Biodegradable Microparticles for Regenerative Medicine: A State of the Art and Trends to Clinical Application
Abstract
1. Introduction
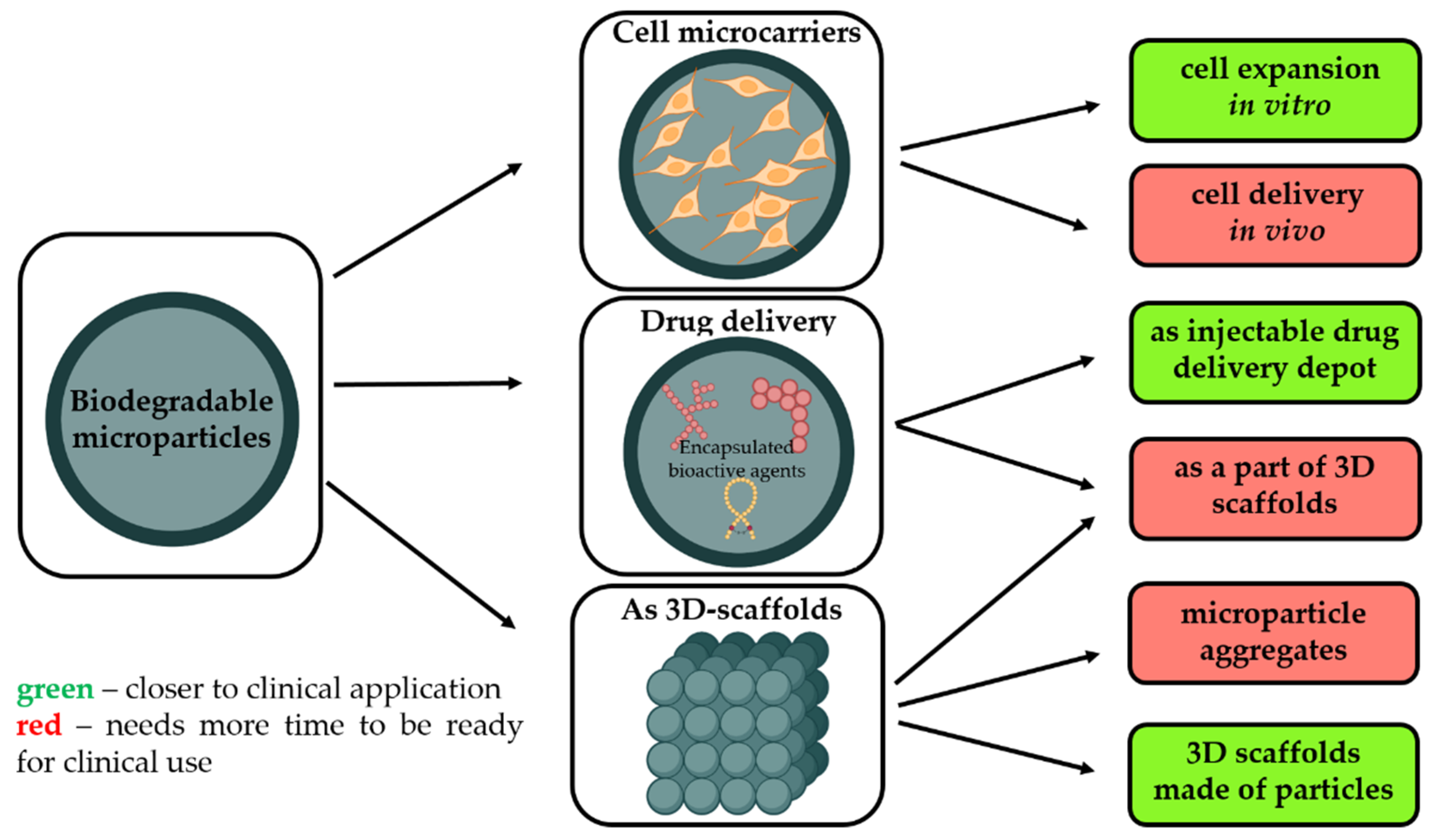
- As a temporary microcarrier to support expansion of cells initially cultivated in vitro [3]. Biodegradability is a crucial characteristic to avoid main technological issues related to cell multiplication on non-degradable microcarriers, i.e., poor yield of cell detachment, contamination related to enzymes requested to harvest cells, and difficulties to separate microparticle debris from free cells. Moreover, as these microcarriers are made from safe and degradable polymers, these cell microcarriers could be injected into the targeted tissues to restore them.
- Without pre-culture with cells in order to provide a sustained and local release of growth factors selected to promote tissue rebuilding while also offering a large surface to enhance in vivo cell adhesion.
- As a part of other types of 3D scaffolds for tissue engineering, including the application of biodegradable microparticles as starting building blocks to generate 3D scaffolds with well-defined architecture, adopting additive technologies or other techniques.
2. Fabrication and Modification of Polymeric-Based Microparticles (MPs)
2.1. Materials for MPs Fabrication
| Material | Chemical Nature, Crystallinity, Thermal Properties | Range of Degradation Rate and the Main Route of Degradation | Functionality | Advantages | Drawbacks | Approval Status | Ref. |
|---|---|---|---|---|---|---|---|
| Synthetic polymers | |||||||
| PCL | Aliphatic polyester; Semi-crystalline; Tg: −60 °C; Tm: 60 °C | >1 year Ester hydrolysis | Hydrophobic material; Limited to aliphatic ester functions; Residual organic solvent content; | Macromolecular features and purity are well-controlled and reliable; Chemical purity is under control; Degradation rate can be easily adjusted in function of the Mw, tacticity, and crystallization %; Easy processability. | Lack of cell adhesion moieties; Release of acidic by-products during degradation. | FDA-approved | [40] |
| PLA | Aliphatic polyester; Semi-crystalline or amorphous; Tg: 40 °C; Tm: 180 °C | >0.6 year Ester hydrolysis | [41] | ||||
| PLGA | Aliphatic polyester; Semi-crystalline or amorphous; Tg: 40 °C; Tm: 180 °C | >0.3 year Ester hydrolysis | [42] | ||||
| Natural polymers | |||||||
| Alginates | Anionic polysaccharides copolymers | Enzymatic degradation pathway | Carboxyl groups; Polyelectrolyte. | Gel-forming ability; Hydrophilicity. | No cell adhesion characteristics; Lack of control of the macromolecular features (Mw, polydispersity, purity). | FDA-approved | [43] |
| Collagen | Natural protein present in the extracellular matrices of tissues | Enzymatic degradation pathway | Carboxyl and amino groups | Cell adhesion and proliferation enhancement; Hydrophilicity. | Risk of allergic reactions; Low mechanical properties. | FDA-approved | [14] |
| Chitosan | Cationic polysaccharides copolymers. | Enzymatic degradation pathway | Primary amino-groups | Positive charge; Cell adhesion enhancement; Hydrophilicity. | Lack of control of the macromolecular features (Mw, polydispersity, purity); Difficulty of processing (not soluble in aqueous medium at neutral pH). | Not approved as pharmaceutical excipient; Under clinical testing as an implant. | [44] |
| PHAs | Polymers with high structural diversity; Semi-crystalline. | Enzymatic and hydrolytic degradation | Ester functions | Cell proliferation stimulation; Hydrophilicity; Controllable mechanical and thermal properties. | Low mechanical properties | Not approved | [45] |
| Silk fibroin | Natural protein isolated from animals. | Enzymatic degradation pathway | Carboxyl and amino groups | Cell proliferation stimulation; Hydrophilicity; Gel-forming material | High risk of allergic reactions. | Not approved | [46] |
2.2. Methods of MPs Fabrication
2.2.1. Emulsions
2.2.2. Spray Drying
2.2.3. Microfluidics
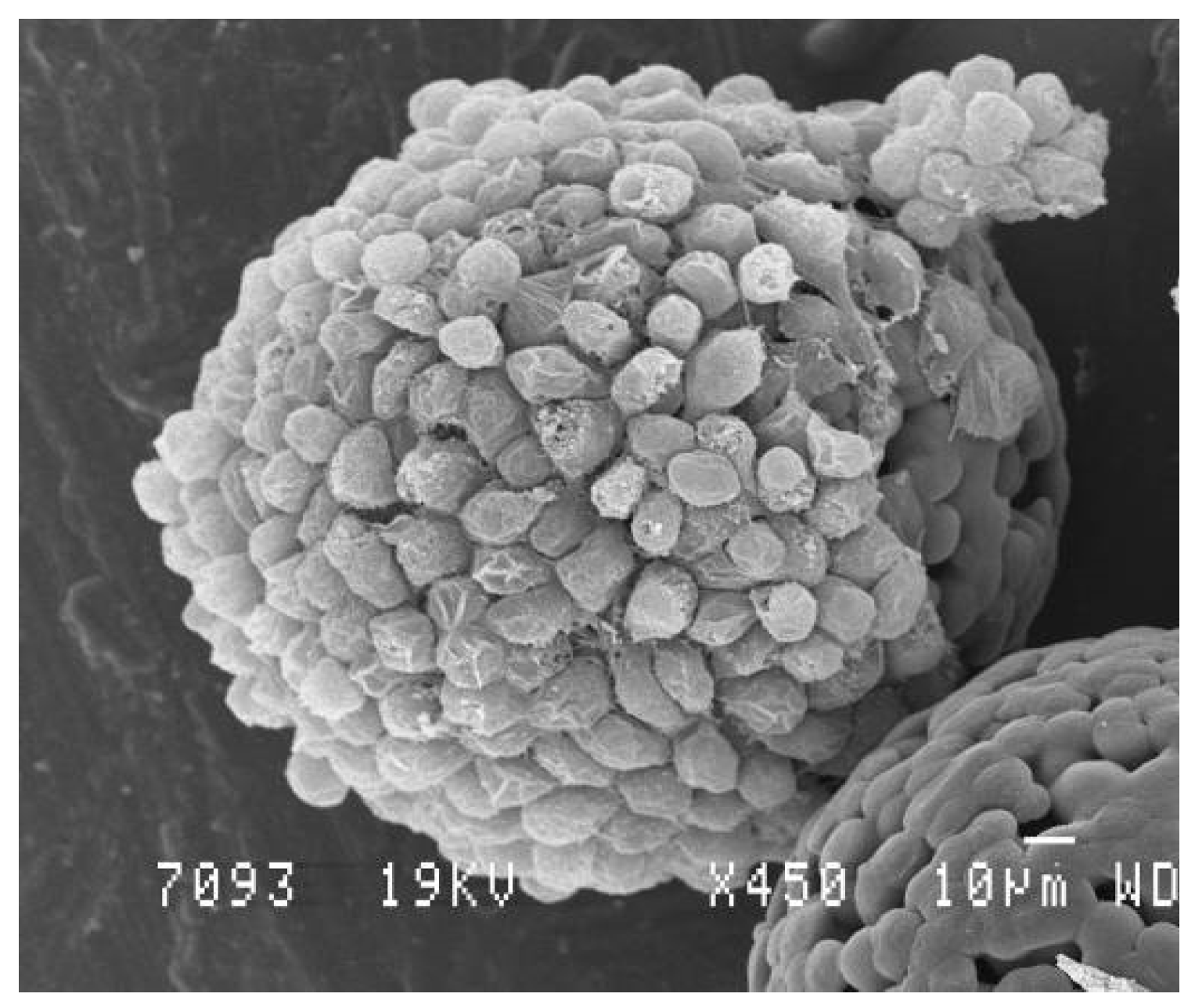
2.3. Microparticle-Based and Microparticle-Contained 3D Structures
- Aggregation of cell-free microparticles via polymer/polymer aggregation or assembly of microparticles with pre-cultured cells through cell/cell interactions;
- Microparticles as filling material to other types of matrices, including the application of them as drug depot, functional fillers to regulate the physico-mechanical properties of the matrix as well as cell-seeded microcarriers within bioinks;
- Microparticles without cells as building blocks for the fabrication of 3D scaffolds.
3. Biomedical Applications of MPs
3.1. Microparticles as Drug Delivery Depot to Promote Tissue Reconstruction
- Their local administration is minimally invasive and feasible into limited accessible sites;
- Their high surface/volume ratio is favorable and reported to be particularly suited as cell supporting microcarriers;
- Being made from well-known biocompatible and biodegradable polyesters, such as PLGA;
- Their degradation rate can be easily adjusted to balance growth factor release kinetics, cell supporting amplification, and mechanical support [107];
- The large scale GMP production of these drug delivery microparticles is already known and applied for several years;
- They are simple products, free of animal cells, and easy to submit to regulatory bodies;
- There is an opportunity to physically combine these microparticles with autologous stem cells just before implantation on a patient.
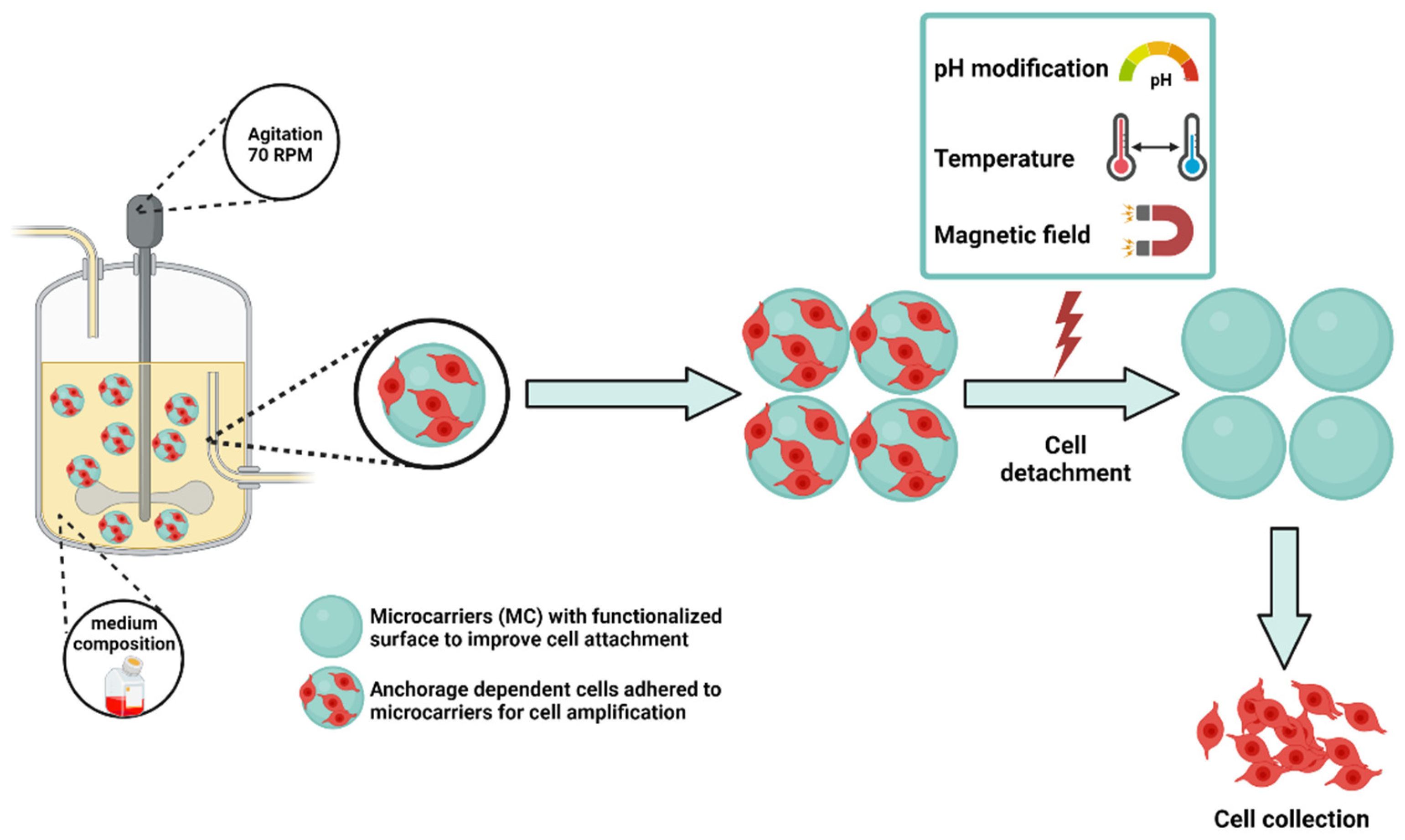
3.2. Microcarriers for Cell Expansion
| Fabrication Technology | Material Component (Matrix) | Types of Particles, Shape, and Dimension | Charge/Surface Area (cm2)/Density | Physicochemical and Biochemical Cues (Surface Coating) | Notes | Ref. |
|---|---|---|---|---|---|---|
| Emulsion solvent/evaporation | Poly (Ԑ-caprolactone) | Size between 224.5–366.3 μm | Pores size of 25.6–84.0 μm. Porosity of 57.4–75.5%. | Coating with hydroxyapatite | MC with surface modification supported very well the adhesion and growth of human fibroblasts. | [144] |
| Microinjection method | Alginate | Size of 421.94 μm. Spherical geometry | NA | Silk fibroin coating | Good adhesion of MSCs within 3 days of culture and preservation of their metabolic activity and multi-lineage differentiation of potential. | [145] |
| Needle/tubing microfluidic device | Polycaprolactone (80 KDa) | NA | NA | ECM coating (combination of fibronectin and poly-l-lysine) | Enhance attachment of human early MSCs at levels equivalent to the commercially available Cytadex 3MC. The cultured cells were able to induce bone formation in ectopic mouse model. | [146] |
| Electrospraying technique | Gelatin-Chitosan | Size of 350 μm | Density between 1.00–1.1 g/cm3. Pore size between 40 and 60 μm. | NA | Demonstration of the usefulness of gelatin-chitosan blends of different weight ratios as suitable material to prepare MC for supporting MSCs attachment and proliferation. | [147] |
| Emulsion-based thermally induced phase separation | Chitosan | Size of about 150 μm | Pores size varying from 20–50 μm. | NA | Excellent biocompatibility and unique pores’ structure, which allows hepatocyte culture in three-dimension space. | [148] |
| Cross-linked reaction | Gelatin | Size of about 250 μm | NA | NA | Report on cross-linked porous gelatin beads (redox-sensitive beads) that afford rapid, stimuli-triggered dissolution for facile cell removal of hMSC. Harvest time was reduced by at least 15-fold in a bioreactor of 3 L. | [149] |
| Emulsion solvent evaporation method | Polylactic-co-glycolic acid (PLGA) | Size about 260 μm | Negatively charged particles (−26.9 and−16.7 mV) | Poly L-Lysine (About 200,000 Dalton) Gelatin | Report on the development of US FDA MC that serves as an adherent platform for human umbilical vein endothelial cells (HUVEC). The cell density was multiplied up to 3.5 fold and healthy morphology. | [150] |
| W/O/W emulsion-based method | Poly-(ɣ-Benzyl-L-glutamate) | Size between 200–400 μm | Pores size above 50 μm | Janus microspheres | Open porous PBLG microcarriers with large pore size were prepared, demonstrating high cellular infiltration and proliferation rate of human adipose derived stem cells (hASCs) | [151] |
| Crystallization (organic solvent free process) | Poly (L-lactide) (PLLA) and poly (ethylene glycol) (PEG) | Size between 100–230 μm | NA | Functionalization with poly (L-ornithine), hyaluronic acid, and bioadhesive RGD peptide | hASCs were able to attach and grow on MCs whatever the surface treatment, but adhesion and proliferation were higher when the MCs were grafted with RGD | [152] |
3.3. Microparticle-Containing 3D Scaffolds for Regenerative Medicine
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunsberger, J.G.; Shupe, T.; Atala, A. An Industry-Driven Roadmap for Manufacturing in Regenerative Medicine. Stem Cells Transl. Med. 2018, 7, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.B.; Mano, J.F. Polymer-Based Microparticles in Tissue Engineering and Regenerative Medicine. Biotechnol. Prog. 2011, 27, 897–912. [Google Scholar] [CrossRef] [PubMed]
- Derakhti, S.; Safiabadi-Tali, S.H.; Amoabediny, G.; Sheikhpour, M. Attachment and Detachment Strategies in Microcarrier-Based Cell Culture Technology: A Comprehensive Review. Mater. Sci. Eng. C 2019, 103, 109782. [Google Scholar] [CrossRef]
- Zhu, M.; Whittaker, A.K.; Han, F.Y.; Smith, M.T. Journey to the Market: The Evolution of Biodegradable Drug Delivery Systems. Appl. Sci. 2022, 12, 935. [Google Scholar] [CrossRef]
- Ratcliffe, E.; Thomas, R.J.; Williams, D.J. Current Understanding and Challenges in Bioprocessing of Stem Cell-Based Therapies for Regenerative Medicine. Br. Med. Bull. 2011, 100, 137–155. [Google Scholar] [CrossRef]
- Krause, M.; Phan, T.G.; Ma, H.; Sobey, C.G.; Lim, R. Cell-Based Therapies for Stroke: Are We There Yet? Front. Neurol. 2019, 10, 656. [Google Scholar] [CrossRef]
- Laso-García, F.; Diekhorst, L.; Gómez-de Frutos, M.C.; Otero-Ortega, L.; Fuentes, B.; Ruiz-Ares, G.; Díez-Tejedor, E.; Gutiérrez-Fernández, M. Cell-Based Therapies for Stroke: Promising Solution or Dead End? Mesenchymal Stem Cells and Comorbidities in Preclinical Stroke Research. Front. Neurol. 2019, 10, 332. [Google Scholar] [CrossRef]
- McKee, C.; Chaudhry, G.R. Advances and Challenges in Stem Cell Culture. Colloids Surf. B Biointerfaces 2017, 159, 62–77. [Google Scholar] [CrossRef]
- Chan, S.W.; Rizwan, M.; Yim, E.K.F. Emerging Methods for Enhancing Pluripotent Stem Cell Expansion. Front. Cell Dev. Biol. 2020, 8, 70. [Google Scholar] [CrossRef]
- Cossu, G.; Birchall, M.; Brown, T.; de Coppi, P.; Culme-Seymour, E.; Gibbon, S.; Hitchcock, J.; Mason, C.; Montgomery, J.; Morris, S.; et al. Lancet Commission: Stem Cells and Regenerative Medicine. Lancet 2018, 391, 883–910. [Google Scholar] [CrossRef]
- Ozdil, D.; Aydin, H.M. Polymers for Medical and Tissue Engineering Applications. J. Chem. Technol. Biotechnol. 2014, 89, 1793–1810. [Google Scholar] [CrossRef]
- Molavi, F.; Barzegar-Jalali, M.; Hamishehkar, H. Polyester Based Polymeric Nano and Microparticles for Pharmaceutical Purposes: A Review on Formulation Approaches. J. Control. Release 2020, 320, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Demina, T.S.; Akopova, T.A.; Zelenetsky, A.N. Materials Based on Chitosan and Polylactide: From Biodegradable Plastics to Tissue Engineering Constructions. Polym. Sci. Ser. C 2021, 63, 219–226. [Google Scholar] [CrossRef]
- Browne, S.; Zeugolis, D.I.; Pandit, A. Collagen: Finding a Solution for the Source. Tissue Eng. Part A 2013, 19, 1491. [Google Scholar] [CrossRef] [PubMed]
- Zakir Hossain, K.M.; Patel, U.; Ahmed, I. Development of Microspheres for Biomedical Applications: A Review. Prog. Biomater. 2014, 4, 1–19. [Google Scholar] [CrossRef]
- Yao, R.; Zhang, R.; Luan, J.; Lin, F. Alginate and Alginate/Gelatin Microspheres for Human Adipose-Derived Stem Cell Encapsulation and Differentiation. Biofabrication 2012, 4, 025007. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Miao, Y.; Tan, H.; Zhou, T.; Ling, Z.; Chen, Y.; Xing, X.; Hu, X. Injectable Alginate/Hydroxyapatite Gel Scaffold Combined with Gelatin Microspheres for Drug Delivery and Bone Tissue Engineering. Mater. Sci. Eng. C 2016, 63, 274–284. [Google Scholar] [CrossRef]
- Mahou, R.; Vlahos, A.E.; Shulman, A.; Sefton, M.V. Interpenetrating Alginate-Collagen Polymer Network Microspheres for Modular Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 3704–3712. [Google Scholar] [CrossRef]
- Bi, Y.-G.; Lin, Z.-T.; Deng, S.-T. Fabrication and Characterization of Hydroxyapatite/Sodium Alginate/Chitosan Composite Microspheres for Drug Delivery and Bone Tissue Engineering. Mater. Sci. Eng. C 2019, 100, 576–583. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Francis Suh, J.K.; Matthew, H.W.T. Application of Chitosan-Based Polysaccharide Biomaterials in Cartilage Tissue Engineering: A Review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [CrossRef]
- Sivashankari, P.R.; Prabaharan, M. Prospects of Chitosan-Based Scaffolds for Growth Factor Release in Tissue Engineering. Int. J. Biol. Macromol. 2016, 93, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Cen, J.; Gibson, E.; Wang, R.; Percival, S.L. An Open Multicenter Comparative Randomized Clinical Study on Chitosan. Wound Repair Regen. 2015, 23, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Hsu, H.J.; Kaunas, R.; Kameoka, J. Collagen Microsphere Production on a Chip. Lab A Chip 2012, 12, 3277–3280. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X.; Zhang, Z.; Wang, Y.; Huang, C.; Yang, C.; Liu, L.; Zhang, Q. Silk Fibroin/Collagen/Hyaluronic Acid Scaffold Incorporating Pilose Antler Polypeptides Microspheres for Cartilage Tissue Engineering. Mater. Sci. Eng. C 2019, 94, 35–44. [Google Scholar] [CrossRef]
- Helary, C.; Browne, S.; Mathew, A.; Wang, W.; Pandit, A. Transfection of Macrophages by Collagen Hollow Spheres Loaded with Polyplexes: A Step towards Modulating Inflammation. Acta Biomater. 2012, 8, 4208–4214. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Tabata, Y. Preparation of Stem Cell Aggregates with Gelatin Microspheres to Enhance Biological Functions. Acta Biomater. 2011, 7, 2797–2803. [Google Scholar] [CrossRef]
- Basu, A.; Domb, A.J. Recent Advances in Polyanhydride Based Biomaterials. Adv. Mater. 2018, 30, 1706815. [Google Scholar] [CrossRef]
- Campos, E.; Branquinho, J.; Carreira, A.S.; Carvalho, A.; Coimbra, P.; Ferreira, P.; Gil, M.H.H.; Campos, E. Designing Polymeric Microparticles for Biomedical and Industrial Applications. Eur. Polym. J. 2013, 49, 2005–2021. [Google Scholar] [CrossRef]
- Wei, D.X.; Dao, J.W.; Chen, G.Q. A Micro-Ark for Cells: Highly Open Porous Polyhydroxyalkanoate Microspheres as Injectable Scaffolds for Tissue Regeneration. Adv. Mater. 2018, 30, 1802273. [Google Scholar] [CrossRef]
- Ray, S.; Kalia, V.C. Biomedical Applications of Polyhydroxyalkanoates. Indian J. Microbiol. 2017, 57, 261. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Pandey, R.; Kumar, S.; Mehrotra, D. Poly Hydroxyalkanoates (PHA): Role in Bone Scaffolds. J. Oral Biol. Craniofacial Res. 2020, 10, 389–392. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Rajkhowa, R.; Wang, X.; Devi, D. Milled Non-Mulberry Silk Fibroin Microparticles as Biomaterial for Biomedical Applications. Int. J. Biol. Macromol. 2015, 81, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, T.K.; Bowles, R.D.; Tainter, D.M.; Bell, R.D.; Kaplan, D.L.; Setton, L.A. Synthesis and Characterization of Silk Fibroin Microparticles for Intra-Articular Drug Delivery. Int. J. Pharm. 2015, 485, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Demina, T.S.; Akopova, T.A.; Vladimirov, L.V.; Zelenetskii, A.N.; Markvicheva, E.A.; Grandfils, C. Polylactide-Based Microspheres Prepared Using Solid-State Copolymerized Chitosan and D, L -Lactide. Mater. Sci. Eng. C 2016, 59, 333–338. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, J.; He, J.; Zhang, J.; Gan, Z. Fabrication, Hydrolysis and Cell Cultivation of Microspheres from Cellulose-Graft-Poly(l-Lactide) Copolymers. RSC Adv. 2016, 6, 17617–17623. [Google Scholar] [CrossRef]
- Demina, T.S.; Drozdova, M.G.; Sevrin, C.; Compère, P.; Akopova, T.A.; Markvicheva, E.; Grandfils, C. Biodegradable Cell Microcarriers Based on Chitosan/Polyester Graft-Copolymers. Molecules 2020, 25, 1949. [Google Scholar] [CrossRef]
- Fu, H.; Rahaman, M.N.; Brown, R.F.; Day, D.E. Evaluation of BSA Protein Release from Hollow Hydroxyapatite Microspheres into PEG Hydrogel. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2245. [Google Scholar] [CrossRef]
- Silva, G.A.; Coutinho, O.P.; Ducheyne, P.; Reis, R.L. Materials in Particulate Form for Tissue Engineering. 2. Applications in Bone. J. Tissue Eng. Regen. Med. 2007, 1, 97–109. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as Biomaterial for Bone Scaffolds: Review of Literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Im, S.H.; Im, D.H.; Park, S.J.; Chung, J.J.; Jung, Y.; Kim, S.H. Stereocomplex Polylactide for Drug Delivery and Biomedical Applications: A Review. Molecules 2021, 26, 2846. [Google Scholar] [CrossRef] [PubMed]
- Cipurković, A.; Horozić, E.; Đonlagić, N.; Marić, S.; Saletović, M.; Ademović, Z. Biodegradable Polymers: Production, Properties and Application in Medicine. Technol. Acta Sci./Prof. J. Chem. Technol. 2018, 11, 25–35. [Google Scholar]
- Fernando, I.P.S.; Lee, W.W.; Han, E.J.; Ahn, G. Alginate-Based Nanomaterials: Fabrication Techniques, Properties, and Applications. Chem. Eng. J. 2020, 391, 123823. [Google Scholar] [CrossRef]
- Ahsan, S.; Bhatnagar, I. Chitosan as Biomaterial in Drug Delivery and Tissue Engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Grigore, M.E.; Grigorescu, R.M.; Iancu, L.; Ion, R.M.; Zaharia, C.; Andrei, E.R. Methods of Synthesis, Properties and Biomedical Applications of Polyhydroxyalkanoates: A Review. J. Biomater. Sci. 2019, 30, 695–712. [Google Scholar] [CrossRef]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk Fibroin as Biomaterial for Bone Tissue Engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- da Costa, R.C.; Pereira, E.D.; Silva, F.M.; de Jesus, E.O.; Souza, F.G. Drug Micro-Carriers Based on Polymers and Their Sterilization. Chem. Chem. Technol. 2018, 12, 473–487. [Google Scholar] [CrossRef]
- Meng, F.; Jiang, Y.; Sun, Z.; Yin, Y.; Li, Y. Electrohydrodynamic Liquid Atomization of Biodegradable Polymer Microparticles: Effect of Electrohydrodynamic Liquid Atomization Variables on Microparticles. J. Appl. Polym. Sci. 2009, 113, 526–534. [Google Scholar] [CrossRef]
- Morais, A.Í.S.; Vieira, E.G.; Afewerki, S.; Sousa, R.B.; Honorio, L.M.C.; Cambrussi, A.N.C.O.; Santos, J.A.; Bezerra, R.D.S.; Furtini, J.A.O.; Silva-Filho, E.C.; et al. Fabrication of Polymeric Microparticles by Electrospray: The Impact of Experimental Parameters. J. Funct. Biomater. 2020, 11, 4. [Google Scholar] [CrossRef]
- Tasci, M.E.; Dede, B.; Tabak, E.; Gur, A.; Sulutas, R.B.; Cesur, S.; Ilhan, E.; Lin, C.C.; Paik, P.; Ficai, D.; et al. Production, Optimization and Characterization of Polylactic Acid Microparticles Using Electrospray with Porous Structure. Appl. Sci. 2021, 11, 5090. [Google Scholar] [CrossRef]
- Bhujel, R.; Maharjan, R.; Kim, N.A.; Jeong, S.H. Practical Quality Attributes of Polymeric Microparticles with Current Understanding and Future Perspectives. J. Drug Deliv. Sci. Technol. 2021, 64, 102608. [Google Scholar] [CrossRef]
- Naidoo, K.; Rolfes, H.; Easton, K.; Moolman, S.; Chetty, A.; Richter, W.; Nilen, R. An Emulsion Preparation for Novel Micro-Porous Polymeric Hemi-Shells. Mater. Lett. 2008, 62, 252–254. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Wang, Y.; Gou, W.; Yuan, X.; Peng, J.; Guo, Q.; Lu, S. Past, Present, and Future of Microcarrier-Based Tissue Engineering. J. Orthop. Transl. 2015, 3, 51–57. [Google Scholar] [CrossRef]
- Vladisavljević, G.T. Structured Microparticles with Tailored Properties Produced by Membrane Emulsification. Adv. Colloid Interface Sci. 2015, 225, 53–87. [Google Scholar] [CrossRef] [PubMed]
- Druel, L.; Kenkel, A.; Baudron, V.; Buwalda, S.; Budtova, T. Cellulose Aerogel Microparticles via Emulsion-Coagulation Technique. Biomacromolecules 2020, 21, 1824–1831. [Google Scholar] [CrossRef]
- Lee, Y.S.; Johnson, P.J.; Robbins, P.T.; Bridson, R.H. Production of Nanoparticles-in-Microparticles by a Double Emulsion Method: A Comprehensive Study. Eur. J. Pharm. Biopharm. 2013, 83, 168–173. [Google Scholar] [CrossRef]
- Giri, T.K.; Choudhary, C.; Ajazuddin; Alexander, A.; Badwaik, H.; Tripathi, D.K. Prospects of Pharmaceuticals and Biopharmaceuticals Loaded Microparticles Prepared by Double Emulsion Technique for Controlled Delivery. Saudi Pharm. J. 2013, 21, 125–141. [Google Scholar] [CrossRef]
- Pacheco, D.P.; Amaral, M.H.; Reis, R.L.; Marques, A.P.; Correlo, V.M. Development of an Injectable PHBV Microparticles-GG Hydrogel Hybrid System for Regenerative Medicine. Int. J. Pharm. 2015, 478, 398–408. [Google Scholar] [CrossRef]
- Nan, K.; Ma, F.; Hou, H.; Freeman, W.R.; Sailor, M.J.; Cheng, L. Porous Silicon Oxide–PLGA Composite Microspheres for Sustained Ocular Delivery of Daunorubicin. Acta Biomater. 2014, 10, 3505–3512. [Google Scholar] [CrossRef]
- Nanaki, S.; Siafaka, P.I.; Zachariadou, D.; Nerantzaki, M.; Giliopoulos, D.J.; Triantafyllidis, K.S.; Kostoglou, M.; Nikolakaki, E.; Bikiaris, D.N. PLGA/SBA-15 Mesoporous Silica Composite Microparticles Loaded with Paclitaxel for Local Chemotherapy. Eur. J. Pharm. Sci. 2017, 99, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Fauer, C.; Hickey, K.; Salifu, M.; Stabenfeldt, S.E. Tunable Delayed Controlled Release Profile from Layered Polymeric Microparticles. J. Mater. Chemistry. B Mater. Biol. Med. 2017, 5, 4487. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, T.; Wang, C.; Wang, Z.; Yang, Y.; Li, P.; Cai, R.; Sun, M.; Yuan, H.; Nie, L. Synthesis and Characterization of Silver Nanoparticles-Doped Hydroxyapatite/Alginate Microparticles with Promising Cytocompatibility and Antibacterial Properties. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124081. [Google Scholar] [CrossRef]
- Shokrolahi, F.; Khodabakhshi, K.; Shokrollahi, P.; Badiani, R.; Moghadam, Z.M. Atorvastatin Loaded PLGA Microspheres: Preparation, HAp Coating, Drug Release and Effect on Osteogenic Differentiation of ADMSCs. Int. J. Pharm. 2019, 565, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Okada, M.; Sawa, H.; Furuzono, T.; Nakamura, Y. Hydroxyapatite Nanoparticles as Particulate Emulsifier: Fabrication of Hydroxyapatite-Coated Biodegradable Microspheres. Langmuir 2009, 25, 9759–9766. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.A. Emulsions Stabilized with Solid Nanoparticles: Pickering Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- ben Cheikh, F.; Mabrouk, A.B.; Magnin, A.; Putaux, J.-L.; Boufi, S. Chitin Nanocrystals as Pickering Stabilizer for O/W Emulsions: Effect of the Oil Chemical Structure on the Emulsion Properties. Colloids Surf. B Biointerfaces 2021, 200, 111604. [Google Scholar] [CrossRef]
- Asfour, M.H.; Elmotasem, H.; Mostafa, D.M.; Salama, A.A.A. Chitosan Based Pickering Emulsion as a Promising Approach for Topical Application of Rutin in a Solubilized Form Intended for Wound Healing: In Vitro and in Vivo Study. Int. J. Pharm. 2017, 534, 325–338. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, D.; Li, Z.; Luo, D.; Hang, L.; Jing, J.; Shah, B.R. Controlled Release of Lysozyme Based Core/Shells Structured Alginate Beads with CaCO3 Microparticles Using Pickering Emulsion Template and in Situ Gelation. Colloids Surf. B: Biointerfaces 2019, 183, 110410. [Google Scholar] [CrossRef]
- Barra, P.A.; Márquez, K.; Gil-Castell, O.; Mujica, J.; Ribes-Greu, A.; Faccini, M. Spray-Drying Performance and Thermal Stability Of. Molecules 2019, 24, 2872. [Google Scholar]
- Encina, C.; Márquez-Ruiz, G.; Holgado, F.; Giménez, B.; Vergara, C.; Robert, P. Effect of Spray-Drying with Organic Solvents on the Encapsulation, Release and Stability of Fish Oil. Food Chem. 2018, 263, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Spray-Drying of Oil-in-Water Emulsions for Encapsulation of Retinoic Acid: Polysaccharide- and Protein-Based Microparticles Characterization and Controlled Release Studies. Food Hydrocoll. 2022, 124, 107193. [Google Scholar] [CrossRef]
- Wu, C.; van de Weert, M.; Baldursdottir, S.G.; Yang, M.; Mu, H. Effect of Excipients on Encapsulation and Release of Insulin from Spray-Dried Solid Lipid Microparticles. Int. J. Pharm. 2018, 550, 439–446. [Google Scholar] [CrossRef]
- Carlan, I.C.; Estevinho, B.N.; Rocha, F. Production of Vitamin B1 Microparticles by a Spray Drying Process Using Different Biopolymers as Wall Materials. Can. J. Chem. Eng. 2020, 98, 1682–1695. [Google Scholar] [CrossRef]
- Ruphuy, G.; Saralegi, A.; Lopes, J.C.; Dias, M.M.; Barreiro, M.F. Spray Drying as a Viable Process to Produce Nano-Hydroxyapatite/Chitosan (n-HAp/CS) Hybrid Microparticles Mimicking Bone Composition. Adv. Powder Technol. 2016, 27, 575–583. [Google Scholar] [CrossRef]
- Gover Antoniraj, M.; Maria Leena, M.; Moses, J.A.; Anandharamakrishnan, C. Cross-Linked Chitosan Microparticles Preparation by Modified Three Fluid Nozzle Spray Drying Approach. Int. J. Biol. Macromol. 2020, 147, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Lins, L.C.; Bazzo, G.C.; Barreto, P.L.M.; Pires, A.T.N. Composite PHB/Chitosan Microparticles Obtained by Spray Drying: Effect of Chitosan Concentration and Crosslinking Agents on Drug Relesase. Artic. J. Braz. Chem. Soc. 2014, 25, 1462–1471. [Google Scholar] [CrossRef]
- Silva, D.M.; Liu, R.; Gonçalves, A.F.; da Costa, A.; Castro Gomes, A.; Machado, R.; Vongsvivut, J.; Tobin, M.J.; Sencadas, V. Design of Polymeric Core-Shell Carriers for Combination Therapies. J. Colloid Interface Sci. 2021, 587, 499–509. [Google Scholar] [CrossRef]
- Spindler, L.M.; Feuerhake, A.; Ladel, S.; Günday, C.; Flamm, J.; Günday-Türeli, N.; Türeli, E.; Tovar, G.E.M.; Schindowski, K.; Gruber-Traub, C. Nano-in-Micro-Particles Consisting of PLGA Nanoparticles Embedded in Chitosan Microparticles via Spray-Drying Enhances Their Uptake in the Olfactory Mucosa. Front. Pharmacol. 2021, 12, 2282. [Google Scholar] [CrossRef]
- Bizeau, J.; Mertz, D. Design and Applications of Protein Delivery Systems in Nanomedicine and Tissue Engineering. Adv. Colloid Interface Sci. 2021, 287, 102334. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.D.; Selomulya, C. On the Spray Drying of Uniform Functional Microparticles. Particuology 2015, 22, 1–12. [Google Scholar] [CrossRef]
- Jo, Y.K.; Lee, D. Biopolymer Microparticles Prepared by Microfluidics for Biomedical Applications. Small 2020, 16, 1903736. [Google Scholar] [CrossRef]
- Xia, H.; Li, J.; Man, J.; Man, L.; Zhang, S.; Li, J. Recent Progress in Preparation of Functional Microparticles Based on Microfluidic Technique. Mater. Today Commun. 2021, 29, 102740. [Google Scholar] [CrossRef]
- Su, H.; Hurd Price, C.A.; Jing, L.; Tian, Q.; Liu, J.; Qian, K. Janus Particles: Design, Preparation, and Biomedical Applications. Mater. Today Bio 2019, 4, 100033. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Zhou, L.; Han, Q.; Chen, X.; Li, S.; Li, L.; Su, Z.; Wang, C. Dual Drug Delivery and Sequential Release by Amphiphilic Janus Nanoparticles for Liver Cancer Theranostics. Biomaterials 2018, 181, 113–125. [Google Scholar] [CrossRef]
- Sun, X.T.; Guo, R.; Wang, D.N.; Wei, Y.Y.; Yang, C.G.; Xu, Z.R. Microfluidic Preparation of Polymer-Lipid Janus Microparticles with Staged Drug Release Property. J. Colloid Interface Sci. 2019, 553, 631–638. [Google Scholar] [CrossRef]
- Wang, J.-T.; Wang, J.; Han, J.-J. Fabrication of Advanced Particles and Particle-Based Materials Assisted by Droplet-Based Microfluidics. Small 2011, 7, 1728–1754. [Google Scholar] [CrossRef]
- Luo, X.; Su, P.; Zhang, W.; Raston, C.L. Microfluidic Devices in Fabricating Nano or Micromaterials for Biomedical Applications. Adv. Mater. Technol. 2019, 4, 1900488. [Google Scholar] [CrossRef]
- Freitas, S.; Merkle, H.P.; Gander, B. Microencapsulation by Solvent Extraction/Evaporation: Reviewing the State of the Art of Microsphere Preparation Process Technology. J. Control. Release 2005, 102, 313–332. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Qiao, F.; Liu, Y.; Zhou, Y.; Li, M.; Ai, M.; Yang, Y.; Sui, L.; Zhou, Z. Polymeric Non-Spherical Coarse Microparticles Fabricated by Double Emulsion-Solvent Evaporation for Simvastatin Delivery. Colloids Surf. B Biointerfaces 2021, 199, 11560. [Google Scholar] [CrossRef]
- Satapathy, S.R.; Sahoo, R.N.; Satapathy, B.; Immani, R.; Panigrahi, L.; Mallick, S. Development and Characterization of Leuprolide Acetate Encapsulated Plga Microspheres for Parenteral Controlled Release Depot Injection. Indian J. Pharm. Educ. Res. 2021, 55, 107–116. [Google Scholar] [CrossRef]
- Panigrahi, D.; Sahu, P.K.; Swain, S.; Verma, R.K. Quality by Design Prospects of Pharmaceuticals Application of Double Emulsion Method for PLGA Loaded Nanoparticles. SN Appl. Sci. 2021, 3, 638. [Google Scholar] [CrossRef]
- Kang, M.K.; Dai, J.; Kim, J.C. Ethylcellulose Microparticles Containing Chitosan and Gelatin: PH-Dependent Release Caused by Complex Coacervation. J. Ind. Eng. Chem. 2012, 18, 355–359. [Google Scholar] [CrossRef]
- Ach, D.; Briançon, S.; Broze, G.; Puel, F.; Rivoire, A.; Galvan, J.M.; Chevalier, Y. Formation of Microcapsules by Complex Coacervation. Can. J. Chem. Eng. 2015, 93, 183–191. [Google Scholar] [CrossRef]
- Scalera, F.; Gervaso, F.; de Benedictis, V.M.; Madaghiele, M.; Demitri, C. Synthesis of Chitosan-Based Sub-Micrometric Particles by Simple Coacervation. IEEE Trans. Nanotechnol. 2016, 15, 884–889. [Google Scholar] [CrossRef]
- Bastos, L.P.H.; de Sá Costa, B.; Siqueira, R.P.; Garcia-Rojas, E.E. Complex Coacervates of β-Lactoglobulin/Sodium Alginate for the Microencapsulation of Black Pepper (Piper Nigrum L.) Essential Oil: Simulated Gastrointestinal Conditions and Modeling Release Kinetics. Int. J. Biol. Macromol. 2020, 160, 861–870. [Google Scholar] [CrossRef]
- Giovagnoli, S.; Mancuso, F.; Vannini, S.; Calvitti, M.; Piroddi, M.; Pietrella, D.; Arato, I.; Falabella, G.; Galli, F.; Moretti, M.; et al. Microparticle-Loaded Neonatal Porcine Sertoli Cells for Cell-Based Therapeutic and Drug Delivery System. J. Control. Release 2014, 192, 249–261. [Google Scholar] [CrossRef]
- Zhou, P.; Wu, J.; Xia, Y.; Yuan, Y.; Zhang, H.; Xu, S.; Lin, K. Loading BMP-2 on Nanostructured Hydroxyapatite Microspheres for Rapid Bone Regeneration. Int. J. Nanomed. 2018, 13, 4083–4092. [Google Scholar] [CrossRef]
- Soni, G.; Yadav, K.S.; Gupta, M.K. QbD Based Approach for Formulation Development of Spray Dried Microparticles of Erlotinib Hydrochloride for Sustained Release. J. Drug Deliv. Sci. Technol. 2020, 57, 101684. [Google Scholar] [CrossRef]
- Joshi, D.J.; Chitre, N.M.; Bansal, A.; Murnane, K.S.; D’Souza, M.J. Formulation and Characterization of Microcapsules Encapsulating PC12 Cells as a Prospective Treatment Approach for Parkinson’s Disease. AAPS PharmSciTech 2021, 22, 149. [Google Scholar] [CrossRef]
- Piacentini, E.; Lakshmi, D.S.; Figoli, A.; Drioli, E.; Giorno, L. Polymeric Microspheres Preparation by Membrane Emulsification-Phase Separation Induced Process. J. Membr. Sci. 2013, 448, 190–197. [Google Scholar] [CrossRef]
- Piacentini, E.; Dragosavac, M.; Giorno, L. Pharmaceutical Particles Design by Membrane Emulsification: Preparation Methods and Applications in Drug Delivery. Curr. Pharm. Des. 2018, 23, 302–318. [Google Scholar] [CrossRef]
- Zhang, M.J.; Zhang, P.; Qiu, L.D.; Chen, T.; Wang, W.; Chu, L.Y. Controllable Microfluidic Fabrication of Microstructured Functional Materials. Biomicrofluidics 2020, 14, 061501. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fontana, F.; Python, A.; Hirvonen, J.T.; Santos, H.A. Microfluidics for Production of Particles: Mechanism, Methodology, and Applications. Small 2020, 16, 1904673. [Google Scholar] [CrossRef]
- Lee, S.; de Rutte, J.; Dimatteo, R.; Koo, D.; di Carlo, D. Scalable Fabrication and Use of 3D Structured Microparticles Spatially Functionalized with Biomolecules. ACS Nano 2021, 16, 38–49. [Google Scholar] [CrossRef]
- Ahrens, C.C.; Dong, Z.; Li, W. Engineering Cell Aggregates through Incorporated Polymeric Microparticles. Acta Biomater. 2017, 62, 64–81. [Google Scholar] [CrossRef]
- Simitzi, C.; Vlahovic, M.; Georgiou, A.; Keskin-Erdogan, Z.; Miller, J.; Day, R.M. Modular Orthopaedic Tissue Engineering With Implantable Microcarriers and Canine Adipose-Derived Mesenchymal Stromal Cells. Front. Bioeng. Biotechnol. 2020, 8, 816. [Google Scholar] [CrossRef]
- Privalova, A.; Markvicheva, E.; Sevrin, C.; Drozdova, M.; Kottgen, C.; Gilbert, B.; Ortiz, M.; Grandfils, C. Biodegradable Polyester-Based Microcarriers with Modified Surface Tailored for Tissue Engineering. J. Biomed. Mater. Res.-Part A 2015, 103, 939–948. [Google Scholar] [CrossRef]
- García Cruz, D.M.; Sardinha, V.; Escobar Ivirico, J.L.; Mano, J.F.; Gómez Ribelles, J.L. Gelatin Microparticles Aggregates as Three-Dimensional Scaffolding System in Cartilage Engineering. J. Mater. Sci. Mater. Med. 2013, 24, 503–513. [Google Scholar] [CrossRef]
- Quinlan, E.; López-Noriega, A.; Thompson, E.; Kelly, H.M.; Cryan, S.A.; O’Brien, F.J. Development of Collagen–Hydroxyapatite Scaffolds Incorporating PLGA and Alginate Microparticles for the Controlled Delivery of RhBMP-2 for Bone Tissue Engineering. J. Control. Release 2015, 198, 71–79. [Google Scholar] [CrossRef]
- DeVolder, R.J.; Kim, I.W.; Kim, E.-S.; Kong, H. Modulating the Rigidity and Mineralization of Collagen Gels Using Poly(Lactic-Co-Glycolic Acid) Microparticles. Tissue Eng. Part A 2012, 18, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Bhagabati, P.; Bhasney, S.M.; Bose, D.; Remadevi, R.; Setty, M.; Rajkhowa, R.; Katiyar, V. Silk and Wool Protein Microparticle-Reinforced Crystalline Polylactic Acid Biocomposites with Improved Cell Interaction for Targeted Biomedical Applications. ACS Appl. Polym. Mater. 2020, 2, 4739–4751. [Google Scholar] [CrossRef]
- Vyas, C.; Zhang, J.; Øvrebø, Ø.; Huang, B.; Roberts, I.; Setty, M.; Allardyce, B.; Haugen, H.; Rajkhowa, R.; Bartolo, P. 3D Printing of Silk Microparticle Reinforced Polycaprolactone Scaffolds for Tissue Engineering Applications. Mater. Sci. Eng. C 2021, 118, 111433. [Google Scholar] [CrossRef]
- Xu, Y.; Peng, J.; Richards, G.; Lu, S.; Eglin, D. Optimization of Electrospray Fabrication of Stem Cell–Embedded Alginate–Gelatin Microspheres and Their Assembly in 3D-Printed Poly(ε-Caprolactone) Scaffold for Cartilage Tissue Engineering. J. Orthop. Transl. 2019, 18, 128–141. [Google Scholar] [CrossRef]
- Levato, R.; Visser, J.; Planell, J.A.; Engel, E.; Malda, J.; Mateos-Timoneda, M.A. Biofabrication of Tissue Constructs by 3D Bioprinting of Cell-Laden Microcarriers. Biofabrication 2014, 6, 035020. [Google Scholar] [CrossRef]
- Leberfinger, A.N.; Ravnic, D.J.; Dhawan, A.; Ozbolat, I.T. Concise Review: Bioprinting of Stem Cells for Transplantable Tissue Fabrication. Stem Cells Transl. Med. 2017, 6, 1940–1948. [Google Scholar] [CrossRef]
- Oberweis, C.V.; Marchal, J.A.; López-Ruiz, E.; Gálvez-Martín, P. A Worldwide Overview of Regulatory Frameworks for Tissue-Based Products. Tissue Eng. Part B Rev. 2020, 26, 181–196. [Google Scholar] [CrossRef]
- Du, L.; Yang, S.; Li, W.; Li, H.; Feng, S.; Zeng, R.; Yu, B.; Xiao, L.; Nie, H.-Y.; Tu, M. Scaffold Composed of Porous Vancomycin-Loaded Poly(Lactide- Co -Glycolide) Microspheres: A Controlled-Release Drug Delivery System with Shape-Memory Effect. Mater. Sci. Eng. C 2017, 78, 1172–1178. [Google Scholar] [CrossRef]
- Yuan, S.; Shen, F.; Chua, C.K.; Zhou, K. Polymeric Composites for Powder-Based Additive Manufacturing: Materials and Applications. Prog. Polym. Sci. 2019, 91, 141–168. [Google Scholar] [CrossRef]
- Mazzoli, A. Selective Laser Sintering in Biomedical Engineering. Med. Biol. Eng. Comput. 2013, 51, 245–256. [Google Scholar] [CrossRef]
- Yan, D.; Zeng, B.; Han, Y.; Dai, H.; Liu, J.; Sun, Y.; Li, F. Preparation and Laser Powder Bed Fusion of Composite Microspheres Consisting of Poly(Lactic Acid) and Nano-Hydroxyapatite. Addit. Manuf. 2020, 34, 101305. [Google Scholar] [CrossRef]
- Krokos, A.; Gazinska, M.; Kryszak, B.; Dzienny, P.; Stepak, B.; Olejarczyk, M.; Gruber, P.; Kwiatkowski, R.; Bondyra, A.; Antonczak, A. Comparison of Thermal, Structural and Morphological Properties of Poly(L-Lactide) and Poly(L-Lactide)/Hydroxyapatite Microspheres for Laser Sintering Processes. Polimery 2020, 65, 605–612. [Google Scholar] [CrossRef]
- McKay, W.F.; Peckham, S.M.; Badura, J.M. A Comprehensive Clinical Review of Recombinant Human Bone Morphogenetic Protein-2 (INFUSE® Bone Graft). Int. Orthop. 2007, 31, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Dimar, J.R.; Glassman, S.D.; Burkus, J.K.; Pryor, P.W.; Hardacker, J.W.; Carreon, L.Y. Clinical and Radiographic Analysis of an Optimized RhBMP-2 Formulation as an Autograft Replacement in Posterolateral Lumbar Spine Arthrodesis. J. Bone Jt. Surg.-Ser. A 2009, 91, 1377–1386. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 7, 469. [Google Scholar] [CrossRef]
- Perez, R.A.; El-Fiqi, A.; Park, J.H.; Kim, T.H.; Kim, J.H.; Kim, H.W. Therapeutic Bioactive Microcarriers: Co-Delivery of Growth Factors and Stem Cells for Bone Tissue Engineering. Acta Biomater. 2014, 10, 520–530. [Google Scholar] [CrossRef]
- Karam, J. Development of Pharmacologically Active Microcarriers Transporting Stem Cells and Releasing Growth Factors for Cardiac Tissue-Engineering. Ph.D. Thesis, HAL, Université d’Angers, Angers, France, 2014. [Google Scholar]
- Barcak, E.A.; Beebe, M.J. Bone Morphogenetic Protein: Is There Still a Role in Orthopedic Trauma in 2017? Orthop. Clin. North Am. 2017, 48, 301–309. [Google Scholar] [CrossRef]
- Krishnakumar, G.S.; Roffi, A.; Reale, D.; Kon, E.; Filardo, G. Clinical Application of Bone Morphogenetic Proteins for Bone Healing: A Systematic Review. Int. Orthop. 2017, 41, 1073–1083. [Google Scholar] [CrossRef]
- Seeherman, H.J.; Berasi, S.P.; Brown, C.T.; Martinez, R.X.; Sean Juo, Z.; Jelinsky, S.; Cain, M.J.; Grode, J.; Tumelty, K.E.; Bohner, M.; et al. A BMP/Activin A Chimera Is Superior to Native BMPs and Induces Bone Repair in Nonhuman Primates When Delivered in a Composite Matrix. Sci. Transl. Med. 2019, 11, eaar4953. [Google Scholar] [CrossRef]
- Somers, P.; Cornelissen, R.; Thierens, H.; van Nooten, G. An Optimized Growth Factor Cocktail for Ovine Mesenchymal Stem Cells. Growth Factors 2012, 30, 37–48. [Google Scholar] [CrossRef]
- Ding, Z.-Y.; Tan, Y.; Peng, Q.; Zuo, J.; Li, N. Novel Applications of Platelet Concentrates in Tissue Regeneration (Review). Exp. Ther. Med. 2021, 21, 226. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Insights, G.T.; Therapy, G.; Rafiq, Q.A. Toward a Scalable and Consistent Manufacturing Process for the Production of Human MSCs. Cell Gene Ther. Insights 2016, 2, 127–140. [Google Scholar] [CrossRef][Green Version]
- Tavassoli, H.; Alhosseini, S.N.; Tay, A.; Chan, P.P.Y.; Weng Oh, S.K.; Warkiani, M.E. Large-Scale Production of Stem Cells Utilizing Microcarriers: A Biomaterials Engineering Perspective from Academic Research to Commercialized Products. Biomaterials 2018, 181, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Van Wezel, A.L. Growth of Cell-strains and Primary Cells on Micro-carriers in Homogeneous Culture. Nature 1967, 216, 64–65. [Google Scholar] [CrossRef]
- Chen, X.Y.; Chen, J.Y.; Tong, X.M.; Mei, J.G.; Chen, Y.F.; Mou, X.Z. Recent Advances in the Use of Microcarriers for Cell Cultures and Their Ex Vivo and in Vivo Applications. Biotechnol. Lett. 2020, 42, 1–10. [Google Scholar] [CrossRef]
- Amer, M.H.; Alvarez-Paino, M.; McLaren, J.; Pappalardo, F.; Trujillo, S.; Wong, J.Q.; Shrestha, S.; Abdelrazig, S.; Stevens, L.A.; Lee, J.B.; et al. Designing Topographically Textured Microparticles for Induction and Modulation of Osteogenesis in Mesenchymal Stem Cell Engineering. Biomaterials 2021, 266, 120450. [Google Scholar] [CrossRef]
- Maciel, M.M.; Correia, T.R.; Henriques, M.; Mano, J.F. Microparticles Orchestrating Cell Fate in Bottom-up Approaches. Curr. Opin. Biotechnol. 2022, 73, 276–281. [Google Scholar] [CrossRef]
- Zheng, S.; Liu, Q.; He, J.; Wang, X.; Ye, K.; Wang, X.; Yan, C.; Liu, P.; Ding, J. Critical Adhesion Areas of Cells on Micro-Nanopatterns. Nano Res. 2022, 15, 1623–1635. [Google Scholar] [CrossRef]
- Darge, H.F.; Chuang, S.H.; Lai, J.Y.; Lin, S.Y.; Tsai, H.C. Preparation of Thermosensitive PNIPAm-Based Copolymer Coated Cytodex 3 Microcarriers for Efficient Nonenzymatic Cell Harvesting during 3D Culturing. Biotechnol. Bioeng. 2021, 118, 4076–4091. [Google Scholar] [CrossRef]
- Narumi, Y.; Iwai, R.; Takagi, M. Recovery of Human Mesenchymal Stem Cells Grown on Novel Microcarrier Coated with Thermoresponsive Polymer. J. Artif. Organs 2020, 23, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qian, Y.; Zhao, S.; Yin, Y.; Li, J. Alginate/PEG Based Microcarriers with Cleavable Crosslinkage for Expansion and Non-Invasive Harvest of Human Umbilical Cord Blood Mesenchymal Stem Cells. Mater. Sci. Eng. C 2016, 64, 43–53. [Google Scholar] [CrossRef]
- Zhou, A.; Ye, Z.; Zhou, Y.; Tan, W.S. Bioactive Poly(ε-Caprolactone) Microspheres with Tunable Open Pores as Microcarriers for Tissue Regeneration. J. Biomater. Appl. 2019, 33, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Perteghella, S.; Martella, E.; de Girolamo, L.; Orfei, C.P.; Pierini, M.; Fumagalli, V.; Pintacuda, D.V.; Chlapanidas, T.; Viganò, M.; Faragò, S.; et al. Fabrication of Innovative Silk/Alginate Microcarriers for Mesenchymal Stem Cell Delivery and Tissue Regeneration. Int. J. Mol. Sci. 2017, 18, 1829. [Google Scholar] [CrossRef]
- Shekaran, A.; Lam, A.; Sim, E.; Jialing, L.; Jian, L.; Wen, J.T.P.; Chan, J.K.Y.; Choolani, M.; Reuveny, S.; Birch, W.; et al. Biodegradable ECM-Coated PCL Microcarriers Support Scalable Human Early MSC Expansion and in Vivo Bone Formation. Cytotherapy 2016, 18, 1332–1344. [Google Scholar] [CrossRef] [PubMed]
- Karimian, S.A.M.; Mashayekhan, S.; Baniasadi, H. Fabrication of Porous Gelatin-Chitosan Microcarriers and Modeling of Process Parameters via the RSM Method. Int. J. Biol. Macromol. 2016, 88, 288–295. [Google Scholar] [CrossRef]
- Huang, L.; Xiao, L.; Jung Poudel, A.; Li, J.; Zhou, P.; Gauthier, M.; Liu, H.; Wu, Z.; Yang, G. Porous Chitosan Microspheres as Microcarriers for 3D Cell Culture. Carbohydr. Polym. 2018, 202, 611–620. [Google Scholar] [CrossRef]
- Dosta, P.; Ferber, S.; Zhang, Y.; Wang, K.; Ros, A.; Uth, N.; Levinson, Y.; Abraham, E.; Artzi, N. Scale-up Manufacturing of Gelatin-Based Microcarriers for Cell Therapy. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2020, 108, 2937–2949. [Google Scholar] [CrossRef]
- Smith, D.; Herman, C.; Razdan, S.; Abedin, M.R.; van Stoecker, W.; Barua, S. Microparticles for Suspension Culture of Mammalian Cells. ACS Appl. Bio Mater. 2019, 2, 2791–2801. [Google Scholar] [CrossRef]
- Xia, P.; Zhang, K.; Fang, J.; Yan, S.; Cui, L.; Chen, X.; Yin, J. A Novel Fabrication of Open Porous Poly-(γ-Benzyl-L-Glutamate) Microcarriers with Large Pore Size to Promote Cellular Infiltration and Proliferation. Mater. Lett. 2017, 206, 136–139. [Google Scholar] [CrossRef]
- Somville, E.; Kumar, A.A.; Guicheux, J.; Halgand, B.; Demoustier-Champagne, S.; des Rieux, A.; Jonas, A.M.; Glinel, K. Green and Tunable Animal Protein-Free Microcarriers for Cell Expansion. ACS Appl. Mater. Interfaces 2020, 12, 50303–50314. [Google Scholar] [CrossRef]
- Roux, R.; Ladavière, C.; Montembault, A.; Delair, T. Particle Assemblies: Toward New Tools for Regenerative Medicine. Mater. Sci. Eng. C 2013, 33, 997–1007. [Google Scholar] [CrossRef]
- Tan, Y.J.; Tan, X.; Yeong, W.Y.; Tor, S.B. Hybrid Microscaffold-Based 3D Bioprinting of Multi-Cellular Constructs with High Compressive Strength: A New Biofabrication Strategy. Sci. Rep. 2016, 6, 39140. [Google Scholar] [CrossRef]
- Liu, S.; Huang, D.; Hu, Y.; Zhang, J.; Chen, B.; Zhang, H.; Dong, X.; Tong, R.; Li, Y.; Zhou, W. Sodium Alginate/Collagen Composite Multiscale Porous Scaffolds Containing Poly(ε-Caprolactone) Microspheres Fabricated Based on Additive Manufacturing Technology. RSC Adv. 2020, 10, 39241–39250. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for Bone Tissue Engineering Scaffolds: A Review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef]
- Grebenik, E.A.; Grinchenko, V.D.; Churbanov, S.N.; Minaev, N.V.; Shavkuta, B.S.; Melnikov, P.A.; Butnaru, D.V.; Rochev, Y.A.; Bagratashvili, V.N.; Timashev, P.S. Osteoinducing Scaffolds with Multi-Layered Biointerface. Biomed. Mater. 2018, 13, 054103. [Google Scholar] [CrossRef]
- Qu, H. Additive Manufacturing for Bone Tissue Engineering Scaffolds. Mater. Today Commun. 2020, 24, 101024. [Google Scholar] [CrossRef]
- Demina, T.S.; Popyrina, T.N.; Minaeva, E.D.; Dulyasova, A.A.; Minaeva, S.A.; Tilkin, R.; Yusupov, V.I.; Grandfils, C.; Akopova, T.A.; Minaev, N.V.; et al. Polylactide Microparticles Stabilized by Chitosan Graft-Copolymer as Building Blocks for Scaffold Fabrication via Surface-Selective Laser Sintering. J. Mater. Res. 2022, 37, 933–942. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Lee, S.H.; Wang, M.; Cheung, W.L.; Ip, W.Y. Selective Laser Sintering of Porous Tissue Engineering Scaffolds from Poly(l-Lactide)/Carbonated Hydroxyapatite Nanocomposite Microspheres. J. Mater. Sci. Mater. Med. 2008, 19, 2535–2540. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-T.; Lee, M.-Y.; Tsai, W.-W.; Wang, H.-C.; Lu, W.-C. Osteogenesis of Adipose-Derived Stem Cells on Polycaprolactone- β -Tricalcium Phosphate Scaffold Fabricated via Selective Laser Sintering and Surface Coating with Collagen Type I. J. Tissue Eng. Regen. Med. 2016, 10, E337–E353. [Google Scholar] [CrossRef]
- Lin, K.; Liu, J.; Wu, J.-M.; Sun, Y.; Li, F.; Zhou, Y.; Shi, Y. Selective Laser Sintered Nano-HA/PDLLA Composite Microspheres for Bone Scaffolds Applications. Rapid Prototyp. J. 2020, 26, 1131–1143. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lee, M.-Y.; Shyu, V.B.-H.; Chen, Y.-C.; Chen, C.-T.; Chen, J.-P. Surface Modification of Polycaprolactone Scaffolds Fabricated via Selective Laser Sintering for Cartilage Tissue Engineering. Mater. Sci. Eng. C 2014, 40, 389–397. [Google Scholar] [CrossRef]
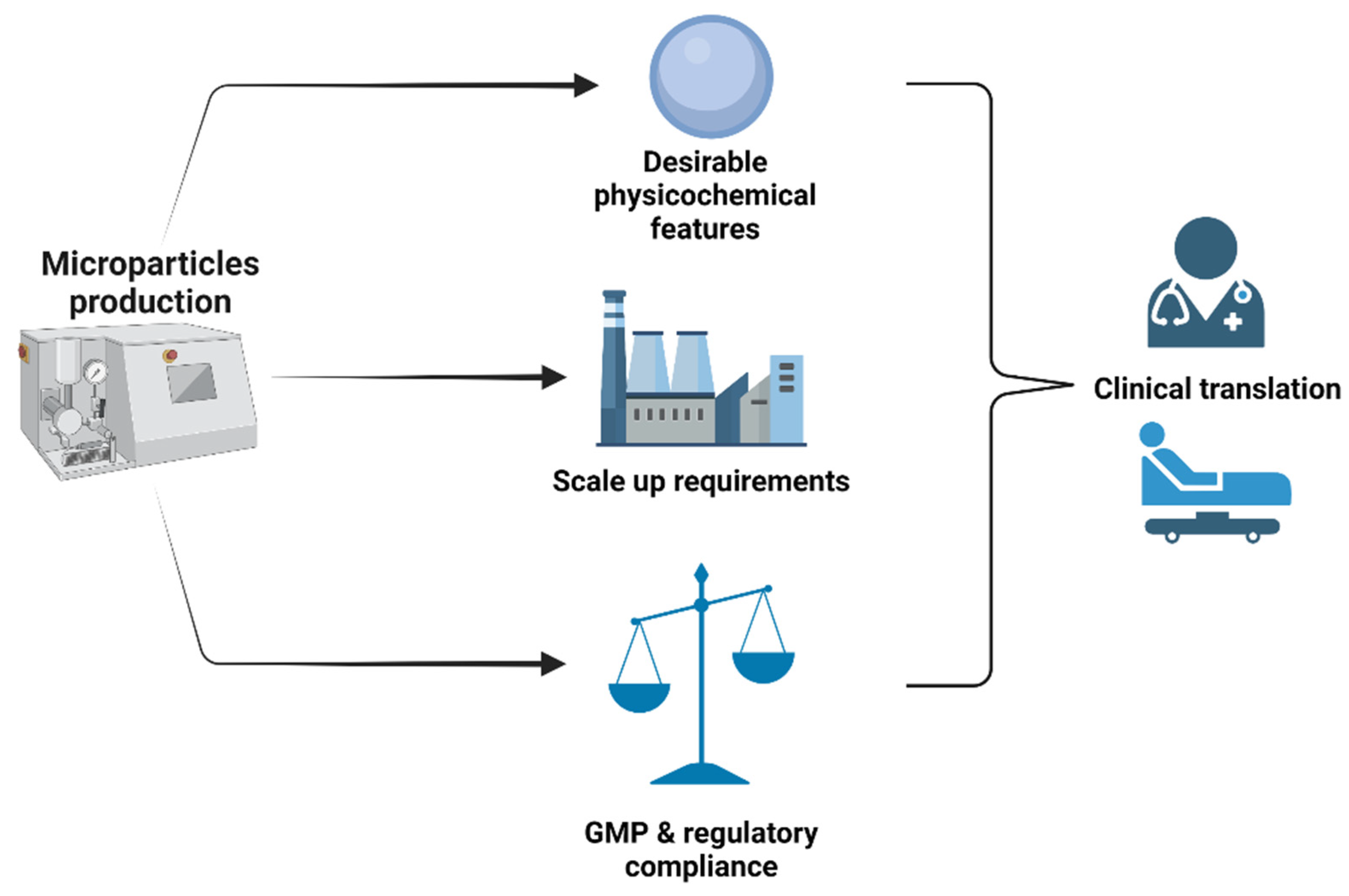


| Fabrication Technology | Critical Process Parameters | Advantages | Drawbacks | Scalability and GMP Compliance | Suitability for Cell Culture and Tissue Engineering | Ref. |
|---|---|---|---|---|---|---|
| Solvent extraction/evaporation based-Methods |
|
|
| Kinam et al., reported recently a continuous in-line emulsification-extraction process capable of processing at flow rate of up to 400 mL/min to produce PLGA microparticles. This system can comply with GMP requirements. | Mesenchymal stem cells, adipose-derived stem cells, cardiac progenitor cells were successfully cultured and evaluated for various applications. All microparticles showed good biocompatibility. | [89,90,91,92] |
| Coacervation |
|
|
| Need to fully understand the impact of each process parameter for possible process scale up. This remains challenging. | Angiogenesis-inducing stem cell, mesenchymal stem cells were cultured for regenerative treatments. | [93,94,95,96] |
| Spray–drying |
|
|
| Attempts of scale-up have been carried out to produce functional microparticles for various pharmaceutical uses. | Cardiac stem cells, neonatal porcine sertoli cells, adrenal pheochromocytoma (P1C12) cells were either cultured or encapsulated into microparticles for various applications. | [97,98,99,100] |
| Membrane emulsification |
|
| Difficult to quantify the interplaying parameters (shear forces, interfacial tensions, etc.) that control the droplet size. | Large scale production can be carried out by transferring meaningful laboratory data for process scale-up. However, low emulsion throughputs and membrane fouling remain the main limitations for scale up. | Embryonic fibroblasts (NIH-3T3 cells), mesenchymal stem cells were investigated. | [55,101,102] |
| Microfluidic |
|
|
| Parallelization of droplet generators in three-dimensional microfluidic devices has been widely proposed for large-scale production of microparticles. However, several challenges remain, especially in the development of systems that increase significantly fluid delivery while maintaining a uniform flow rate. | Mesenchymal stem cells were successfully expanded or encapsulated in various types of microparticles for tissue engineering constructs. | [103,104,105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sherstneva, A.A.; Demina, T.S.; Monteiro, A.P.F.; Akopova, T.A.; Grandfils, C.; Ilangala, A.B. Biodegradable Microparticles for Regenerative Medicine: A State of the Art and Trends to Clinical Application. Polymers 2022, 14, 1314. https://doi.org/10.3390/polym14071314
Sherstneva AA, Demina TS, Monteiro APF, Akopova TA, Grandfils C, Ilangala AB. Biodegradable Microparticles for Regenerative Medicine: A State of the Art and Trends to Clinical Application. Polymers. 2022; 14(7):1314. https://doi.org/10.3390/polym14071314
Chicago/Turabian StyleSherstneva, Anastasia A., Tatiana S. Demina, Ana P. F. Monteiro, Tatiana A. Akopova, Christian Grandfils, and Ange B. Ilangala. 2022. "Biodegradable Microparticles for Regenerative Medicine: A State of the Art and Trends to Clinical Application" Polymers 14, no. 7: 1314. https://doi.org/10.3390/polym14071314
APA StyleSherstneva, A. A., Demina, T. S., Monteiro, A. P. F., Akopova, T. A., Grandfils, C., & Ilangala, A. B. (2022). Biodegradable Microparticles for Regenerative Medicine: A State of the Art and Trends to Clinical Application. Polymers, 14(7), 1314. https://doi.org/10.3390/polym14071314









