Chitosan Hydrogels Based on the Diels–Alder Click Reaction: Rheological and Kinetic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of N-Furfuryl-Chitosan (FCs)
2.3. Fourier Transformed Infrared Spectroscopy (FTIR)
2.4. Nuclear Magnetic Resonance (1H-NMR)
2.5. Rheological Studies
3. Results and Discussion
3.1. Diels–Alder Click Reaction for Chemical Crosslinking of Chitosan
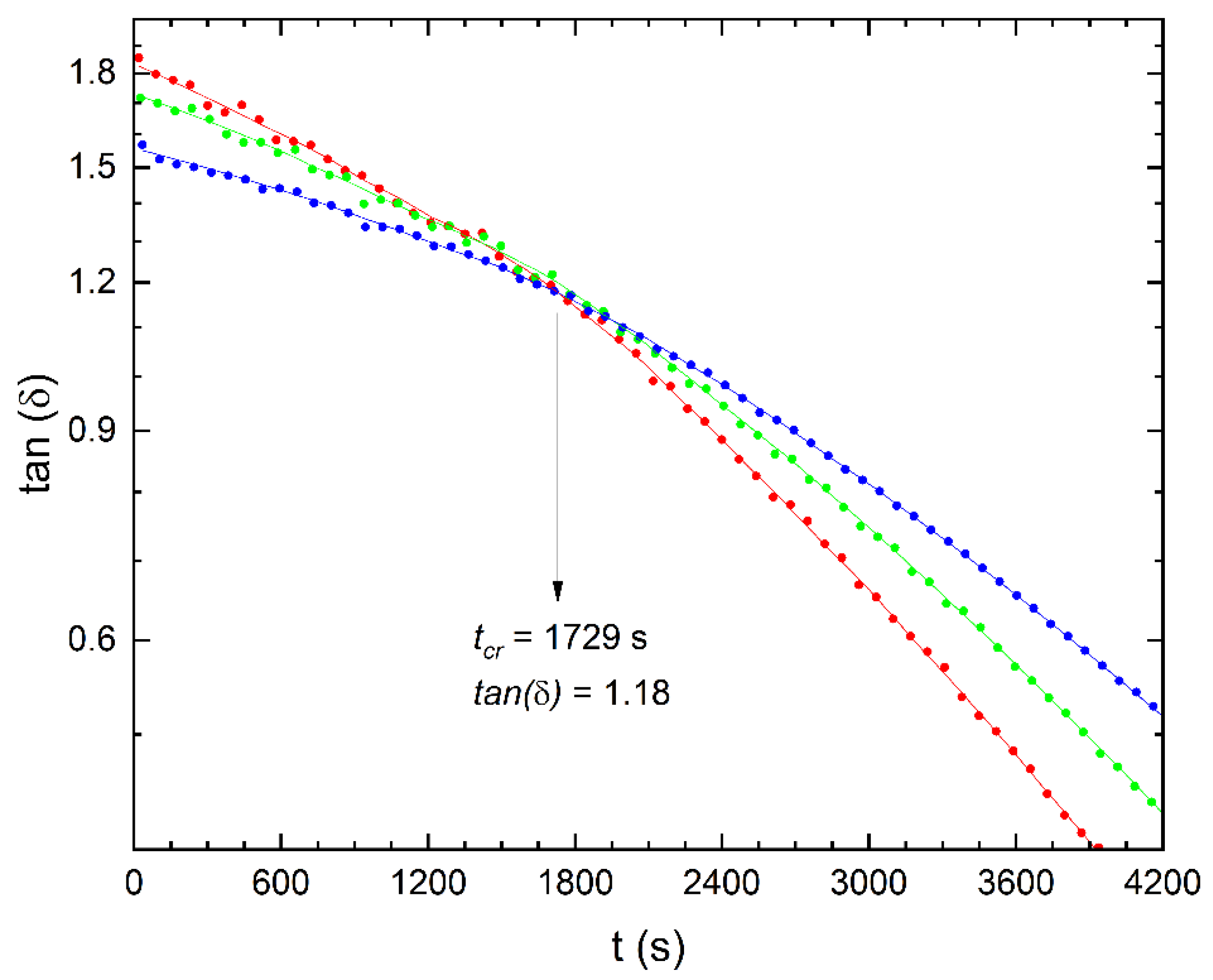
3.2. Rheological Analysis of the Influence of Temperature on Diels–Alder Cycloaddition for Chemical Crosslinking
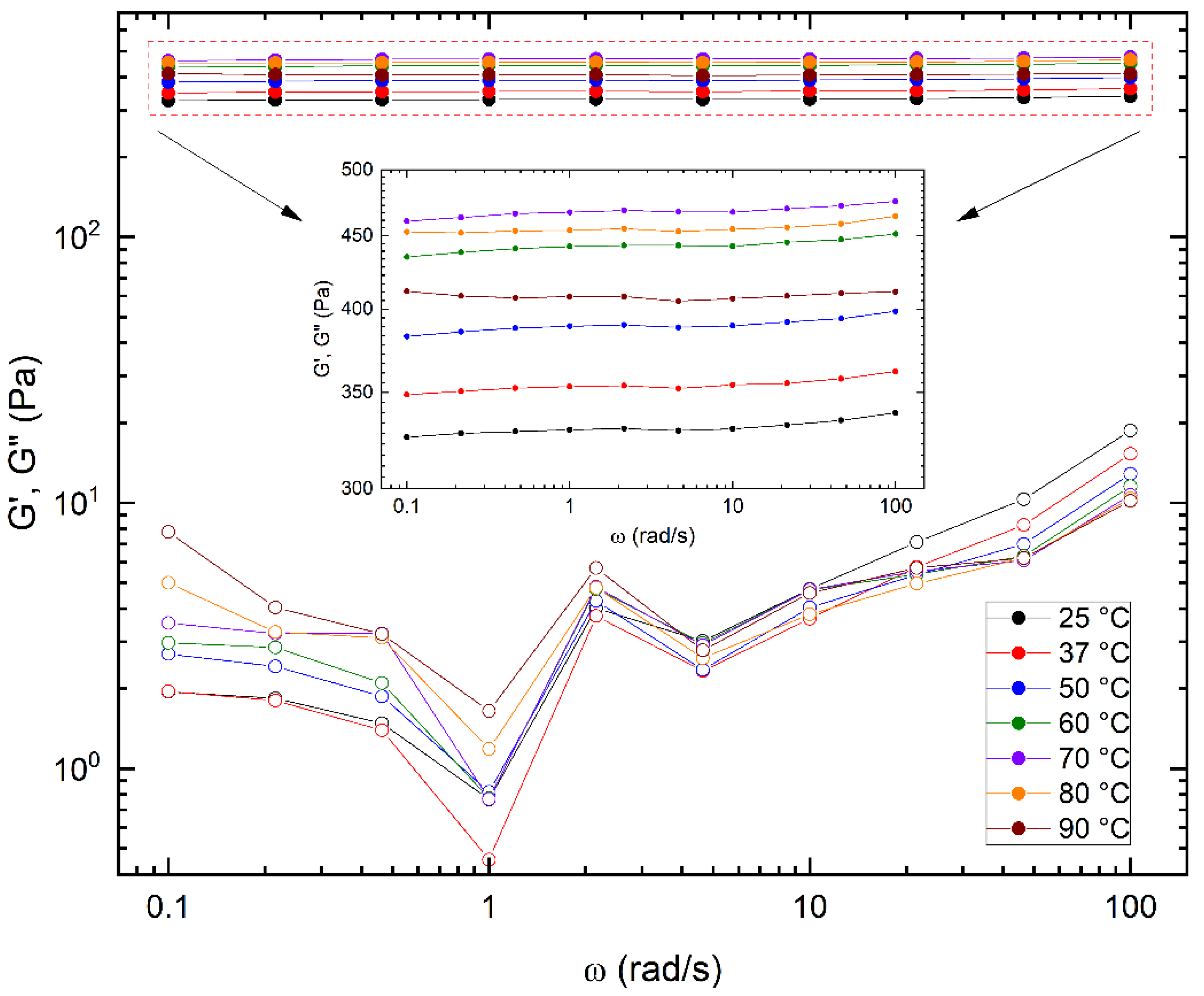
3.3. Viscoelastic Characterization of the Hydrogels Obtained at Different Temperatures
3.4. Influence of Other Eexperimental Parameters: Concentration of Chitosan, Maleimide to Furan Molar Ratio, and Characteristics of the Maleimide Crosslinker
3.5. Influence of the Temperature on the Mechanical Properties of a Diels–Alder Hydrogel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumirska, J.; Weinhold, M.X.; Thöming, J.; Stepnowski, P. Biomedical Activity of Chitin/Chitosan Based Materials—Influence of Physicochemical Properties Apart from Molecular Weight and Degree of N-Acetylation. Polymers 2011, 3, 1875–1901. [Google Scholar] [CrossRef]
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018, 2018, 1708172. [Google Scholar] [CrossRef]
- Argüelles-Monal, W.; Goycoolea, F.M.; Peniche, C.; Higuera-Ciapara, I. Rheological Study of the Chitosan/Glutaraldehyde Chemical Gel System. Polym. Gels Netw. 1998, 6, 429–440. [Google Scholar] [CrossRef]
- Moura, M.J.; Figueiredo, M.M.; Gil, M.H. Rheological Study of Genipin Cross-Linked Chitosan Hydrogels. Biomacromolecules 2007, 8, 3823–3829. [Google Scholar] [CrossRef] [Green Version]
- Espinosa-García, B.M.; Argüelles-Monal, W.M.; Hernández, J.; Félix-Valenzuela, L.; Acosta, N.; Goycoolea, F.M. Molecularly Imprinted Chitosan–Genipin Hydrogels with Recognition Capacity toward O-Xylene. Biomacromolecules 2007, 8, 3355–3364. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Herrera, M.; Gandini, A.; Goycoolea, F.M.; Jacobsen, N.E.; Lizardi-Mendoza, J.; Recillas-Mota, M.; Argüelles-Monal, W.M. N-(Furfural) Chitosan Hydrogels Based on Diels–Alder Cycloadditions and Application as Microspheres for Controlled Drug Release. Carbohydr. Polym. 2015, 128, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Herrera, M.; Gandini, A.; Goycoolea, F.M.; Jacobsen, N.E.; Lizardi-Mendoza, J.; Recillas-Mota, M.T.; Argüelles-Monal, W.M. Furan–Chitosan Hydrogels Based on Click Chemistry. Iran. Polym. J. 2015, 24, 349–357. [Google Scholar] [CrossRef]
- Nimmo, C.M.; Owen, S.C.; Shoichet, M.S. Diels−Alder Click Cross-Linked Hyaluronic Acid Hydrogels for Tissue Engineering. Biomacromolecules 2011, 12, 824–830. [Google Scholar] [CrossRef] [Green Version]
- Guaresti, O.; García–Astrain, C.; Palomares, T.; Alonso–Varona, A.; Eceiza, A.; Gabilondo, N. Synthesis and Characterization of a Biocompatible Chitosan–Based Hydrogel Cross–Linked via ‘Click’ Chemistry for Controlled Drug Release. Int. J. Biol. Macromol. 2017, 102, 1–9. [Google Scholar] [CrossRef]
- Papadimitriou, S.A.; Achilias, D.S.; Bikiaris, D.N. Chitosan-g-PEG Nanoparticles Ionically Crosslinked with Poly(Glutamic Acid) and Tripolyphosphate as Protein Delivery Systems. Int. J. Pharm. 2012, 430, 318–327. [Google Scholar] [CrossRef]
- Natesan, S.; Pandian, S.; Ponnusamy, C.; Palanichamy, R.; Muthusamy, S.; Kandasamy, R. Co-Encapsulated Resveratrol and Quercetin in Chitosan and Peg Modified Chitosan Nanoparticles: For Efficient Intra Ocular Pressure Reduction. Int. J. Biol. Macromol. 2017, 104, 1837–1845. [Google Scholar] [CrossRef]
- Casettari, L.; Vllasaliu, D.; Castagnino, E.; Stolnik, S.; Howdle, S.; Illum, L. PEGylated Chitosan Derivatives: Synthesis, Characterizations and Pharmaceutical Applications. Prog. Polym. Sci. 2012, 37, 659–685. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Lahann, J. (Ed.) Click Chemistry for Biotechnology and Materials Science; John Wiley & Sons, Ltd.: Chichester, UK, 2009. [Google Scholar]
- Han, J.; Wang, X.; Liu, L.; Li, D.; Suyaola, S.; Wang, T.; Baigude, H. “Click” Chemistry Mediated Construction of Cationic Curdlan Nanocarriers for Efficient Gene Delivery. Carbohydr. Polym. 2017, 163, 191–198. [Google Scholar] [CrossRef]
- Evans, D.A.; Johnson, J.S. Diels-Alder Reactions. In Comprehensive Asymmetric Catalysis; Jacobsen, E.N., Pfaltz, A., Yamamoto, H., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1999; Volume III, pp. 1177–1235. [Google Scholar]
- Gandini, A.; Lacerda, T.M. From Monomers to Polymers from Renewable Resources: Recent Advances. Prog. Polym. Sci. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Gandini, A. The Application of the Diels-Alder Reaction to Polymer Syntheses Based on Furan/Maleimide Reversible Couplings. Polímeros Ciênc. Tecnol. 2005, 15, 95–101. [Google Scholar] [CrossRef]
- Gandini, A. The Furan/Maleimide Diels–Alder Reaction: A Versatile Click–Unclick Tool in Macromolecular Synthesis. Prog. Polym. Sci. 2013, 38, 1–29. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2001. [Google Scholar]
- Orozco, F.; Niyazov, Z.; Garnier, T.; Migliore, N.; Zdvizhkov, A.T.; Raffa, P.; Moreno-Villoslada, I.; Picchioni, F.; Bose, R.K. Maleimide Self-Reaction in Furan/Maleimide-Based Reversibly Crosslinked Polyketones: Processing Limitation or Potential Advantage? Molecules 2021, 26, 2230. [Google Scholar] [CrossRef]
- Liu, X.; Du, P.; Liu, L.; Zheng, Z.; Wang, X.; Joncheray, T.; Zhang, Y. Kinetic Study of Diels–Alder Reaction Involving in Maleimide–Furan Compounds and Linear Polyurethane. Polym. Bull. 2013, 70, 2319–2335. [Google Scholar] [CrossRef]
- Goussé, C.; Gandini, A. Diels–Alder Polymerization of Difurans with Bismaleimides. Polym. Int. 1999, 48, 723–731. [Google Scholar] [CrossRef]
- García-Astrain, C.; Gandini, A.; Coelho, D.; Mondragon, I.; Retegi, A.; Eceiza, A.; Corcuera, M.A.; Gabilondo, N. Green Chemistry for the Synthesis of Methacrylate-Based Hydrogels Crosslinked through Diels–Alder Reaction. Eur. Polym. J. 2013, 49, 3998–4007. [Google Scholar] [CrossRef]
- Tung, C.-Y.M.; Dynes, P.J. Relationship between Viscoelastic Properties and Gelation in Thermosetting Systems. J. Appl. Polym. Sci. 1982, 27, 569–574. [Google Scholar] [CrossRef]
- Winter, H.H.; Chambon, F. Analysis of Linear Viscoelasticity of a Crosslinking Polymer at the Gel Point. J. Rheol. 1986, 30, 367–382. [Google Scholar] [CrossRef]
- Chambon, F.; Winter, H.H. Linear Viscoelasticity at the Gel Point of a Crosslinking PDMS with Imbalanced Stoichiometry. J. Rheol. 1987, 31, 683–697. [Google Scholar] [CrossRef]
- Holly, E.E.; Venkataraman, S.K.; Chambon, F.; Winter, H.H. Fourier Transform Mechanical Spectroscopy of Viscoelastic Materials with Transient Structure. J. Non-Newton. Fluid Mech. 1988, 27, 17–26. [Google Scholar] [CrossRef]
- Winter, H.H. Evolution of rheology during chemical gelation. In Permanent and Transient Networks; Progress in Colloid and Polymer Science; Steinkopff: Darmstadt, Germany, 1987; Volume 75, pp. 104–110. [Google Scholar]
- Winter, H.H.; Mours, M. Rheology of Polymers near Liquid-Solid Transitions. Adv. Polym. Sci. 1997, 134, 165–234. [Google Scholar]
- Winter, H.H. Can the Gel Point of a Cross-Linking Polymer Be Detected by the G′–G″ Crossover? Polym. Eng. Sci. 1987, 27, 1698–1702. [Google Scholar] [CrossRef]
- Gough, L.J.; Smith, I.T. A Gel Point Method for the Estimation of Overall Apparent Activation Energies of Polymerization. J. Appl. Polym. Sci. 1960, 3, 362–364. [Google Scholar] [CrossRef]
- Ross-Murphy, S.B. Reversible and Irreversible Biopolymer Gels—Structure and Mechanical Properties. Ber. Bunsenges. Phys. Chem. 1998, 102, 1534–1539. [Google Scholar] [CrossRef]
- Nnyigide, O.S.; Hyun, K. The Rheological Properties and Gelation Kinetics of Corn Starch/Bovine Serum Albumin Blend. Korea-Aust. Rheol. J. 2020, 32, 71–78. [Google Scholar] [CrossRef]
- Dewar, M.J.S.; Pierini, A.B. Mechanism of the Diels-Alder Reaction. Studies of the Addition of Maleic Anhydride to Furan and Methylfurans. J. Am. Chem. Soc. 1984, 106, 203–208. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Hsieh, C.-Y.; Chen, Y.-W. Thermally Reversible Cross-Linked Polyamides and Thermo-Responsive Gels by Means of Diels–Alder Reaction. Polymer 2006, 47, 2581–2586. [Google Scholar] [CrossRef]
- Gandini, A.; Coelho, D.; Silvestre, A.J.D. Reversible Click Chemistry at the Service of Macromolecular Materials. Part 1: Kinetics of the Diels–Alder Reaction Applied to Furan–Maleimide Model Compounds and Linear Polymerizations. Eur. Polym. J. 2008, 44, 4029–4036. [Google Scholar] [CrossRef]
- Tian, Q.; Rong, M.Z.; Zhang, M.Q.; Yuan, Y.C. Synthesis and Characterization of Epoxy with Improved Thermal Remendability Based on Diels-Alder Reaction. Polym. Int. 2010, 59, 1339–1345. [Google Scholar] [CrossRef]
- Park, J.S.; Yun, D.H.; Ko, T.W.; Park, Y.S.; Woo, J.W. Kinetic Study of the Diels-Alder Reaction of Cyclopentadiene with Bis(2-Ethylhexyl) Maleate. Adv. Mater. Res. 2013, 634–638, 541–545. [Google Scholar]
- Liu, S.; Liu, X.; He, Z.; Liu, L.; Niu, H. Thermoreversible Cross-Linking of Ethylene/Propylene Copolymers Based on Diels–Alder Chemistry: The Cross-Linking Reaction Kinetics. Polym. Chem. 2020, 11, 5851–5860. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Hsieh, C.-Y. Crosslinked Epoxy Materials Exhibiting Thermal Remendablility and Removability from Multifunctional Maleimide and Furan Compounds. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 905–913. [Google Scholar] [CrossRef]
- Engberts, J.B.F.N. Diels-Alder Reactions in Water: Enforced Hydrophobic Interaction and Hydrogen Bonding. Pure Appl. Chem. 1995, 67, 823–828. [Google Scholar] [CrossRef] [Green Version]
- Fringuelli, F.; Taticchi, A. Diels–Alder Reaction in Unconventional Reaction Media. In The Diels–Alder Reaction; John Wiley & Sons, Ltd.: Chichester, UK, 2001; pp. 251–300. [Google Scholar]
- Graziano, G. Rate Enhancement of Diels–Alder Reactions in Aqueous Solutions. J. Phys. Org. Chem. 2004, 17, 100–101. [Google Scholar] [CrossRef]
- Kwart, H.; Burchuk, I. Isomerism and Adduct Stability in the Diels—Alder Reaction. 1a I. The Adducts of Furan and Maleimide. J. Am. Chem. Soc. 1952, 74, 3094–3097. [Google Scholar] [CrossRef]
- Martin, J.G.; Hill, R.K. Stereochemistry of the Diels-Alder Reaction. Chem. Rev. 1961, 61, 537–562. [Google Scholar] [CrossRef]
- Rulíšek, L.; Šebek, P.; Havlas, Z.; Hrabal, R.; Čapek, P.; Svatoš, A. An Experimental and Theoretical Study of Stereoselectivity of Furan–Maleic Anhydride and Furan–Maleimide Diels–Alder Reactions. J. Org. Chem. 2005, 70, 6295–6302. [Google Scholar] [CrossRef]
- Cioc, R.C.; Lutz, M.; Pidko, E.A.; Crockatt, M.; van der Waal, J.C.; Bruijnincx, P.C.A. Direct Diels–Alder Reactions of Furfural Derivatives with Maleimides. Green Chem. 2021, 23, 367–373. [Google Scholar] [CrossRef]
- Buonerba, A.; Lapenta, R.; Ortega Sánchez, S.; Capacchione, C.; Milione, S.; Grassi, A. A Comprehensive Depiction of the Furan-Maleimide Coupling via Kinetic and Thermodynamic Investigations of the Diels-Alder Reaction of Poly(Styrene–co-2-Vinylfuran) with Maleimides. ChemistrySelect 2017, 2, 1605–1612. [Google Scholar] [CrossRef]
- Cuvellier, A.; Verhelle, R.; Brancart, J.; Vanderborght, B.; Assche, G.V.; Rahier, H. The Influence of Stereochemistry on the Reactivity of the Diels–Alder Cycloaddition and the Implications for Reversible Network Polymerization. Polym. Chem. 2019, 10, 473–485. [Google Scholar] [CrossRef]
- Mangialetto, J.; Verhelle, R.; Van Assche, G.; Van den Brande, N.; Van Mele, B. Time-Temperature-Transformation, Temperature-Conversion-Transformation, and Continuous-Heating-Transformation Diagrams of Reversible Covalent Polymer Networks. Macromolecules 2021, 54, 412–425. [Google Scholar] [CrossRef]
- Defize, T.; Thomassin, J.-M.; Alexandre, M.; Gilbert, B.; Riva, R.; Jérôme, C. Comprehensive Study of the Thermo-Reversibility of Diels–Alder Based PCL Polymer Networks. Polymer 2016, 84, 234–242. [Google Scholar] [CrossRef]
- Guigo, N.; Sbirrazzuoli, N.; Vyazovkin, S. Gelation on Heating of Supercooled Gelatin Solutions. Macromol. Rapid Commun. 2012, 33, 698–702. [Google Scholar] [CrossRef]
- Chen, K.; Baker, A.N.; Vyazovkin, S. Concentration Effect on Temperature Dependence of Gelation Rate in Aqueous Solutions of Methylcellulose. Macromol. Chem. Phys. 2009, 210, 211–216. [Google Scholar] [CrossRef]
- Pescosolido, L.; Feruglio, L.; Farra, R.; Fiorentino, S.; Colombo, I.; Coviello, T.; Matricardi, P.; Hennink, W.E.; Vermonden, T.; Grassi, M. Mesh Size Distribution Determination of Interpenetrating Polymer Network Hydrogels. Soft Matter 2012, 8, 7708–7715. [Google Scholar] [CrossRef]
- Kjøniksen, A.-L.; Nyström, B. Effects of Polymer Concentration and Cross-Linking Density on Rheology of Chemically Cross-Linked Poly(Vinyl Alcohol) near the Gelation Threshold. Macromolecules 1996, 29, 5215–5222. [Google Scholar] [CrossRef]
- Elling, B.R.; Dichtel, W.R. Reprocessable Cross-Linked Polymer Networks: Are Associative Exchange Mechanisms Desirable? ACS Cent. Sci. 2020, 6, 1488–1496. [Google Scholar] [CrossRef] [PubMed]







| Identification | f1 | Substitution 2 (%) | Molecular Weight (Polydispersity) 3 | Purity 4 (%) |
|---|---|---|---|---|
| PEG(mal)2-2000 | 2 | 97.9 | 2084 (1.04) | 99.2 |
| PEG(mal)2-7500 | 2 | 98.1 | 7408 (1.03) | 100 |
| PEG(mal)4-10,000 | 4 | 94.7 | 10,160 (1.04) | 98.0 |
| Tgel (°C) | tgel (s) | S (Pa s1/2) |
|---|---|---|
| 50 | 1729 | 1.15 |
| 60 | 590 | 0.94 |
| 70 | 292 | 0.87 |
| 80 | 158 | 0.75 |
| 90 | 68 | - |
| Tgel. (°C) | G’ (Pa) | ρx (mol∙cm−3) | ξa (nm) |
|---|---|---|---|
| 50 | 499.8 | 2.02 × 10−7 | 25 |
| 60 | 380.5 | 1.53 × 10−7 | 27 |
| 70 | 294.7 | 1.19 × 10−7 | 30 |
| 80 | 233.7 | 0.94 × 10−7 | 32 |
| 90 | 191.9 | 0.77 × 10−7 | 34 |
| PEG(mal)2-2000 | PEG(mal)2-7500 | PEG(mal)4-10,000 | ||
|---|---|---|---|---|
| [FCs] (wt.%) | R | Crosslinking Density (mol/cm3) | ||
| 1.8 | 0.5 | 5.09 × 10−7 | 8.33 × 10−7 | 8.17 × 10−7 |
| 0.25 | 1.97 × 10−7 | 6.28 × 10−7 | 8.34 × 10−7 | |
| 1.0 | 0.5 | 0.61 × 10−7 | 2.28 × 10−7 | 4.84 × 10−7 |
| 0.25 | 0.07 × 10−7 | 1.19 × 10−7 | 2.24 × 10−7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Pardo, C.; Silva-Gutiérrez, L.; Lizardi-Mendoza, J.; López-Franco, Y.; Peniche-Covas, C.; Argüelles-Monal, W. Chitosan Hydrogels Based on the Diels–Alder Click Reaction: Rheological and Kinetic Study. Polymers 2022, 14, 1202. https://doi.org/10.3390/polym14061202
Ruiz-Pardo C, Silva-Gutiérrez L, Lizardi-Mendoza J, López-Franco Y, Peniche-Covas C, Argüelles-Monal W. Chitosan Hydrogels Based on the Diels–Alder Click Reaction: Rheological and Kinetic Study. Polymers. 2022; 14(6):1202. https://doi.org/10.3390/polym14061202
Chicago/Turabian StyleRuiz-Pardo, Cinthya, Luisa Silva-Gutiérrez, Jaime Lizardi-Mendoza, Yolanda López-Franco, Carlos Peniche-Covas, and Waldo Argüelles-Monal. 2022. "Chitosan Hydrogels Based on the Diels–Alder Click Reaction: Rheological and Kinetic Study" Polymers 14, no. 6: 1202. https://doi.org/10.3390/polym14061202
APA StyleRuiz-Pardo, C., Silva-Gutiérrez, L., Lizardi-Mendoza, J., López-Franco, Y., Peniche-Covas, C., & Argüelles-Monal, W. (2022). Chitosan Hydrogels Based on the Diels–Alder Click Reaction: Rheological and Kinetic Study. Polymers, 14(6), 1202. https://doi.org/10.3390/polym14061202







