Biodegradable, Stretchable and Transparent Plastic Films from Modified Waterborne Polyurethane Dispersions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the PU/PVP Hybrid Films
2.2. Optical and Chemical Spectroscopy
2.3. Scanning Electron Microscopy (SEM) and Atomic Force Microscopy (AFM)
2.4. Thermal Characteristics Measurements
2.5. Measurement of Film Mechanical Properties
2.6. Wetting, Water Absorption and Swelling Properties
2.7. Biodegradation Measurements: Biological Oxygen Demand (BOD) Tests
3. Results
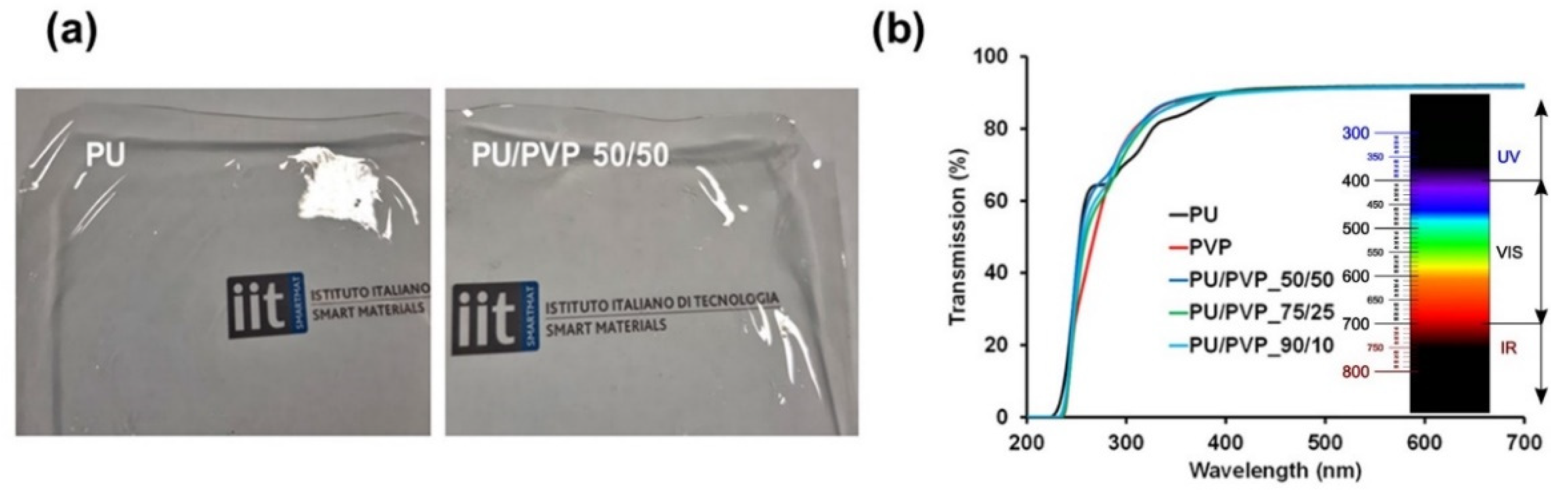
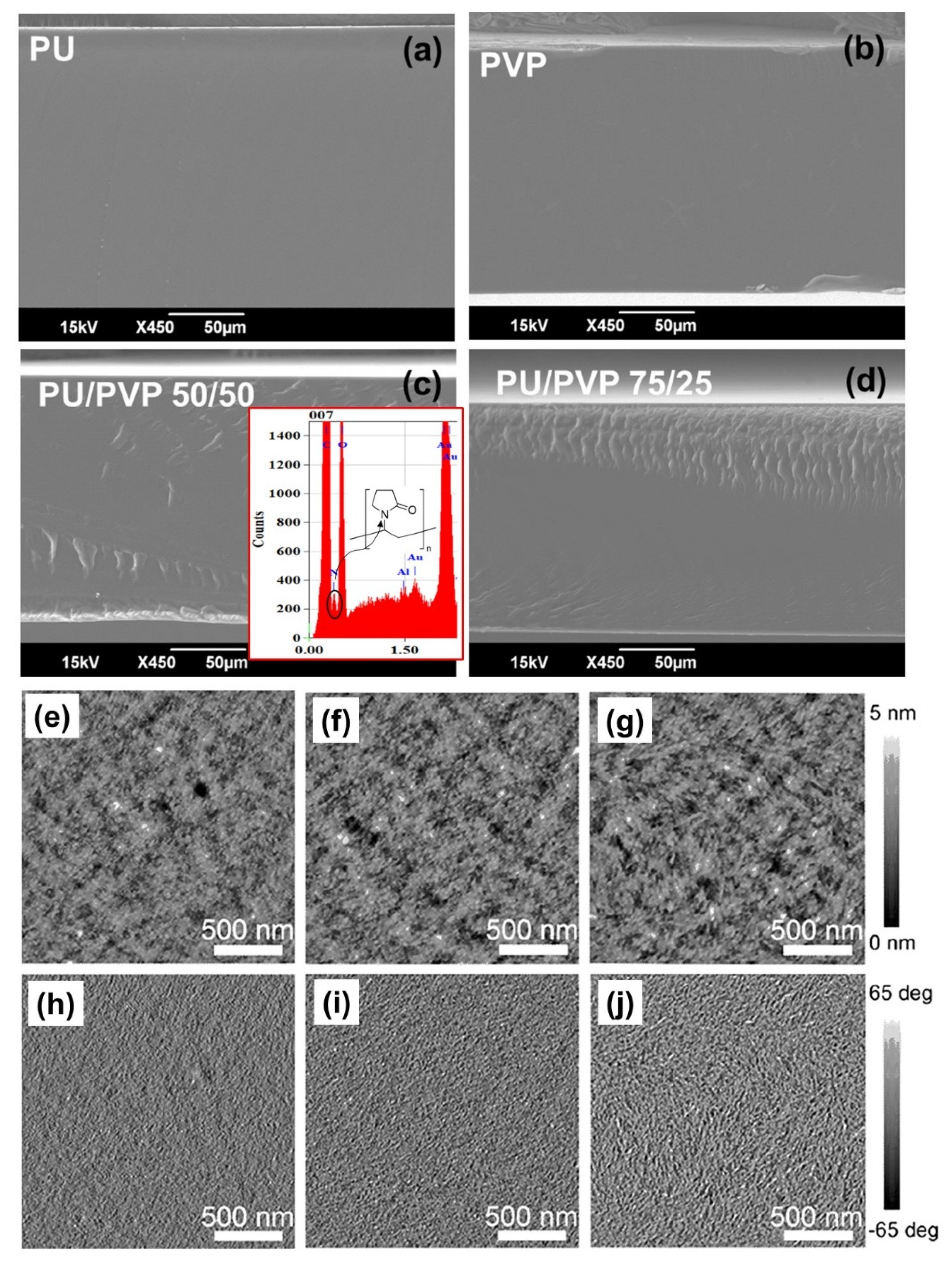
3.1. Film Morphology and Transparency
3.2. Chemical Properties of the Films
3.3. Thermal Properties of the PU/PVP Hybrids
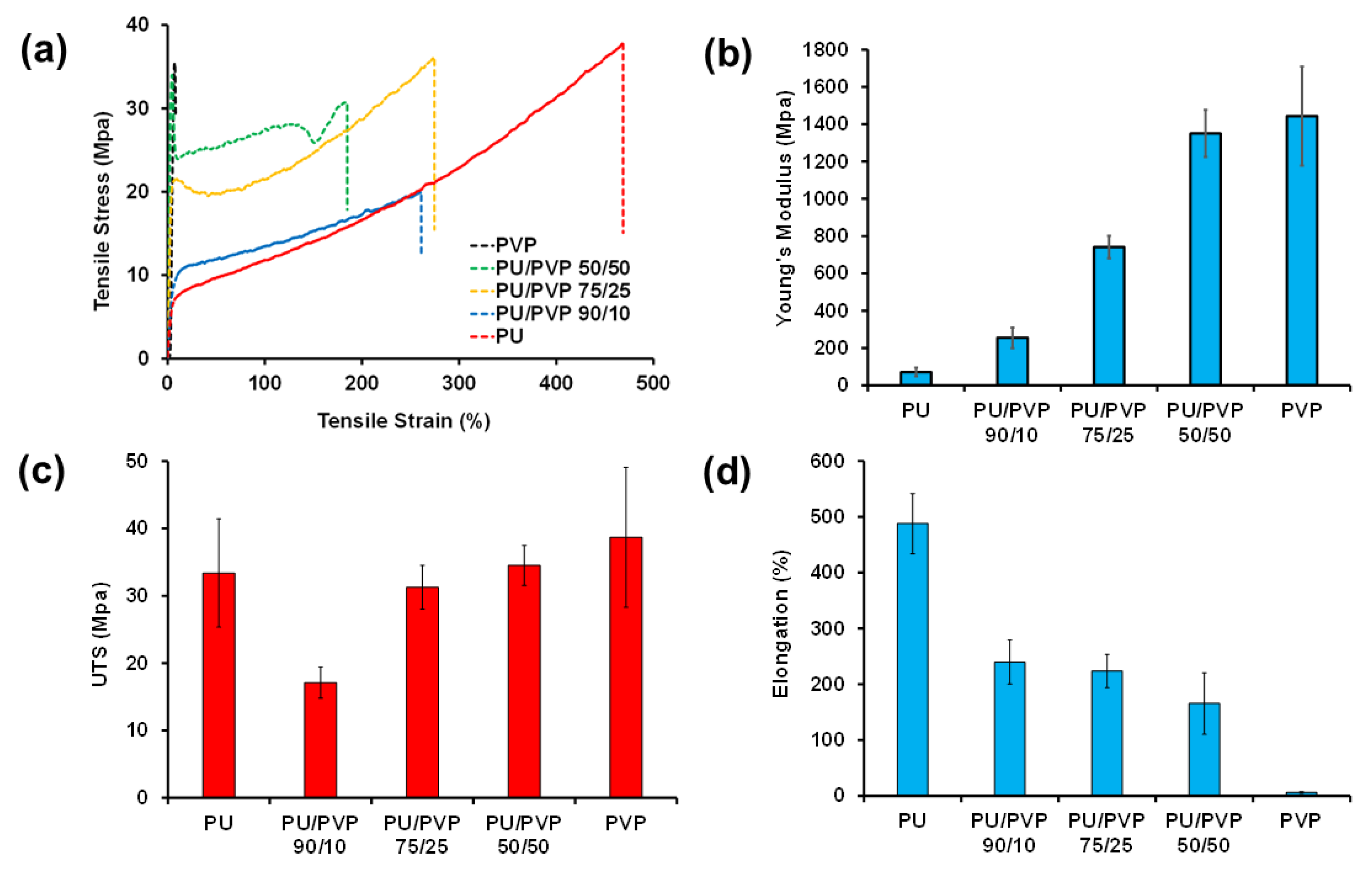
3.4. Mechanical Properties of the Films
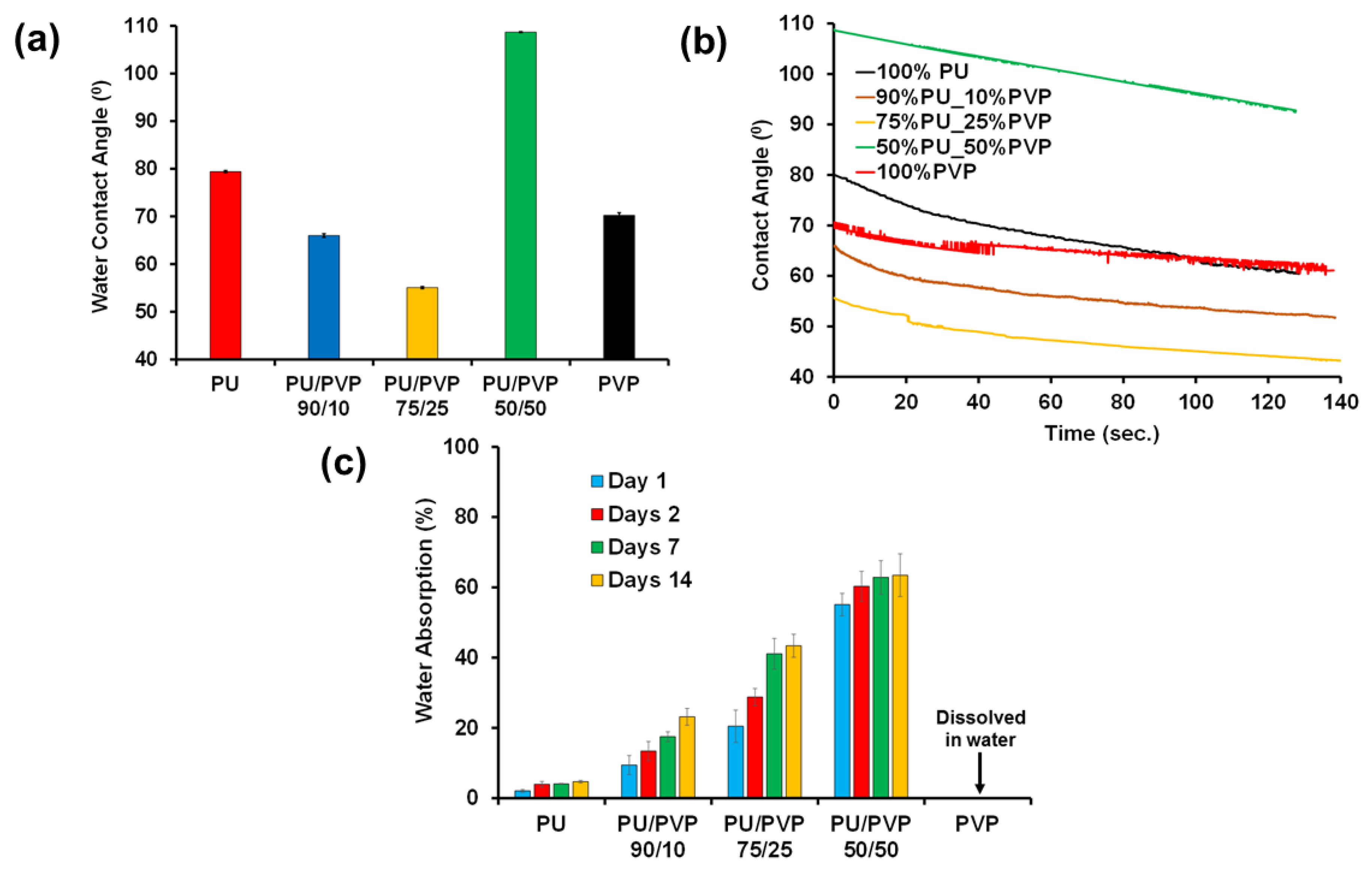
3.5. Wetting and Long-Term Water Uptake Properties
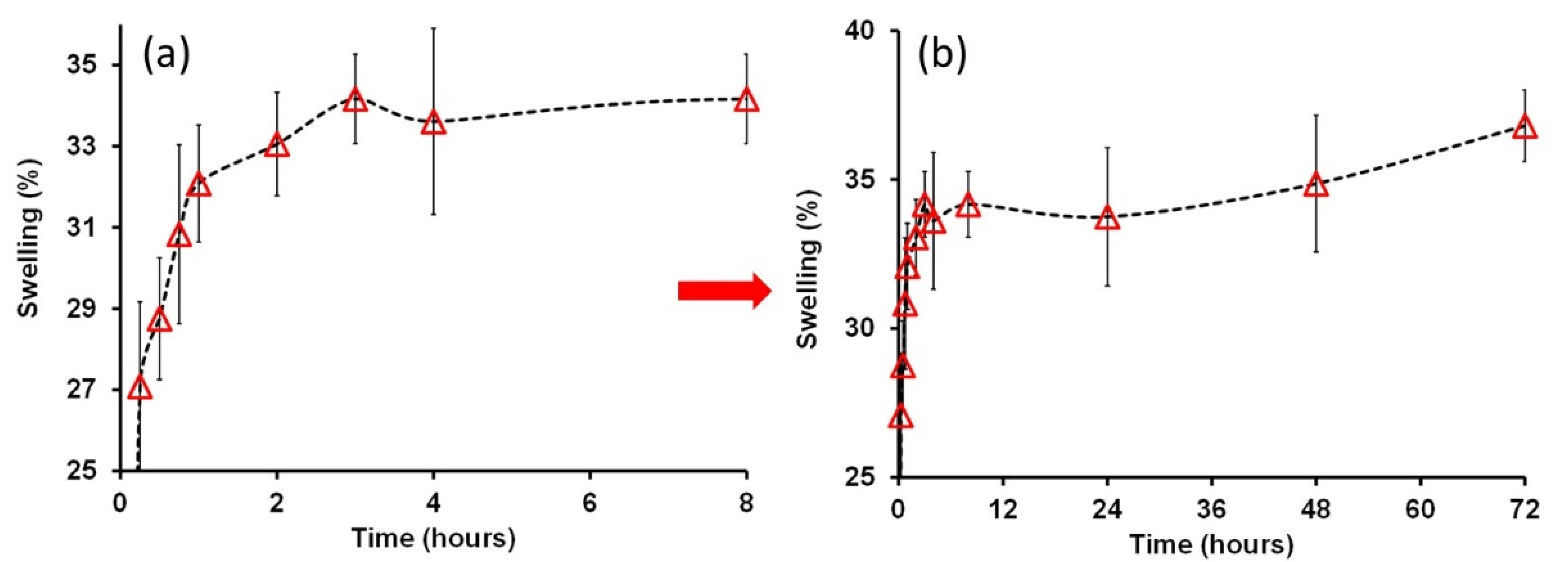
3.6. Swelling and Mechanical Film Recovery after Wet/Dry Cycles
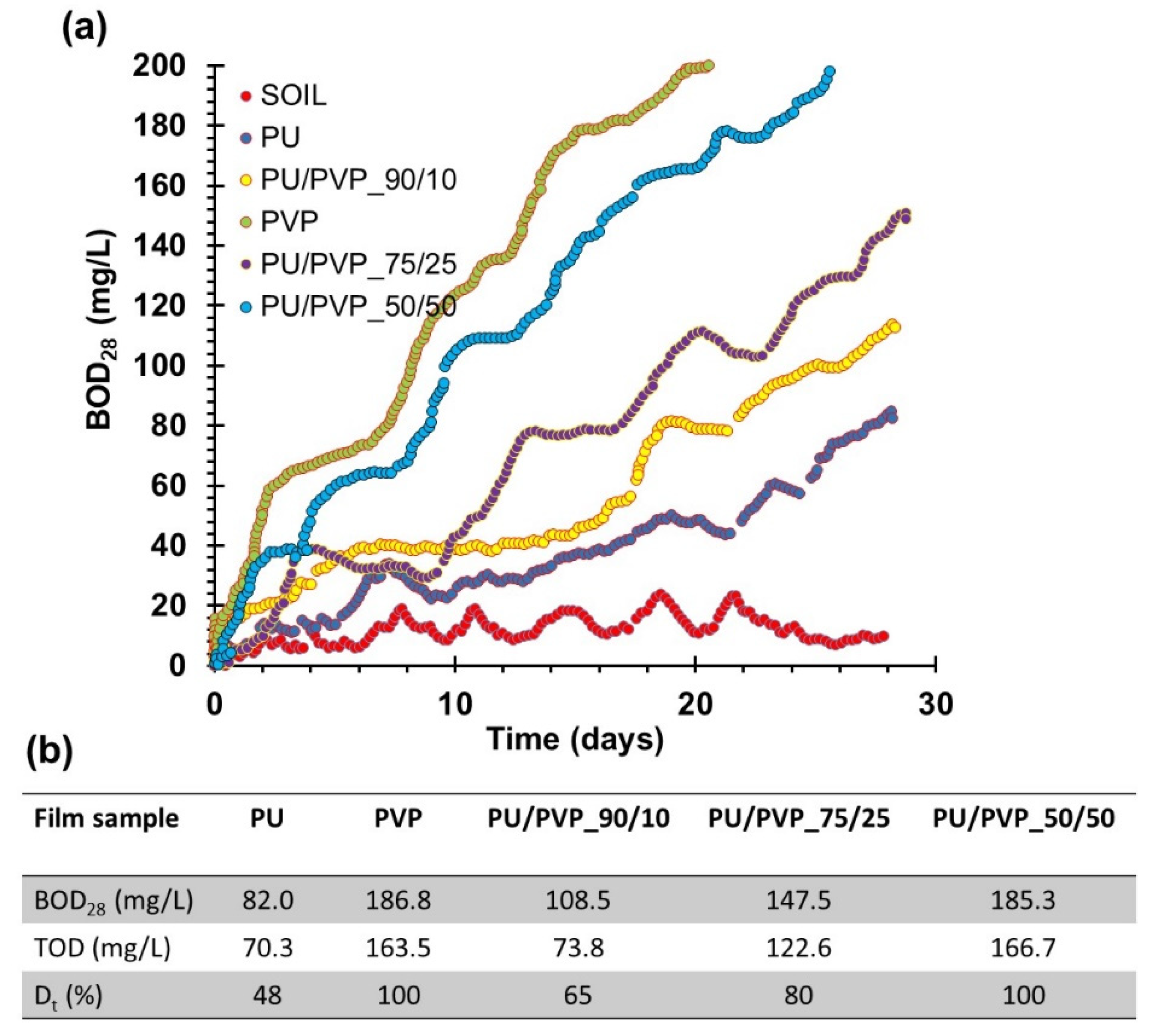
3.7. Biochemical Oxygen Demand (BOD) Analysis of the Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Noguchi, T.; Miyashita, M.; Inagaki, Y.; Watanabe, H. A new recycling system for expanded polystyrene using a natural solvent. Part 1. A new recycling technique. Packag. Technol. Sci. Int. J. 1998, 11, 19–27. [Google Scholar] [CrossRef]
- Liu, X.; Hong, W.; Chen, X. Continuous production of water-borne polyurethanes: A review. Polymers 2020, 12, 2875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wei, L.; Ma, Z.; Fan, Q.; Ma, J. Advances in waterborne polymer/carbon material composites for electromagnetic interference shielding. Carbon 2021, 177, 412–426. [Google Scholar] [CrossRef]
- Zhenova, A. Challenges in the development of new green solvents for polymer dissolution. Polym. Int. 2020, 69, 895–901. [Google Scholar] [CrossRef]
- Noble, K.L. Waterborne polyurethanes. Prog. Org. Coat. 1997, 32, 131–136. [Google Scholar] [CrossRef]
- Wang, L.; Shen, Y.; Lai, X.; Li, Z.; Liu, M. Synthesis and properties of crosslinked waterborne polyurethane. J. Polym. Res. 2011, 18, 469–476. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y.; Fang, C.; Li, S.; Cheng, Y.; Lei, W.; Meng, X. Recent advances in synthesis of waterborne polyurethane and their application in water-based ink: A review. J. Mater. Sci. Technol. 2015, 31, 708–722. [Google Scholar] [CrossRef]
- Scrinzi, E.; Rossi, S.; Deflorian, F.; Zanella, C. Evaluation of aesthetic durability of waterborne polyurethane coatings applied on wood for interior applications. Prog. Org. Coat. 2011, 72, 81–87. [Google Scholar] [CrossRef]
- García-Pacios, V.; Costa, V.; Colera, M.; Martín-Martínez, J.M. Waterborne polyurethane dispersions obtained with polycarbonate of hexanediol intended for use as coatings. Prog. Org. Coat. 2011, 71, 136–146. [Google Scholar] [CrossRef]
- Kim, M.G.; Jo, K.I.; Kim, E.; Park, J.H.; Ko, J.W.; Lee, J.H. Preparation of Polydimethylsiloxane-Modified Waterborne Polyurethane Coatings for Marine Applications. Polymers 2021, 13, 4283. [Google Scholar] [CrossRef]
- Chang, C.W.; Liao, J.Y.; Lu, K.T. Syntheses and Characteristics of Urushiol-Based Waterborne UV-Cured Wood Coatings. Polymers 2021, 13, 4005. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Shi, Y.; Zhao, D.; He, M. Synthesis of epoxidatied castor oil and its effect on the properties of waterborne polyurethane. Procedia Eng. 2011, 18, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Bullermann, J.; Friebel, S.; Salthammer, T.; Spohnholz, R. Novel polyurethane dispersions based on renewable raw materials-Stability studies by variations of DMPA content and degree of neutralisation. Prog. Org. Coat. 2013, 76, 609–615. [Google Scholar] [CrossRef]
- Panda, S.S.; Panda, B.P.; Nayak, S.K.; Mohanty, S. A review on waterborne thermosetting polyurethane coatings based on castor oil: Synthesis, characterization, and application. Polym.-Plast. Technol. Eng. 2018, 57, 500–522. [Google Scholar] [CrossRef]
- Fang, Y.; Du, X.; Jiang, Y.; Du, Z.; Pan, P.; Cheng, X.; Wang, H. Thermal-driven self-healing and recyclable waterborne polyurethane films based on reversible covalent interaction. ACS Sustain. Chem. Eng. 2018, 6, 14490–14500. [Google Scholar] [CrossRef]
- Ghosh, B.; Gogoi, S.; Thakur, S.; Karak, N. Bio-based waterborne polyurethane/carbon dot nanocomposite as a surface coating material. Prog. Org. Coat. 2016, 90, 324–330. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, C.; Kessler, M.R. Anionic waterborne polyurethane dispersion from a bio-based ionic segment. RSC Adv. 2014, 4, 35476–35483. [Google Scholar] [CrossRef]
- Ren, L.; Ma, X.; Zhang, J.; Qiang, T. Preparation of gallic acid modified waterborne polyurethane made from bio-based polyol. Polymer 2020, 194, 122370. [Google Scholar] [CrossRef]
- Poussard, L.; Lazko, J.; Mariage, J.; Raquez, J.M.; Dubois, P. Biobased waterborne polyurethanes for coating applications: How fully biobased polyols may improve the coating properties. Prog. Org. Coat. 2016, 97, 175–183. [Google Scholar] [CrossRef]
- Lei, L.; Zhong, L.; Lin, X.; Li, Y.; Xia, Z. Synthesis and characterization of waterborne polyurethane dispersions with different chain extenders for potential application in waterborne ink. Chem. Eng. J. 2014, 253, 518–525. [Google Scholar] [CrossRef]
- Otts, D.B.; Urban, M.W. Heterogeneous crosslinking of waterborne two-component polyurethanes (WB 2K-PUR); stratification processes and the role of water. Polymer 2005, 46, 2699–2709. [Google Scholar] [CrossRef]
- Liu, Y.; Glenn, J.; Tran, K.; Zhang, M.; Zhou, H.; Soleimani, M.; Lucas, F.; Winnik, M.A. Film Formation of Waterborne 2K Polyurethanes: Effect of Polyols Containing Different Carboxylic Acid Content. Macromolecules 2021, 54, 7943–7954. [Google Scholar] [CrossRef]
- Madbouly, S.A. Waterborne polyurethane dispersions and thin films: Biodegradation and antimicrobial behaviors. Molecules 2021, 26, 961. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, G. Effects of soft segment characteristics on the properties of biodegradable amphiphilic waterborne polyurethane prepared by a green process. J. Mater. Sci. 2020, 55, 3139–3156. [Google Scholar] [CrossRef]
- Hsu, S.H.; Hsieh, C.T.; Sun, Y.M. Synthesis and characterization of waterborne polyurethane containing poly (3-hydroxybutyrate) as new biodegradable elastomers. J. Mater. Chem. B 2015, 3, 9089–9097. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.H.; Hsu, S.C.; Hsu, S.H.; Chang, S.W. Molecular structures and mechanisms of waterborne biodegradable polyurethane nanoparticles. Comput. Struct. Biotechnol. J. 2019, 17, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wang, D.; Zheng, Y.; Zhao, L.; Xu, T.; Guo, Z.; Jiang, H. A novel waterborne polyurethane with biodegradability and high flexibility for 3D printing. Biofabrication 2020, 12, 035015. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, B.K. Covalent incorporation of starch derivative into waterborne polyurethane for biodegradability. Carbohydr. Polym. 2012, 87, 1803–1809. [Google Scholar] [CrossRef]
- Gogoi, S.; Karak, N. Biobased biodegradable waterborne hyperbranched polyurethane as an ecofriendly sustainable material. ACS Sustain. Chem. Eng. 2014, 2, 2730–2738. [Google Scholar] [CrossRef]
- Lee, T.J.; Kwon, S.H.; Kim, B.K. Biodegradable sol-gel coatings of waterborne polyurethane/gelatin chemical hybrids. Prog. Org. Coat. 2014, 77, 1111–1116. [Google Scholar] [CrossRef]
- Dai, M.; Wang, J.; Zhang, Y. Improving water resistance of waterborne polyurethane coating with high transparency and good mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 124994. [Google Scholar] [CrossRef]
- Yoo, H.J.; Kim, H.D. Synthesis and properties of waterborne polyurethane hydrogels for wound healing dressings. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 85, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Tighzert, L.; Berzin, F.; Rondot, S. Innovative plasticized starch films modified with waterborne polyurethane from renewable resources. Carbohydr. Polym. 2005, 61, 174–182. [Google Scholar] [CrossRef]
- ISO 17556::2019 Standard; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in Soil by Measuring the Oxygen Demand in a Respirometer or the Amount of Carbon Dioxide Evolved. International Organization for Standardization: Geneva, Switzerland, 2019.
- Buonanno, M.; Welch, D.; Shuryak, I.; Brenner, D.J. Far-UVC light (222 nm) efficiently and safely inactivates airborne human coronaviruses. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Quilez-Molina, A.I.; Marini, L.; Athanassiou, A.; Bayer, I.S. UV-blocking, transparent, and antioxidant polycyanoacrylate films. Polymers 2020, 12, 2011. [Google Scholar] [CrossRef] [PubMed]
- Skarja, G.A.; Woodhouse, K.A. Synthesis and characterization of degradable polyurethane elastomers containing an amino acid-based chain extender. Journal of biomaterials science. Polym. Ed. 1998, 9, 271–295. [Google Scholar]
- Guan, J.; Sacks, M.S.; Beckman, E.J.; Wagner, W.R. Synthesis, characterization, and cytocompatibility of elastomeric, biodegradable poly (ester-urethane) ureas based on poly (caprolactone) and putrescine. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 61, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Cakić, S.M.; Ristić, I.S.; Krakovský, I.; Stojiljković, D.T.; Bělský, P.; Kollová, L. Crystallization and thermal properties in waterborne polyurethane elastomers: Influence of mixed soft segment block. Mater. Chem. Phys. 2014, 144, 31–40. [Google Scholar] [CrossRef]
- Pukánszky, B.; Tüdõs, F. Miscibility and mechanical properties of polymer blends. In Makromolekulare Chemie. Macromolecular Symposia; Hüthig & Wepf Verlag: Basel, Switzerland, 1990; Volume 38, pp. 221–231. [Google Scholar]
- Siviour, C.R.; Jordan, J.L. High strain rate mechanics of polymers: A review. J. Dyn. Behav. Mater. 2016, 2, 15–32. [Google Scholar] [CrossRef] [Green Version]
- Attanasio, A.; Bayer, I.S.; Ruffilli, R.; Ayadi, F.; Athanassiou, A. Surprising high hydrophobicity of polymer networks from hydrophilic components. ACS Appl. Mater. Interfaces 2013, 5, 5717–5726. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Saha, N.; Kitano, T.; Saha, P. Novel hydrogels of PVP-CMC and their swelling effect on viscoelastic properties. J. Appl. Polym. Sci. 2010, 117, 1703–1710. [Google Scholar] [CrossRef] [Green Version]
- Matveev, Y.I.; Grinberg, V.Y.; Tolstoguzov, V.B. The plasticizing effect of water on proteins, polysaccharides and their mixtures. Glassy state of biopolymers, food and seeds. Food Hydrocoll. 2000, 14, 425–437. [Google Scholar] [CrossRef]
- Godbillot, L.; Dole, P.; Joly, C.; Rogé, B.; Mathlouthi, M. Analysis of water binding in starch plasticized films. Food Chem. 2006, 96, 380–386. [Google Scholar] [CrossRef]
- Contardi, M.; Kossyvaki, D.; Picone, P.; Summa, M.; Guo, X.; Heredia-Guerrero, J.A.; Bayer, I.S. Electrospun polyvinylpyrrolidone (PVP) hydrogels containing hydroxycinnamic acid derivatives as potential wound dressings. Chem. Eng. J. 2021, 409, 128144. [Google Scholar] [CrossRef]
- Contardi, M.; Montano, S.; Galli, P.; Mazzon, G.; Mah’d Moh’d Ayyoub, A.; Seveso, D.; Bayer, I.S. Marine Fouling Characteristics of Biocomposites in a Coral Reef Ecosystem. Adv. Sustain. Syst. 2021, 5, 2100089. [Google Scholar] [CrossRef]
- Kim, Y.D.; Kim, S.C. Effect of chemical structure on the biodegradation of polyurethanes under composting conditions. Polym. Degrad. Stab. 1998, 62, 343–352. [Google Scholar] [CrossRef]
- Hung, C.S.; Barlow, D.E.; Varaljay, V.A.; Drake, C.A.; Crouch, A.L.; Russell, J.N., Jr.; Biffinger, J.C. The biodegradation of polyester and polyester polyurethane coatings using Papiliotrema laurentii. Int. Biodeterior. Biodegrad. 2019, 139, 34–43. [Google Scholar] [CrossRef]
- Schmidt, J.; Wei, R.; Oeser, T.; Dedavid e Silva, L.A.; Breite, D.; Schulze, A.; Zimmermann, W. Degradation of polyester polyurethane by bacterial polyester hydrolases. Polymers 2017, 9, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penn, M.R.; Pauer, J.J.; Mihelcic, J.R. Biochemical oxygen demand. Environ. Ecol. Chem. 2009, 2, 278–297. [Google Scholar]
- Baker, J.R.; Milke, M.W.; Mihelcic, J.R. Relationship between chemical and theoretical oxygen demand for specific classes of organic chemicals. Water Res. 1999, 33, 327–334. [Google Scholar] [CrossRef]
- Masood, M.T.; Zahid, M.; Goldoni, L.; Ceseracciu, L.; Athanassiou, A.; Bayer, I.S. Highly transparent polyethylcyanoacrylates from approved eco-friendly fragrance materials demonstrating excellent fog-harvesting and anti-wear properties. ACS Appl. Mater. Interfaces 2018, 10, 34573–34584. [Google Scholar] [CrossRef] [PubMed]
- Nanni, G.; Heredia-Guerrero, J.A.; Paul, U.C.; Dante, S.; Caputo, G.; Canale, C.; Bayer, I.S. Poly (furfuryl alcohol)-polycaprolactone blends. Polymers 2019, 11, 1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Sample | PVP (wt.%) | PU (wt.%) |
|---|---|---|
| PU | 0.0 | 100.0 |
| PVP | 100 | 0.0 |
| PU/PVP_50/50 | 50 | 50 |
| PU/PVP_75/25 | 25 | 75 |
| PU/PVP_90/10 | 10 | 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, U.C.; Bayer, G.; Grasselli, S.; Malchiodi, A.; Bayer, I.S. Biodegradable, Stretchable and Transparent Plastic Films from Modified Waterborne Polyurethane Dispersions. Polymers 2022, 14, 1199. https://doi.org/10.3390/polym14061199
Paul UC, Bayer G, Grasselli S, Malchiodi A, Bayer IS. Biodegradable, Stretchable and Transparent Plastic Films from Modified Waterborne Polyurethane Dispersions. Polymers. 2022; 14(6):1199. https://doi.org/10.3390/polym14061199
Chicago/Turabian StylePaul, Uttam C., Gözde Bayer, Silvia Grasselli, Annalisa Malchiodi, and Ilker S. Bayer. 2022. "Biodegradable, Stretchable and Transparent Plastic Films from Modified Waterborne Polyurethane Dispersions" Polymers 14, no. 6: 1199. https://doi.org/10.3390/polym14061199
APA StylePaul, U. C., Bayer, G., Grasselli, S., Malchiodi, A., & Bayer, I. S. (2022). Biodegradable, Stretchable and Transparent Plastic Films from Modified Waterborne Polyurethane Dispersions. Polymers, 14(6), 1199. https://doi.org/10.3390/polym14061199







