The Structural Characteristics of an Acidic Water-Soluble Polysaccharide from Bupleurum chinense DC and Its In Vivo Anti-Tumor Activity on H22 Tumor-Bearing Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of BCP
2.3. BCP Characterization
2.3.1. Chemical Components Analysis
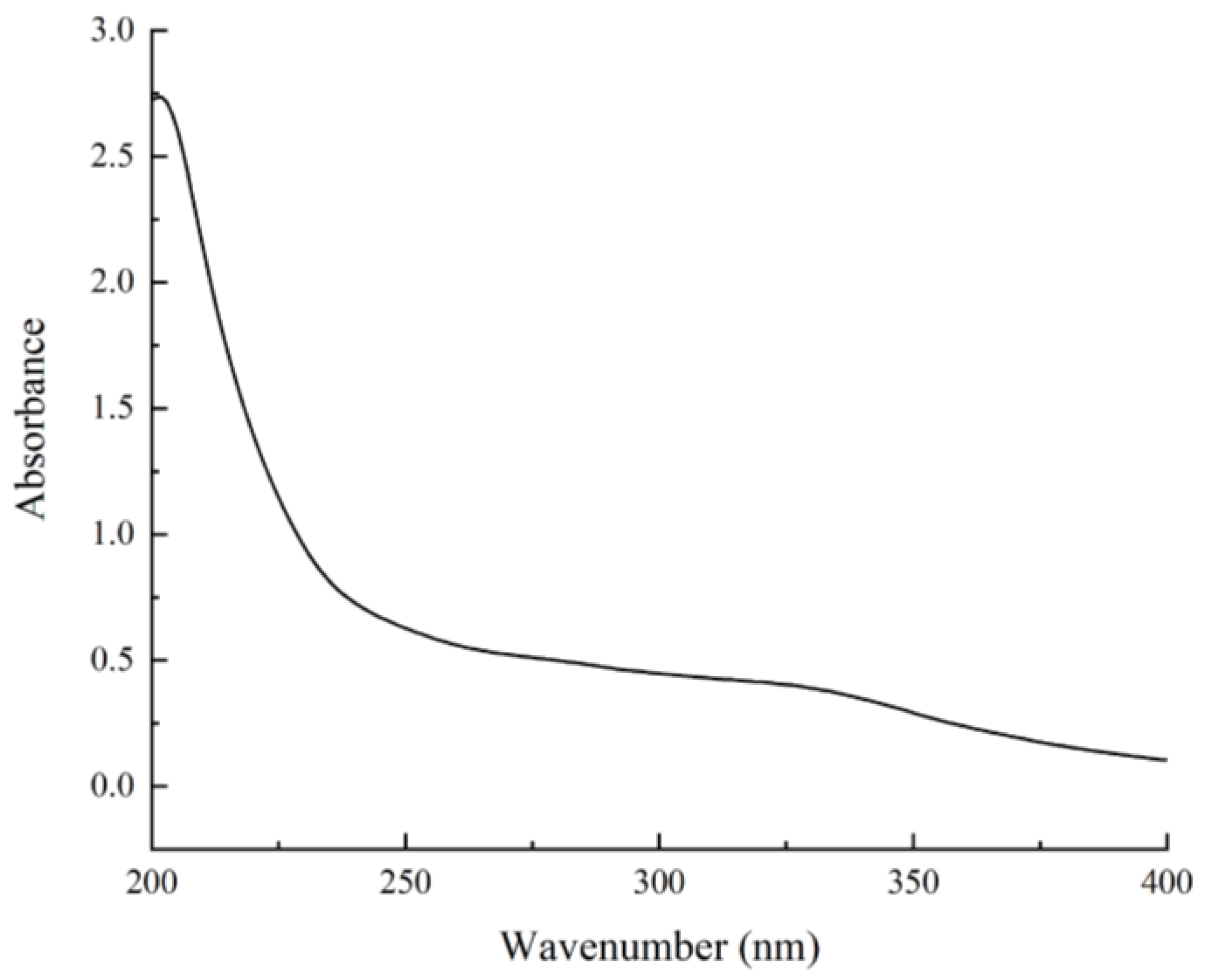
2.3.2. UV Spectroscopy Analysis
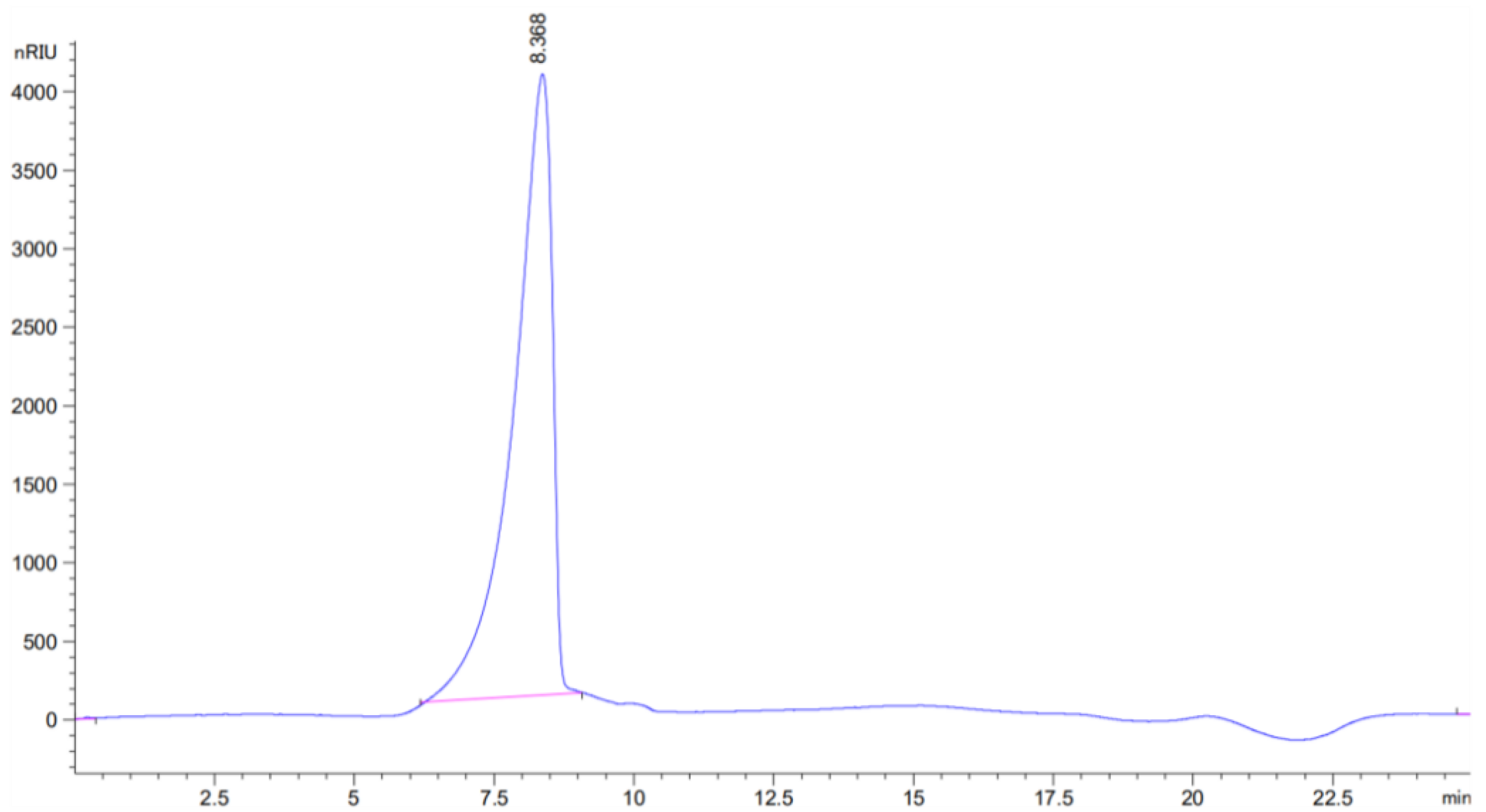
2.3.3. Molecular Weight Analysis by HPGP
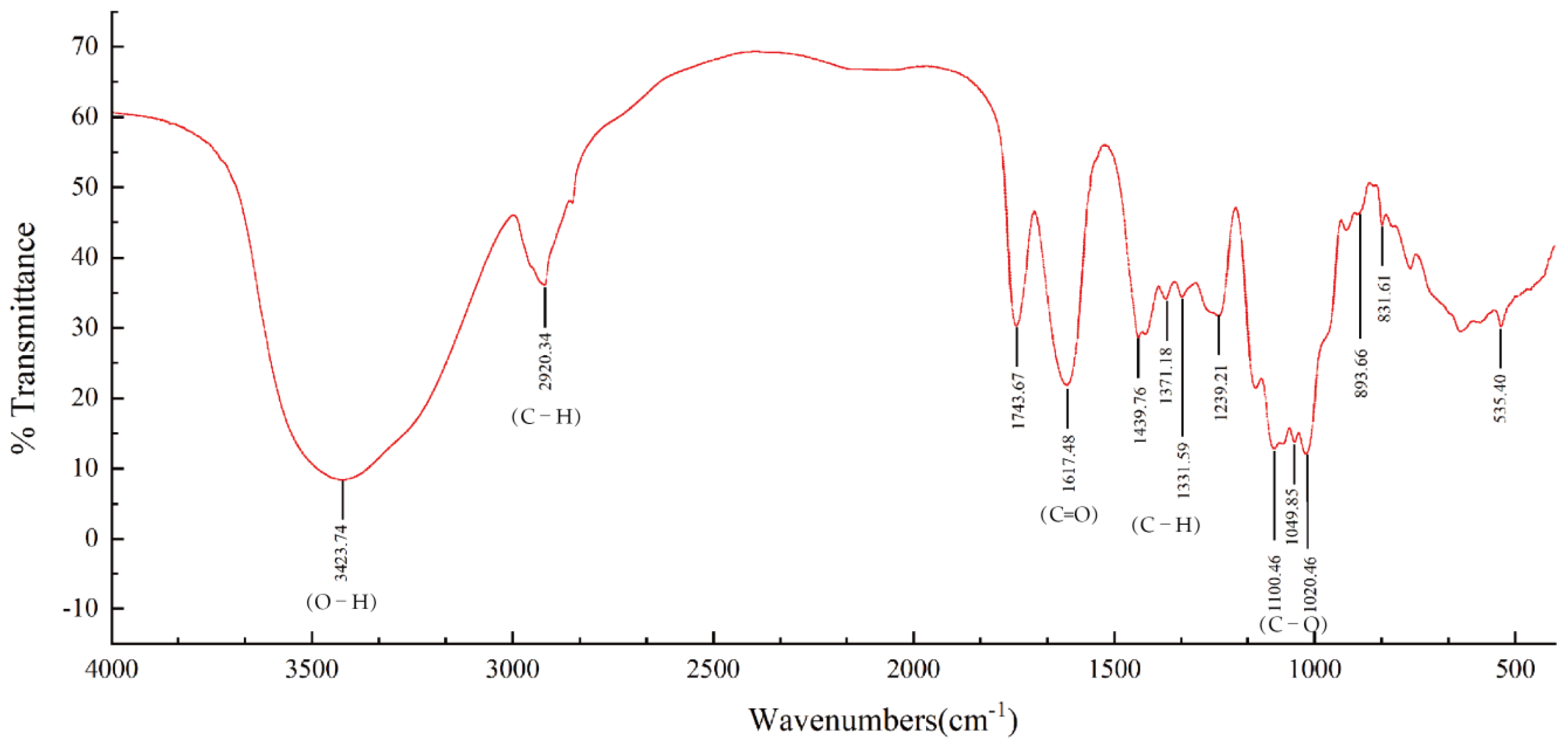
2.3.4. FT-IR Spectrum Analysis
2.3.5. Monosaccharide Composition Analysis by IC
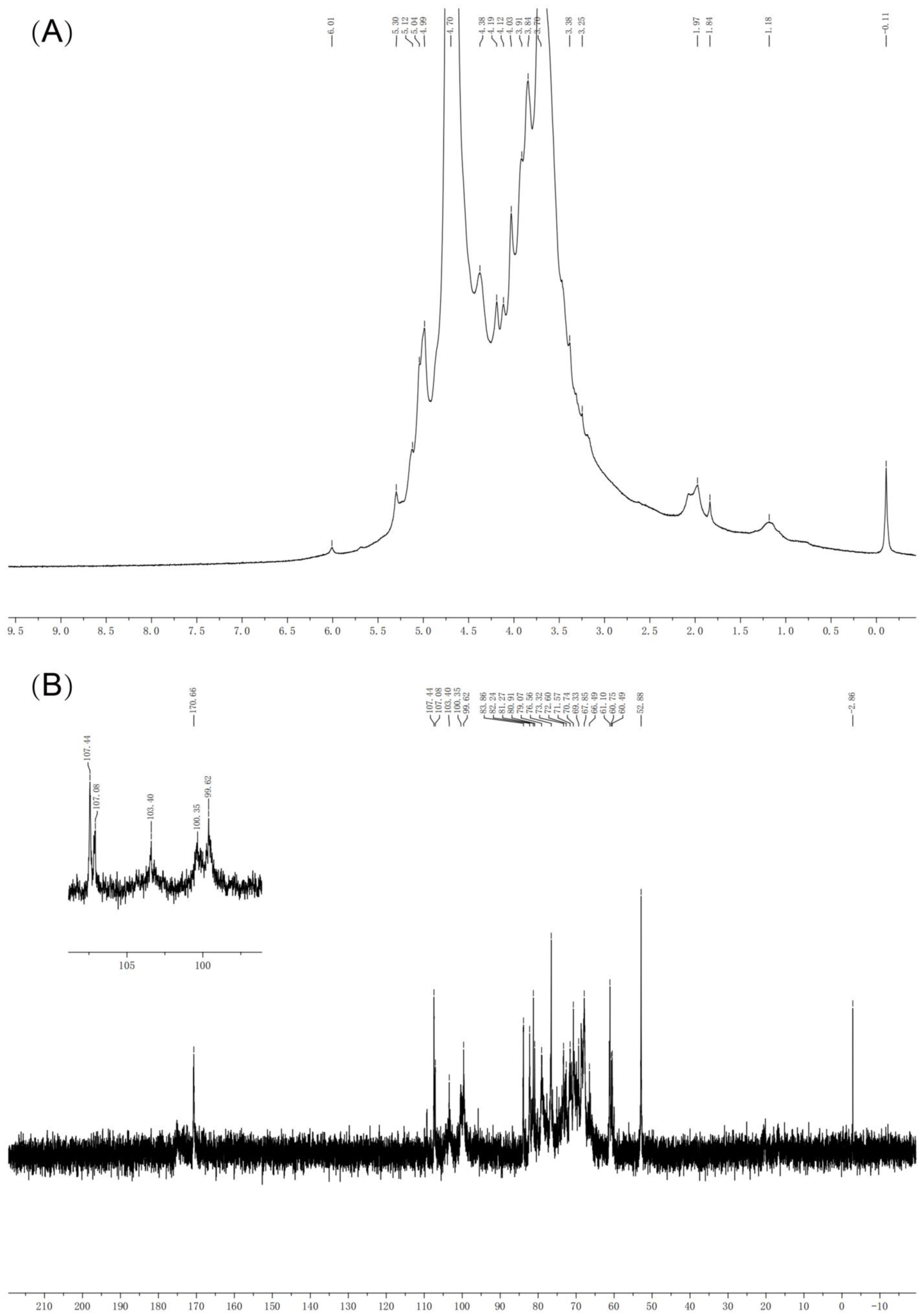
2.3.6. NMR Spectroscopy Analysis
2.3.7. Scanning Electron Microscopy Analysis
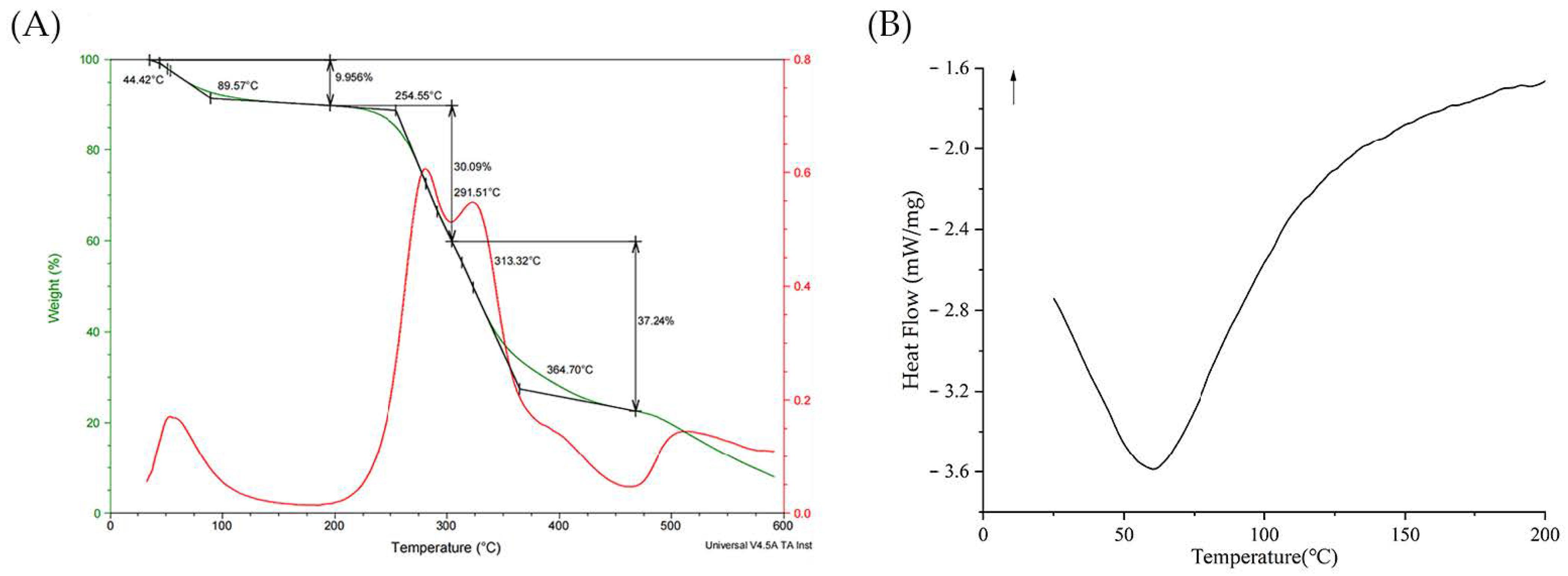
2.3.8. Thermal Analysis by TGA and DSC
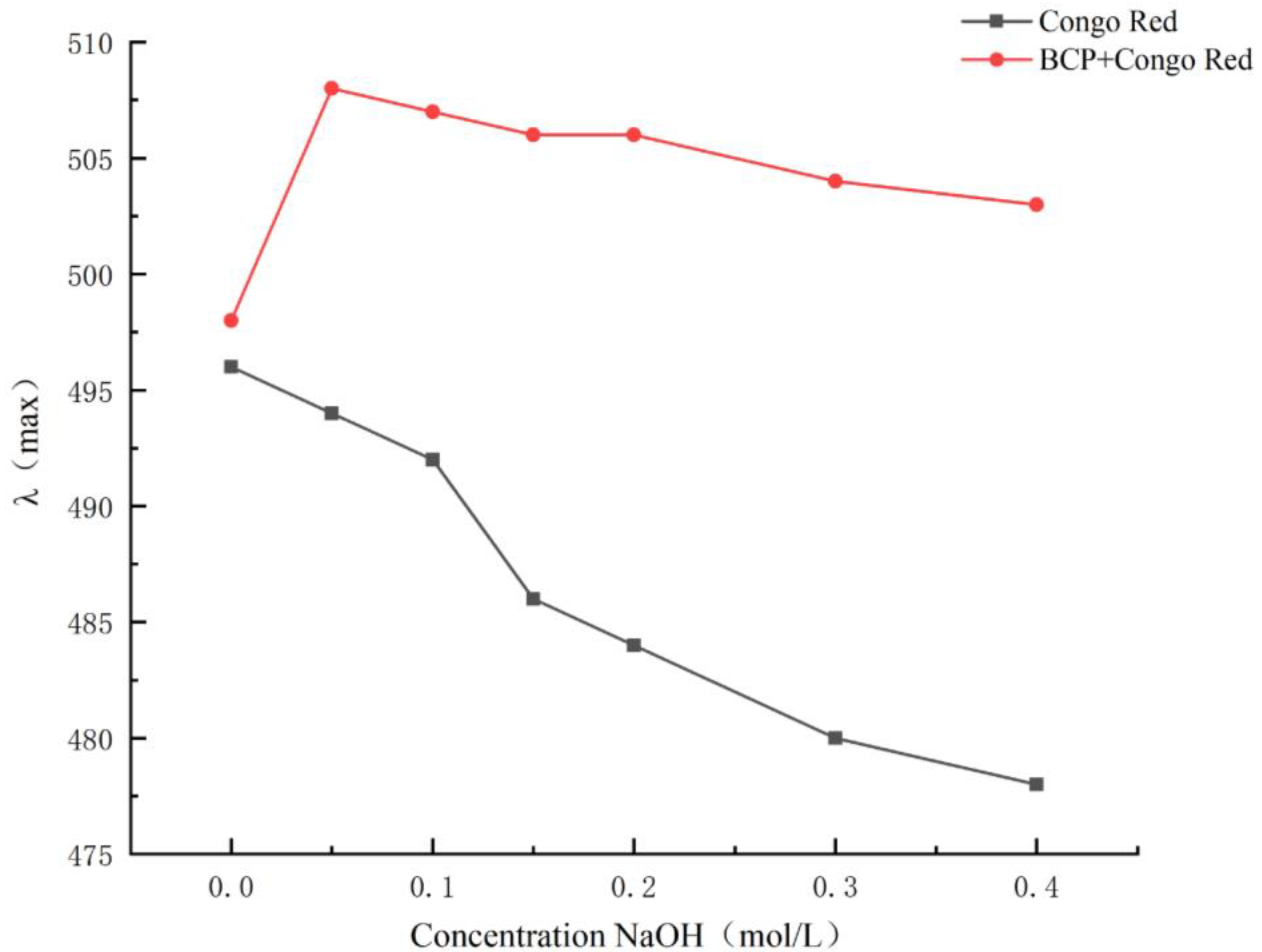
2.3.9. Congo Red Analysis
2.4. BCP Anti-Tumor Activity on H22 Tumor-Bearing Mice
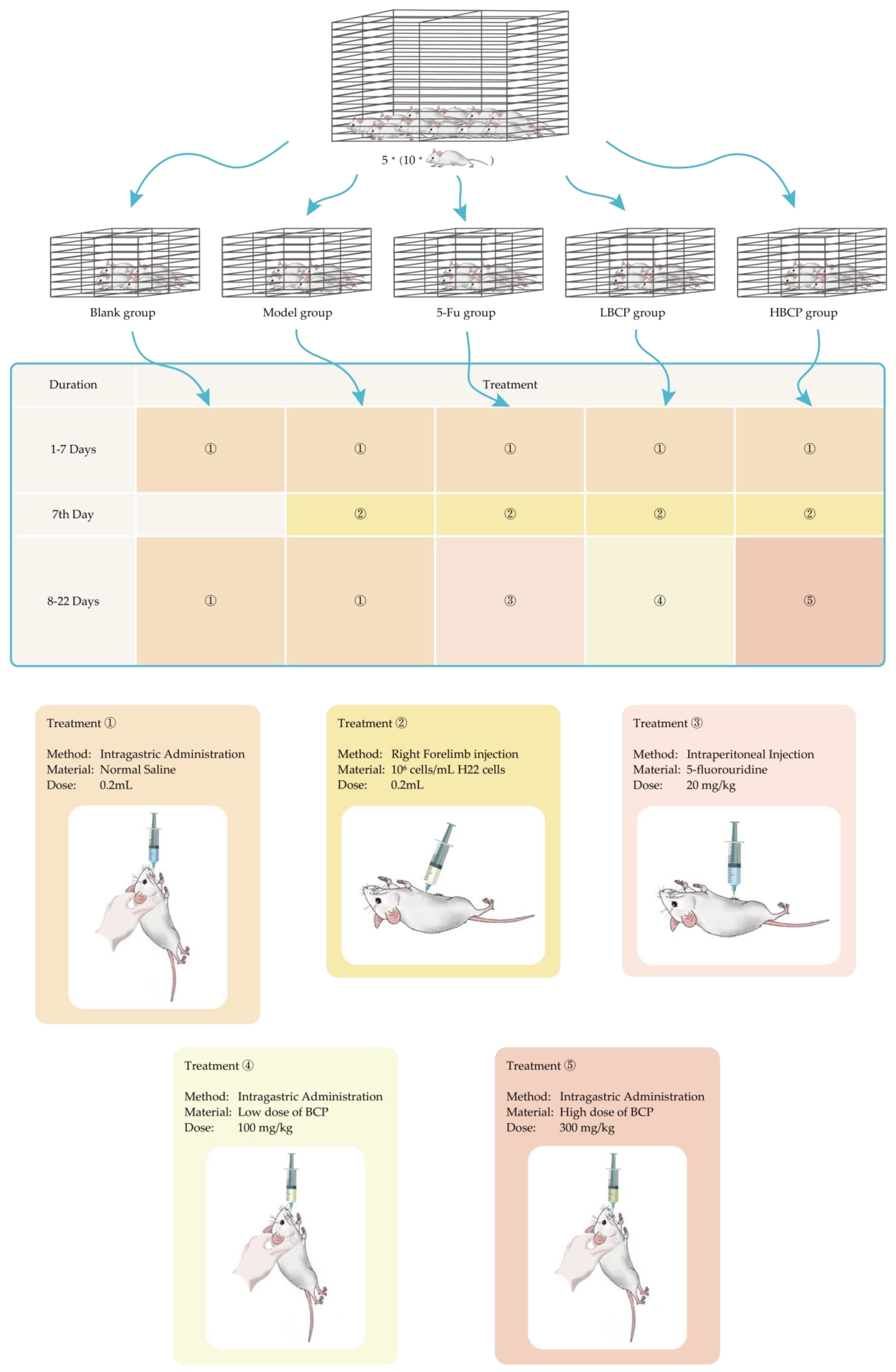
2.4.1. The Materials and Conditions of Animals Experimental
2.4.2. Establishment of H22 Tumor-Bearing Mouse Model
- Blank group;
- Model group;
- 5-Fu group: Injection of 5-fluorouracil as a positive control group (20 mg/kg);
- LBCP group: Low dose of BCP treatment group (100 mg/kg);
- HBCP group: High dose of BCP treatment group (300 mg/kg).
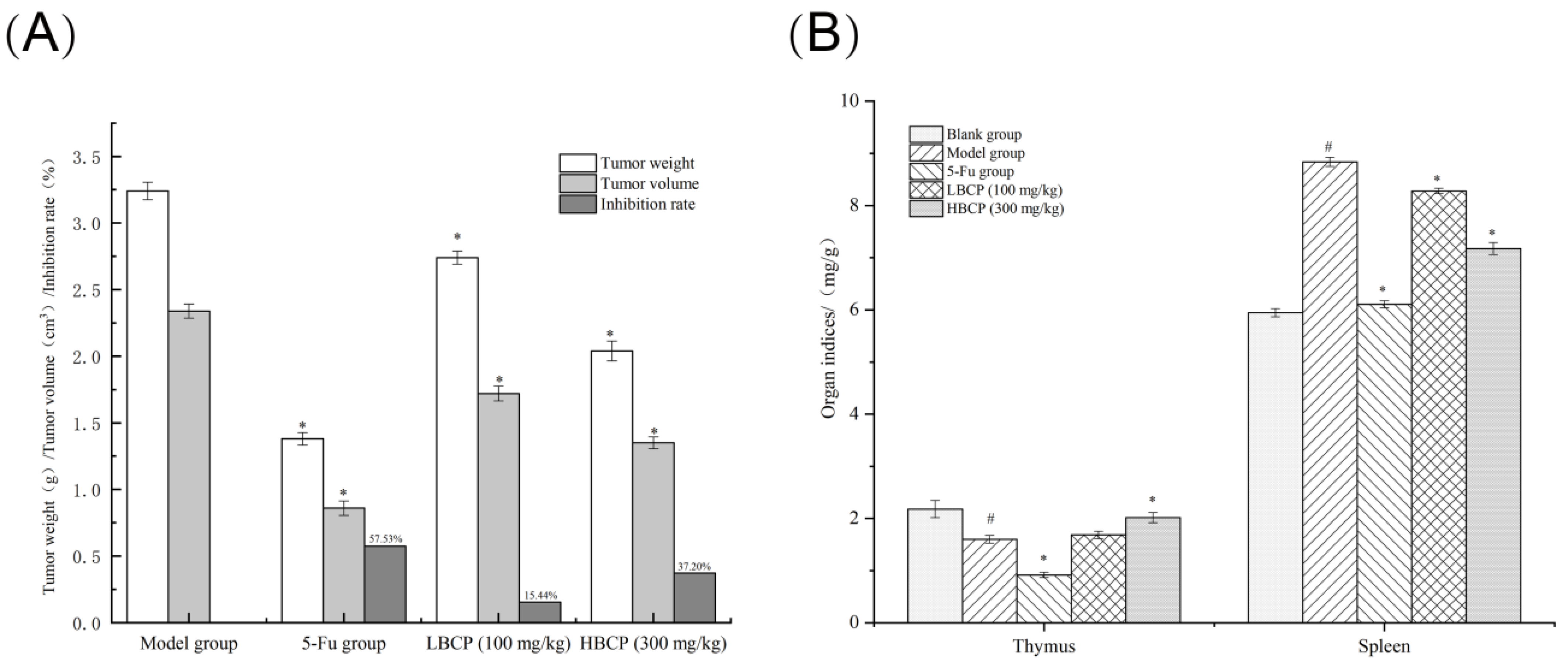
2.4.3. Solid Tumors and Immune Organ Indices
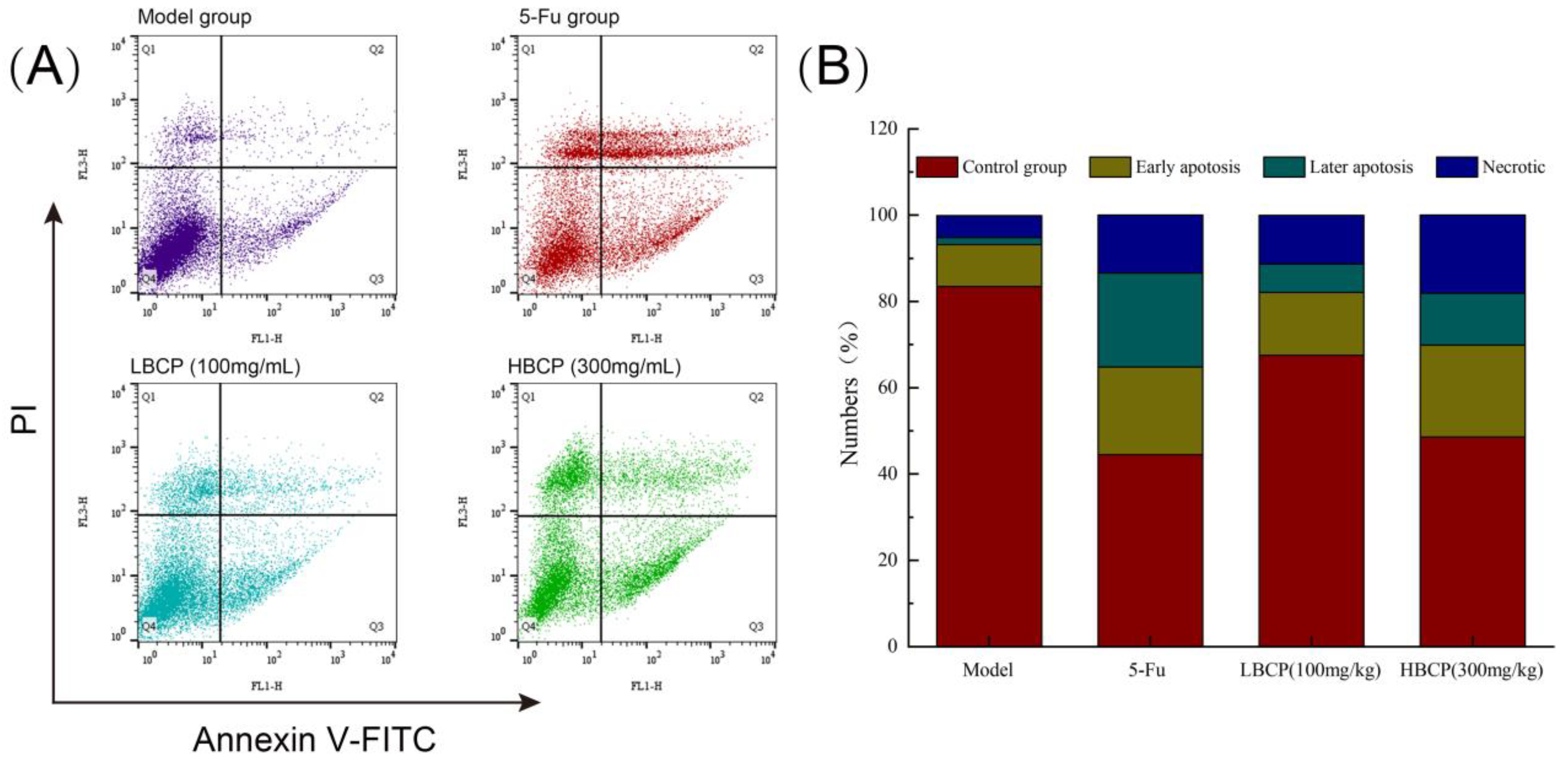
2.4.4. FITC-AnnexinV/PI Double Staining Detection
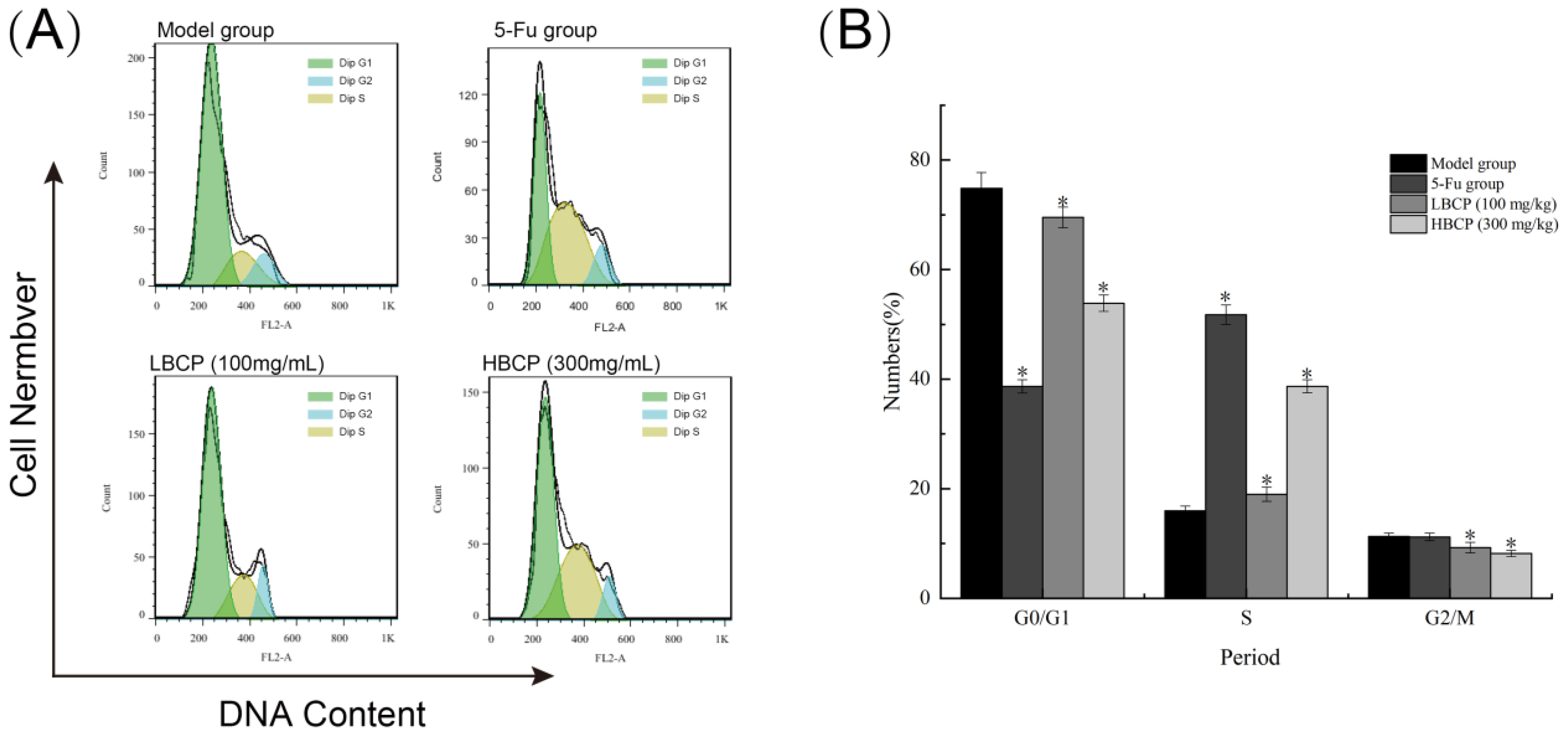
2.4.5. Cell Cycle Distribution Detection
2.4.6. Assay of Mitochondrial Membrane Potential (∆Ψm)
2.5. Statistical Analysis
3. Results
3.1. The Basic Chemical Components and UV-Visible Spectrum Analysis of BCP
3.2. HPGPC and FT-IR Analysis of BCP
3.3. Monosaccharide Composition Analysis of BCP
3.4. NMR Results of BCP
3.5. The Molecular Morphology of BCP
3.6. Thermal Analysis of BCP
3.7. Congo Red Analysis of BCP
3.8. Anti-Tumor Activities In Vivo of BCP on H22-bearing Mice
3.8.1. Weight, Immune Organ Indices, and Tumor Inhibition Rate
3.8.2. Cell Apoptosis Analysis by FITC-AnnexinV/PI
3.8.3. Cell Cycle Analysis
3.8.4. Mitochondrial Membrane Potential (MMP) Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Gingold, J.A.; Zhu, D.D.; Lee, D.F.; Kaseb, A.; Chen, J. Genomic Profiling and Metabolic Homeostasis in Primary Liver Cancers. Trends Mol. Med. 2018, 24, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Schultink, A.; Suleiman, A.A.; Schellens, J.H.M.; Beijnen, J.H.; Huitema, A.D.R. Pharmacodynamic modeling of adverse effects of anti-cancer drug treatment. Eur. J. Clin. Pharmacol. 2016, 72, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Jin, M.L.; Morris, G.A.; Zha, X.Q.; Chen, H.Q.; Yi, Y.; Li, J.E.; Wang, Z.J.; Gao, J.; Nie, S.P.; et al. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. S1), S60–S84. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Peng, Q.; Yuan, Y.; Shen, J.; Xie, X.; Wang, M. Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphus jujuba Mill.): A review. Food Chem. 2017, 227, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Zong, A.; Cao, H.; Wang, F. Anticancer polysaccharides from natural resources: A review of recent research. Carbohydr. Polym. 2012, 90, 1395–1410. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Z.Y.; Liu, P.; Huang, J.; Algradi, A.M.; Pan, J.; Guan, W.; Zhou, Y.Y.; Yang, B.Y.; Kuang, H.X. New flavonoids from the aerial part of Bupleurum chinense DC. Fitoterapia 2020, 147, 104739. [Google Scholar] [CrossRef]
- Li, X.Q.; Song, A.H.; Li, W.; Chen, X.H.; Bi, K.S. Analysis of the fatty acid from Bupleurum chinense DC in China by GC-MS and GC-FID. Chem. Pharm. Bull. 2005, 53, 1613–1617. [Google Scholar] [CrossRef]
- Tong, H.B.; Zheng, X.L.; Song, J.X.; Liu, J.; Ren, T.; Zhang, X.; Huang, L.Q.; Wu, M.J. Radical scavenging activity of sulfated Bupleurum chinense polysaccharides and their effects against oxidative stress-induced senescence. Carbohydr. Polym. 2018, 192, 143–149. [Google Scholar] [CrossRef]
- Feng, Y.; Weng, H.; Ling, L.; Zeng, T.; Zhang, Y.; Chen, D.; Li, H. Modulating the gut microbiota and inflammation is involved in the effect of Bupleurum polysaccharides against diabetic nephropathy in mice. Int. J. Biol. Macromol. 2019, 132, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Feng, K.; Jiang, R.; Chen, J.; Zhao, Y.; Ma, R.; Tong, H. Water-soluble polysaccharide from Bupleurum chinense DC: Isolation, structural features and antioxidant activity. Carbohydr. Polym. 2010, 79, 180–183. [Google Scholar] [CrossRef]
- Wang, Y.X.; Liu, Q.Y.; Zhang, M.; Yang, Z.; Pei, X.; Wu, X.; Chen, X.; Hong, J.; Xu, K.Z. Polysaccharides from Bupleurum Induce Immune Reversal in Late Sepsis. Shock 2018, 49, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-D.; Li, H.; Wan, F.; Su, X.-Y.; Lu, Y.; Chen, D.-F.; Zhang, Y.-Y. Polysaccharides extracted from the roots of Bupleurum chinense DC modulates macrophage functions. Chin. J. Nat. Med. 2017, 15, 889–898. [Google Scholar] [CrossRef]
- Song, X.; Ren, T.; Zheng, Z.; Lu, T.; Wang, Z.; Du, F.; Tong, H. Anti-tumor and immunomodulatory activities induced by an alkali-extracted polysaccharide BCAP-1 from Bupleurum chinense via NF-kappaB signaling pathway. Int. J. Biol. Macromol. 2017, 95, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Jiang, G.; Qi, D.; Bi, J.; Tian, D.; Guan, X.; Zheng, S.; Sun, X. Bupleurum chinense polysaccharide inhibit adhesion of human melanoma cells via blocking beta1 integrin function. Carbohydr. Polym. 2017, 156, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, J.J.; Yue, S.Q.; Zhang, L.Y.; Dou, K.F. Antioxidant activity and hepatoprotective effect of a polysaccharide from Bei Chaihu (Bupleurum chinense DC). Carbohydr. Polym. 2012, 89, 448–452. [Google Scholar] [CrossRef]
- Xie, J.Y.; Di, H.Y.; Li, H.; Cheng, X.Q.; Zhang, Y.Y.; Chen, D.F. Bupleurum chinense DC polysaccharides attenuates lipopolysaccharide-induced acute lung injury in mice. Phytomedicine 2012, 19, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Sun, X.B.; Hanawa, T.; Kodaira, H.; Ishii, K.; Yamada, H. Effect of the antiulcer polysaccharide fraction from Bupleurum falcatum L. on the healing of gastric ulcer induced by acetic acid in rats. Phytother. Res. 2002, 16, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ji, H.Y.; Liu, C.; Liu, A.J. The structural characteristics of an acid-soluble polysaccharide from Grifola frondosa and its antitumor effects on H22-bearing mice. Int. J. Biol. Macromol. 2020, 158, 1288–1298. [Google Scholar] [CrossRef]
- Chen, X.; Ji, H.; Zhang, C.; Yu, J.; Liu, A. Structural characterization and antitumor activity of a novel polysaccharide from Grifola frondosa. J. Food Meas. Charact. 2019, 14, 272–282. [Google Scholar] [CrossRef]
- Benson, J.D.; Chen, Y.N.; Cornell-Kennon, S.A.; Dorsch, M.; Kim, S.; Leszczyniecka, M.; Sellers, W.R.; Lengauer, C. Validating cancer drug targets. Nature 2006, 441, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhu, L.Y.; Yang, B.S.; Shi, L.J.; Liu, Y.; Jiang, A.M.; Zhao, L.L.; Song, G.; Liu, T.F. Antitumor and immunomodulating activity of a polysaccharide from Sophora flavescens Ait. Int. J. Biol. Macromol. 2012, 51, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, X.; Xue, Y.; Wang, N.; Liu, W. Anti-hepatoma activity and mechanism of corn silk polysaccharides in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2014, 64, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, W.; Song, W.; Chen, H.; Teng, A.; Liu, A. Partial characterization and anti-tumor activity of an acidic polysaccharide from Gracilaria lemaneiformis. Carbohydr. Polym. 2012, 88, 1313–1318. [Google Scholar] [CrossRef]
- Riemann, M.; Andreas, N.; Fedoseeva, M.; Meier, E.; Weih, D.; Freytag, H.; Schmidt-Ullrich, R.; Klein, U.; Wang, Z.Q.; Weih, F. Central immune tolerance depends on crosstalk between the classical and alternative NF-kappaB pathways in medullary thymic epithelial cells. J. Autoimmun. 2017, 81, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; He, M.L.; Gu, C.K.; Wei, D.; Liang, Y.Q.; Yan, J.M.; Wang, C.H. Extraction Optimization, Purification, Antioxidant Activity, and Preliminary Structural Characterization of Crude Polysaccharide from an Arctic Chlorella sp. Polymers 2018, 10, 292. [Google Scholar] [CrossRef]
- Kong, L.S.; Yu, L.; Feng, T.; Yin, X.J.; Liu, T.J.; Dong, L. Physicochemical characterization of the polysaccharide from Bletilla striata: Effect of drying method. Carbohydr. Polym. 2015, 125, 1–8. [Google Scholar] [CrossRef]
- Borovkova, V.S.; Malyar, Y.N.; Sudakova, I.G.; Chudina, A.I.; Skripnikov, A.M.; Fetisova, O.Y.; Kazachenko, A.S.; Miroshnikova, A.V.; Zimonin, D.V.; Ionin, V.A.; et al. Molecular Characteristics and Antioxidant Activity of Spruce (Picea abies) Hemicelluloses Isolated by Catalytic Oxidative Delignification. Molecules 2022, 27, 266. [Google Scholar] [CrossRef]
- Li, H.; Gu, L.; Zhong, Y.; Chen, Y.; Zhang, L.; Zhang, A.R.; Sobol, R.W.; Chen, T.; Li, J. Administration of polysaccharide from Panax notoginseng prolonged the survival of H22 tumor-bearing mice. Onco Targets Ther. 2016, 9, 3433–3441. [Google Scholar]
- Nair, J.J.; van Staden, J. Cell cycle modulatory effects of Amaryllidaceae alkaloids. Life Sci. 2018, 213, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, J.J.; Zhu, J.H.; Huang, C.H.; Bi, S.X.; Song, L.Y.; Hu, X.J.; Yu, R.M. Structural characterization and immunomodulatory activity of a novel polysaccharide from Ficus carica. Food Funct. 2018, 9, 3930–3943. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T. Retention behavior in ion chromatography using stationary phases modified with ionic polysaccharides. Bunseki Kagaku 1999, 48, 69–75. [Google Scholar] [CrossRef][Green Version]
- Kazachenko, A.S.; Malyar, Y.N.; Vasilyeva, N.Y.; Fetisova, O.Y.; Chudina, A.I.; Sudakova, I.G.; Antonov, A.V.; Borovkova, V.S.; Kuznetsova, S.A. Isolation and sulfation of galactoglucomannan from larch wood (Larix sibirica). Wood Sci. Technol. 2021, 55, 1091–1107. [Google Scholar] [CrossRef]
- Jing, Y.S.; Zhang, R.J.; Wu, L.F.; Zhang, D.S.; Zheng, Y.G. Structural characteristics and antioxidant activity of polysaccharide-iron complex from Glehniae Radix. Int. J. Food Prop. 2020, 23, 894–907. [Google Scholar] [CrossRef]
- Arab, K.; Ghanbarzadeh, B.; Ayaseh, A.; Jahanbin, K. Extraction, purification, physicochemical properties and antioxidant activity of a new polysaccharide from Ocimum album L. seed. Int. J. Biol. Macromol. 2021, 180, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Kang, J.; Xu, Z.Y.; Guo, Q.B.; Zhang, L.F.; Ning, H.F.; Cui, S.W. Triple-helix polysaccharides: Formation mechanisms and analytical methods. Carbohydr. Polym. 2021, 262, 117962. [Google Scholar] [CrossRef] [PubMed]
- Semedo, M.C.; Karmali, A.; Fonseca, L. A high throughput colorimetric assay of beta-1,3-D-glucans by Congo red dye. J. Microbiol. Methods 2015, 109, 140–148. [Google Scholar] [CrossRef]
- Liu, J.Y.; Hou, X.X.; Li, Z.Y.; Shan, S.H.; Chang, M.C.; Feng, C.P.; Wei, Y. Isolation and structural characterization of a novel polysaccharide from Hericium erinaceus fruiting bodies and its arrest of cell cycle at S-phage in colon cancer cells. Int. J. Biol. Macromol. 2020, 157, 288–295. [Google Scholar] [CrossRef]
- Han, X.; Liu, A.J.; Zhao, X.H.; Li, Y.D.; Zheng, G.Q.; Zhang, G.R. Immunizing effects of cocultures of H22 hepatocarcinoma cells and cartilage polysaccharide on murine H22 hepatocarcinoma. J. Food Sci. 2010, 75, H265–H273. [Google Scholar] [CrossRef]
- Chen, D.; Sun, S.; Cai, D.; Kong, G. Induction of mitochondrial-dependent apoptosis in T24 cells by a selenium (Se)-containing polysaccharide from Ginkgo biloba L. leaves. Int. J. Biol. Macromol. 2017, 101, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Di, H.Y.; Zhang, Y.Y.; Chen, D.F. An anti-complementary polysaccharide from the roots of Bupleurum chinense. Int. J. Biol. Macromol. 2013, 58, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Di, H.Y.; Zhang, Y.Y.; Chen, D.F. Isolation of an anti-complementary polysaccharide from the root of Bupleurum chinense and identification of its targets in complement activation cascade. Chin. J. Nat. Med. 2013, 11, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Xu, J.L.; Wang, X.; Lu, J.A.O.; Chan, M.K.; Zhou, Y.F.; Sun, L. Structural Characterization of Pectic Polysaccharides from Bupleurum chinense DC. Nat. Prod. Commun. 2020, 15, 1934578X20931654. [Google Scholar] [CrossRef]
- Zhao, Y.; Wan, P.; Wang, J.Q.; Li, P.; Hu, Q.H.; Zhao, R.Z. Polysaccharide from vinegar baked radix bupleuri as efficient solubilizer for water-insoluble drugs of Chinese medicine. Carbohydr. Polym. 2020, 229, 115473. [Google Scholar] [CrossRef]
- Tong, H.B.; Wu, S.Y.; Song, K.X.; Liu, J.; Song, X.D.; Zhang, X.; Huang, L.Q.; Wu, M.J. Characterization of a P-selectin-binding moiety from Bupleurum chinense polysaccharide and its antagonistic effect against P-selectin-mediated function. Carbohydr. Polym. 2018, 196, 110–116. [Google Scholar] [CrossRef]
- Chen, P.; Liu, H.P.; Ji, H.H.; Sun, N.X.; Feng, Y.Y. A cold-water soluble polysaccharide isolated from Grifola frondosa induces the apoptosis of HepG2 cells through mitochondrial passway. Int. J. Biol. Macromol. 2019, 125, 1232–1241. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, L.; Hou, A.J.; Zhang, J.X.; Wang, S.; Man, W.J.; Zheng, S.W.; Yu, H.; Wang, X.J.; Yang, B.Y.; et al. Botany, traditional uses, phytochemistry, analytical methods, processing, pharmacology and pharmacokinetics of Bupleuri Radix: A systematic review. Biomed. Pharmacother. 2020, 131, 110679. [Google Scholar] [CrossRef]
- Dang, Z.; Li, Q.; Sun, S.; Wang, Y.; Lin, R.; Zhang, Y.; Dai, J.; Zheng, N. The Medicinal Plant Pair Bupleurum chinense-Scutellaria baicalensis-Metabolomics and Metallomics Analysis in a Model for Alcoholic Liver Injury. Front. Pharmacol. 2019, 10, 254. [Google Scholar] [CrossRef]
- Wang, Z.B.; Liu, Y.Q.; Cui, Y.F. Pathways to caspase activation. Cell Biol. Int. 2005, 29, 489–496. [Google Scholar] [CrossRef]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Lyu, F.Z.; Xu, X.J.; Zhang, L.N. Recent Advances in Chain Conformation and Bioactivities of Triple-Helix Polysaccharides. Biomacromolecules 2020, 21, 1653–1677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tian, M.; Row, K.H. Optimized Extraction Conditions of Polysaccharides from Different Plants. Asian J. Chem. 2013, 25, 9679–9682. [Google Scholar] [CrossRef]
- Li, S.; Wang, A.; Liu, L.; Tian, G.; Xu, F. Effect of deproteinization methods on the antioxidant activity of polysaccharides extracted from Lentinus edodes stipe. J. Food Meas. Charact. 2019, 13, 1382–1389. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Wang, X.; Zhang, L.; Cheung, P.C.K. Advances in lentinan: Isolation, structure, chain conformation and bioactivities. Food Hydrocoll. 2011, 25, 196–206. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.; Lee, Y.C. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef]
- Wang, Y.G.; Li, Y.L.; Ma, X.Q.; Ren, H.W.; Fan, W.G.; Leng, F.F.; Yang, M.J.; Wang, X.L. Extraction, purification, and bioactivities analyses of polysaccharides from Glycyrrhiza uralensis. Ind. Crops Prod. 2018, 122, 596–608. [Google Scholar] [CrossRef]
- Guo, M.Z.; Meng, M.; Duan, S.Q.; Feng, C.C.; Wang, C.L. Structure characterization, physicochemical property and immunomodulatory activity on RAW264.7 cells of a novel triple-helix polysaccharide from Craterellus cornucopioides. Int. J. Biol. Macromol. 2019, 126, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Nawrocka, A.; Szymanska-Chargot, M.; Mis, A.; Wilczewska, A.Z.; Markiewicz, K.H. Effect of dietary fibre polysaccharides on structure and thermal properties of gluten proteins-A study on gluten dough with application of FT-Raman spectroscopy, TGA and DSC. Food Hydrocoll. 2017, 69, 410–421. [Google Scholar] [CrossRef]
- Hou, F.; Wu, Y.W.; Kan, L.N.; Li, Q.; Xie, S.S.; Ouyang, J. Effects of Ultrasound on the Physicochemical Properties and Antioxidant Activities of Chestnut Polysaccharide. Int. J. Food Eng. 2016, 12, 439–449. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, Y.N.; Patil, M.P.; Cho, Y.J.; Kim, G.D.; Park, Y.B.; Woo, H.C.; Chun, B.S. Hydrothermal degradation of seaweed polysaccharide: Characterization and biological activities. Food Chem. 2018, 268, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Jiang, Z.; Sun, T.; Wang, C.; Chen, Y.Y.; Yang, Z.Y.; Du, B.; Liu, C.Y. Comparison of structural, antioxidant and immuno-stimulating activities of polysaccharides from Tremella fuciformis in two different regions of China. Int. J. Food Sci. Technol. 2018, 53, 1942–1953. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhang, L.N.; Xu, X.J. Morphologies and conformation transition of Lentinan in aqueous NaOH solution. Biopolymers 2004, 75, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.X.; Song, R.Z.; Cai, J.J.; Xu, J.J.; Tang, X.Z.; Li, N.Y. Ginger polysaccharides induced cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells. Int. J. Biol. Macromol. 2019, 123, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Chow, P.S.; Landhausser, S.M. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef]
- Xu, J.; Tan, Z.C.; Shen, Z.Y.; Shen, X.J.; Tang, S.M. Cordyceps cicadae polysaccharides inhibit human cervical cancer hela cells proliferation via apoptosis and cell cycle arrest. Food Chem. Toxicol. 2021, 148, 111971. [Google Scholar] [CrossRef]
- Brunelle, J.L.; Green, R. Coomassie blue staining. Methods Enzymol. 2014, 541, 161–167. [Google Scholar]
- Song, J.-M.; Im, J.-H.; Kang, J.-H.; Kang, D.-J. A simple method for hyaluronic acid quantification in culture broth. Carbohydr. Polym. 2009, 78, 633–634. [Google Scholar] [CrossRef]
- Zhao, P.; Li, X.; Wang, Y.; Yan, L.; Guo, L.; Huang, L.; Gao, W. Characterisation and saccharide mapping of polysaccharides from four common Polygonatum spp. Carbohydr. Polym. 2020, 233, 115836. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef]
- Coimbra, M.A.; Barros, A.; Barros, M.; Rutledge, D.N.; Delgadillo, I. Multivariate analysis of uronic acid and neutral sugars in whole pectic samples by FT-IR spectroscopy. Carbohydr. Polym. 1998, 37, 241–248. [Google Scholar] [CrossRef]
- Ji, X.; Liu, F.; Peng, Q.; Wang, M. Purification, structural characterization, and hypolipidemic effects of a neutral polysaccharide from Ziziphus Jujuba cv. Muzao. Food Chem. 2018, 245, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Singthong, J.; Cui, S.; Ningsanond, S.; Douglasgoff, H. Structural characterization, degree of esterification and some gelling properties of Krueo Ma Noy (Cissampelos pareira) pectin. Carbohydr. Polym. 2004, 58, 391–400. [Google Scholar] [CrossRef]
- Chen, H.X.; Zhang, M.; Xie, B.J. Quantification of uronic acids in tea polysaccharide conjugates and their antioxidant properties. J. Agric. Food Chem. 2004, 52, 3333–3336. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.H.; Liu, Y.; Wu, X.L.; Liu, L.Z.; Fu, W.; Song, D.D. Isolation, purification, characterization and immunostimulatory activity of polysaccharides derived from American ginseng. Carbohydr. Polym. 2017, 156, 9–18. [Google Scholar] [CrossRef]
- Gu, S.; Sun, H.; Zhang, X.; Huang, F.; Pan, L.; Zhu, Z. Structural characterization and inhibitions on alpha-glucosidase and alpha-amylase of alkali-extracted water-soluble polysaccharide from Annona squamosa residue. Int. J. Biol. Macromol. 2021, 166, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, H.; Ma, L.; Liu, A. Polysaccharides from the peels of Citrus aurantifolia induce apoptosis in transplanted H22 cells in mice. Int. J. Biol. Macromol. 2017, 101, 680–689. [Google Scholar] [CrossRef]
- Tian, W.T.; Zhang, X.W.; Liu, H.P.; Wen, Y.H.; Li, H.R.; Gao, J. Structural characterization of an acid polysaccharide from Pinellia ternata and its induction effect on apoptosis of Hep G2 cells. Int. J. Biol. Macromol. 2020, 153, 451–460. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Zhu, Z.Y.; Sun, H.Q.; Chen, L.J. Structural characterization and inhibition on alpha-glucosidase activity of acidic polysaccharide from Annona squamosa. Carbohydr. Polym. 2017, 174, 1–12. [Google Scholar] [CrossRef]
- Wang, Q.; Niu, L.L.; Liu, H.P.; Wu, Y.R.; Li, M.Y.; Jia, Q. Structural characterization of a novel polysaccharide from Pleurotus citrinopileatus and its antitumor activity on H22 tumor-bearing mice. Int. J. Biol. Macromol. 2021, 168, 251–260. [Google Scholar] [CrossRef]
- Su, Y.; Li, L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr. Polym. 2020, 229, 115407. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Prasad, K.N.; Jiang, Y. Structure identification of a polysaccharide purified from litchi (Litchi chinensis Sonn.) pulp. Carbohydr. Polym. 2016, 137, 570–575. [Google Scholar] [CrossRef] [PubMed]




| Group | Blank | Model | 5-Fu | LBCP | HBCP |
|---|---|---|---|---|---|
| Death Date (d) | - | 20 | 12, 18 | 9 | - |
| Number of deaths | 0 | 1 | 2 | 1 | 0 |
| Survival Rate (%) | 100 | 90 | 80 | 90 | 100 |
| Group | Dose (mg/kg) | Body Weight (g) | Number of Mice Start/End | |||
|---|---|---|---|---|---|---|
| 0 | 7 Days | 14 Days | 21 Days | |||
| Blank | 0 | 21.98 ± 0.63 | 26.89 ± 0.69 | 30.06 ± 0.39 | 33.10 ± 0.35 | 10/10 |
| Model | 0 | 22.02 ± 0.28 | 27.11 ± 0.53 | 29.17 ± 0.30 # | 30.54 ± 0.48 # | 10/9 |
| 5-Fu | 20 | 21.91 ± 0.45 | 26.97 ± 1.76 | 26.49 ± 0.51 * | 25.20 ± 0.33 * | 10/8 |
| LBCP | 100 | 21.90 ± 0.48 | 26.59 ± 0.82 | 29.82 ± 1.67 | 31.97 ± 0.54 * | 10/9 |
| HBCP | 300 | 22.06 ± 0.63 | 26.68 ± 0.42 | 29.36 ± 1.19 | 31.05 ± 0.57 * | 10/10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, S.; Chang, M.; Liu, H.; Ding, S.; Yan, Z.; Si, K.; Gong, T. The Structural Characteristics of an Acidic Water-Soluble Polysaccharide from Bupleurum chinense DC and Its In Vivo Anti-Tumor Activity on H22 Tumor-Bearing Mice. Polymers 2022, 14, 1119. https://doi.org/10.3390/polym14061119
Shi S, Chang M, Liu H, Ding S, Yan Z, Si K, Gong T. The Structural Characteristics of an Acidic Water-Soluble Polysaccharide from Bupleurum chinense DC and Its In Vivo Anti-Tumor Activity on H22 Tumor-Bearing Mice. Polymers. 2022; 14(6):1119. https://doi.org/10.3390/polym14061119
Chicago/Turabian StyleShi, Shuyuan, Mengli Chang, Huiping Liu, Suyun Ding, Zhiqian Yan, Kai Si, and Tingting Gong. 2022. "The Structural Characteristics of an Acidic Water-Soluble Polysaccharide from Bupleurum chinense DC and Its In Vivo Anti-Tumor Activity on H22 Tumor-Bearing Mice" Polymers 14, no. 6: 1119. https://doi.org/10.3390/polym14061119
APA StyleShi, S., Chang, M., Liu, H., Ding, S., Yan, Z., Si, K., & Gong, T. (2022). The Structural Characteristics of an Acidic Water-Soluble Polysaccharide from Bupleurum chinense DC and Its In Vivo Anti-Tumor Activity on H22 Tumor-Bearing Mice. Polymers, 14(6), 1119. https://doi.org/10.3390/polym14061119







