Development and Evaluation of Nanoparticles-in-Film Technology to Achieve Extended In Vivo Exposure of MK-2048 for HIV Prevention
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production and Characterization of the MK-2048-Loaded PLGA Nanoparticles (PNP)
2.3. High-Performance Liquid Chromatography (HPLC) Method for the Analysis of MK-2048
2.4. Liquid Chromatography-Mass Spectrometry (LC/LC-MS) Method for the Bioanalysis of MK-2048
2.5. Preparation and Characterization of PNP-Incorporated Polymeric Films
2.6. PNP Film Compatibility with Lactobacillus
2.7. Cell Culture
2.8. PNP Film Efficacy Study against HIV-1BaL in the TMZ-bl Antiviral Assay
2.9. PNP Permeability Study Using Transporter-Overexpressed Cell Lines
2.10. PNP Film Permeability Study Using the Ex-Vivo Ectocervical Tissue Model in an In-Line Set-Up
2.11. In Vivo Pharmacokinetic (PK) Evaluation of PNP Films in a Macaque Model
2.12. Statistical Analysis
3. Results
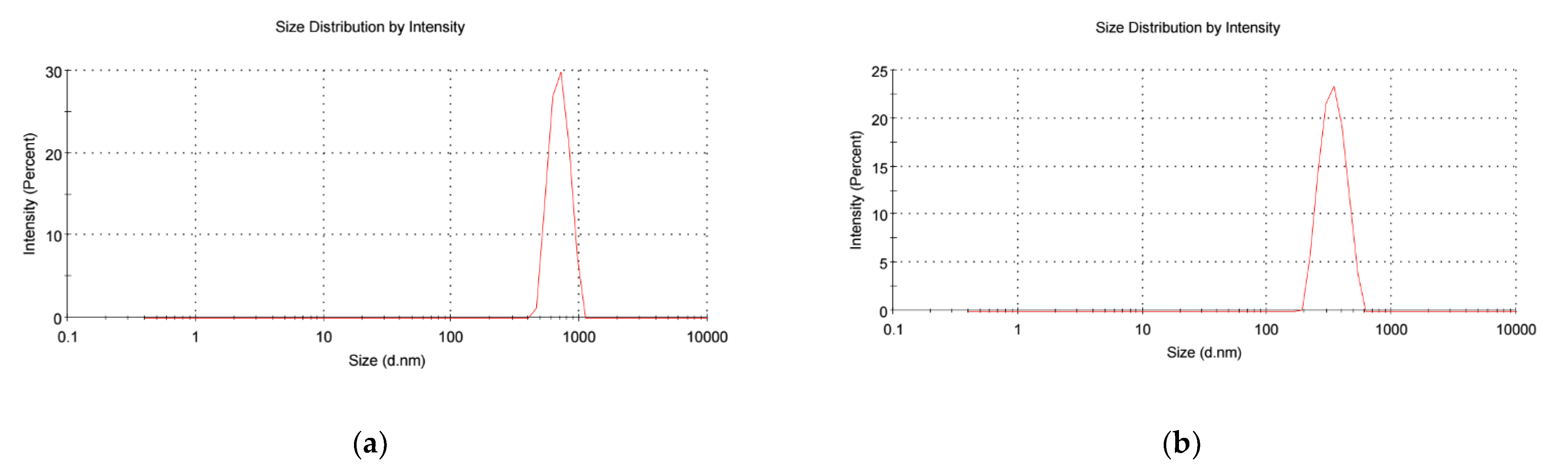
3.1. Characterization of MK-2048-Loaded PLGA Nanoparticles (PNPs)
3.2. Characterization of PNP-Incorporated Polymeric Films
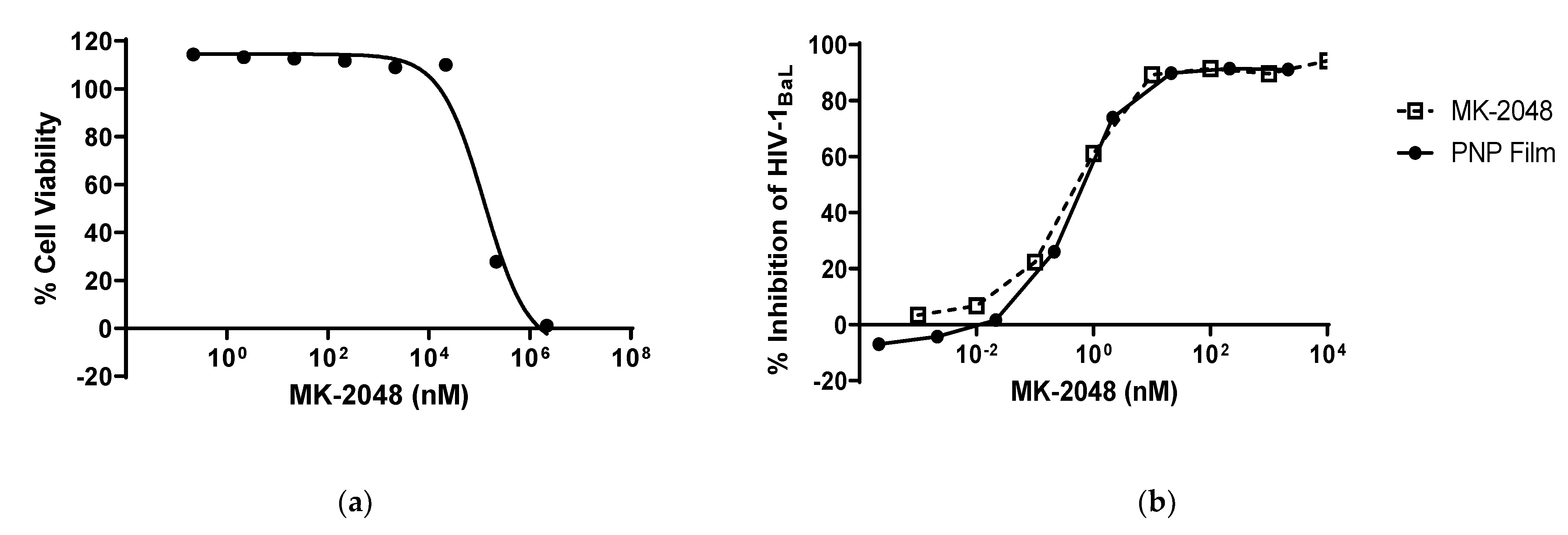
3.3. PNP Film Efficacy against HIV-1
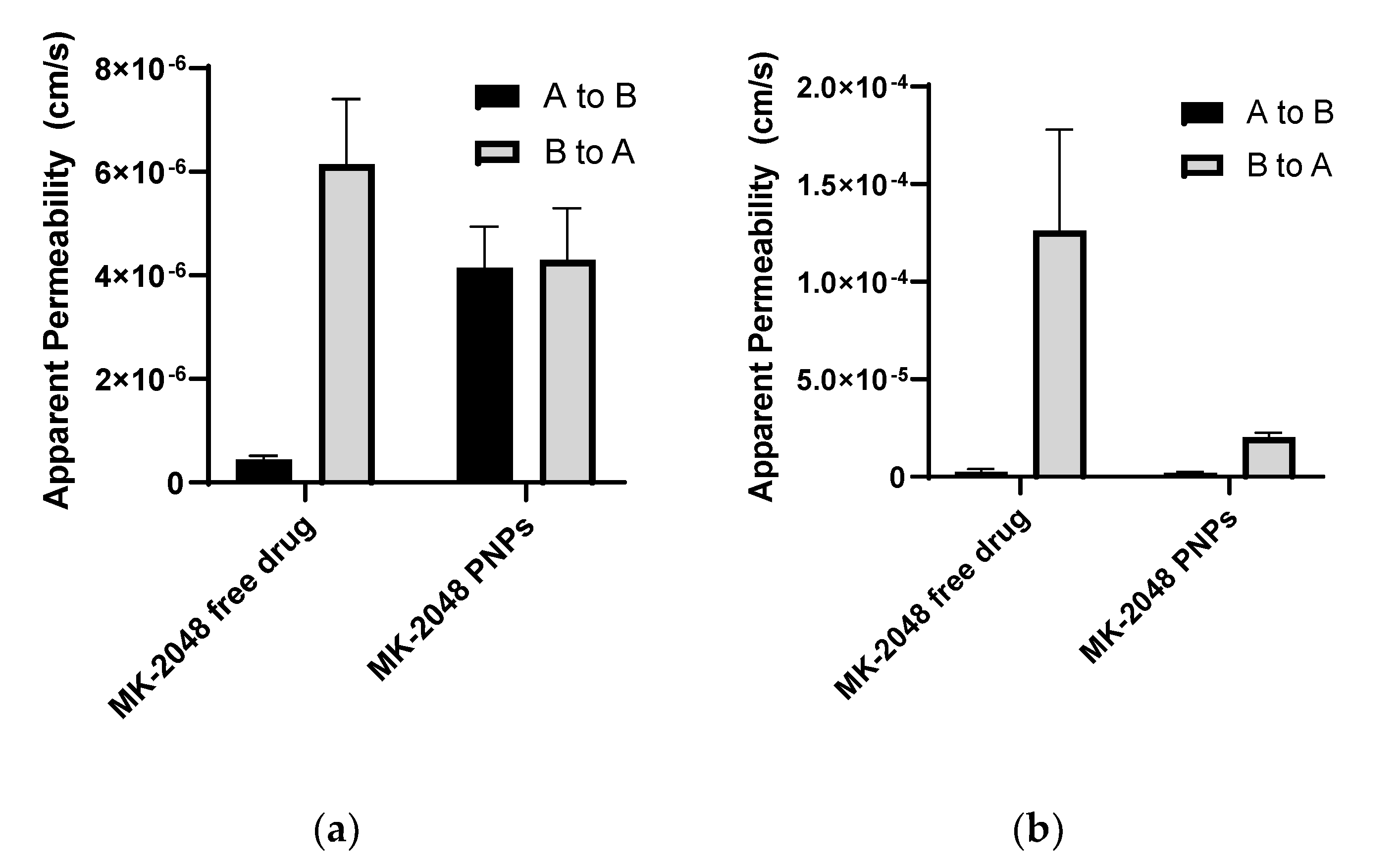
3.4. MK-2048 in PNPs Evades Transporters
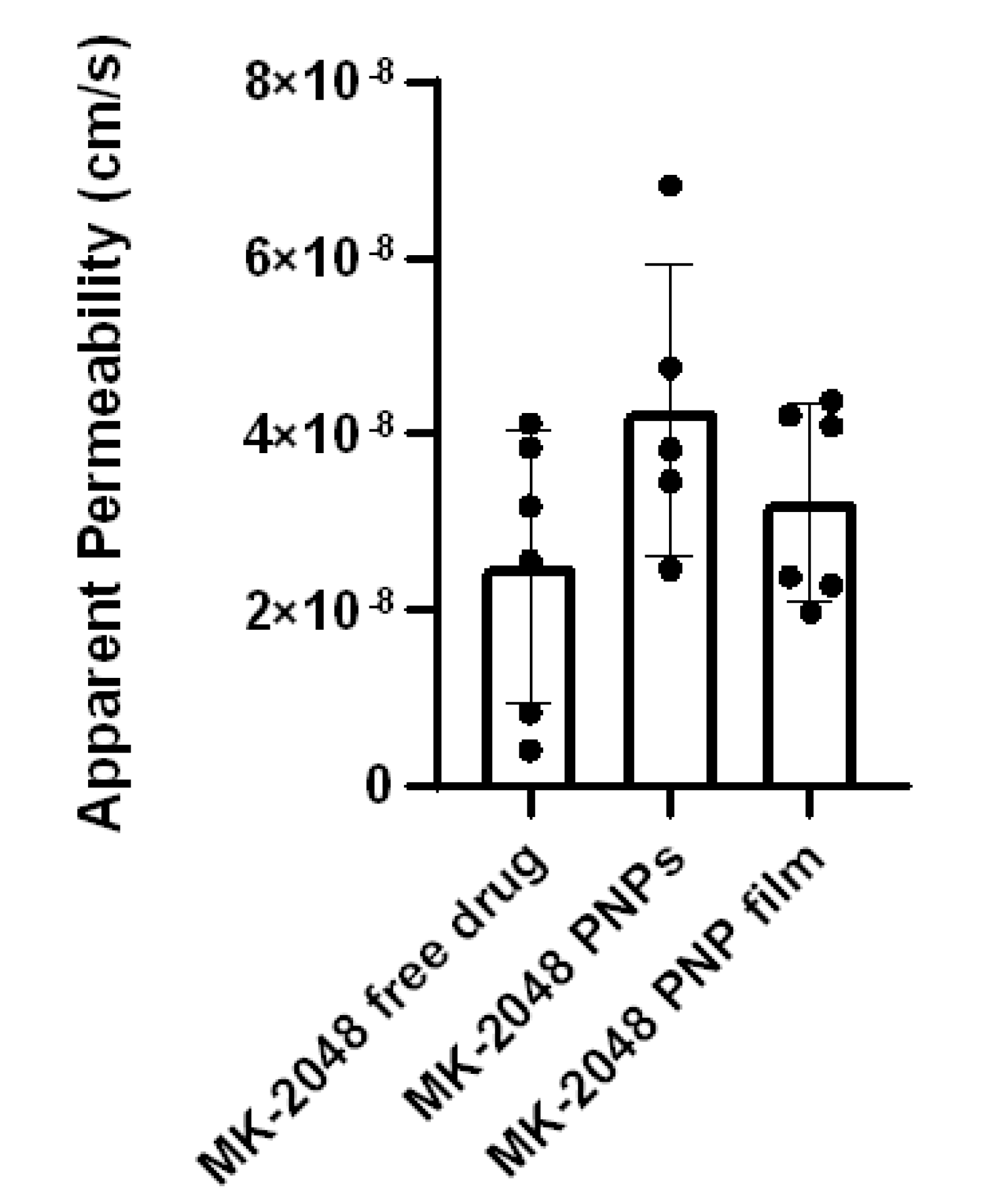
3.5. Ex Vivo Permeability in Ectocervical Tissues
3.6. MK-2048 Delivered by PNP Films Sustained in the Macaque Vagina
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. People Living with HIV/AIDS. Available online: https://www.who.int/data/gho/data/themes/hiv-aids (accessed on 22 January 2022).
- UNAIDS. Global HIV & AIDS Statistics—Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 22 January 2022).
- Naicker, N.; Kharsany, A.B.; Werner, L.; van Loggerenberg, F.; Mlisana, K.; Garrett, N.; Abdool Karim, S.S. Risk Factors for HIV Acquisition in High Risk Women in a Generalised Epidemic Setting. AIDS Behav. 2015, 19, 1305–1316. [Google Scholar] [CrossRef]
- Bradley, E.; Forsberg, K.; Betts, J.E.; DeLuca, J.B.; Kamitani, E.; Porter, S.E.; Sipe, T.A.; Hoover, K.W. Factors Affecting Pre-Exposure Prophylaxis Implementation for Women in the United States: A Systematic Review. J. Womens Health 2019, 28, 1272–1285. [Google Scholar] [CrossRef] [PubMed]
- Thomson, K.A.; Baeten, J.M.; Mugo, N.R.; Bekker, L.G.; Celum, C.L.; Heffron, R. Tenofovir-based oral preexposure prophylaxis prevents HIV infection among women. Curr. Opin. HIV AIDS 2016, 11, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Hensen, B.; Machingura, F.; Busza, J.; Birdthistle, I.; Chabata, S.T.; Chiyaka, T.; Floyd, S.; Jamali, G.; Mushati, P.; Hargreaves, J.; et al. How Can We Support the Use of Oral PrEP Among Young Women Who Sell Sex? A PrEP Cascade Analysis. J. Acquir. Immune Defic. Syndr. 2021, 88, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.J.; Petrozzino, J.J. An evidence-based review of treatment-related determinants of patients’ nonadherence to HIV medications. AIDS Patient Care STDS 2009, 23, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Chesney, M.A. Factors affecting adherence to antiretroviral therapy. Clin. Infect. Dis. 2000, 30 (Suppl. S2), S171–S176. [Google Scholar] [CrossRef] [PubMed]
- Baeten, J.M.; Palanee-Phillips, T.; Brown, E.R.; Schwartz, K.; Soto-Torres, L.E.; Govender, V.; Mgodi, N.M.; Matovu Kiweewa, F.; Nair, G.; Mhlanga, F.; et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. N. Engl. J. Med. 2016, 375, 2121–2132. [Google Scholar] [CrossRef]
- Kiser, P.F.; Johnson, T.J.; Clark, J.T. State of the art in intravaginal ring technology for topical prophylaxis of HIV infection. AIDS Rev. 2012, 14, 62–77. [Google Scholar]
- Rohan, L.C.; Sassi, A.B. Vaginal drug delivery systems for HIV prevention. AAPS J. 2009, 11, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.J.; Machado, R.; Ribeiro, A.; Goncalves, H.; Real Oliveira, M.; Viseu, T.; das Neves, J.; Lucio, M. Rational Development of Liposomal Hydrogels: A Strategy for Topical Vaginal Antiretroviral Drug Delivery in the Context of HIV Prevention. Pharmaceutics 2019, 11, 485. [Google Scholar] [CrossRef]
- Mahalingam, A.; Simmons, A.P.; Ugaonkar, S.R.; Watson, K.M.; Dezzutti, C.S.; Rohan, L.C.; Buckheit, R.W., Jr.; Kiser, P.F. Vaginal microbicide gel for delivery of IQP-0528, a pyrimidinedione analog with a dual mechanism of action against HIV-1. Antimicrob. Agents Chemother. 2011, 55, 1650–1660. [Google Scholar] [CrossRef]
- Ham, A.S.; Rohan, L.C.; Boczar, A.; Yang, L.; Buckheit, K.W.; Buckheit, R.W., Jr. Vaginal film drug delivery of the pyrimidinedione IQP-0528 for the prevention of HIV infection. Pharm. Res. 2012, 29, 1897–1907. [Google Scholar] [CrossRef] [PubMed]
- Sassi, A.B.; Cost, M.R.; Cole, A.L.; Cole, A.M.; Patton, D.L.; Gupta, P.; Rohan, L.C. Formulation development of retrocyclin 1 analog RC-101 as an anti-HIV vaginal microbicide product. Antimicrob. Agents Chemother. 2011, 55, 2282–2289. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.M.; Mitchnick, L.B.; Risha, P.; Muungo, L.T.; Norick, P.M. Acceptability of vaginal film, soft-gel capsule, and tablet as potential microbicide delivery methods among African women. J. Womens Health 2011, 20, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.M.; Palmeira-de-Oliveira, A.; Martinez-De-Oliveira, J.; Palmeira-de-Oliveira, R. Vaginal films for drug delivery. J. Pharm. Sci. 2013, 102, 2069–2081. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.D.; Kramzer, L.F.; Hillier, S.L.; Chang, J.C.; Meyn, L.A.; Rohan, L.C. Preferred Physical Characteristics of Vaginal Film Microbicides for HIV Prevention in Pittsburgh Women. Arch. Sex. Behav. 2017, 46, 1111–1119. [Google Scholar] [CrossRef]
- Hoesley, C.J.; Chen, B.A.; Anderson, P.L.; Dezzutti, C.S.; Strizki, J.; Sprinkle, C.; Heard, F.; Bauermeister, J.; Hall, W.; Jacobson, C.; et al. Phase 1 Safety and Pharmacokinetics Study of MK-2048/Vicriviroc (MK-4176)/MK-2048A Intravaginal Rings. Clin. Infect. Dis. 2019, 68, 1136–1143. [Google Scholar] [CrossRef]
- Liu, A.Y.; Zhang, J.; Anderson, P.L.; Wagner, T.; Pan, Z.; Peda, M.; Gomez, K.; Beamer, M.; Jacobson, C.; Strizki, J.; et al. Phase 1 Pharmacokinetic Trial of 2 Intravaginal Rings Containing Different Dose Strengths of Vicriviroc (MK-4176) and MK-2048. Clin. Infect. Dis. 2019, 68, 1129–1135. [Google Scholar] [CrossRef]
- Van Wesenbeeck, L.; Rondelez, E.; Feyaerts, M.; Verheyen, A.; van der Borght, K.; Smits, V.; Cleybergh, C.; De Wolf, H.; van Baelen, K.; Stuyver, L.J. Cross-resistance profile determination of two second-generation HIV-1 integrase inhibitors using a panel of recombinant viruses derived from raltegravir-treated clinical isolates. Antimicrob. Agents Chemother. 2011, 55, 321–325. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Minuesa, G.; Arimany-Nardi, C.; Erkizia, I.; Cedeno, S.; Molto, J.; Clotet, B.; Pastor-Anglada, M.; Martinez-Picado, J. P-glycoprotein (ABCB1) activity decreases raltegravir disposition in primary CD4+P-gphigh cells and correlates with HIV-1 viral load. J. Antimicrob. Chemother. 2016, 71, 2782–2792. [Google Scholar] [CrossRef]
- Hoque, M.T.; Kis, O.; De Rosa, M.F.; Bendayan, R. Raltegravir permeability across blood-tissue barriers and the potential role of drug efflux transporters. Antimicrob. Agents Chemother. 2015, 59, 2572–2582. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, Y.; Hamada, A.; Shinohara, T.; Tsuchiya, K.; Jono, H.; Saito, H. Role of P-glycoprotein in the efflux of raltegravir from human intestinal cells and CD4+ T-cells as an interaction target for anti-HIV agents. Biochem. Biophys. Res. Commun. 2013, 439, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Zembruski, N.C.; Buchel, G.; Jodicke, L.; Herzog, M.; Haefeli, W.E.; Weiss, J. Potential of novel antiretrovirals to modulate expression and function of drug transporters in vitro. J. Antimicrob. Chemother. 2011, 66, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Chavanpatil, M.D.; Khdair, A.; Gerard, B.; Bachmeier, C.; Miller, D.W.; Shekhar, M.P.; Panyam, J. Surfactant-polymer nanoparticles overcome P-glycoprotein-mediated drug efflux. Mol. Pharm. 2007, 4, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.; Rao, S.; Thomas, N.; Boulos, R.A.; Prestidge, C.A. Ramizol((R)) encapsulation into extended release PLGA micro- and nanoparticle systems for subcutaneous and intramuscular administration: In vitro and in vivo evaluation. Drug. Dev. Ind. Pharm. 2018, 44, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Reis, C.; Machado, A.; Barreiros, L.; Araujo, F.; Nunes, R.; Seabra, V.; Ferreira, D.; Segundo, M.A.; Sarmento, B.; das Neves, J. Nanoparticles-in-film for the combined vaginal delivery of anti-HIV microbicide drugs. J. Control. Release 2016, 243, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Cunha-Reis, C.; Araujo, F.; Nunes, R.; Seabra, V.; Ferreira, D.; das Neves, J.; Sarmento, B. Development and in vivo safety assessment of tenofovir-loaded nanoparticles-in-film as a novel vaginal microbicide delivery system. Acta Biomater. 2016, 44, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Minnis, A.M.; Gandham, S.; Richardson, B.A.; Guddera, V.; Chen, B.A.; Salata, R.; Nakabiito, C.; Hoesley, C.; Justman, J.; Soto-Torres, L.; et al. Adherence and acceptability in MTN 001: A randomized cross-over trial of daily oral and topical tenofovir for HIV prevention in women. AIDS Behav. 2013, 17, 737–747. [Google Scholar] [CrossRef][Green Version]
- Marrazzo, J.M.; Ramjee, G.; Richardson, B.A.; Gomez, K.; Mgodi, N.; Nair, G.; Palanee, T.; Nakabiito, C.; van der Straten, A.; Noguchi, L.; et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 2015, 372, 509–518. [Google Scholar] [CrossRef]
- Moncla, B.J.; Hillier, S.L. Why nonoxynol-9 may have failed to prevent acquisition of Neisseria gonorrhoeae in clinical trials. Sex. Transm. Dis. 2005, 32, 491–494. [Google Scholar] [CrossRef]
- Wei, X.; Decker, J.M.; Liu, H.; Zhang, Z.; Arani, R.B.; Kilby, J.M.; Saag, M.S.; Wu, X.; Shaw, G.M.; Kappes, J.C. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 2002, 46, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- Rohan, L.C.; Moncla, B.J.; Kunjara Na Ayudhya, R.P.; Cost, M.; Huang, Y.; Gai, F.; Billitto, N.; Lynam, J.D.; Pryke, K.; Graebing, P.; et al. In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PLoS ONE 2010, 5, e9310. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Guide for the Care and Use of Laboratory Animals: Eighth Edition; The National Academies Press: Washington DC, USA, 2011; p. 246. [Google Scholar]
- Rossi, S.; Vigani, B.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Recent advances in the mucus-interacting approach for vaginal drug delivery: From mucoadhesive to mucus-penetrating nanoparticles. Expert. Opin. Drug. Deliv. 2019, 16, 777–781. [Google Scholar] [CrossRef]
- Li, J.; Regev, G.; Patel, S.K.; Patton, D.; Sweeney, Y.; Graebing, P.; Grab, S.; Wang, L.; Sant, V.; Rohan, L.C. Rational Design of a Multipurpose Bioadhesive Vaginal Film for Co-Delivery of Dapivirine and Levonorgestrel. Pharmaceutics 2019, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Serrao, E.; Odde, S.; Ramkumar, K.; Neamati, N. Raltegravir, elvitegravir, and metoogravir: The birth of “me-too” HIV-1 integrase inhibitors. Retrovirology 2009, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Gomez, G.; Pinon-Segundo, E.; Mendoza-Munoz, N.; Zambrano-Zaragoza, M.L.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Approaches in Polymeric Nanoparticles for Vaginal Drug Delivery: A Review of the State of the Art. Int. J. Mol. Sci. 2018, 19, 1549. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.S.; Xu, Q.; Boylan, N.J.; Chisholm, J.; Tang, B.C.; Schuster, B.S.; Henning, A.; Ensign, L.M.; Lee, E.; Adstamongkonkul, P.; et al. Nanoparticles that do not adhere to mucus provide uniform and long-lasting drug delivery to airways following inhalation. Sci. Adv. 2017, 3, e1601556. [Google Scholar] [CrossRef]
- Ensign, L.M.; Cone, R.; Hanes, J. Nanoparticle-based drug delivery to the vagina: A review. J. Control. Release 2014, 190, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Whaley, K.J.; Hanes, J.; Shattock, R.; Cone, R.A.; Friend, D.R. Novel approaches to vaginal delivery and safety of microbicides: Biopharmaceuticals, nanoparticles, and vaccines. Antivir. Res. 2010, 88 (Suppl. S1), S55–S66. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.Y.; Hida, K.; Cone, R.; Hanes, J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc. Natl. Acad. Sci. USA 2010, 107, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Crater, J.S.; Carrier, R.L. Barrier properties of gastrointestinal mucus to nanoparticle transport. Macromol. Biosci. 2010, 10, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, J.; Patel, S.K.; Palmer, K.E.; Devlin, B.; Rohan, L.C. Design of Poly(lactic-co-glycolic Acid) (PLGA) Nanoparticles for Vaginal Co-Delivery of Griffithsin and Dapivirine and Their Synergistic Effect for HIV Prophylaxis. Pharmaceutics 2019, 11, 184. [Google Scholar] [CrossRef] [PubMed]
- Linhares, I.M.; Summers, P.R.; Larsen, B.; Giraldo, P.C.; Witkin, S.S. Contemporary perspectives on vaginal pH and lactobacilli. Am. J. Obstet. Gynecol. 2011, 204, e121–e125. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.I.; van den Broek, M.; Balzarini, J.; Vanderleyden, J.; Lebeer, S. Vaginal microbiota and its role in HIV transmission and infection. FEMS Microbiol. Rev. 2013, 37, 762–792. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; Hida, K.; Shukair, S.; Wang, Y.Y.; Figueiredo, A.; Cone, R.; Hope, T.J.; Hanes, J. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J. Virol. 2009, 83, 11196–11200. [Google Scholar] [CrossRef] [PubMed]
- Lichtenwalner, A.B.; Patton, D.L.; Klebanoff, S.J.; Headley, C.M.; Hillier, S.L. Vaginal myeloperoxidase and flora in the pig-tailed macaque. J. Med. Primatol. 2000, 29, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Yacobi, N.R.; Demaio, L.; Xie, J.; Hamm-Alvarez, S.F.; Borok, Z.; Kim, K.J.; Crandall, E.D. Polystyrene nanoparticle trafficking across alveolar epithelium. Nanomedicine 2008, 4, 139–145. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Bernkop-Schnurch, A. Thiomers: A new generation of mucoadhesive polymers. Adv. Drug Deliv. Rev. 2005, 57, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Kollner, S.; Dunnhaupt, S.; Waldner, C.; Hauptstein, S.; Pereira de Sousa, I.; Bernkop-Schnurch, A. Mucus permeating thiomer nanoparticles. Eur. J. Pharm. Biopharm. 2015, 97, 265–272. [Google Scholar] [CrossRef]
- Mohideen, M.; Quijano, E.; Song, E.; Deng, Y.; Panse, G.; Zhang, W.; Clark, M.R.; Saltzman, W.M. Degradable bioadhesive nanoparticles for prolonged intravaginal delivery and retention of elvitegravir. Biomaterials 2017, 144, 144–154. [Google Scholar] [CrossRef] [PubMed]



 : NHP#1,
: NHP#1,  : NHP#2,
: NHP#2,  : NHP#3,
: NHP#3,  : NHP#4.
: NHP#4.
 : NHP#1,
: NHP#1,  : NHP#2,
: NHP#2,  : NHP#3,
: NHP#3,  : NHP#4.
: NHP#4.
| Lactobacillus Strain | Film–Buffer Log Difference |
|---|---|
| L. crispatus CTV05 | 0.062 |
| L. crispatus ATCC 33197 | 0.560 |
| L. jensenii ATCC 25258 | 0.041 |
| L. jensenii LBP 28Ab | −0.214 |
| ER | MK-2048 Free Drug | MK-2048 Nanoparticles |
|---|---|---|
| BCRP | 13.77 | 1.04 |
| P-gp | 48.38 | 10.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, X.; Patel, S.K.; Li, J.; Patton, D.; Xu, E.; Anderson, P.L.; Parikh, U.; Sweeney, Y.; Strizki, J.; Hillier, S.L.; et al. Development and Evaluation of Nanoparticles-in-Film Technology to Achieve Extended In Vivo Exposure of MK-2048 for HIV Prevention. Polymers 2022, 14, 1196. https://doi.org/10.3390/polym14061196
Tong X, Patel SK, Li J, Patton D, Xu E, Anderson PL, Parikh U, Sweeney Y, Strizki J, Hillier SL, et al. Development and Evaluation of Nanoparticles-in-Film Technology to Achieve Extended In Vivo Exposure of MK-2048 for HIV Prevention. Polymers. 2022; 14(6):1196. https://doi.org/10.3390/polym14061196
Chicago/Turabian StyleTong, Xin, Sravan Kumar Patel, Jing Li, Dorothy Patton, Elaine Xu, Peter L. Anderson, Urvi Parikh, Yvonne Sweeney, Julie Strizki, Sharon L. Hillier, and et al. 2022. "Development and Evaluation of Nanoparticles-in-Film Technology to Achieve Extended In Vivo Exposure of MK-2048 for HIV Prevention" Polymers 14, no. 6: 1196. https://doi.org/10.3390/polym14061196
APA StyleTong, X., Patel, S. K., Li, J., Patton, D., Xu, E., Anderson, P. L., Parikh, U., Sweeney, Y., Strizki, J., Hillier, S. L., & Rohan, L. C. (2022). Development and Evaluation of Nanoparticles-in-Film Technology to Achieve Extended In Vivo Exposure of MK-2048 for HIV Prevention. Polymers, 14(6), 1196. https://doi.org/10.3390/polym14061196








