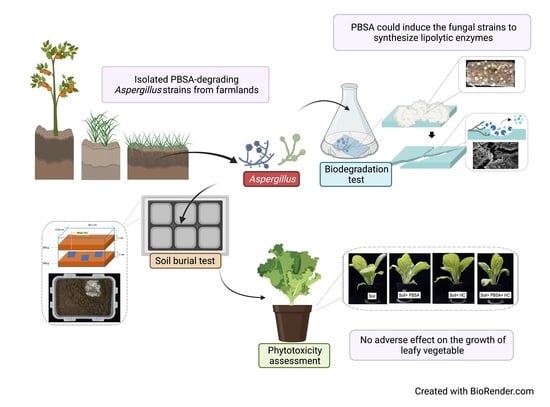
Biodegradation of PBSA Films by Elite Aspergillus Isolates and Farmland Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plastic Material
2.2. Screening and Isolation of PBSA-Degrading Microorganisms from Soils
2.3. Phylogenetic Analysis of ITS Gene Sequences
2.4. Determination of the PBSA Film Degradation Ability of Isolated Fungal Strains
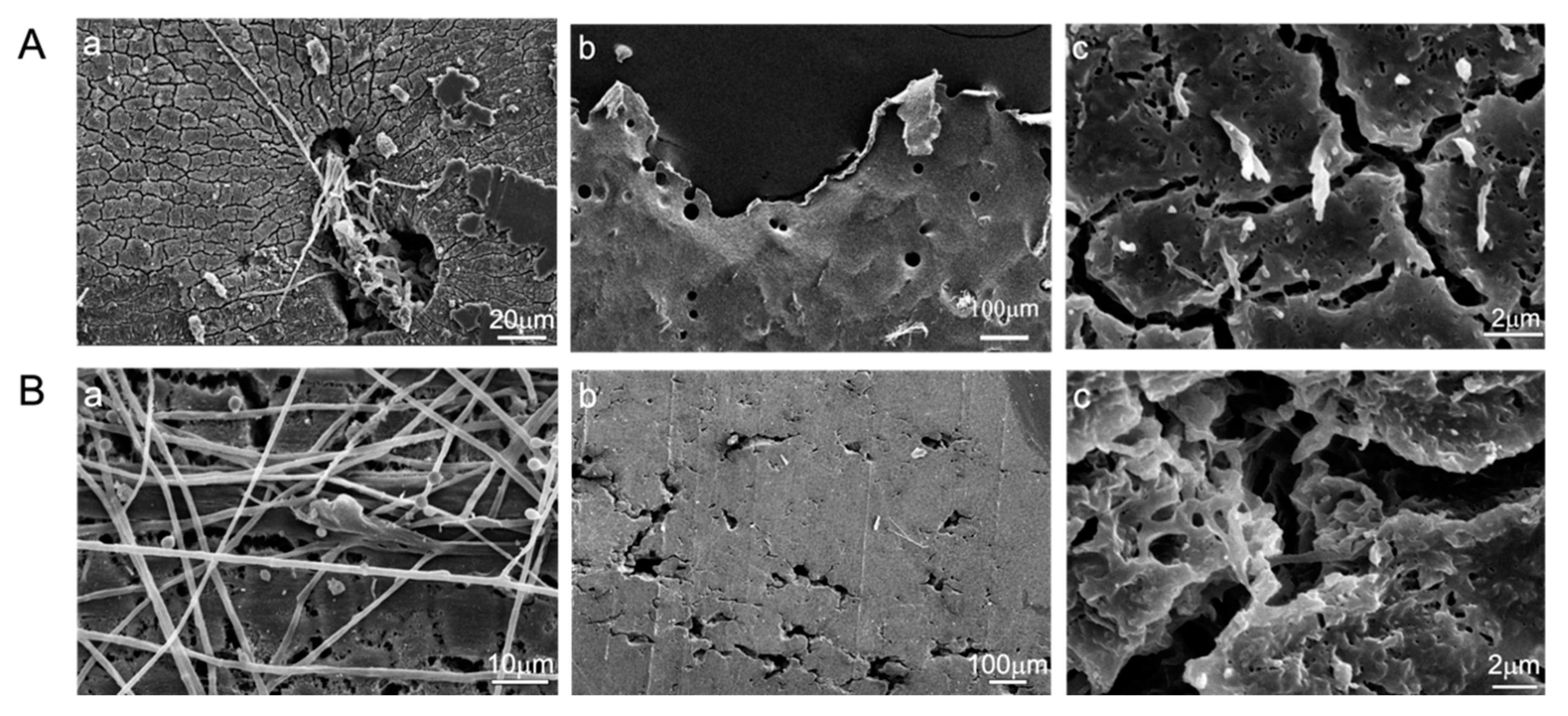
2.5. Scanning Electron Microscopic Analysis
2.6. NMR Analysis
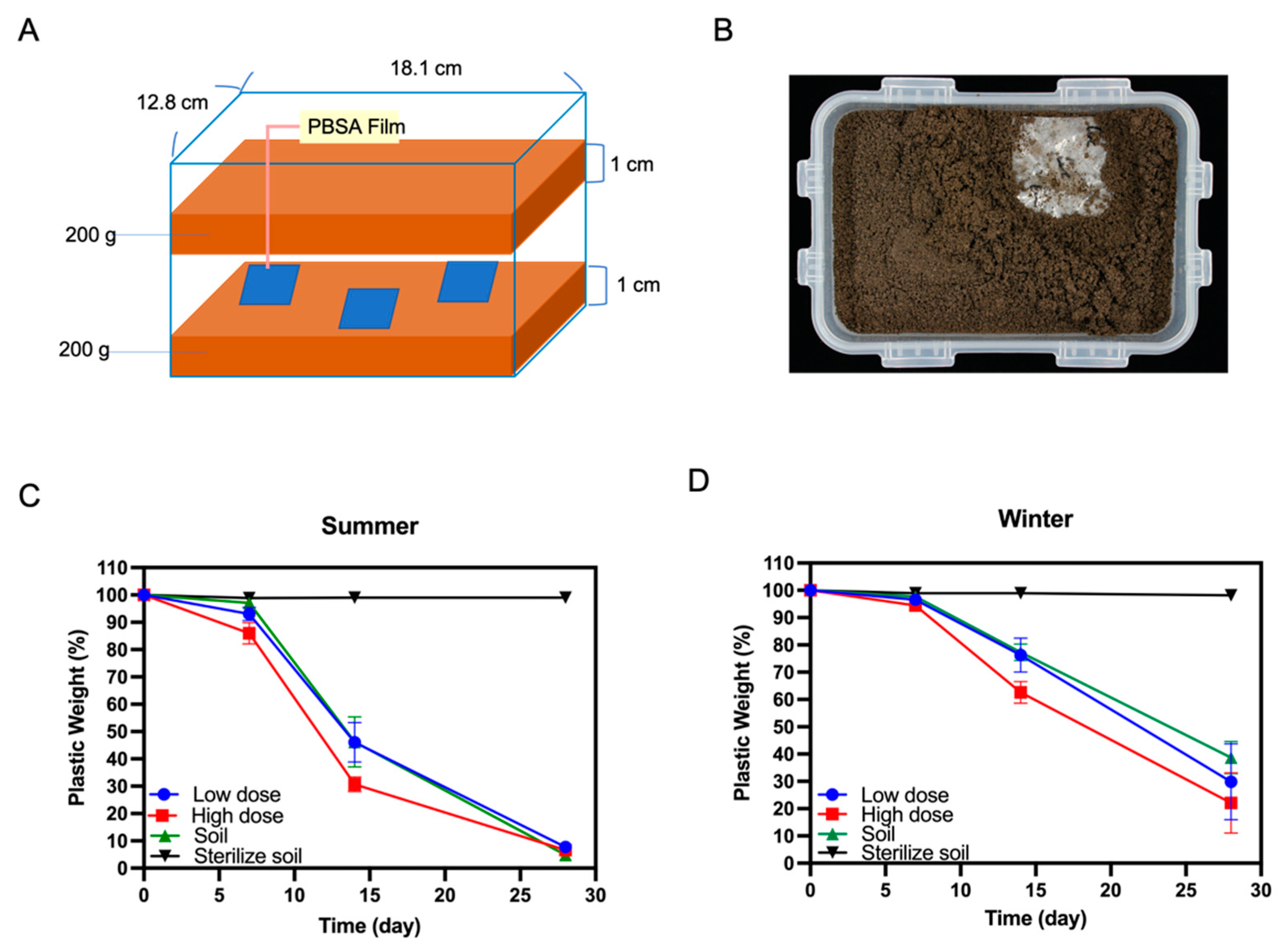
2.7. Soil Burial Tests of PBSA Plastic Films
2.8. Analysis of Lipolytic Enzyme Activities in Culture Supernatant
2.9. Analysis of Lipolytic Enzyme Activities in Soil
2.10. Phytotoxicity Assessment of the Soil Containing PBSA Residues
3. Results
3.1. Isolation and Identification of Two Elite PBSA-Degrading Fungal Strains
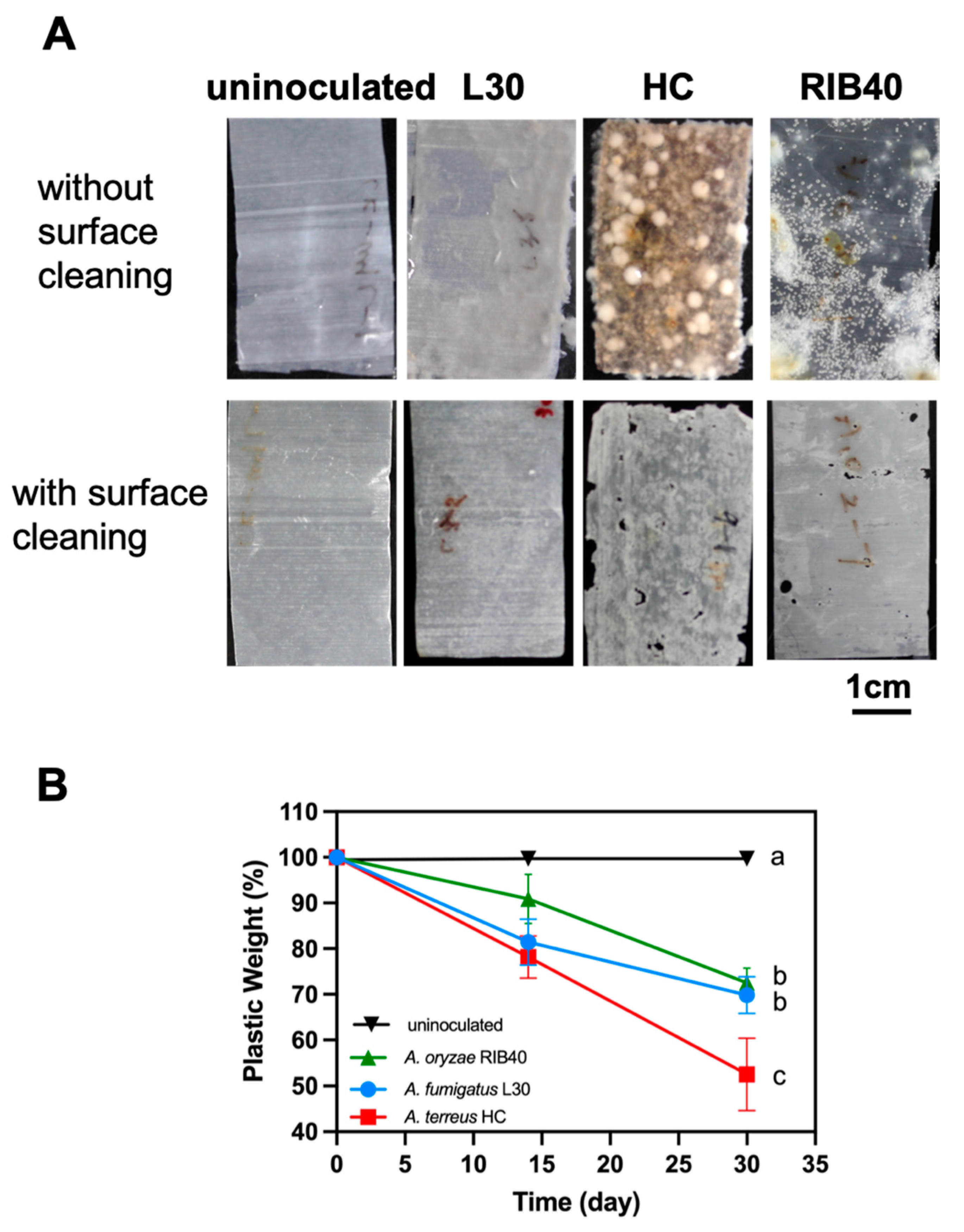
3.2. Two Elite Fungal Strains Showed High PBSA Biodegradation Efficacy
3.3. Effect of Replacement or Addition of Fresh Medium on PBSA Plastic Film Degradation
3.4. Appearance of PBSA Plastic Films Degraded by Two Elite Aspergillus Strains
3.5. Changes in the Chemical Composition of PBSA Films during Biodegradation
3.6. Biodegradation of PBSA Films in Farmland Soil
3.7. Lipolytic Enzyme Activities of A. terreus HC and Soil
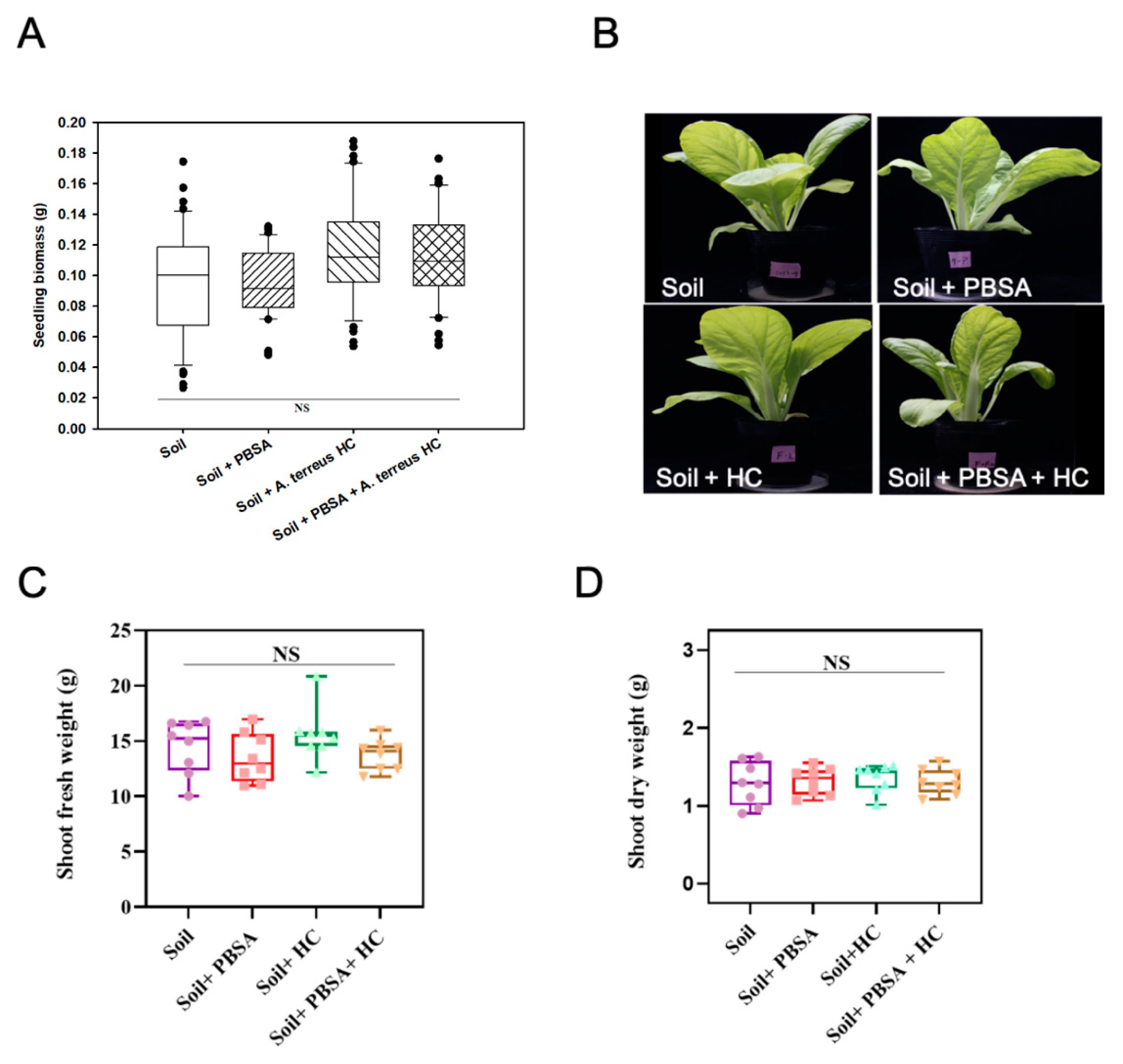
3.8. Phytotoxicity Assessment
4. Discussion
4.1. Individual Aspergillus Strains Showed Unique PBSA Degradation Characteristics
4.2. PBSA Degradation Efficiency of the Winter Soil Can Be Improved by Addition of Fungal Mycelia
4.3. PBSA Could Induce the of Lipolytic Enzyme Activities in Farmland Soil
4.4. No Observed Adverse Effect on the Growth of Leafy Vegetable by PBSA Degradation or the Addition of Elite Fungal Culture in Farmland Soil
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bishop, G.; Styles, D.; Lens, P.N. Recycling of European plastic is a pathway for plastic debris in the ocean. Environ. Int. 2020, 142, 105893. [Google Scholar] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [PubMed] [Green Version]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [PubMed] [Green Version]
- Serrano-Ruiz, H.; Martin-Closas, L.; Pelacho, A.M. Biodegradable plastic mulches: Impact on the agricultural biotic environment. Sci. Total Environ. 2021, 750, 141228. [Google Scholar] [CrossRef] [PubMed]
- Sintim, H.Y.; Flury, M. Is Biodegradable Plastic Mulch the Solution to Agriculture’s Plastic Problem? Environ. Sci. Technol. 2017, 51, 1068–1069. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.-S. Characterization of a novel sound absorption material derived from waste agricultural film. Constr. Build. Mater. 2017, 157, 237–243. [Google Scholar]
- Feuilloley, P.; Cesar, G.; Benguigui, L.; Grohens, Y.; Pillin, I.; Bewa, H.; Lefaux, S.; Jamal, M. Degradation of polyethylene designed for agricultural purposes. J. Polym. Environ. 2005, 13, 349–355. [Google Scholar] [CrossRef]
- Zhang, G.S.; Liu, Y.F. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef]
- Brodhagen, M.; Peyron, M.; Miles, C.; Inglis, D.A. Biodegradable plastic agricultural mulches and key features of microbial degradation. Appl. Microbiol. Biotechnol. 2015, 99, 1039–1056. [Google Scholar] [CrossRef]
- Kitamoto, H.K.; Shinozaki, Y.; Cao, X.-H.; Morita, T.; Konishi, M.; Tago, K.; Kajiwara, H.; Koitabashi, M.; Yoshida, S.; Watanabe, T. Phyllosphere yeasts rapidly break down biodegradable plastics. AMB Express 2011, 1, 44. [Google Scholar]
- Zhao, J.-H.; Wang, X.-Q.; Zeng, J.; Yang, G.; Shi, F.-H.; Yan, Q. Biodegradation of poly(butylene succinate-co-butylene adipate) by Aspergillus versicolor. Polym. Degrad. Stab. 2005, 90, 173–179. [Google Scholar] [CrossRef]
- Puchalski, M.; Szparaga, G.; Biela, T.; Gutowska, A.; Sztajnowski, S.; Krucińska, I. Molecular and supramolecular changes in polybutylene succinate (PBS) and polybutylene succinate adipate (PBSA) copolymer during degradation in various environmental conditions. Polymers 2018, 10, 251. [Google Scholar]
- Palai, B.; Mohanty, S.; Nayak, S.K. Synergistic effect of polylactic acid (PLA) and Poly (butylene succinate-co-adipate)(PBSA) based sustainable, reactive, super toughened eco-composite blown films for flexible packaging applications. Polym. Test. 2020, 83, 106130. [Google Scholar]
- Lee, S.-M.; Lee, J.-W. Characterization and processing of biodegradable polymer blends of poly (lactic acid) with poly (butylene succinate adipate). Korea-Aust. Rheol. J. 2005, 17, 71–77. [Google Scholar]
- Koitabashi, M.; Sameshima–Yamashita, Y.; Watanabe, T.; Shinozaki, Y.; Kitamoto, H. Phylloplane fungal enzyme accelerate decomposition of biodegradable plastic film in agricultural settings. Jpn. Agric. Res. Q. 2016, 50, 229–234. [Google Scholar]
- Yamamoto-Tamura, K.; Hiradate, S.; Watanabe, T.; Koitabashi, M.; Sameshima-Yamashita, Y.; Yarimizu, T.; Kitamoto, H. Contribution of soil esterase to biodegradation of aliphatic polyester agricultural mulch film in cultivated soils. AMB Express 2015, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Haider, T.P.; Volker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Kovacic, F.; Babic, N.; Krauss, U.; Jaeger, K. Classification of lipolytic enzymes from bacteria. Aerob. Util. Hydrocarb. Oils Lipids 2019, 24, 255–289. [Google Scholar]
- Ahmad, A.; Tsutsui, A.; Iijima, S.; Suzuki, T.; Shah, A.A.; Nakajima-Kambe, T. Gene structure and comparative study of two different plastic-degrading esterases from Roseateles depolymerans strain TB-87. Polym. Degrad. Stab. 2019, 164, 109–117. [Google Scholar]
- Tsutsumi, C.; Hayase, N.; Nakagawa, K.; Tanaka, S.; Miyahara, Y. The enzymatic degradation of commercial biodegradable polymers by some lipases and chemical degradation of them. Macromol. Symp. 2003, 197, 431–442. [Google Scholar]
- Hoshino, A.; Isono, Y. Degradation of aliphatic polyester films by commercially available lipases with special reference to rapid and complete degradation of poly(L-lactide) film by lipase PL derived from Alcaligenes sp. Biodegradation 2002, 13, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, Y.; Morita, T.; Cao, X.H.; Yoshida, S.; Koitabashi, M.; Watanabe, T.; Suzuki, K.; Sameshima-Yamashita, Y.; Nakajima-Kambe, T.; Fujii, T.; et al. Biodegradable plastic-degrading enzyme from Pseudozyma antarctica: Cloning, sequencing, and characterization. Appl. Microbiol. Biotechnol. 2013, 97, 2951–2959. [Google Scholar] [CrossRef] [PubMed]
- Tserki, V.; Matzinos, P.; Pavlidou, E.; Vachliotis, D.; Panayiotou, C. Biodegradable aliphatic polyesters. Part I. Properties and biodegradation of poly(butylene succinate-co-butylene adipate). Polym. Degrad. Stab. 2006, 91, 367–376. [Google Scholar] [CrossRef]
- Hajighasemi, M.; Tchigvintsev, A.; Nocek, B.; Flick, R.; Popovic, A.; Hai, T.; Khusnutdinova, A.N.; Brown, G.; Xu, X.; Cui, H. Screening and characterization of novel polyesterases from environmental metagenomes with high hydrolytic activity against synthetic polyesters. Environ. Sci. Technol. 2018, 52, 12388–12401. [Google Scholar]
- Nakajima-Kambe, T.; Toyoshima, K.; Saito, C.; Takaguchi, H.; Akutsu-Shigeno, Y.; Sato, M.; Miyama, K.; Nomura, N.; Uchiyama, H. Rapid monomerization of poly (butylene succinate)-co-(butylene adipate) by Leptothrix sp. J. Biosci. Bioeng. 2009, 108, 513–516. [Google Scholar]
- Waring, S.; Bremner, J. Ammonium production in soil under waterlogged conditions as an index of nitrogen availability. Nature 1964, 201, 951–952. [Google Scholar]
- Sato, S.; Saika, A.; Shinozaki, Y.; Watanabe, T.; Suzuki, K.; Sameshima-Yamashita, Y.; Fukuoka, T.; Habe, H.; Morita, T.; Kitamoto, H. Degradation profiles of biodegradable plastic films by biodegradable plastic-degrading enzymes from the yeast Pseudozyma antarctica and the fungus Paraphoma sp. B47-9. Polym. Degrad. Stab. 2017, 141, 26–32. [Google Scholar]
- Hu, X.; Gao, Z.; Wang, Z.; Su, T.; Yang, L.; Li, P. Enzymatic degradation of poly (butylene succinate) by cutinase cloned from Fusarium solani. Polym. Degrad. Stab. 2016, 134, 211–219. [Google Scholar]
- Merugu, R. Studies on PHB (polyhydroxybutyrate) degradation by some species of Aspergillus. Int. J. ChemTech Res. 2012, 4, 1111–1113. [Google Scholar]
- Vasile, C.; Pamfil, D.; Rapa, M.; Darie-Nita, R.N.; Mitelut, A.C.; Popa, E.E.; Popescu, P.A.; Draghici, M.C.; Popa, M.E. Study of the soil burial degradation of some PLA/CS biocomposites. Compos. Part B Eng. 2018, 142, 251–262. [Google Scholar] [CrossRef]
- Jung, H.-W.; Yang, M.-K.; Su, R.-C. Purification, characterization, and gene cloning of an Aspergillus fumigatus polyhydroxybutyrate depolymerase used for degradation of polyhydroxybutyrate, polyethylene succinate, and polybutylene succinate. Polym. Degrad. Stab. 2018, 154, 186–194. [Google Scholar]
- Nishida, H.; Tokiwa, Y. Distribution of poly (β-hydroxybutyrate) and poly (ε-caprolactone) aerobic degrading microorganisms in different environments. J. Environ. Polym. Degrad. 1993, 1, 227–233. [Google Scholar]
- Yang, J.; Yang, Y.; Wu, W.M.; Zhao, J.; Jiang, L. Evidence of Polyethylene Biodegradation by Bacterial Strains from the Guts of Plastic-Eating Waxworms. Environ. Sci. Technol. 2014, 48, 13776–13784. [Google Scholar] [CrossRef] [PubMed]
- Hoang, K.C.; Tseng, M.; Shu, W.J. Degradation of polyethylene succinate (PES) by a new thermophilic Microbispora strain. Biodegradation 2007, 18, 333–342. [Google Scholar] [CrossRef]
- Manter, D.K.; Vivanco, J.M. Use of the ITS primers, ITS1F and ITS4, to characterize fungal abundance and diversity in mixed-template samples by qPCR and length heterogeneity analysis. J. Microbiol. Methods 2007, 71, 7–14. [Google Scholar]
- Ragonezi, C.; Caldeira, A.T.; Rosário Martins, M.; Salvador, C.; Santos-Silva, C.; Ganhão, E.; Klimaszewska, K.; Zavattieri, A. Molecular approach to characterize ectomycorrhizae fungi from Mediterranean pine stands in Portugal. Braz. J. Microbiol. 2013, 44, 657–665. [Google Scholar]
- Hall, T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- La Mantia, F.P.; Ascione, L.; Mistretta, M.C.; Rapisarda, M.; Rizzarelli, P. Comparative investigation on the soil burial degradation behaviour of polymer films for agriculture before and after photo-oxidation. Polymers 2020, 12, 753. [Google Scholar]
- Popovic, A.; Hai, T.; Tchigvintsev, A.; Hajighasemi, M.; Nocek, B.; Khusnutdinova, A.N.; Brown, G.; Glinos, J.; Flick, R.; Skarina, T. Activity screening of environmental metagenomic libraries reveals novel carboxylesterase families. Sci. Rep. 2017, 7, 44103. [Google Scholar]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly (ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar]
- Sander, D.; Yu, Y.; Sukul, P.; Schäkermann, S.; Bandow, J.E.; Mukherjee, T.; Mukhopadhyay, S.K.; Leichert, L.I. Metaproteomic Discovery and Characterization of a Novel Lipolytic Enzyme from an Indian Hot Spring. Front. Microbiol. 2021, 12, 1136. [Google Scholar]
- Hwang, B.-Y.; Kim, J.-H.; Kim, J.; Kim, B.-G. Screening of Exiguobacterium acetylicum from soil samples showing enantioselective and alkalotolerant esterase activity. Biotechnol. Bioprocess Eng. 2005, 10, 367. [Google Scholar]
- Gilham, D.; Lehner, R. Techniques to measure lipase and esterase activity in vitro. Methods 2005, 36, 139–147. [Google Scholar]
- Margesin, R.; Feller, G.; Hammerle, M.; Stegner, U.; Schinner, F. A colorimetric method for the determination of lipase activity in soil. Biotechnol. Lett. 2002, 24, 27–33. [Google Scholar] [CrossRef]
- Souza, P.M.S.; Sommaggio, L.R.D.; Marin-Morales, M.A.; Morales, A.R. PBAT biodegradable mulch films: Study of ecotoxicological impacts using Allium cepa, Lactuca sativa and HepG2/C3A cell culture. Chemosphere 2020, 256, 126985. [Google Scholar] [PubMed]
- Wong, W.-T.; Tseng, C.-H.; Hsu, S.-H.; Lur, H.-S.; Mo, C.-W.; Huang, C.-N.; Hsu, S.-C.; Lee, K.-T.; Liu, C.-T. Promoting effects of a single Rhodopseudomonas palustris inoculant on plant growth by Brassica rapa chinensis under low fertilizer input. Microbes Environ. 2014, 29, 303–313. [Google Scholar]
- Bano, K.; Kuddus, M.; Zaheer, M.R.; Zia, Q.; Khan, M.F.; Ashraf, G.M.; Gupta, A.; Aliev, G. Microbial Enzymatic Degradation of Biodegradable Plastics. Curr. Pharm. Biotechnol. 2017, 18, 429–440. [Google Scholar] [CrossRef]
- Maeda, H.; Yamagata, Y.; Abe, K.; Hasegawa, F.; Machida, M.; Ishioka, R.; Gomi, K.; Nakajima, T. Purification and characterization of a biodegradable plastic-degrading enzyme from Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2005, 67, 778–788. [Google Scholar] [CrossRef]
- Kliem, S.; Kreutzbruck, M.; Bonten, C. Review on the Biological Degradation of Polymers in Various Environments. Materials 2020, 13, 4586. [Google Scholar] [CrossRef]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Wilkes, R.A.; Aristilde, L. Degradation and metabolism of synthetic plastics and associated products by Pseudomonas sp.: Capabilities and challenges. J. Appl. Microbiol. 2017, 123, 582–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottone, E.J.; Nagarsheth, N.; Chiu, K. Evidence of self-inhibition by filamentous fungi accounts for unidirectional hyphal growth in colonies. Can. J. Microbiol. 1998, 44, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Urra, A.B.; Jimenez, C.; Duenas, M.; Ugalde, U. Bicarbonate gradients modulate growth and colony morphology in Aspergillus nidulans. FEMS Microbiol. Lett. 2009, 300, 216–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ugalde, U. Autoregulatory Signals in Mycelial Fungi. In Growth, Differentiation and Sexuality; Springer: Cham, Switzerland, 2016; Volume 1, pp. 185–202. [Google Scholar]
- Hoshino, A.; Sawada, H.; Yokota, M.; Tsuji, M.; Fukuda, K.; Kimura, M. Influence of weather conditions and soil properties on degradation of biodegradable plastics in soil. Soil Sci. Plant Nutr. 2001, 47, 35–43. [Google Scholar]
- Ishii, N.; Inoue, Y.; Tagaya, T.; Mitomo, H.; Nagai, D.; Kasuya, K.-I. Isolation and characterization of poly (butylene succinate)-degrading fungi. Polym. Degrad. Stab. 2008, 93, 883–888. [Google Scholar]
- Bandopadhyay, S.; Sintim, H.Y.; DeBruyn, J.M. Effects of biodegradable plastic film mulching on soil microbial communities in two agroecosystems. PeerJ 2020, 8, e9015. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Moore-Kucera, J.; Lee, J.; Corbin, A.; Brodhagen, M.; Miles, C.; Inglis, D. Effects of biodegradable mulch on soil quality. Appl. Soil Ecol. 2014, 79, 59–69. [Google Scholar] [CrossRef]
- Fritz, J.; Sandhofer, M.; Stacher, C.; Braun, R. Strategies for detecting ecotoxicological effects of biodegradable polymers in agricultural applications. Macromol. Symp. 2003, 197, 397–409. [Google Scholar] [CrossRef]
- Muroi, F.; Tachibana, Y.; Kobayashi, Y.; Sakurai, T.; Kasuya, K. Influences of poly(butylene adipate-co-terephthalate) on soil microbiota and plant growth. Polym. Degrad. Stab. 2016, 129, 338–346. [Google Scholar] [CrossRef]
- Qi, Y.L.; Yang, X.M.; Pelaez, A.M.; Lwanga, E.H.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro- plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Rychter, P.; Biczak, R.; Herman, B.; Smylla, A.; Kurcok, P.; Adamus, G.; Kowalczuk, M. Environmental degradation of polyester blends containing atactic poly(3-hydroxybutyrate). Biodegradation in soil and ecotoxicological impact. Biomacromolecules 2006, 7, 3125–3131. [Google Scholar] [CrossRef] [PubMed]
- Sforzini, S.; Oliveri, L.; Chinaglia, S.; Viarengo, A. Application of Biotests for the Determination of Soil Ecotoxicity after Exposure to Biodegradable Plastics. Front. Environ. Sci. 2016, 4, 68. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chien, H.-L.; Tsai, Y.-T.; Tseng, W.-S.; Wu, J.-A.; Kuo, S.-L.; Chang, S.-L.; Huang, S.-J.; Liu, C.-T. Biodegradation of PBSA Films by Elite Aspergillus Isolates and Farmland Soil. Polymers 2022, 14, 1320. https://doi.org/10.3390/polym14071320
Chien H-L, Tsai Y-T, Tseng W-S, Wu J-A, Kuo S-L, Chang S-L, Huang S-J, Liu C-T. Biodegradation of PBSA Films by Elite Aspergillus Isolates and Farmland Soil. Polymers. 2022; 14(7):1320. https://doi.org/10.3390/polym14071320
Chicago/Turabian StyleChien, Hsiao-Lin, Yi-Ting Tsai, Wei-Sung Tseng, Jin-An Wu, Shin-Liang Kuo, Sheng-Lung Chang, Shu-Jiuan Huang, and Chi-Te Liu. 2022. "Biodegradation of PBSA Films by Elite Aspergillus Isolates and Farmland Soil" Polymers 14, no. 7: 1320. https://doi.org/10.3390/polym14071320
APA StyleChien, H.-L., Tsai, Y.-T., Tseng, W.-S., Wu, J.-A., Kuo, S.-L., Chang, S.-L., Huang, S.-J., & Liu, C.-T. (2022). Biodegradation of PBSA Films by Elite Aspergillus Isolates and Farmland Soil. Polymers, 14(7), 1320. https://doi.org/10.3390/polym14071320