Modification of Polydiallyldimethylammonium Chloride with Sodium Polystyrenesulfonate Dramatically Changes the Resistance of Polymer-Based Coatings towards Wash-Off from Both Hydrophilic and Hydrophobic Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. IPEC Preparation
2.3. PDADMAC and IPEC Coatings Wash-Off Procedure
2.4. Preparation of IPEC Coatings for the Microscopy Analysis
2.5. Methods
3. Results
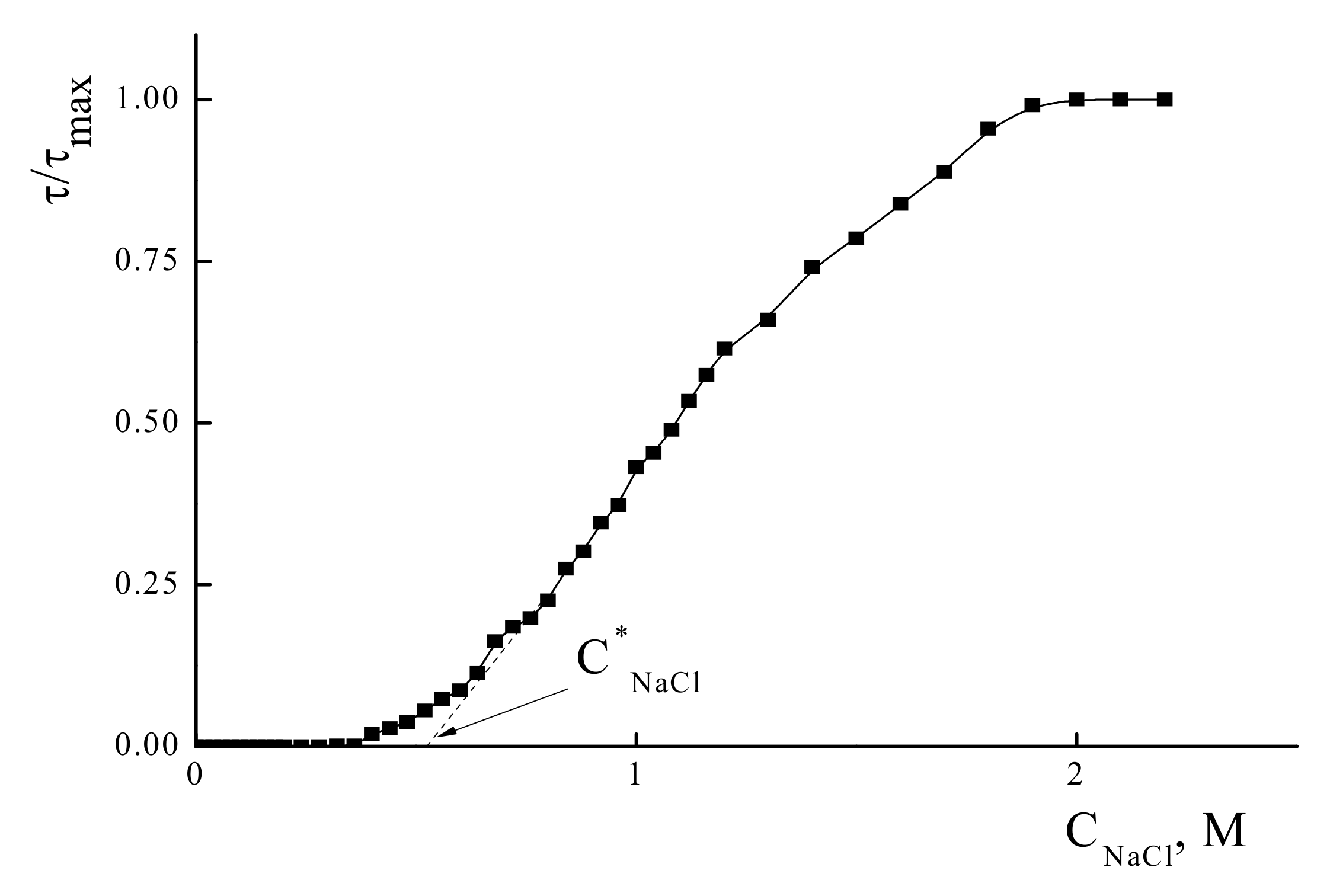
3.1. Formation of Water-Soluble IPEC
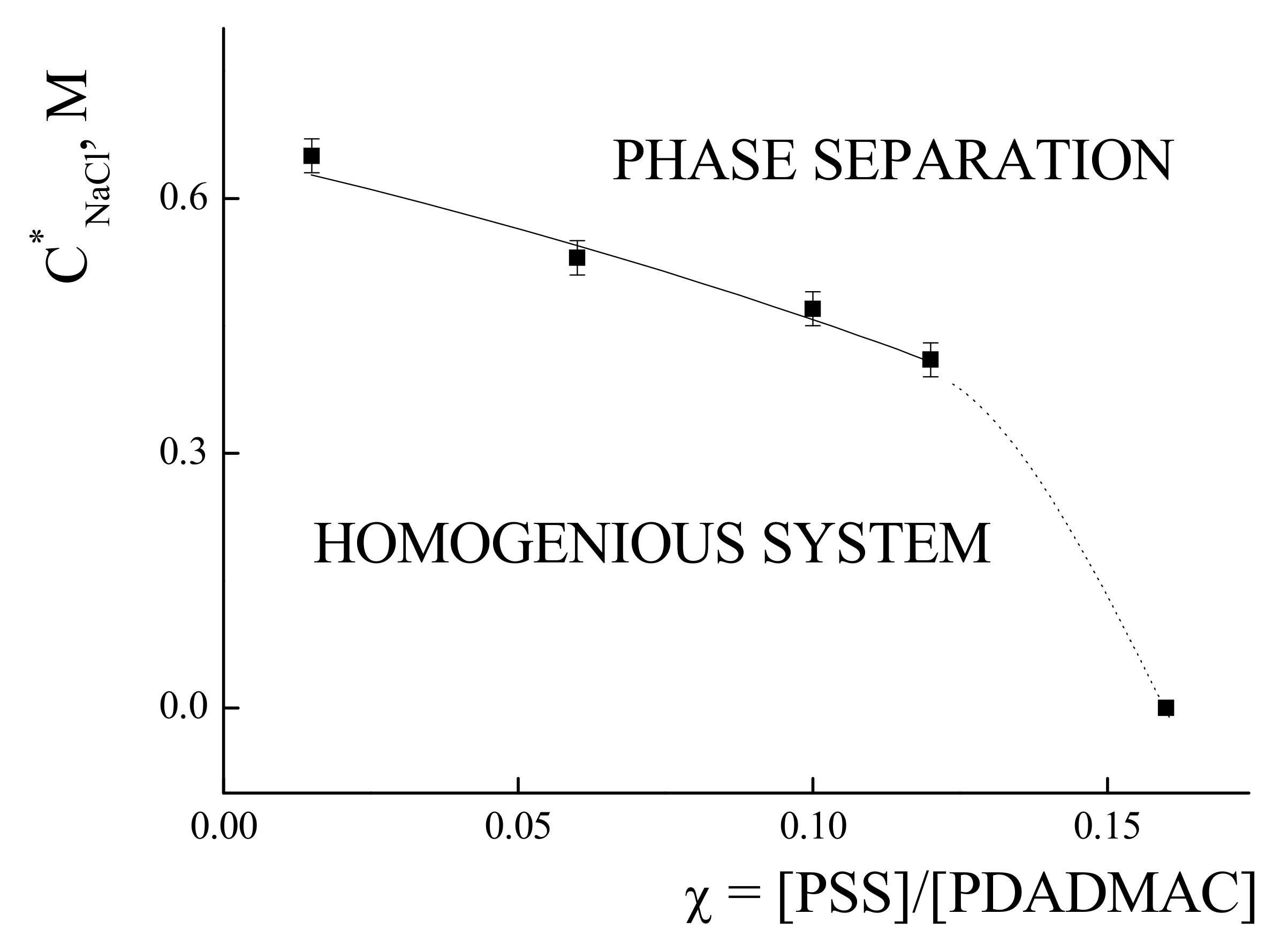
3.2. Phase Sepatation in IPEC Water-Salt Solutions
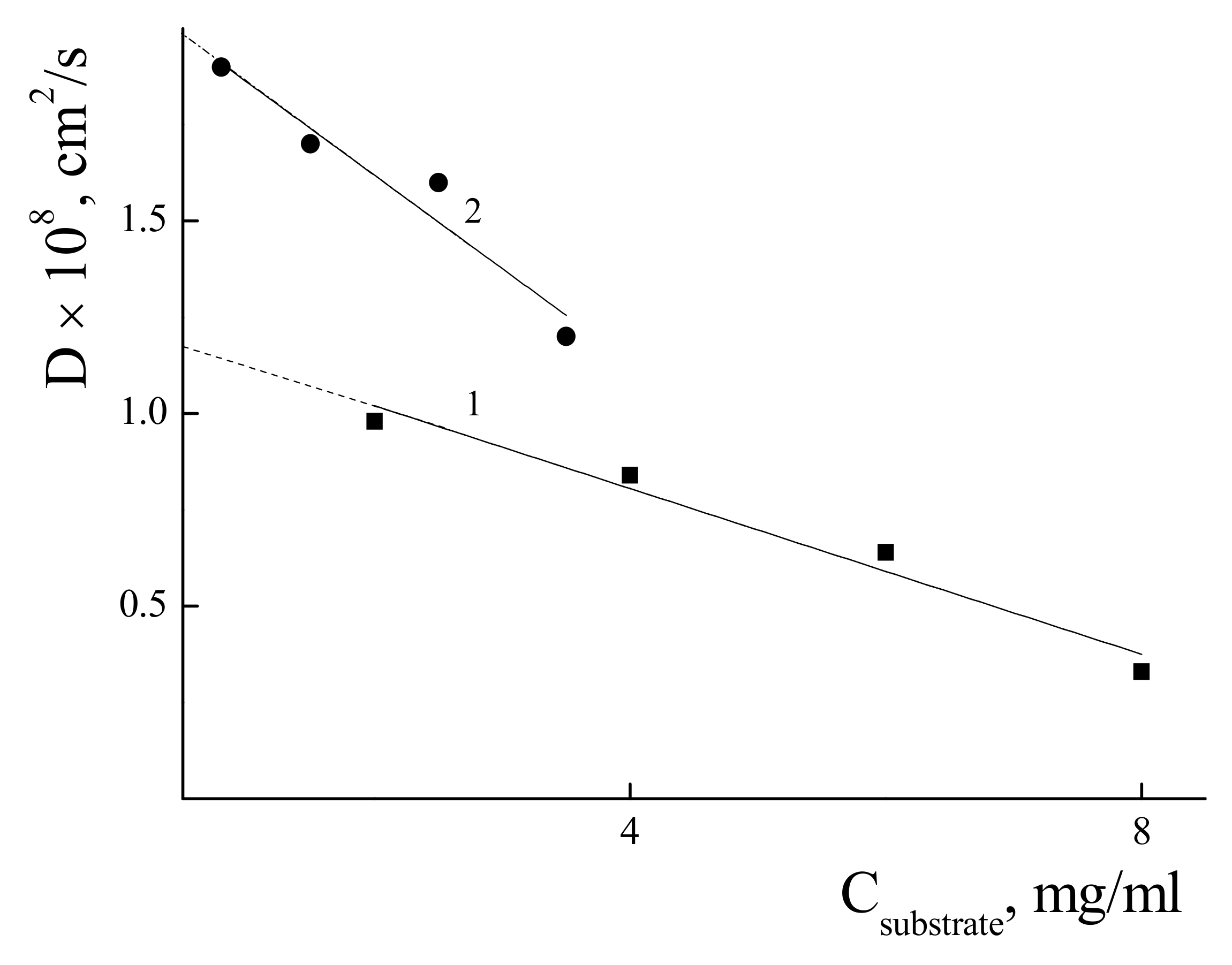
3.3. Characterization of the IPEC with χ = 0.06 in Water-Salt Media
3.4. Stucture and Properties of the IPEC Coatings
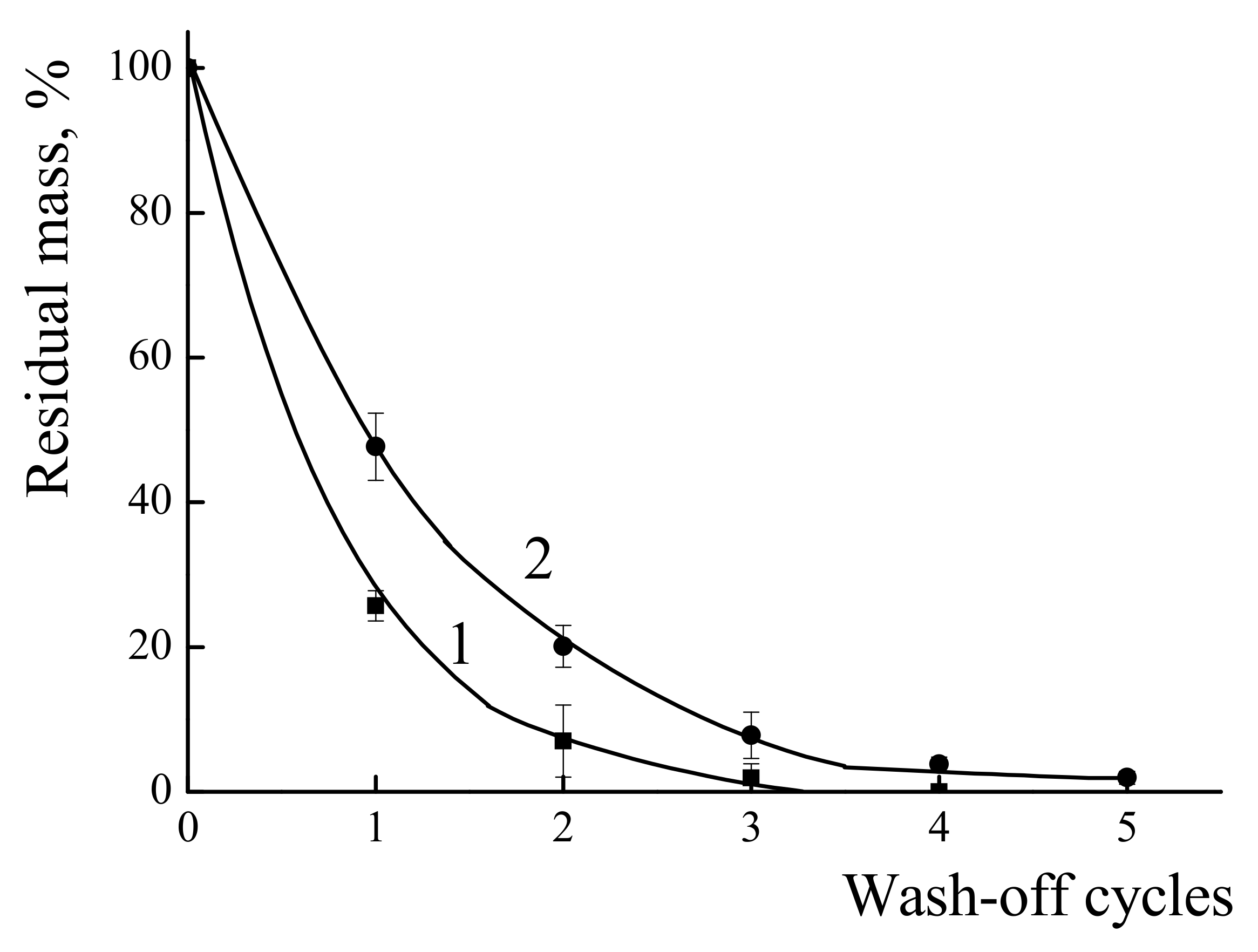
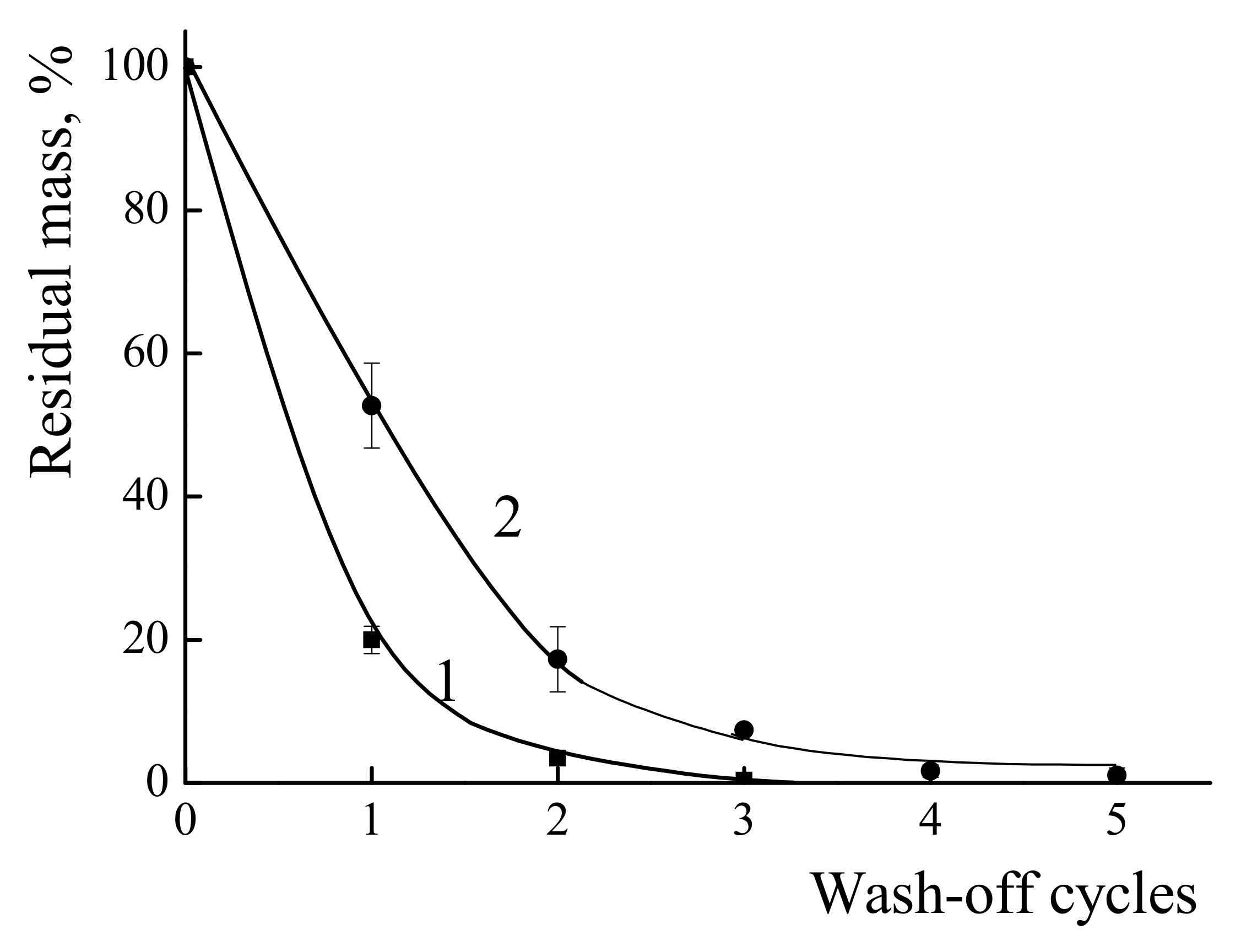
3.4.1. The Resistance of the Polymer Coatings towards Wash-Off with Water
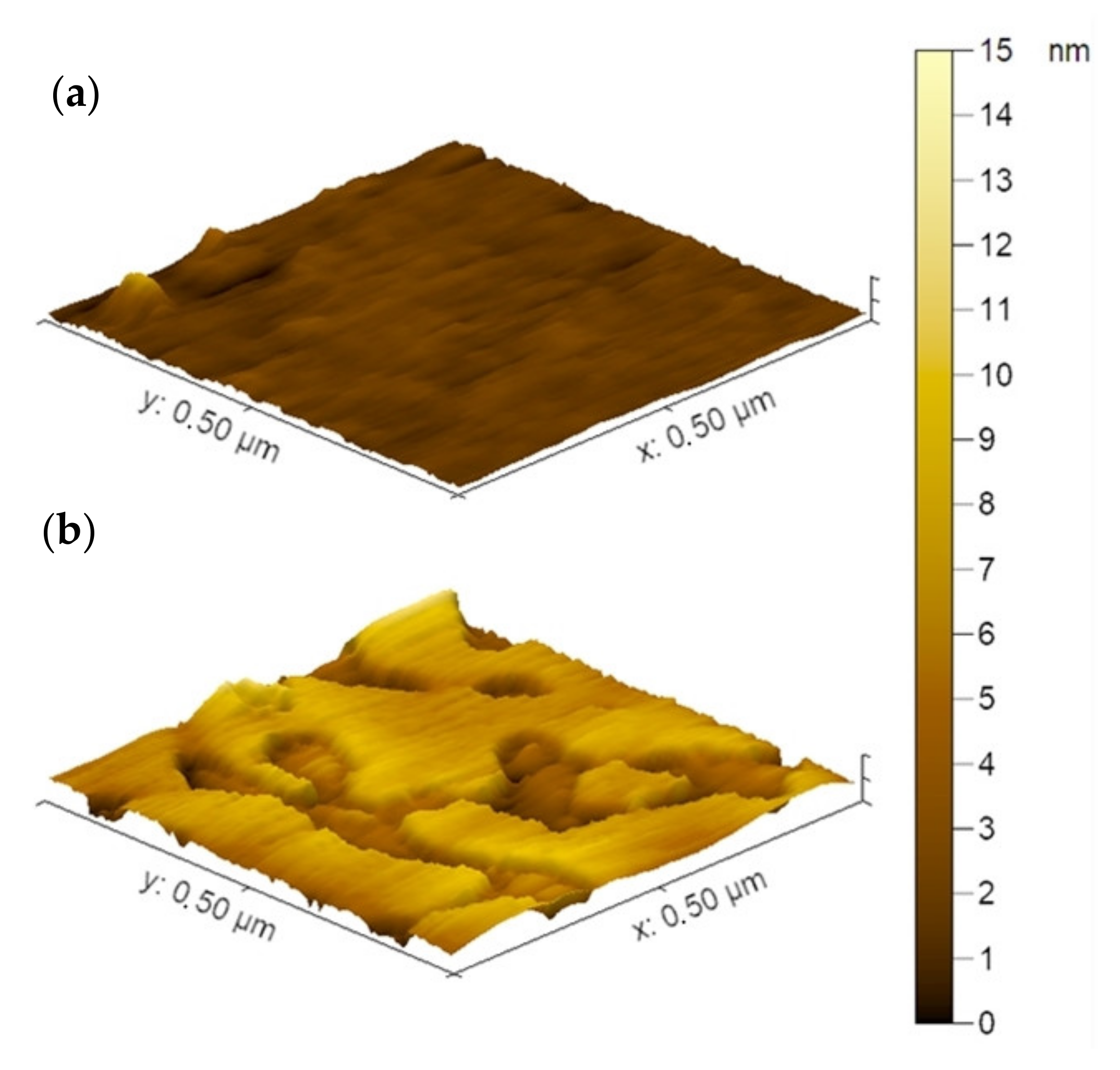
3.4.2. The Structure of the IPEC Coatings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bandl, C.; Krempl, N.; Berger-Weber, G.; Kern, W.; Friesenbichler, W. Application of organosilane coatings for improved anti-adhesive properties enabling facilitated demolding in polymer processing. J. Appl. Polym. Sci. 2021, 138, e50714. [Google Scholar] [CrossRef]
- Faccini, M.; Bautista, L.; Soldi, L.; Escobar, A.M.; Altavilla, M.; Calvet, M.; Domènech, A.; Domínguez, E. Environmentally Friendly Anticorrosive Polymeric Coatings. Appl. Sci. 2021, 11, 3446. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Wang, L.; Zuo, W.; Zhou, L.; Hu, X.; Bao, D. Effect of Terminal Groups on Thermomechanical and Dielectric Properties of Silica–Epoxy Composite Modified by Hyperbranched Polyester. Polymers 2021, 13, 2451. [Google Scholar] [CrossRef] [PubMed]
- Lan, P.; Nunez, E.E.; Polycarpou, A.A. Advanced Polymeric Coatings and Their Applications: Green Tribology. In Encyclopedia of Renewable and Sustainable Materials; Hashmi, S., Choudhury, I.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 4, pp. 345–358. [Google Scholar]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Bai, S.; Li, X.; Zhao, Y.; Ren, L.; Yuan, X. Antifogging/Antibacterial Coatings Constructed by N-Hydroxyethylacrylamide and Quaternary Ammonium-Containing Copolymers. ACS Appl. Mater. Interfaces 2020, 12, 12305–12316. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Wang, X.; Dong, X.; Zheng, J.; Li, S. Renewable antibacterial and antifouling polysulfone membranes incorporating a PEO-grafted amphiphilic polymer and N-chloramine functional groups. J. Colloid Interface Sci. 2019, 554, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Cattò, C.; Villa, F.; Cappitelli, F. Recent progress in bio-inspired biofilm-resistant polymeric surfaces. Crit. Rev. Microbiol. 2018, 44, 633–652. [Google Scholar] [CrossRef]
- Vaz, J.M.; Pezzoli, D.; Chevallier, P.; Campelo, C.S.; Candiani, G.; Mantovani, D. Antibacterial Coatings Based on Chitosan for Pharmaceutical and Biomedical Applications. Curr. Pharm. Des. 2018, 24, 866–885. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, L.; Zhang, J.; Hu, J.; Duan, G.; Liu, X.; Li, Y.; Gu, Z. Polydopamine antibacterial materials. Mater. Horiz. 2021, 8, 1618–1633. [Google Scholar] [CrossRef]
- Dayyoub, E.; Frant, M.; Pinnapireddy, S.R.; Liefeith, K.; Bakowsky, U. Antibacterial and anti-encrustation biodegradable polymer coating for urinary catheter. Int. J. Pharm. 2017, 531, 205–214. [Google Scholar] [CrossRef]
- Klimov, D.I.; Zezina, E.A.; Lipik, V.C.; Abramchuk, S.S.; Yaroslavov, A.A.; Feldman, V.I.; Sybachin, A.V.; Spiridonov, V.V.; Zezin, A.A. Radiation-induced preparation of metal nanostructures in coatings of interpolyelectrolyte complexes. Rad. Phys. Chem. 2019, 162, 23–30. [Google Scholar] [CrossRef]
- Zhang, R.; Jones, M.M.; Moussa, H.; Keskar, M.; Huo, N.; Zhang, Z.; Visser, M.B.; Sabatini, C.; Swihart, M.T.; Cheng, C. Polymer-antibiotic conjugates as antibacterial additives in dental resins. Biomater. Sci. 2018, 7, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Yin, X.Q.; Tan, P.F.; Gu, Z.P.; Liu, Z.M.; Tan, L. Polymeric antibacterial materials: Design, platforms and applications. J. Mater. Chem B. 2021, 9, 2802–2815. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Nishimura, N.; Kamata, H.; Furue, K.; Ono, Y.; Hosomi, M.; Terada, A. Antibacterial and anti-biofilm efficacy of fluoropolymer coating by a 2,3,5,6-tetrafluoro-p-phenylenedimethanol structure. Colloids Surf. B Biointerfaces 2017, 151, 363–371. [Google Scholar] [CrossRef]
- Hernández-Rivas, M.; Guzmán, E.; Fernández-Peña, L.; Akanno, A.; Greaves, A.; Léonforte, F.; Ortega, F.; Rubio, R.G.; Luengo, G.S. Deposition of Synthetic and Bio-Based Polycations onto Negatively Charged Solid Surfaces: Effect of the Polymer Cationicity, Ionic Strength, and the Addition of an Anionic Surfactant. Colloids Interfaces 2020, 4, 33. [Google Scholar] [CrossRef]
- Dirain, C.O.; Silva, R.C.; Antonelli, P.J. Prevention of biofilm formation by polyquaternary polymer. Int. J. Pediatr. Otorhinolaryngol. 2016, 88, 157–162. [Google Scholar] [CrossRef]
- Mertz, G.; Bour, J.; Toniazzo, V.; Ruch, D.; Ball, V. Deposition of polyelectrolyte multilayer films made from poly(diallyldimethyl ammonium chloride) and poly(4-styrene sulfonate): Influence of the NaCl concentration for films obtained by alternated spraying and alternated dipping. Colloids Surf. A 2012, 415, 77–85. [Google Scholar] [CrossRef]
- Azinfar, A.; Neuber, S.; Vancova, M.; Sterba, J.; Stranak, V.; Helm, C.A. Self-Patterning Polyelectrolyte Multilayer Films: Influence of Deposition Steps and Drying in a Vacuum. Langmuir 2021, 37, 10490–10498. [Google Scholar] [CrossRef]
- Lefort, M.; Boulmedais, F.; Jierry, L.; Gonthier, E.; Voegel, J.C.; Hemmerlé, J.; Lavalle, P.; Ponche, A.; Schaaf, P. Simultaneous spray coating of interacting species: General rules governing the poly(styrene sulfonate)/poly(allylamine) system. Langmuir 2011, 27, 4653–4660. [Google Scholar] [CrossRef] [Green Version]
- Pavan, C.; Santalucia, R.; Leinardi, R.; Fabbiani, M.; Yakoub, Y.; Uwambayinema, F.; Ugliengo, P.; Tomatis, M.; Martra, G.; Turci, F.; et al. Nearly free surface silanols are the critical molecular moieties that initiate the toxicity of silica particles. Proc. Natl. Acad. Sci. USA 2020, 117, 27836–27846. [Google Scholar] [CrossRef]
- Sayama, D.; Hatanaka, M.; Miyasaks, M. Effects of hydrophilic/hydrophobic surfaces on polymer-complexation kinetics. Appl. Polym. Sci. 2018, 135, 46493. [Google Scholar] [CrossRef]
- Kennemur, J.G. Poly(vinylpyridine) Segments in Block Copolymers: Synthesis, Self-Assembly, and Versatility. Macromolecules 2019, 52, 1254–1370. [Google Scholar] [CrossRef] [Green Version]
- Oda, Y.; Yasuhara, K.; Kanaoka, S.; Sato, T.; Aoshima, S.; Kuroda, K. Aggregation of Cationic Amphiphilic Block and Random Copoly(vinyl ether)s with Antimicrobial Activity. Polymers 2018, 10, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izumrudov, V.A.; Mussabayeva, B.K.; Kassymova, Z.S.; Klivenko, A.N.; Orazzhanova, L.K. Interpolyelectrolyte complexes: Advances and prospects of application. Russ. Chem. Rev. 2019, 88, 1046–1062. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Vanjari, Y.H.; Sancheti, K.H.; Patel, H.M.; Belgamwar, V.S.; Surana, S.J.; Pardeshi, C.V. Polyelectrolyte complexes: Mechanisms, critical experimental aspects, and applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1615–1625. [Google Scholar] [CrossRef] [Green Version]
- Panova, I.G.; Sybachin, A.V.; Spiridonov, V.V.; Kydralieva, K.; Jorobekova, S.; Zezin, A.B.; Yaroslavov, A.A. Non-stoichiometric interpolyelectrolyte complexes: Promising candidates for protection of soils. Geoderma 2017, 307, 91–97. [Google Scholar] [CrossRef]
- Van der Gucht, J.; Spruijt, E.; Lemmers, M.; Cohen Stuart, M.A. Polyelectrolyte complexes: Bulk phases and colloidal systems. J Colloid Interface Sci. 2011, 361, 407–422. [Google Scholar] [CrossRef]
- Izumrudov, V.A.; Paraschuk, V.V.; Sybachin, A.V. Controlled phase separations in solutions of polyelectrolyte complexes-potential for gene delivery. J. Drug Deliv. Sci. Technol. 2006, 16, 267–274. [Google Scholar] [CrossRef]
- Jha, P.K.; Desai, P.S.; Li, J.; Larson, R.G. pH and Salt Effects on the Associative Phase Separation of Oppositely Charged Polyelectrolytes. Polymers 2014, 6, 1414–1436. [Google Scholar] [CrossRef]
- Van Hees, I.A.; Hofman, A.H.; Dompe, M.; van der Gucht, J.; Kamperman, M. Temperature-responsive polyelectrolyte complexes for bio-inspired underwater adhesives. Eur. Polym. J. 2020, 141, 110034. [Google Scholar] [CrossRef]
- Proftos, D.; Tirrell, M. Phase behaviour and complex coacervation of aqueous polypeptide solutions. Soft Matter 2012, 8, 9396. [Google Scholar] [CrossRef]
- Mengarelli, V.; Zeghal, M.; Auvray, L.; Clemens, D. Phase behavior and structure of stable complexes between a long polyanion and a branched polycation. Phys. Rev. E Stat. Nonlin. Soft. Matter Phys. 2011, 84, 021805. [Google Scholar] [CrossRef] [PubMed]
- Izumrudov, V.A.; Sybachin, A.V. Phase separation in solutions of polyelectrolyte complexes: The decisive effect of a host polyion. Polym. Sci. Ser. A 2006, 48, 1098–1104. [Google Scholar] [CrossRef]
- Sybachin, A.V.; Efimova, A.A.; Litmanovich, E.A.; Menger, F.M.; Yaroslavov, A.A. Complexation of polycations to anionic liposomes: Composition and structure of the interfacial complexes. Langmuir 2007, 23, 10034–10039. [Google Scholar] [CrossRef]
- Machinskaya, A.E.; Leclercq, L.; Boustta, M.; Vert, M.; Vasilevskaya, V.V. Salt Effects on Macrophase Separations in Non-Stoichiometric Mixtures of Oppositely Charged Macromolecules: Theory and Experiment. J. Pol. Sci. B Pol. Phys. 2016, 54, 1717–1730. [Google Scholar] [CrossRef]
- Dautzenberg, H. Polyelectrolyte complex formation in highly aggregating systems. 1. Effect of salt: Polyelectrolyte complex formation in the presence of NaCl. Macromolecules 1997, 30, 7810–7815. [Google Scholar] [CrossRef]
- Jukić, J.; Kovačević, D.; Cindro, N.; Fink, R.; Oder, M.; Milisav, A.M.; Požar, J. Predicting the outcomes of interpolyelectrolyte neutralization at surfaces on the basis of complexation experiments and vice versa. Soft Matter 2022, 18, 744–754. [Google Scholar] [CrossRef]
- Karibyants, N.; Dautzenberg, H. Preferential Binding with Regard to Chain Length and Chemical Structure in the Reactions of Formation of Quasi-Soluble Polyelectrolyte Complexes. Langmuir 1998, 14, 4427–4434. [Google Scholar] [CrossRef]
- Decher, G.; Schlenoff, J.B. (Eds.) Multilayer Thin Films: Sequential Assembly of Nanocomposite Materials, 1st ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Zezin, A.B.; Mikheikin, S.V.; Rogacheva, V.B.; Zansokhova, M.F.; Sybachin, A.V.; Yaroslavov, A.A. Polymeric stabilizers for protection of soil and ground against wind and water erosion. Adv. Colloid Interface Sci. 2015, 226, 17–23. [Google Scholar] [CrossRef]
- Rumyantsev, A.M.; Potemkin, I.I. Explicit description of complexation between oppositely charged polyelectrolytes as an advantage of the random phase approximation over the scaling approach. Phys. Chem. Chem. Phys. 2017, 19, 27580–27592. [Google Scholar] [CrossRef] [Green Version]
- Manning, G.S. Limiting laws and counterion condensation in polyelectrolyte solutions. 8. Mixtures of counterions, species selectivity, and valence selectivity. J. Phys. Chem. 1984, 88, 6654–6661. [Google Scholar]
- Qiao, B.; Cerdà, J.J.; Holm, C. Poly(Styrenesulfonate)-Poly(Diallyldimethylammonium) Mixtures: Toward the Understanding of Polyelectrolyte Complexes and Multilayers via Atomistic Simulations. Macromolecules 2010, 43, 7828–7838. [Google Scholar] [CrossRef]
- Dautzenberg, H.; Kritz, J. Response of Polyelectrolyte Complexes to Subsequent Addition of Salts with Different Cations. Langmuir 2003, 19, 5204–5211. [Google Scholar] [CrossRef]
- Jukić, J.; Korade, K.; Milisav, A.-M.; Marion, I.D.; Kovačević, D. Ion-Specific and Solvent Effects on PDADMA–PSS Complexation and Multilayer Formation. Colloids Interfaces 2021, 5, 38. [Google Scholar] [CrossRef]
- Sánchez, P.A.; Vögele, M.; Smiatek, J.; Qiao, B.; Sega, M.; Holm, C. PDADMAC/PSS Oligoelectrolyte Multilayers: Internal Structure and Hydration Properties at Early Growth Stages from Atomistic Simulations. Molecules 2020, 25, 1848. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pigareva, V.A.; Senchikhin, I.N.; Bolshakova, A.V.; Sybachin, A.V. Modification of Polydiallyldimethylammonium Chloride with Sodium Polystyrenesulfonate Dramatically Changes the Resistance of Polymer-Based Coatings towards Wash-Off from Both Hydrophilic and Hydrophobic Surfaces. Polymers 2022, 14, 1247. https://doi.org/10.3390/polym14061247
Pigareva VA, Senchikhin IN, Bolshakova AV, Sybachin AV. Modification of Polydiallyldimethylammonium Chloride with Sodium Polystyrenesulfonate Dramatically Changes the Resistance of Polymer-Based Coatings towards Wash-Off from Both Hydrophilic and Hydrophobic Surfaces. Polymers. 2022; 14(6):1247. https://doi.org/10.3390/polym14061247
Chicago/Turabian StylePigareva, Vladislava A., Ivan N. Senchikhin, Anastasia V. Bolshakova, and Andrey V. Sybachin. 2022. "Modification of Polydiallyldimethylammonium Chloride with Sodium Polystyrenesulfonate Dramatically Changes the Resistance of Polymer-Based Coatings towards Wash-Off from Both Hydrophilic and Hydrophobic Surfaces" Polymers 14, no. 6: 1247. https://doi.org/10.3390/polym14061247
APA StylePigareva, V. A., Senchikhin, I. N., Bolshakova, A. V., & Sybachin, A. V. (2022). Modification of Polydiallyldimethylammonium Chloride with Sodium Polystyrenesulfonate Dramatically Changes the Resistance of Polymer-Based Coatings towards Wash-Off from Both Hydrophilic and Hydrophobic Surfaces. Polymers, 14(6), 1247. https://doi.org/10.3390/polym14061247






