Conductivity Behaviour under Pressure of Copper Micro-Additive/Polyurethane Composites (Experiment and Modelling)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
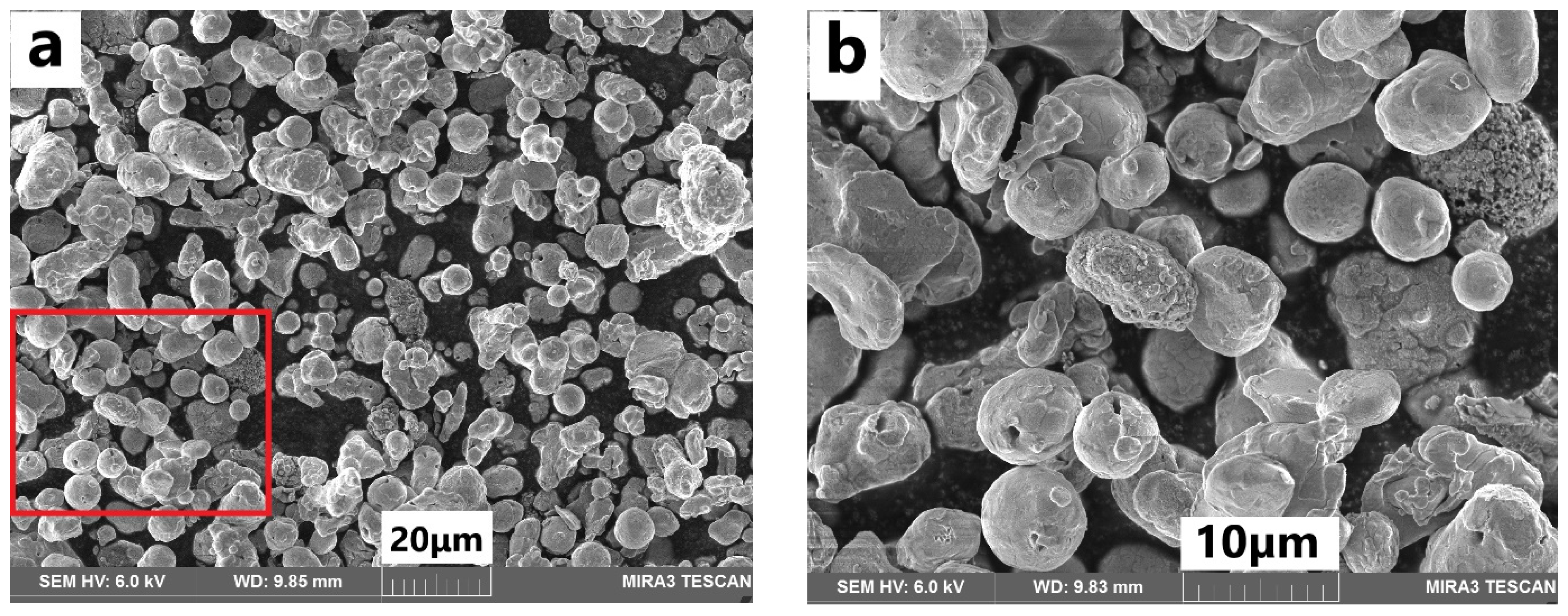
2.2. Preparation of Micro-Copper Polyurethane Films
2.3. Characterization
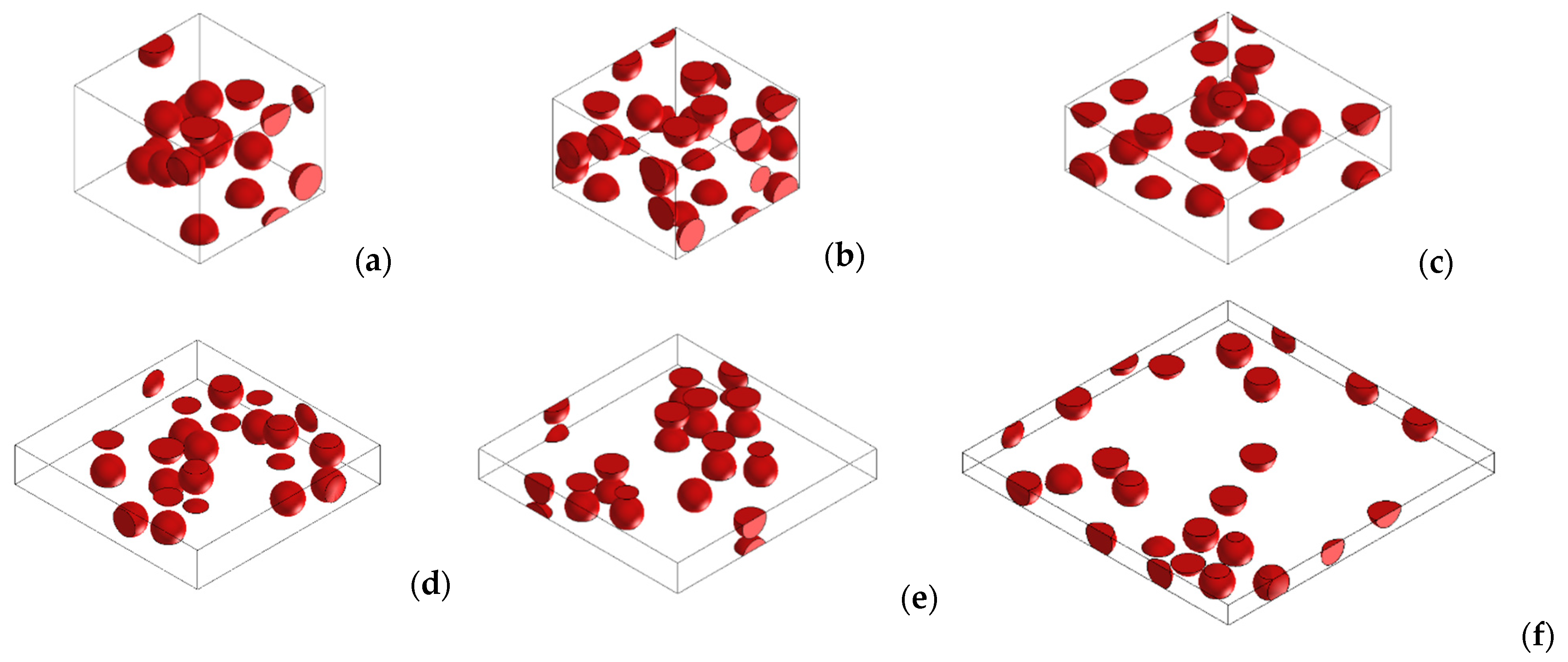
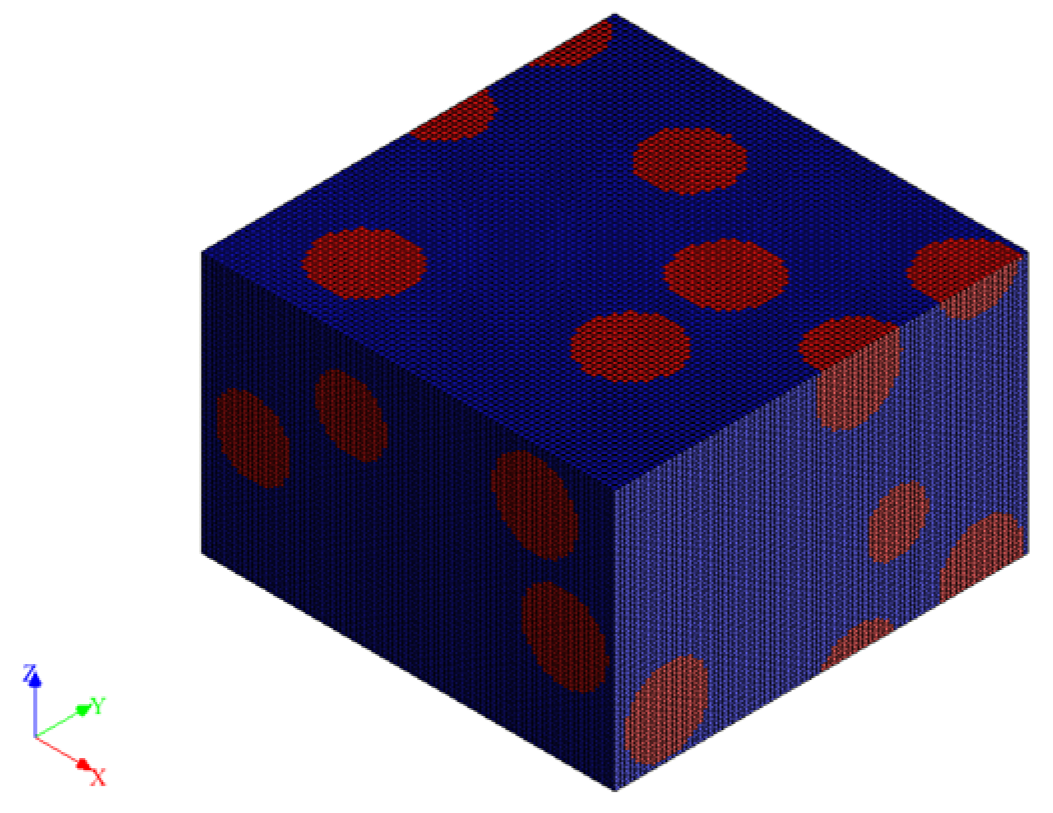
2.4. Simulation
3. Results and Discussion
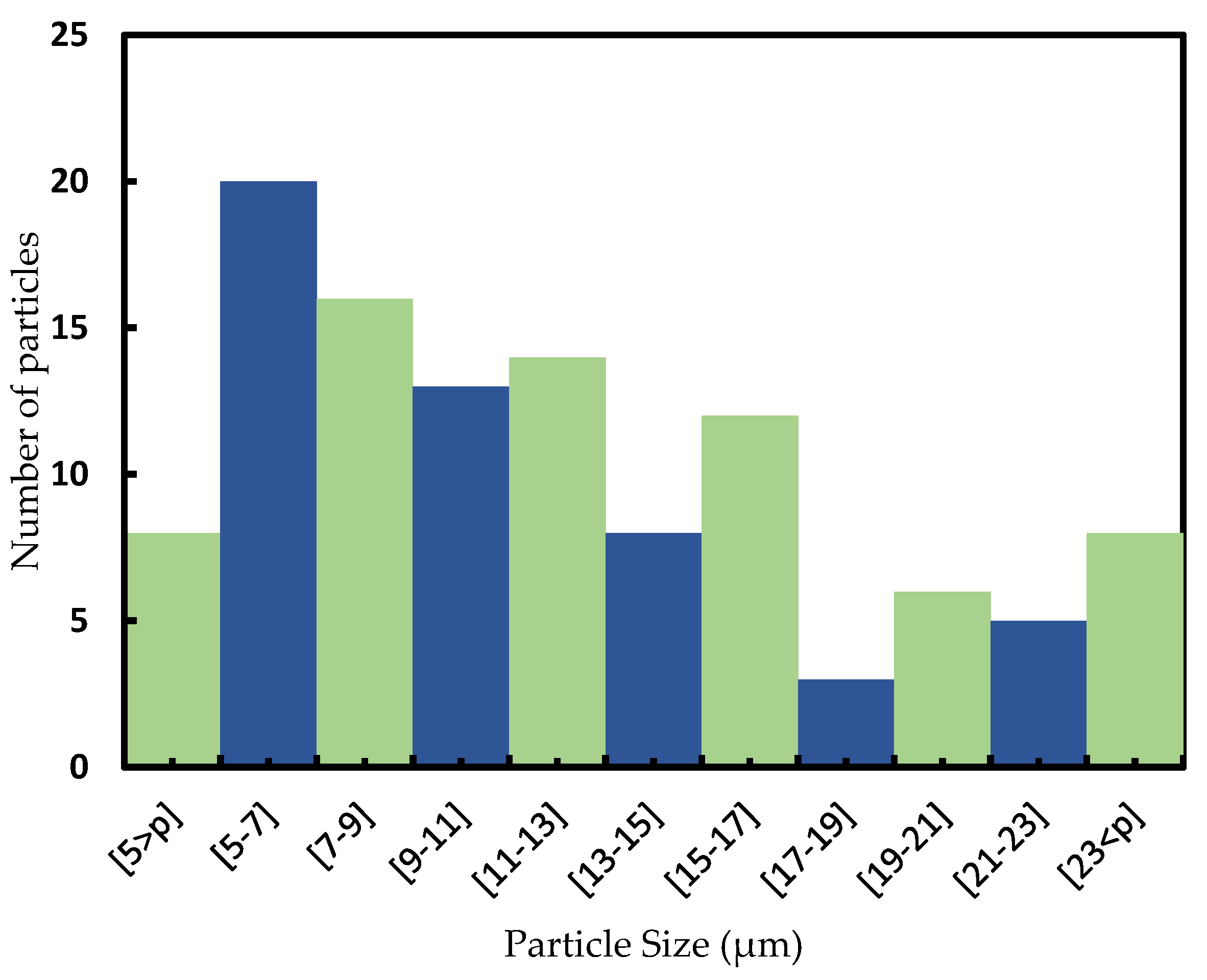
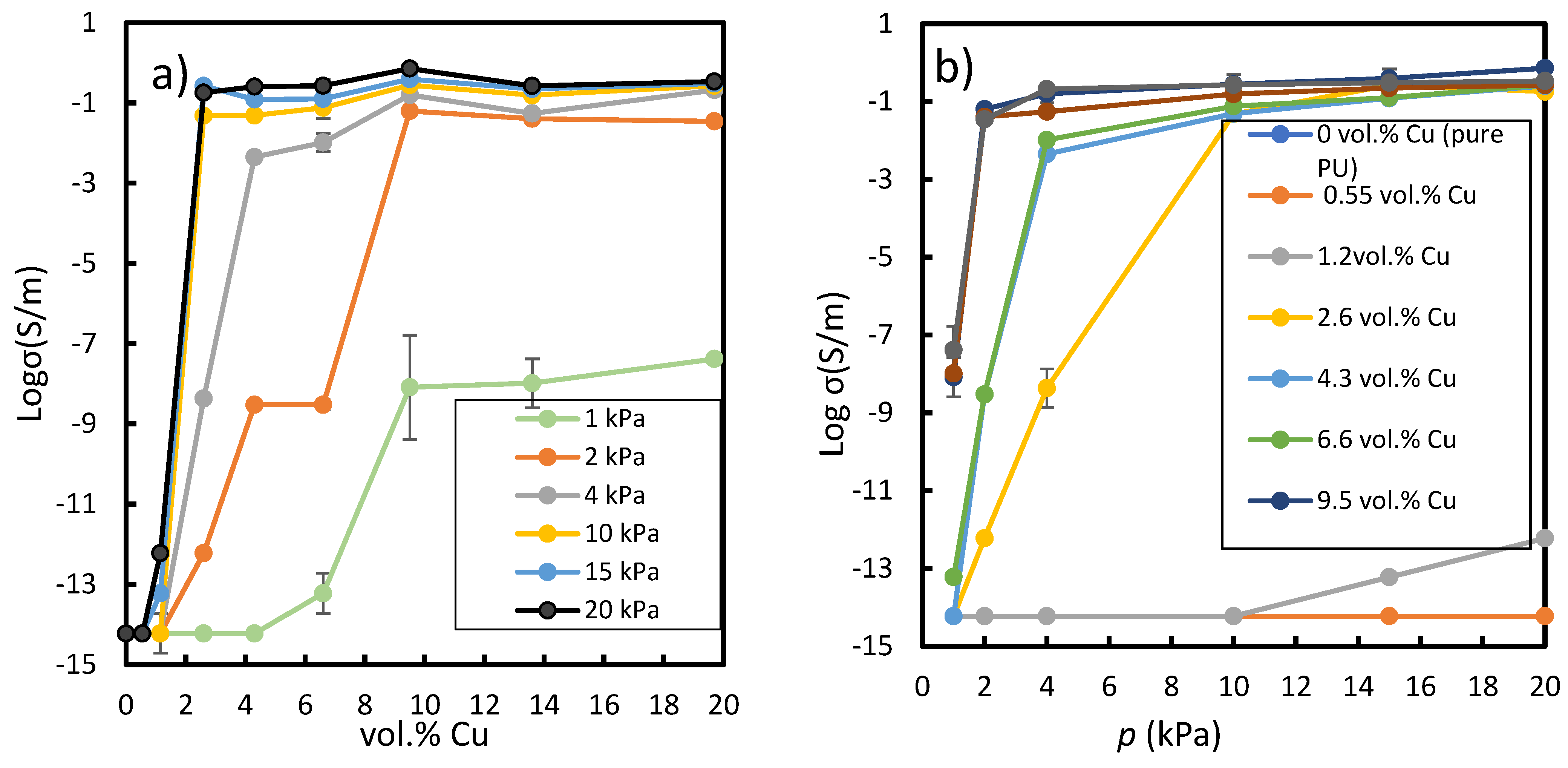
3.1. Characterization and Electrical Conductivity
3.2. Analytical Model
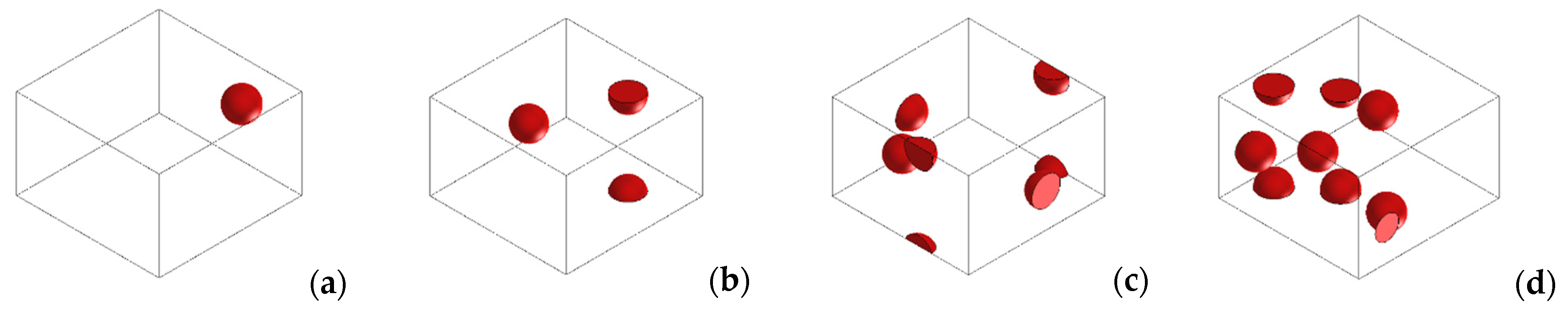
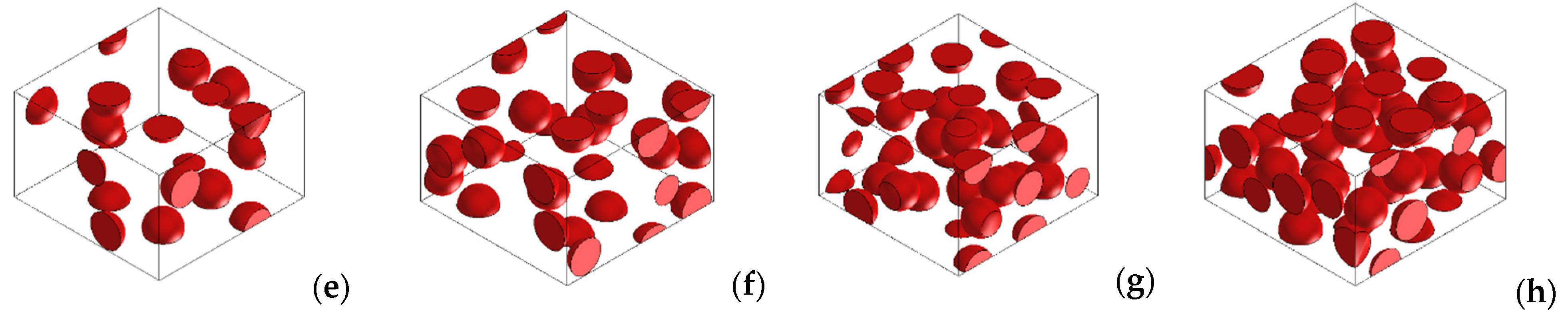
3.3. Numerical Simulations
4. Conclusions
- (a)
- The obtained Cu-PU composite films could be used as a basic material for flip-chips, switching devices, and tactile sensors, as they are flexible, easy to prepare, lightweight, conductive, and their conductivity can change from 10−14 to 3.5 10−1 S·m−1 depending on the applied pressure (1 to 20 kPa);
- (b)
- The FE-RVE simulation could be used to describe the change in the electrical conductivity of polymer–metal composites subjected to different pressures. This also includes the dominating electron transport mechanisms before and after the percolation threshold. This research could help to reduce the number of expensive and time-consuming experiments carried out. Further investigation would be required to find out more about the exhaustive coverage of this method for a wide range of composites, including carbonaceous–polymer, hybrid fillers and reinforced composites.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, D.; Sharma, R.C. Advances in conductive polymers. Eur. Polym. J. 1998, 34, 1053–1060. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Bauhofer, W.; Kovacs, J.Z. A review and analysis of electrical percolation in carbon nanotube polymer composites. Compos. Sci. Technol. 2009, 69, 1486–1498. [Google Scholar] [CrossRef]
- Forintos, N.; Czigany, T. Multifunctional application of carbon fiber reinforced polymer composites: Electrical properties of the reinforcing carbon fibers—A short review. Compos. Part B Eng. 2019, 162, 331–343. [Google Scholar] [CrossRef]
- Balberg, I. A comprehensive picture of the electrical phenomena in carbon black-polymer composites. Carbon 2002, 40, 139–143. [Google Scholar] [CrossRef]
- Sengupta, R.; Bhattacharya, M.; Bandyopadhyay, S.; Bhowmick, A.K. A review on the mechanical and electrical properties of graphite and modified graphite reinforced polymer composites. Prog. Polym. Sci. 2011, 36, 638–670. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.-T.; Hui, D.; Bhattacharyya, D. Graphene-based materials and their composites: A review on production, applications and product limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Mamunya, Y.P.; Davydenko, V.V.; Pissis, P.; Lebedev, E.V. Electrical and thermal conductivity of polymers filled with metal powders. Eur. Polym. J. 2002, 38, 1887–1897. [Google Scholar] [CrossRef]
- Sannicolo, T.; Lagrange, M.; Cabos, A.; Celle, C.; Simonato, J.-P.; Bellet, D. Metallic Nanowire-Based Transparent Electrodes for Next Generation Flexible Devices: A Review. Small 2016, 12, 6052–6075. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, Q.; Cui, X.; Dong, M.; Zhang, J.; Wang, C.; Fan, J.; Zhu, Y.; Guo, Z. An overview of stretchable strain sensors from conductive polymer nanocomposites. J. Mater. Chem. C 2019, 7, 11710–11730. [Google Scholar] [CrossRef]
- Zhao, C.; Jia, X.; Shu, K.; Yu, C.; Wallace, G.G.; Wang, C. Conducting polymer composites for unconventional solid-state supercapacitors. J. Mater. Chem. A 2020, 8, 4677–4699. [Google Scholar] [CrossRef]
- Xie, J.; Gu, P.; Zhang, Q. Nanostructured Conjugated Polymers: Toward High-Performance Organic Electrodes for Rechargeable Batteries. ACS Energy Lett. 2017, 2, 1985–1996. [Google Scholar] [CrossRef]
- Wan, S.; Miao, C.-H.; Wang, R.-M.; Zhang, Z.-F.; Dong, Z.-H. Enhanced corrosion resistance of copper by synergetic effects of silica and BTA codoped in polypyrrole film. Prog. Org. Coat. 2019, 129, 187–198. [Google Scholar] [CrossRef]
- Jin, Y.; Sun, Y.; Wang, K.; Chen, Y.; Liang, Z.; Xu, Y.; Xiao, F. Long-term stable silver nanowire transparent composite as bottom electrode for perovskite solar cells. Nano Res. 2018, 11, 1998–2011. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, X.; Zhang, G.; Wang, S.; Zhu, S.; Wu, X.; Wang, Y.; Wang, Q.; Hu, C. Conducting polymer/silver nanowires stacking composite films for high-performance electrochromic devices. Sol. Energy Mater. Sol. Cells 2019, 200, 109919. [Google Scholar] [CrossRef]
- Naghdi, S.; Rhee, K.Y.; Hui, D.; Park, S.J. A Review of Conductive Metal Nanomaterials as Conductive, Transparent, and Flexible Coatings, Thin Films, and Conductive Fillers: Different Deposition Methods and Applications. Coatings 2018, 8, 278. [Google Scholar] [CrossRef]
- Liu, H.; Li, Q.; Zhang, S.; Yin, R.; Liu, X.; He, Y.; Dai, K.; Shan, C.; Guo, J.; Liu, C.; et al. Electrically conductive polymer composites for smart flexible strain sensors: A critical review. J. Mater. Chem. C 2018, 6, 12121–12141. [Google Scholar] [CrossRef]
- Matula, R.A. Electrical resistivity of copper, gold, palladium, and silver. J. Phys. Chem. Ref. Data 1979, 8, 1147–1298. [Google Scholar] [CrossRef]
- Joseph, H.; Koo, S.D. Polymer Nanocomposites: Processing, Characterization, and Applications, 2nd ed.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Kabir, E.; Kumar, V.; Kim, K.-H.; Yip, A.C.K.; Sohn, J.R. Environmental impacts of nanomaterials. J. Environ. Manag. 2018, 225, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.-S. Conductive Elastomers for Stretchable Electronics, Sensors and Energy Harvesters. Polymers 2016, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Larmagnac, A.; Eggenberger, S.; Janossy, H.; Vörös, J. Stretchable electronics based on Ag-PDMS composites. Sci. Rep. 2014, 4, 7254. [Google Scholar] [CrossRef]
- Vaithylingam, R.; Ansari, M.N.M.; Shanks, R.A. Recent Advances in Polyurethane-Based Nanocomposites: A Review. Polym.-Plast. Technol. Eng. 2017, 56, 1528–1541. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Huang, X.-W.; Song, X.; Hu, M.; Tang, L.; Xue, H.; Gao, J. Superhydrophobic and superelastic conductive rubber composite for wearable strain sensors with ultrahigh sensitivity and excellent anti-corrosion property. J. Mater. Chem. A 2018, 6, 24523–24533. [Google Scholar] [CrossRef]
- Kirkpatrick, S. Percolation and Conduction. Rev. Mod. Phys. 1973, 45, 574–588. [Google Scholar] [CrossRef]
- Folorunso, O.; Hamam, Y.; Sadiku, R.; Ray, S.S.; Joseph, A.G. Parametric Analysis of Electrical Conductivity of Polymer-Composites. Polymers 2019, 11, 1250. [Google Scholar] [CrossRef]
- Taherian, R. Experimental and analytical model for the electrical conductivity of polymer-based nanocomposites. Compos. Sci. Technol. 2016, 123, 17–31. [Google Scholar] [CrossRef]
- Krupa, I.; Chodák, I. Physical properties of thermoplastic/graphite composites. Eur. Polym. J. 2001, 37, 2159–2168. [Google Scholar] [CrossRef]
- Zeng, Q.H.; Yu, A.B.; Lu, G.Q. Multiscale modeling and simulation of polymer nanocomposites. Prog. Polym. Sci. 2008, 33, 191–269. [Google Scholar] [CrossRef]
- Zamani, S.M.M.; Behdinan, K. A molecular dynamics study of the mechanical and electrical properties of Polydimethylsiloxane-Ni conductive nanocomposites. Compos. Sci. Technol. 2020, 200, 108463. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, J.; Chen, X.; Weng, G.J. Calculating the Electrical Conductivity of Graphene Nanoplatelet Polymer Composites by a Monte Carlo Method. Nanomaterials 2020, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- Baştuğ, T.; Kuyucak, S. Memory effects in Brownian dynamics simulations of ion transport. Chem. Phys. Lett. 2005, 401, 175–179. [Google Scholar] [CrossRef]
- Zhou, J.; Schmid, F. Dielectric response of nanoscopic spherical colloids in alternating electric fields: A dissipative particle dynamics simulation. J. Phys. Condens. Matter 2012, 24, 464112. [Google Scholar] [CrossRef][Green Version]
- Seidel, G.D.; Lagoudas, D.C. A Micromechanics Model for the Electrical Conductivity of Nanotube-Polymer Nanocomposites. J. Compos. Mater. 2009, 43, 917–941. [Google Scholar] [CrossRef]
- Castellino, M.; Chiolerio, A.; Shahzad, M.I.; Jagdale, P.V.; Tagliaferro, A. Electrical conductivity phenomena in an epoxy resin-carbon-based materials composite. Compos. Part A Appl. Sci. Manuf. 2014, 61, 108–114. [Google Scholar] [CrossRef]
- Wang, G.; Wang, C.; Zhang, F.; Yu, X. Electrical percolation of nanoparticle-polymer composites. Comput. Mater. Sci. 2018, 150, 102–106. [Google Scholar] [CrossRef]
- Tamayo-Vegas, S.; Muhsan, A.; Tarfaoui, M.; Lafdi, K.; Chang, L. Effect of CNT additives on the electrical properties of derived nanocomposites (experimentally and numerical investigation). Mater. Today Proc. 2021, 52, 199–205. [Google Scholar] [CrossRef]
- Tserpes, K.; Tzatzadakis, V.; Bachmann, J. Electrical Conductivity and Electromagnetic Shielding Effectiveness of Bio-Composites. J. Compos. Sci. 2020, 4, 28. [Google Scholar] [CrossRef]
- Yaman, K.; Taga, Ö. Thermal and Electrical Conductivity of Unsaturated Polyester Resin Filled with Copper Filler Composites. Int. J. Polym. Sci. 2018, 2018, 8190190. [Google Scholar] [CrossRef]
- Luyt, A.S.; Molefi, J.A.; Krump, H. Thermal, mechanical and electrical properties of copper powder filled low-density and linear low-density polyethylene composites. Polym. Degrad. Stab. 2006, 91, 1629–1636. [Google Scholar] [CrossRef]
- Poblete, V.H.; Álvarez, M.P.; Fuenzalida, V.M. Conductive copper-PMMA nanocomposites: Microstructure, electrical behavior, and percolation threshold as a function of metal filler concentration. Polym. Compos. 2009, 30, 328–333. [Google Scholar] [CrossRef]
- Misiura, A.I.; Mamunya, Y.P.; Kulish, M.P. Metal-Filled Epoxy Composites: Mechanical Properties and Electrical/Thermal Conductivity. J. Macromol. Sci. Part B 2020, 59, 121–136. [Google Scholar] [CrossRef]
- Mamunya, Y.P.; Zois, H.; Apekis, L.; Lebedev, E.V. Influence of pressure on the electrical conductivity of metal powders used as fillers in polymer composites. Powder Technol. 2004, 140, 49–55. [Google Scholar] [CrossRef]
- Mamunya, Y.; Matzui, L.; Vovchenko, L.; Maruzhenko, O.; Oliynyk, V.; Pusz, S.; Kumanek, B.; Szeluga, U. Influence of conductive nano- and microfiller distribution on electrical conductivity and EMI shielding properties of polymer/carbon composites. Compos. Sci. Technol. 2019, 170, 51–59. [Google Scholar] [CrossRef]
- Boudenne, A.; Ibos, L.; Fois, M.; Majesté, J.C.; Géhin, E. Electrical and thermal behavior of polypropylene filled with copper particles. Compos. Part A Appl. Sci. Manuf. 2005, 36, 1545–1554. [Google Scholar] [CrossRef]
- Slater, C.; Davis, C.; Strangwood, M. Compression set of thermoplastic polyurethane under different thermal-mechanical-moisture conditions. Polym. Degrad. Stab. 2011, 96, 2139–2144. [Google Scholar] [CrossRef]
- Amoabeng, D.; Velankar, S.S. A review of conductive polymer composites filled with low melting point metal alloys. Polym. Eng. Sci. 2018, 58, 1010–1019. [Google Scholar] [CrossRef]
- Schmidt, R.H.; Kinloch, I.A.; Burgess, A.N.; Windle, A.H. The Effect of Aggregation on the Electrical Conductivity of Spin-Coated Polymer/Carbon Nanotube Composite Films. Langmuir 2007, 23, 5707–5712. [Google Scholar] [CrossRef]
- Putson, C.; Lebrun, L.; Guyomar, D.; Muensit, N.; Cottinet, P.J.; Seveyrat, L.; Guiffard, B. Effects of copper filler sizes on the dielectric properties and the energy harvesting capability of nonpercolated polyurethane composites. J. Appl. Phys. 2011, 109, 024104. [Google Scholar] [CrossRef]
- Bloor, D.; Donnelly, K.; Hands, P.J.; Laughlin, P.; Lussey, D. A metal-polymer composite with unusual properties. J. Phys. D Appl. Phys. 2005, 38, 2851–2860. [Google Scholar] [CrossRef]
- Chelidze, T.; Gueguen, Y. Pressure-induced percolation transitions in composites. J. Phys. D Appl. Phys. 1998, 31, 2877–2885. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Ismail, H.; Saeed, A.M. A Review: Metal Filled Thermoplastic Composites. Polym.-Plast. Technol. Mater. 2021, 60, 1033–1050. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Chiu, S.-S. Electrical Properties of Copper-Filled Electrically Conductive Adhesives and Pressure-Dependent Conduction Behavior of Copper Particles. J. Adhes. Sci. Technol. 2008, 22, 1673–1697. [Google Scholar] [CrossRef]
- Fang, Q.; Lafdi, K. Effect of nanofiller morphology on the electrical conductivity of polymer nanocomposites. Nano Express 2021, 2, 010019. [Google Scholar] [CrossRef]


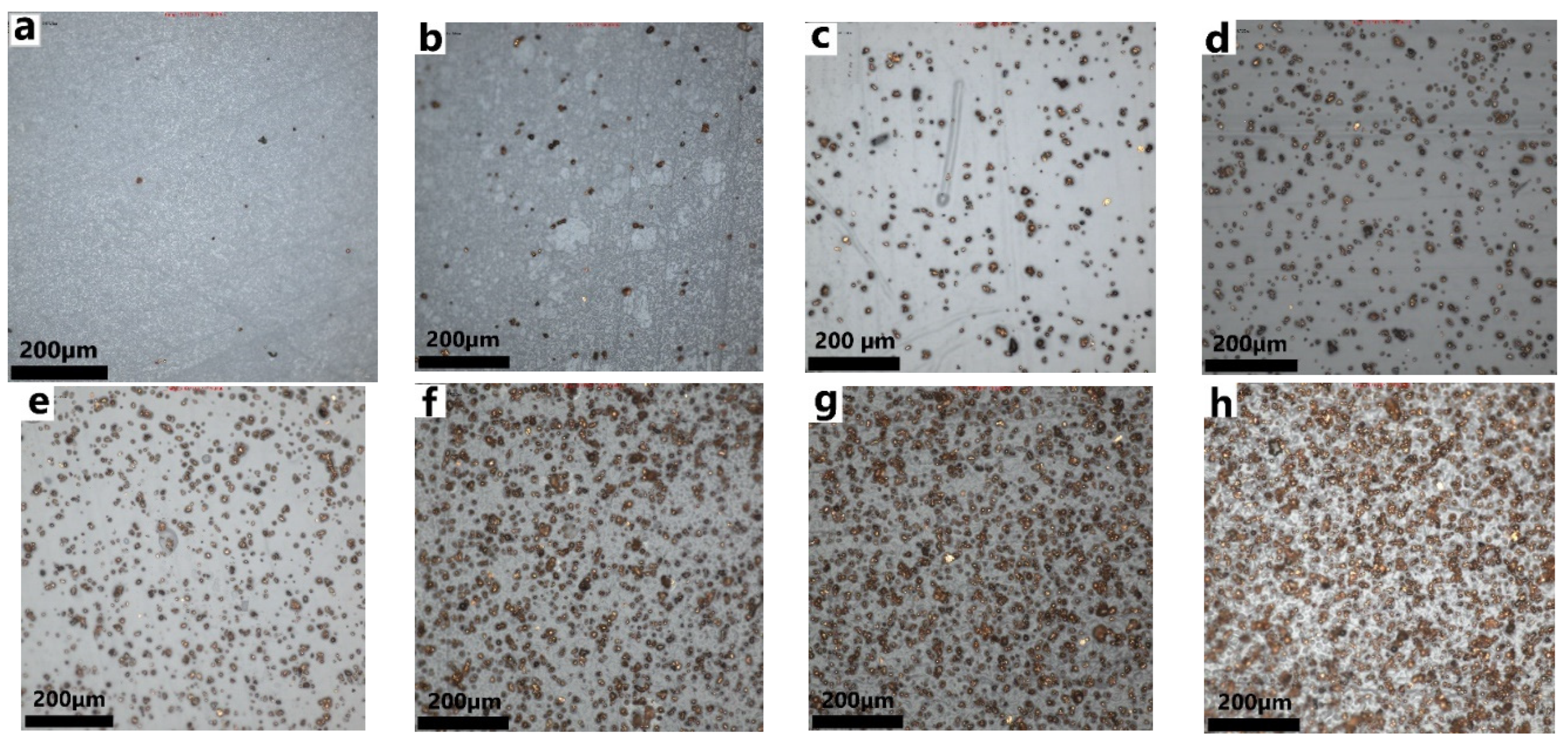



| Concentration of Copper in PU | ||||||||
|---|---|---|---|---|---|---|---|---|
| wt.% | 5 | 10 | 20 | 30 | 40 | 50 | 60 | 70 |
| vol.% | 0.55 | 1.2 | 2.6 | 4.3 | 6.6 | 9.5 | 14 | 20 |
| Material | Conductivity (S·m−1) | Density (Kg/m3) |
|---|---|---|
| PU | 10−14 | 1110 |
| Cu | 5.97 × 107 | 8940 |
| P (kPa) | B | α | n | (vol.%) | σc,max (S/m) | s | |
|---|---|---|---|---|---|---|---|
| 1 | 6.545 | 115.7 | 3899 | 0.0710 | 7.10 | 3.5∙10−8 | 0.17 |
| 2 | 13.15 | 71.89 | 14.77 | 0.0370 | 3.70 | 1.4∙10−1 | 0.73 |
| 4 | 13.09 | 117.7 | 16.74 | 0.0240 | 2.40 | 1.3∙10−1 | 0.39 |
| 10 | 13.35 | 371.1 | 528.3 | 0.0170 | 1.70 | 2.3∙10−1 | 0.27 |
| 15 | 13.55 | 723.1 | 9076 | 0.0126 | 1.26 | 3.5∙10−1 | 0.33 |
| 20 | 13.75 | 313.3 | 69.82 | 0.0135 | 1.35 | 5.6∙10−1 | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehvari, S.; Sanchez-Vicente, Y.; González, S.; Lafdi, K. Conductivity Behaviour under Pressure of Copper Micro-Additive/Polyurethane Composites (Experiment and Modelling). Polymers 2022, 14, 1287. https://doi.org/10.3390/polym14071287
Mehvari S, Sanchez-Vicente Y, González S, Lafdi K. Conductivity Behaviour under Pressure of Copper Micro-Additive/Polyurethane Composites (Experiment and Modelling). Polymers. 2022; 14(7):1287. https://doi.org/10.3390/polym14071287
Chicago/Turabian StyleMehvari, Saeid, Yolanda Sanchez-Vicente, Sergio González, and Khalid Lafdi. 2022. "Conductivity Behaviour under Pressure of Copper Micro-Additive/Polyurethane Composites (Experiment and Modelling)" Polymers 14, no. 7: 1287. https://doi.org/10.3390/polym14071287
APA StyleMehvari, S., Sanchez-Vicente, Y., González, S., & Lafdi, K. (2022). Conductivity Behaviour under Pressure of Copper Micro-Additive/Polyurethane Composites (Experiment and Modelling). Polymers, 14(7), 1287. https://doi.org/10.3390/polym14071287









