonine-Based Polyelectrolyte Shell
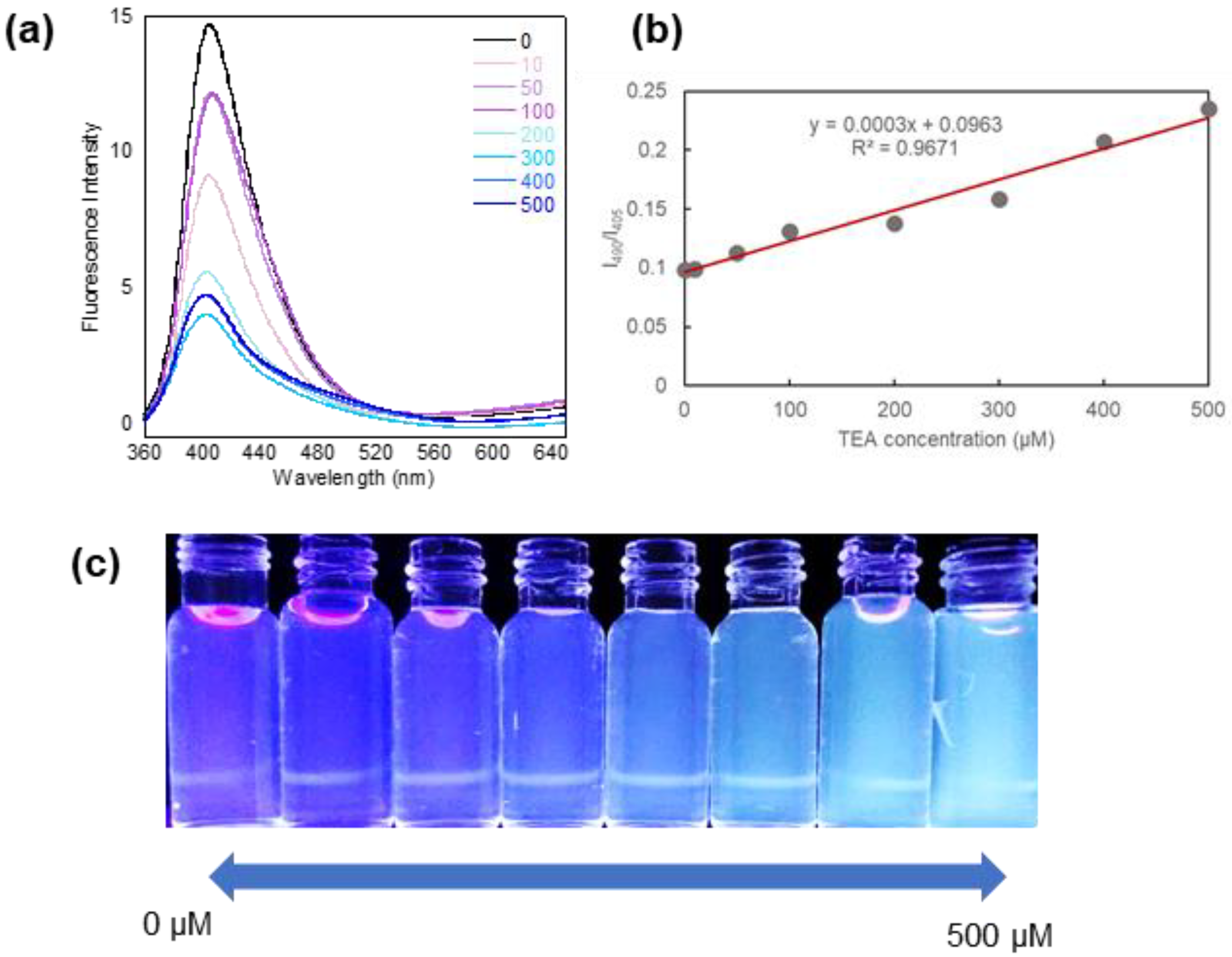
2.4. Detection of Amine Compounds
2.5. Instrumentation
3. Results
3.1. Synthesis of Threonine-Containing Amphiphilic Block Copolymer
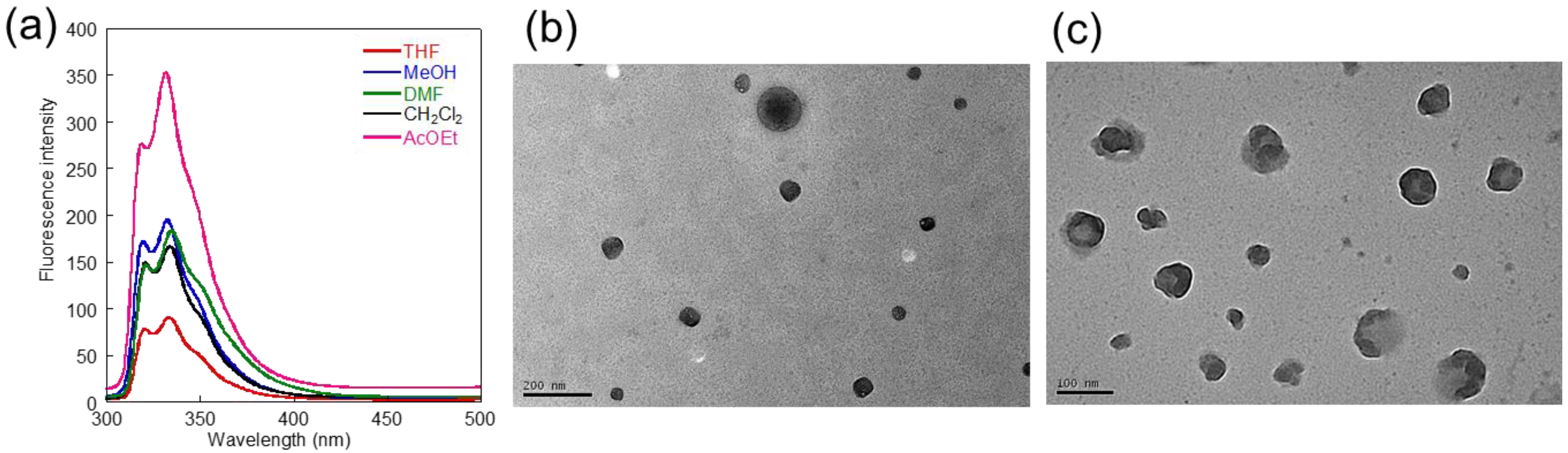
3.2. Synthesis and Fluorescent Properties of Core-Cross-Linked NPs
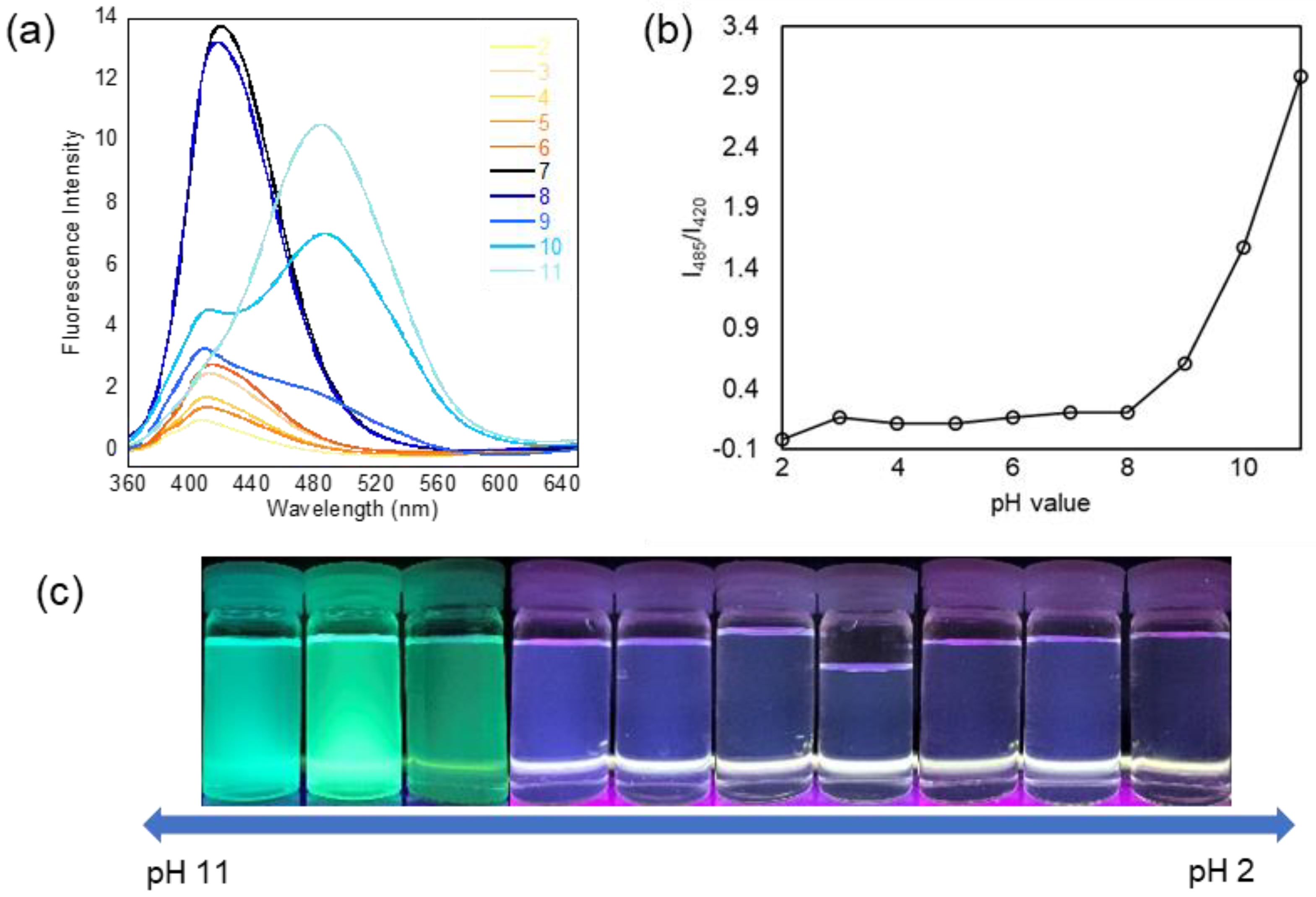
3.3. pH-Dependent Fluorescence and Detection for Amine Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pecher, J.; Mecking, S. Nanoparticles of Conjugated Polymers. Chem. Rev. 2010, 110, 6260–6279. [Google Scholar] [CrossRef] [PubMed]
- Grigalevicius, S.; Forster, M.; Ellinger, S.; Landfester, K.; Scherf, U. Excitation energy transfer from semi-conducting polymer nanoparticles to surface-bound fluorescent dyes. Macromol. Rapid Commun. 2006, 27, 200–202. [Google Scholar] [CrossRef]
- Wu, C.; Peng, H.; Jiang, Y.; McNeill, J. Energy transfer mediated fluorescence from blended conjugated polymer nanoparticles. J. Phys. Chem. B 2006, 110, 14148–14154. [Google Scholar] [CrossRef]
- Wu, C.; Szymanski, C.; McNeill, J. Preparation and encapsulation of highly fluorescent conjugated polymer nanoparticles. Langmuir 2006, 22, 2956–2960. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, B. Polymer-encapsulated organic nanoparticles for fluorescence and photoacoustic imaging. Chem. Soc. Rev. 2014, 43, 6570–6597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuncel, D.; Demir, H.V. Conjugated polymer nanoparticles. Nanoscale 2010, 2, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Kietzke, T.; Neher, D.; Landfester, K.; Montenegro, R.; Guntner, R.; Scherf, U. Novel approaches to polymer blends based on polymer nanoparticles. Nat. Mater. 2003, 2, 408–412. [Google Scholar] [CrossRef]
- Fisslthaler, E.; Bluemel, A.; Landfester, K.; Scherf, U.; List, E.J.W. Printing functional nanostructures: A novel route towards nanostructuring of organic electronic devices via soft embossing, inkjet printing and colloidal self assembly of semiconducting polymer nanospheres. Soft Matter 2008, 4, 2448–2453. [Google Scholar] [CrossRef]
- Mauthner, G.; Landfester, K.; Köck, A.; Brückl, H.; Kast, M.; Stepper, C.; List, E.J.W. Inkjet printed surface cell light-emitting devices from a water-based polymer dispersion. Org. Electron. 2008, 9, 164–170. [Google Scholar] [CrossRef]
- Wu, C.; Szymanski, C.; Cain, Z.; McNeill, J. Conjugated Polymer Dots for Multiphoton Fluorescence Imaging. J. Am. Chem. Soc. 2007, 129, 12904–12905. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.; Wu, W.; Wan, W.; Li, A.D.Q. Single-Chromophore-Based Photoswitchable Nanoparticles Enable Dual-Alternating-Color Fluorescence for Unambiguous Live Cell Imaging. J. Am. Chem. Soc. 2009, 131, 4245–4252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Wu, W.; Zhu, M.-Q.; Han, J.J.; Hurst, J.K.; Li, A.D.Q. Reversibly photoswitchable dual-color fluorescent nanoparticles as new tools for live-cell imaging. J. Am. Chem. Soc. 2007, 129, 3524–3526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, J.H.; McDaniel, W.; MacLean, P.; Hancock, L.F. Live-cell-permeable poly(p-phenylene ethynylene). Angew. Chem. Int. Ed. 2007, 46, 8223–8225. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-Q.; Zhu, L.; Han, J.J.; Wu, W.; Hurst, J.K.; Li, A.D.Q. Spiropyran-Based Photochromic Polymer Nanoparticles with Optically Switchable Luminescence. J. Am. Chem. Soc. 2006, 128, 4303–4309. [Google Scholar] [CrossRef] [Green Version]
- Fischer, C.S.; Baier, M.C.; Mecking, S. Enhanced Brightness Emission-Tuned Nanoparticles from Heterodifunctional Polyfluorene Building Blocks. J. Am. Chem. Soc. 2013, 135, 1148–1154. [Google Scholar] [CrossRef]
- Chen, C.-P.; Huang, Y.-C.; Liou, S.-Y.; Wu, P.-J.; Kuo, S.-Y.; Chan, Y.-H. Near-Infrared Fluorescent Semiconducting Polymer Dots with High Brightness and Pronounced Effect of Positioning Alkyl Chains on the Comonomers. ACS Appl. Mater. Interfaces 2014, 6, 21585–21595. [Google Scholar] [CrossRef]
- Yang, C.; Liu, H.; Zhang, Y.; Xu, Z.; Wang, X.; Cao, B.; Wang, M. Hydrophobic-Sheath Segregated Macromolecular Fluorophores: Colloidal Nanoparticles of Polycaprolactone-Grafted Conjugated Polymers with Bright Far-Red/Near-Infrared Emission for Biological Imaging. Biomacromolecules 2016, 17, 1673–1683. [Google Scholar] [CrossRef]
- Yang, C.; Huang, S.; Wang, X.; Wang, M. Theranostic unimolecular micelles of highly fluorescent conjugated polymer bottlebrushes for far red/near infrared bioimaging and efficient anticancer drug delivery. Polym. Chem. 2016, 7, 7455–7468. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, K.; Huang, G.; Hensley, C.; Huang, X.; Ma, X.; Zhao, T.; Sumer, B.D.; DeBerardinis, R.J.; Gao, J. A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nat. Mater. 2014, 13, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Ng, K.K.; Zheng, G. Molecular Interactions in Organic Nanoparticles for Phototheranostic Applications. Chem. Rev. 2015, 115, 11012–11042. [Google Scholar] [CrossRef]
- Canfarotta, F.; Whitcombe, M.J.; Piletsky, S.A. Polymeric nanoparticles for optical sensing. Biotechnol. Adv. 2013, 31, 1585–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Liu, S. Polymeric assemblies and nanoparticles with stimuli-responsive fluorescence emission characteristics. Chem. Commun. 2012, 48, 3262–3278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Yin, M. Design and development of fluorescent nanostructures for bioimaging. Prog. Polym. Sci. 2014, 39, 365–395. [Google Scholar] [CrossRef]
- Hasseb, A.A.; Ghani, N.d.T.A.; Shehab, O.R.; El Nashar, R.M. Application of molecularly imprinted polymers for electrochemical detection of some important biomedical markers and pathogens. Curr. Opin. Electrochem. 2022, 31, 100848. [Google Scholar] [CrossRef]
- Ziai, Y.; Petronella, F.; Rinoldi, C.; Nakielski, P.; Zakrzewska, A.; Kowalewski, T.A.; Augustyniak, W.; Li, X.; Calogero, A.; Sabala, I.; et al. Chameleon-inspired multifunctional plasmonic nanoplatforms for biosensing applications. NPG Asian Mater. 2022, 14, 18. [Google Scholar] [CrossRef]
- Schaeferling, M. The Art of Fluorescence Imaging with Chemical Sensors. Angew. Chem. Int. Ed. 2012, 51, 3532–3554. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Ge, J.; Zhang, H.; Wang, P. New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem. Soc. Rev. 2011, 40, 3483–3495. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Zheng, K.; He, L.; Huang, W. Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chem. Soc. Rev. 2013, 42, 622–661. [Google Scholar] [CrossRef]
- Sathiskumar, U.; Easwaramoorthi, S. Red-Emitting Ratiometric Fluorescence Chemodosimeter for the Discriminative Detection of Aromatic and Aliphatic Amines. ChemistrySelect 2019, 4, 7486–7494. [Google Scholar] [CrossRef]
- Mallick, A.; El-Zohry, A.M.; Shekhah, O.; Yin, J.; Jia, J.; Aggarwal, H.; Emwas, A.-H.; Mohammed, O.F.; Eddaoudi, M. Unprecedented Ultralow Detection Limit of Amines using a Thiadiazole-Functionalized Zr(IV)-Based Metal-Organic Framework. J. Am. Chem. Soc. 2019, 141, 7245–7249. [Google Scholar] [CrossRef] [Green Version]
- Oliveri, I.P.; Di Bella, S. Sensitive Fluorescent Detection and Lewis Basicity of Aliphatic Amines. J. Phys. Chem. A 2011, 115, 14325–14330. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Nonoguchi, M.; Aya, T.; Ogoshi, H. Molecular recognition of alpha, omega-diamines by metalloporphyrin dimer. Tetrahedron Lett. 1997, 38, 1603–1606. [Google Scholar] [CrossRef]
- Fu, Y.; Yao, J.; Xu, W.; Fan, T.; He, Q.; Zhu, D.; Cao, H.; Cheng, J. Reversible and “fingerprint” fluorescence differentiation of organic amine vapours using a single conjugated polymer probe. Polym. Chem. 2015, 6, 2179–2182. [Google Scholar] [CrossRef]
- Gao, M.; Li, S.; Lin, Y.; Geng, Y.; Ling, X.; Wang, L.; Qin, A.; Tang, B.Z. Fluorescent Light-Up Detection of Amine Vapors Based on Aggregation-Induced Emission. ACS Sens. 2016, 1, 179–184. [Google Scholar] [CrossRef]
- Gao, L.-f.; Lin, X.; Hai, X.; Chen, X.-w.; Wang, J.-h. Polymeric Ionic Liquid-Based Fluorescent Amphiphilic Block Copolymer Micelle for Selective and Sensitive Detection of p-Phenylenediamine. ACS Appl. Mater. Interfaces 2018, 10, 43049–43056. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Duan, J.; Mao, Q.; Zhang, S.; Lv, J. Direct chemiluminescent sensing of para-Phenylenediamine over its isomers and analogues via luminol diazotization. Sens. Actuators B Chem. 2019, 287, 173–179. [Google Scholar] [CrossRef]
- Zhao, Y.-J.; Miao, K.; Zhu, Z.; Fan, L.-J. Fluorescence Quenching of a Conjugated Polymer by Synergistic Amine-Carboxylic Acid and pi-pi Interactions for Selective Detection of Aromatic Amines in Aqueous Solution. ACS Sens. 2017, 2, 842–847. [Google Scholar] [CrossRef]
- Rheingans, O.; Hugenberg, N.; Harris, J.R.; Fischer, K.; Maskos, M. Nanoparticles Built of Cross-Linked Heterotelechelic, Amphiphilic Poly(dimethylsiloxane)-b-poly(ethylene oxide) Diblock Copolymers. Macromolecules 2000, 33, 4780–4790. [Google Scholar] [CrossRef]
- Henselwood, F.; Liu, G. Water-soluble nanospheres of poly(2-cinnamoylethyl methacrylate)-block-poly(acrylic acid). Macromolecules 1997, 30, 488–493. [Google Scholar] [CrossRef]
- Guo, A.; Liu, G.; Tao, J. Star Polymers and Nanospheres from Cross-Linkable Diblock Copolymers. Macromolecules 1996, 29, 2487–2493. [Google Scholar] [CrossRef]
- Chen, D.; Peng, H.; Jiang, M. A Novel One-Step Approach to Core-Stabilized Nanoparticles at High Solid Contents. Macromolecules 2003, 36, 2576–2578. [Google Scholar] [CrossRef]
- Bronich, T.K.; Keifer, P.A.; Shlyakhtenko, L.S.; Kabanov, A.V. Polymer Micelle with Cross-Linked Ionic Core. J. Am. Chem. Soc. 2005, 127, 8236–8237. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Hoogenboom, R.; Leenen, M.A.M.; Guillet, P.; Jonas, A.M.; Schubert, U.S.; Gohy, J.-F. Solvent-Induced Morphological Transition in Core-Cross-Linked Block Copolymer Micelles. J. Am. Chem. Soc. 2006, 128, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Nagasaki, Y.; Okada, T.; Kato, M.; Kataoka, K. Core-Polymerized Reactive Micelles from Heterotelechelic Amphiphilic Block Copolymers. Macromolecules 1999, 32, 1140–1146. [Google Scholar] [CrossRef]
- Pioge, S.; Nesterenko, A.; Brotons, G.; Pascual, S.; Fontaine, L.; Gaillard, C.; Nicol, E. Core Cross-Linking of Dynamic Diblock Copolymer Micelles: Quantitative Study of Photopolymerization Efficiency and Micelle Structure. Macromolecules 2011, 44, 594–603. [Google Scholar] [CrossRef]
- Anger, C.; Deubel, F.; Salzinger, S.; Stohrer, J.; Halbach, T.; Jordan, R.; Veinot, J.G.C.; Rieger, B. Organic-Inorganic Hybrid Nanoparticles via Photoinduced Micellation and Siloxane Core Cross-Linking of Stimuli-Responsive Copolymers. ACS Macro Lett. 2013, 2, 121–124. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Oya, H.; Mori, H. Cross-linked core-shell nanoparticles based on amphiphilic block copolymers by RAFT polymerization and palladium-catalyzed suzuki coupling reaction. Macromolecules 2012, 45, 3197–3204. [Google Scholar] [CrossRef]
- Mori, H.; Takano, K.; Endo, T. RAFT Polymerization of vinylthiophene derivatives and synthesis of block copolymers having cross-linkable segments. Macromolecules 2009, 42, 7342–7352. [Google Scholar] [CrossRef]
- Abiko, Y.; Matsumura, A.; Nakabayashi, K.; Mori, H. Thermoresponsive core–shell nanoparticles with cross-linked π-conjugate core based on amphiphilic block copolymers by RAFT polymerization and palladium-catalyzed coupling reactions. Polymer 2014, 55, 6025–6035. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Noda, D.; Watanabe, Y.; Mori, H. Rylene bisimide-based nanoparticles with cross-linked core and thermoresponsive shell using poly(vinyl amine)-based block copolymers. Polymer 2015, 68, 17–24. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Noda, D.; Takahashi, T.; Mori, H. Design of stimuli-responsive nanoparticles with optoelectronic cores by post-assembly cross-linking and self-assembly of functionalized block copolymers. Polymer 2016, 86, 56–68. [Google Scholar] [CrossRef]
- Rudolph, T.; Schacher, F.H. Selective crosslinking or addressing of individual domains within block copolymer nanostructures. Eur. Polym. J. 2016, 80, 317–331. [Google Scholar] [CrossRef]
- Lo, C.-T.; Watanabe, Y.; Oya, H.; Nakabayashi, K.; Mori, H.; Chen, W.-C. Non-volatile transistor memory devices using charge storage cross-linked core-shell nanoparticles. Chem. Commun. 2016, 52, 7269–7272. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, K.; Takahashi, T.; Sugawara, R.; Lo, C.-T.; Mori, H. Benzothiadiazole-based donor-acceptor nanoparticles with solvatochromic and thermoresponsive properties. React. Funct. Polym. 2018, 131, 350–360. [Google Scholar] [CrossRef]
- Lo, C.-T.; Watanabe, Y.; Murakami, D.; Shih, C.-C.; Nakabayashi, K.; Mori, H.; Chen, W.-C. Donor-Acceptor Core-Shell Nanoparticles and Their Application in Non-Volatile Transistor Memory Devices. Macromol. Rapid Commun. 2019, 40, 1900115. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, M.; Nakabayashi, K.; Mori, H. Aggregation-induced multicolor luminescent nanoparticles with adaptive and fixed cores derived from brominated tetraphenylethene-containing block copolymer. J. Polym. Sci. 2021, 59, 532–546. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Takata, M.; Furukawa, M.; Mori, H. Luminescent core-shell nanoparticles with crosslinked aggregation-induced emission core structures: Emission both in solution and solid states. J. Polym. Sci. 2020, 58, 852–859. [Google Scholar] [CrossRef]
- Zhao, Y.; Higashihara, T.; Sugiyama, K.; Hirao, A. Synthesis of functionalized asymmetric star polymers containing conductive polyacetylene segments by living anionic polymerization. J. Am. Chem. Soc. 2005, 127, 14158–14159. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Abiko, Y.; Mori, H. RAFT Polymerization of S-vinyl sulfide derivatives and synthesis of block copolymers having two distinct optoelectronic functionalities. Macromolecules 2013, 46, 5998–6012. [Google Scholar] [CrossRef]
- Shoji, K.; Nakayama, M.; Koseki, T.; Nakabayashi, K.; Mori, H. Threonine-based chiral homopolymers with multi-stimuli-responsive property by RAFT polymerization. Polymer 2016, 97, 20–30. [Google Scholar] [CrossRef]
- Chiefari, J.; Mayadunne, R.T.A.; Moad, C.L.; Moad, G.; Rizzardo, E.; Postma, A.; Skidmore, M.A.; Thang, S.H. Thiocarbonylthio compounds (S=C(Z)S-R) in free radical polymerization with reversible addition-fragmentation chain transfer (RAFT polymerization). Effect of the activating group Z. Macromolecules 2003, 36, 2273–2283. [Google Scholar] [CrossRef]
- Mori, H.; Nakano, S.; Endo, T. Controlled Synthesis of Poly(N-ethyl-3-vinylcarbazole) and Block Copolymers via RAFT Polymerization. Macromolecules 2005, 38, 8192–8201. [Google Scholar] [CrossRef]
- Kanto, R.; Qiao, Y.; Masuko, K.; Furusawa, H.; Yano, S.; Nakabayashi, K.; Mori, H. Synthesis, Assembled Structures, and DNA Complexation of Thermoresponsive Lysine-Based Zwitterionic and Cationic Block Copolymers. Langmuir 2019, 35, 4646–4659. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.J.; Peng, J.; Xu, Z.; Fang, R.; Zhang, H.-r.; Xu, X.; Li, L.; Gao, J.; Wong, M.S. AIE-Active Fluorene Derivatives for Solution-Processable Nondoped Blue Organic Light-Emitting Devices (OLEDs). ACS Appl. Mater. Interfaces 2015, 7, 28156–28165. [Google Scholar] [CrossRef]
- Naeem, K.C.; Neenu, K.; Nair, V.C. Effect of Differential Self-Assembly on Mechanochromic Luminescence of Fluorene-Benzothiadiazole-Based Fluorophores. ACS Omega 2017, 2, 9118–9126. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zhang, R.; Liang, X.; Guo, K.; Han, Z.; Lu, X.; Xie, J.; Li, J.; Li, D.; Tian, X. 1, 3-Indanedione functionalized fluorene luminophores: Negative solvatochromism, nanostructure-morphology determined AIE and mechanoresponsive luminescence turn-on. Dye. Pigment. 2018, 155, 225–232. [Google Scholar] [CrossRef]
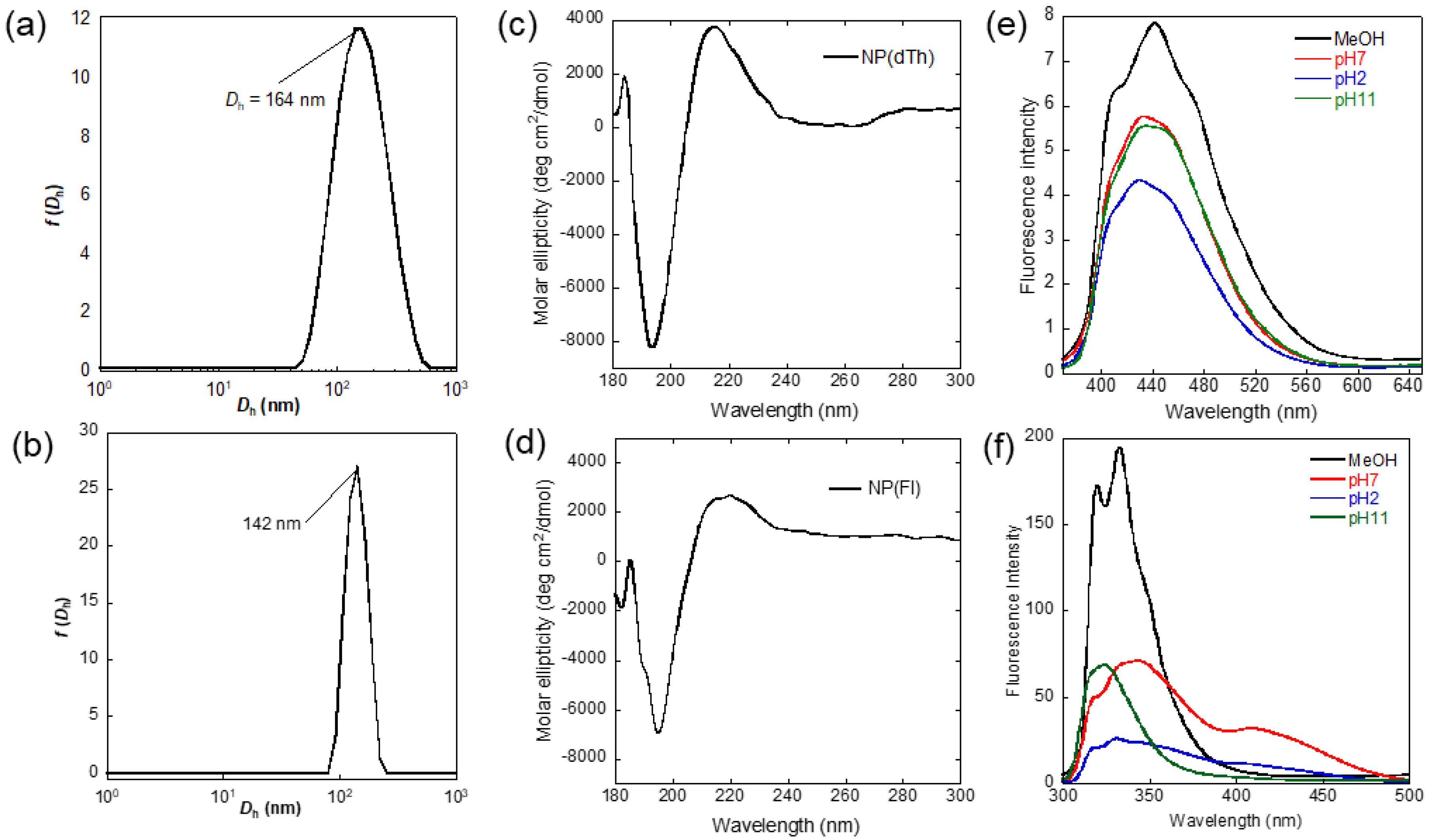


| Product | Yield a | Solvent | Dhb (nm) | Zeta Potential | λmaxabs c | λmaxem d | Φ e |
|---|---|---|---|---|---|---|---|
| NP(dTh) | 54 | CH3OH | 164 | - | 340 | 446 | 0.04 |
| H2O (pH2) | 142 | −2.0 | 341 | 430 | 0.14 | ||
| H2O (pH7) | 220 | −47.7 | 341 | 434 | 0.16 | ||
| H2O (pH11) | 142 | −44.5 | 349 | 435 | 0.04 | ||
| NP(Fl) | 35 | CH3OH | 142 | - | 279 | 333 | 0.22 |
| H2O (pH2) | 122 | −2.1 | 267 | 333 | 0.06 | ||
| H2O (pH7) | 190 | −46.6 | 274 | 343 | 0.58 | ||
| H2O (pH11) | 220 | −45.8 | 267 | 324 | 0.03 |
| Solvent | λmaxabsa | λmaxemb | Φ c |
|---|---|---|---|
| THF | 276 | 334 | 0.04 |
| CH3OH | 280 | 333 | 0.22 |
| DMF | 280 | 335 | 0.23 |
| CH2Cl2 | 290 | 334 | 0.31 |
| AcOEt | 290 | 332 | 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kataoka, K.; Nakabayashi, K.; Lo, C.-T.; Mori, H. Threonine-Based Stimuli-Responsive Nanoparticles with Aggregation-Induced Emission-Type Fixed Cores for Detection of Amines in Aqueous Solutions. Polymers 2022, 14, 1362. https://doi.org/10.3390/polym14071362
Kataoka K, Nakabayashi K, Lo C-T, Mori H. Threonine-Based Stimuli-Responsive Nanoparticles with Aggregation-Induced Emission-Type Fixed Cores for Detection of Amines in Aqueous Solutions. Polymers. 2022; 14(7):1362. https://doi.org/10.3390/polym14071362
Chicago/Turabian StyleKataoka, Keita, Kazuhiro Nakabayashi, Chen-Tsyr Lo, and Hideharu Mori. 2022. "Threonine-Based Stimuli-Responsive Nanoparticles with Aggregation-Induced Emission-Type Fixed Cores for Detection of Amines in Aqueous Solutions" Polymers 14, no. 7: 1362. https://doi.org/10.3390/polym14071362
APA StyleKataoka, K., Nakabayashi, K., Lo, C.-T., & Mori, H. (2022). Threonine-Based Stimuli-Responsive Nanoparticles with Aggregation-Induced Emission-Type Fixed Cores for Detection of Amines in Aqueous Solutions. Polymers, 14(7), 1362. https://doi.org/10.3390/polym14071362







