Current Research and Applications of Starch-Based Biodegradable Films for Food Packaging
Abstract
1. Introduction
2. Starch as a Biodegradable Packaging Material
2.1. Sources of Starch
2.2. Effects of Starch as a Biodegradable Film
2.3. Production and Processing of Starch
2.4. Extraction of Starch
2.5. Previous Reviews on the Application of Starch-Based Biodegradable Material
2.6. Application of Starch-Based Nanomaterials
3. Evaluation of the Shelf Life of Foods
Types of Shelf Life Evaluation and Design
4. Challenges Facing Starch-Based Biodegradable Films for Food Packaging
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sadeghizadeh-Yazdi, J.; Habibi, M.; Kamali, A.A.; Banaei, M. Application of edible and biodegradable starch-based films in food packaging: A systematic review and meta-analysis. Curr. Res. Nutr. Food Sci. 2019, 7, 624–637. [Google Scholar] [CrossRef]
- Making Plastic Environmental Friendly; EPI Environmental Products Inc Vancouver Canada. 2014. Available online: https://www.foresightusa.com/single-post/2014/11/04/biodegradable-plastics-let-s-clear-up-the-confusion (accessed on 28 January 2022).
- Sorrentino, A.; Gorrasi, G.; Vittoria, V. Potential perspectives of bio nanocomposites for food packaging applications. Trends Food Sci. Technol. 2007, 18, 84–95. [Google Scholar] [CrossRef]
- Xu, Y.X.; Kim, K.M.; Hanna, M.A.; Nag, D. Chitosane starch composite film: Preparation and characterization. Ind. Crops Process 2005, 21, 185–192. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics: The Facts 2015. An Analysis of European Plastics Production, Demand and Waste Data; Plastics Europe: Brussel, Belgium, 2015. [Google Scholar]
- Kalia, V.C.; Raizada, N.; Sonakya, V. Bioplastics. Sci. Ind. Res. 2000, 59, 433–445. [Google Scholar]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and Biodegradable Starch Films: A Review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Galus, S.; Kadzi_nska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Malathi, A.N.; Santhosh, K.S.; Nidoni, U. Recent trends of biodegradable polymer: Biodegradable films for food packaging and application of Nanoparticles in biodegradable food packaging. Curr. Trends Technol. Sci. 2014, 3, 73–79. [Google Scholar]
- Issa, A.; Ibrahim, S.A.; Tahergorabi, R. Sweet potato starch/clay nanocomposite: New material for emerging biodegradable food packaging. MOJ Food Process Technol. 2016, 3, 313–315. [Google Scholar]
- Primožič, M.; Knez, Ž.; Leitgeb, M. (Bio)Nanotechnology in Food Science—Food Packaging. Nanomaterials 2021, 11, 292. [Google Scholar] [CrossRef]
- Huang, Y.; Mei, L.; Chen, X.; Wang, Q. Recent Developments in Food Packaging Based on Nanomaterials. Nanomaterials 2018, 8, 830. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Agyepong, J.K.; Barimah, J. Physiochemical properties of starches extracted from local cassava varieties with the aid of crude pectolytc enzymes from Saccharomyces cerevisiae (ATCC 52712). Afr. J. Food Sci. 2018, 12, 151–164. [Google Scholar]
- Altemimi, A.B. Extraction and optimization of potato starch and its application as a stabilizer in yogurt manufacturing. Foods 2018, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Ramírez, Y.I.; Martínez-Cruz, O.; Del Toro-Sánchez, C.L.; Wong-Corral, F.J.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J. The structural characteristics of starches and their functional properties. CyTA-J. Food 2018, 16, 1003–1017. [Google Scholar] [CrossRef]
- Horstmann, S.W.; Lynch, K.M.; Arendt, E.K. Starch Characteristics Linked to Gluten-Free Products. Foods 2017, 6, 29. [Google Scholar] [CrossRef]
- Kolybaba, M.; Tabil, L.; Panigrahi, S.; Crerar, W.; Powell, T.; Wang, B. Biodegradable Polymers: Past, Present, and Future; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2006. [Google Scholar]
- Pelissari, F.M.; Grossmann, M.V.E.; Yamashita, F. Antimicrobial, mechanical, and barrier properties of cassava starch–chitosan films incorporated with oregano essential oil. J. Agric. Food Chem. 2009, 57, 7499–7504. [Google Scholar] [CrossRef]
- Flieger, M.; Kantorova, M.; Prell, A.; Rezanka, T. Biodegradable plastics from renewable sources. Folia Microbiol. 2003, 48, 27–44. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Nassiri, R.; Sheibani, S. Preparation and characterization of bio nanocomposite films filled with Nano rod-rich zinc oxide. Carbohydr. Polym. 2013, 96, 233–239. [Google Scholar] [CrossRef]
- Park, H.W.; Lee, W.K.; Park, C.Y. Environmentally friendly polymer hybrids: Part, I. Mechanical, thermal, and barrier properties of thermoplastic starch/clay nanocomposites. J. Mater. Sci. 2003, 38, 909–915. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Debeaufort, F.; Karbowiak, T. Bioactive edible films for food applications: Mechanisms of antimicrobial and antioxidant activity. Crit. Rev. Food Sci. Nutr. 2019, 59, 3431–3455. [Google Scholar] [CrossRef]
- Han, J.H. Edible films and coatings: A review. In Innovations in Food Packaging; Academic Press: San Diego, CA, USA, 2014; pp. 213–255. [Google Scholar]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Properties of wheat starch film-forming dispersions and filmsas affected by chitosan addition. J. Food Eng. 2013, 114, 303–312. [Google Scholar]
- Chakraborty, S.; Pal, U. Towards growth and sustainable researches in phase change materials. J. Phase Chang. Mater. 2021, 1, 1. Available online: https://j-pcm.org/index.php/jpcm/article/view/9 (accessed on 27 February 2022).
- De Paola, M.G.; Paletta, R.; Lopresto, C.G.; Lio, G.E.; De Luca, A.; Chakraborty, S.; Calabrò, V. Stability of film forming dispersions: Affect the morphology and optical properties of polymeric films. Polymers 2021, 13, 1464. [Google Scholar] [CrossRef]
- Ma, X.; Chang, P.R.; Yu, J. Properties of biodegradable thermoplastic pea starch/carboxymethyl cellulose and pea starch/microcrystalline cellulose composites. Carbohydr. Polym. 2008, 72, 369–375. [Google Scholar] [CrossRef]
- Sabbah, M.; Esposito, M. Insight into Zeta Potential Measurements in Biopolymer Film Preparation. J. Biotechnol. Biomater. 2016, 6, 2–4. [Google Scholar] [CrossRef]
- De Paola, M.G.; Arcuri, N.; Calabrò, V.; De Simone, M. Thermal and stability investigation of phase change material dispersions for thermal energy storage by T-history and optical methods. Energies 2017, 10, 354. [Google Scholar] [CrossRef]
- De Paola, M.G.; Lopresto, C.G.; Arcuri, N.; Calabrò, V. Crossed analysis by T-history and optical light scattering method for the performance evaluation of Glauber’s salt-based phase change materials. J. Dispers. Sci. Technol. 2020, 1, 1–9. [Google Scholar] [CrossRef]
- Jane, J. Structural features of starch granules II. In Starch: Chemistry and Chemical Technology, 3rd ed.; Royal Society of Chemistry: London, UK, 2009. [Google Scholar]
- Miranda, J.; Lasa, A.; Bustamante, M.A.; Churruca, I.; Simon, E. Nutritional Differences between a Gluten-free Diet and a Diet Containing Equivalent Products with Gluten. Plant Food Hum. Nutr. 2014, 69, 182–187. [Google Scholar] [CrossRef]
- López, A.C.B.; Pereira, A.J.G.; Junqueira, R.G. Flour mixture of rice flour, corn and cassava starch in the production of gluten-free white bread. Braz. Arch. Biol. Technol. 2004, 47, 63–70. [Google Scholar] [CrossRef]
- Sabanis, D.; Lebesi, D.; Tzia, C. Effect of dietary fibre enrichment on selected properties of gluten-free bread. LWT-Food Sci. Technol. 2009, 42, 1380–1389. [Google Scholar] [CrossRef]
- Van Riemsdijk, L.E.; van der Goot, A.J.; Hamer, R.J.; Boom, R.M. Preparation of gluten-free bread using a meso-structured whey protein particle system. J. Cereal. Sci. 2011, 53, 355–361. [Google Scholar] [CrossRef]
- Milde, L.B.; Ramallo, L.A.; Puppo, M.C. Gluten-free bread based on tapioca starch: Texture and sensory studies. Food Bioprocess Technol. 2012, 5, 888–896. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Wang, J.; Ying, W.B.; Zhu, J. Fully bio-based poly (propylene succinate-co-propylene furandicarboxylate) co-polyesters with proper mechanical, degradation and barrier properties for green packaging applications. Eur. Poly. J. 2018, 102, 101–110. [Google Scholar] [CrossRef]
- Cerqueira, M.; Teixeira, J.; Vicente, A. “Edible Packaging Today”, Edible Food Packaging: Materials and Processing Technologies; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Cano, A.I.; Cháfer, M.; Chiralt AGonzález-Martínez, C. Physical and microstructural properties of biodegradable films based on pea starch and PVA. J. Food Eng. 2015, 167, 59–64. [Google Scholar] [CrossRef]
- Sajjan, A.M.; Naik, M.L.; Kulkarni, A.S. Preparation and characterization of PVA-Ge/PEG-400biodegradable plastic blend films for Packaging applications. Chem. Data Coll. 2020, 26, 100338. [Google Scholar]
- Zhou, X.; Yang, R.; Wang BChen, K. Development and characterization of bilayer films based on pea starch/polylactic acid and use in the cherry tomatoes packaging. Carbohydr. Polym 2019, 222, 114912–114917. [Google Scholar] [CrossRef]
- Aung, S.H.; Shein, H.H.; Aye, K.N.; Nwe, N. Environment-friendly biopolymers for food packaging: Starch, protein, and poly-lactic acid (PLA). In Bio-Based Materials for Food Packaging; Springer: Singapore, 2018; pp. 173–195. [Google Scholar]
- Mali, S.; Grossmann, M.V.; Garcia, M.A.; Martino, M.N.; Zaritzky, N.E. Microstructural characterization of yam starch films. Carbohydr. Polym. 2002, 50, 379–386. [Google Scholar] [CrossRef]
- Vaezi, K.; Asadpour, G.; Sharifi, H. Effect of ZnO nanoparticles on the mechanical, barrier and optical properties of thermoplastic cationic starch/montmorillonite biodegradable films. Int. J. Biol. Macromol. 2018, 124, 519–529. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Effect of re-crystallization on tensile, optical and water vapour barrier properties of corn starch films containing fatty acids. Food Hydrocoll. 2012, 26, 302–310. [Google Scholar] [CrossRef]
- Yu, Z.; Li, B.; Chu, J.; Zhang, P. Silica in situ enhanced PVA/chitosan biodegradable films for food packages. Carbohydr. Polym. 2018, 184, 214–220. [Google Scholar] [CrossRef]
- Liu, X.; Yu, L.; Liu, H.; Chen, L.; Li, L. Thermal Decomposition of Corn Starch with Different Amylose/Amylopectin Ratios in Open and Sealed Systems. Cereal Chem. 2009, 86, 383–385. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Yu, L.; Tong, Z.; Chen, L.; Liu, H.; Li, X. Thermal degradation and stability of starch under different processing conditions. Starch-Stärke 2012, 65, 48–60. [Google Scholar] [CrossRef]
- Liu, X.; Ma, H.; Yu, L.; Chen, L.; Tong, Z.; Chen, P. Thermal-oxidative degradation of high-amylose corn starch. J. Therm. Anal. 2013, 115, 659–665. [Google Scholar] [CrossRef]
- Wen, L.; Rodis, P.; Wasserman, B. Starch fragmentation and protein in solubilisation during twin-screw extrusion of corn meal. Cereal Chem. 1990, 67, 268–275. [Google Scholar]
- Davidson, V.J.; Paton, D.; Diosady, L.L.; Larocque, G. Degradation of Wheat Starch in a Single Screw Extruder: Characteristics of Extruded Starch Polymers. J. Food Sci. 1984, 49, 453–458. [Google Scholar] [CrossRef]
- Willett, J.; Millard, M.; Jasberg, B. Extrusion of waxy maize starch: Melt rheology and molecular weight degradation of amylopectin. Polymer 1997, 38, 5983–5989. [Google Scholar] [CrossRef]
- Carvalho, A.; Zambon, M.; Curvelo, A.; Gandini, A. Size exclusion chromatography characterization of thermoplastic starch composites 1. Influence of plasticizer and fibre content. Polym. Degrad. Stab. 2003, 79, 133–138. [Google Scholar] [CrossRef]
- Olkku, J.; Rha, C. Gelatinisation of starch and wheat flour starch—A review. Food Chem. 1978, 3, 293–317. [Google Scholar] [CrossRef]
- Lim, M.H.; Wu, H.; Reid, D.S. The effect of starch gelatinization and solute concentrations on Tg′ of starch model system. J. Sci. Food Agric. 2000, 80, 1757–1762. [Google Scholar] [CrossRef]
- Tester, R.F.; Debon, S.J. Annealing of starch. A. review. Int. J. Biol. Macromol. 2000, 27, 1–12. [Google Scholar] [CrossRef]
- Andrade, L.A.; Barbosa, N.A.; Pereira, J. Extraction and properties of starches from the non-traditional vegetables Yam and Taro. Polímeros 2017, 27, 151–157. [Google Scholar] [CrossRef]
- Tejavathi, D.; Sujatha, B.; Karigar, C. Physicochemical properties of starch obtained from Curcuma karnatakensis—A new botanical source for high amylose content. Heliyon 2020, 6, e03169. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, S. Extraction of starches from tuber crops using ammonia. Carbohydr. Polym. 1991, 16, 391–398. [Google Scholar] [CrossRef]
- Ramli, R.N.; Lee, C.K.; Kassim, M.A. Extraction and Characterization of Starch from Microalgae and Comparison with Commercial Corn Starch. IOP Conf. Ser. Mater. Sci. Eng. 2020, 716, 012012. [Google Scholar] [CrossRef]
- Patkar, M.S.; Prasad, R.R.; Rahate, K.S.; Shetye, P.A.; Sinha, R.A.; Chitnis, K.S. Extraction and characterisation of starch from tuber and its application as bioplastic. World J. Environ. Biosci. 2020, 9, 1–5. [Google Scholar]
- Corke, H.; Faubion, J.; Seetheraman, K.; Wrigley, C. Encyclopedia of Food Grains; Academic Press: Cambridge, UK, 2016. [Google Scholar]
- Rodrigues, A.A.M.; da Costa, R.R.; Santos, L.F.; de Melo Silva, S.; de Britto, D.; de Lima, M.A. Propertiesand characterisation of biodegradable films obtained from different starch sources. Food Sci. Technol. 2021, 476, 482. [Google Scholar]
- Molaee Aghaee, E.; Kamkar, A.; Akhondzadeh Basti, A.; Khanjari, A.; Kontominas, M. Effect of packaging with Chitosan biodegradable films formulated with Garlic essential oil (Allium sativum L.) on the chemical properties of chicken fillet. Iran. J. Health Environ. 2015, 8, 379–390. [Google Scholar]
- Soukoulis, C.; Singh, P.; Macnaughtan, W.; Parmenter, C.; Fisk, I.D. Compositional and physicochemical factors governing the viability of Lactobacillus rhamnosus GG embedded in starch-protein based edible films. Food Hydrocoll. 2015, 52, 876–887. [Google Scholar] [CrossRef]
- Escamilla-García, M.; Reyes-Basurto, A.; García-Almendárez, B.; Hernández-Hernández, E.; Calderón-Domínguez, G.; Rossi-Marquez, G.; Regalado-González, C. Modified Starch-Chitosan Edible Films: Physicochemical and Mechanical Characterization. Coatings 2017, 7, 224. [Google Scholar] [CrossRef]
- Gomes, M.A.; Palmiro, D.; Ascheri, R.; Campos, A. Characterization of edible films of Swartziaburchelli phosphate starches and development of coatings for post-harvest application to cherry tomatoes. Semin. Ciências Agrárias 2016, 37, 1897–1910. [Google Scholar] [CrossRef]
- Adjouman, Y.D.; Nindjin, C.; Tetchi, F.A.; Dalcq, A.C.; Amani, N.G.; Sindic, M. Water Vapor Permeability of Edible Films Based on Improved Cassava (Manihot esculenta Crantz) Native Starches. J. Food Process. Technol. 2017, 8, 665. [Google Scholar] [CrossRef]
- Song, X.; Cheng, L.; Tan, L. Edible iron yam and maize starch convenient food flavoring packaging films with lemon essential oil as plasticization. Food Sci. Technol. 2019, 39, 971–979. [Google Scholar] [CrossRef]
- He, X.; Deng, H.; Hwang, H.-M. The current application of nanotechnology in food and agriculture. J. Food Drug Anal. 2019, 27, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K.; Rather, I.A. Application of nanotechnology in food science: Perception and overview. Front. Microbiol. 2017, 8, 1501. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nano emulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A.; Garaguso, I.; Britti, M.S.; Mengheri, E. Zinc oxide protects cultured enterocytes from the damage induced by Escherichia coli. J. Nutr. 2003, 133, 4077–4082. [Google Scholar] [CrossRef]
- Mohamadian, N.; Ghorbani, H.; Wood, D.A.; Khoshmardan, M.A. A hybrid nanocomposite of poly (styrene-methyl methacrylateacrylic acid)/clay as a novel rheology-improvement additive for drilling fluids. J. Polym. Res. 2019, 26, 33. [Google Scholar] [CrossRef]
- Samadi, A.; Klingberg, H.; Jauffred, L.; Kjær, A.; Bendix, P.M.; Oddershede, L.B. Platinum nanoparticles: A non-toxic, effective and thermally stable alternative plasmonic material for cancer therapy and bioengineering. Nanoscale 2018, 10, 9097–9107. [Google Scholar] [CrossRef]
- Kuswandi, B.; Moradi, M. Improvement of food packaging based on functional nanomaterial. In Nanotechnology: Applications in Energy, Drug and Food; Siddiquee, S., Melvin, G.J.H., Rahman, M.M., Eds.; Springer: Cham, Switzerland, 2019; pp. 309–344. ISBN 978-3-319-99602-8. [Google Scholar]
- Joye, I.J.; Davidov-Pardo, G.; McClements, D.J. Nanotechnology in food processing. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 49–55. ISBN 978-0-12-384953-3. [Google Scholar]
- Khare, S.; Williams, K.; Gokulan, K. Nanotechnology. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 893–900. ISBN 978-0-12-384733-1. [Google Scholar]
- Yoksan, R.; Chirachanchai, S. Silver nanoparticle-loaded chitosan-starch based films: Fabrication and evaluation of tensile, barrier and antimicrobial properties. Mater. Sci. Eng. C 2010, 30, 891–897. [Google Scholar] [CrossRef]
- Brody, A.L. Case studies on nanotechnologies for food packaging. Food Technol. 2007, 61, 102–107. [Google Scholar]
- Flore, S.; Famá, L.; Rojas, A.M.; Goyanes, S.; Gerschenson, L. Physical properties of tapioca-starch edible films: Influence of filmmaking and potassium sorbate. Food Res. Int. 2007, 6, 257–265. [Google Scholar] [CrossRef]
- Goudarzi VShahabi-Ghahfarrokhi, I.; Babaei-Ghazvini, A. Preparation of eco-friendly UV-protective food packaging material by starch/TiO2 bio-nanocomposite: Characterization. Int. J. Biol. Macromol. 2017, 95, 306–313. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, Y.; Xiao, L.; Lin, D.; Yang, Y.; Wang, H.; Yang, Y.; Wu, D.; Chen, H.; Zhang, Q.; et al. Physical properties and structural characterization of starch/polyvinyl alcohol/graphene oxide composite films. Int. J. Biol. Macromol. 2018, 123, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Aqlil, M.; Moussemba Nzenguet, A.; Essamlali, Y.; Snik, A.; Larzek, M.; Zahouily, M. Graphene oxide filled lignin/starch polymer bio nanocomposite: Structural, physical, and mechanical studies. J. Agric. Food Chem. 2017, 65, 10571–10581. [Google Scholar] [CrossRef] [PubMed]
- Shahbezi, M.; Rajabzadeh, G.; Sotoodeh, S. Functional characteristics, wettability properties and cytotoxic effect of starch film incorporated with multi-walled and hydroxylated multi-walled carbon nanotubes. Int. J. Biol. Macromol. 2017, 104, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Oleyaei, S.A.; Zahedi, Y.; Ghanbarzadeh, B.; Moayedi, A.A. Modification of physicochemical and thermal properties of starch films by incorporation of TiO2 nanoparticles. Int. J. Biol. Macromol. 2016, 89, 256–264. [Google Scholar] [CrossRef]
- Dai, L.; Qiu, C.; Xiong, L.; Sun, Q. Characterisation of corn starch-based films reinforced with taro starch nanoparticles. Food Chem. 2015, 174, 82–88. [Google Scholar] [CrossRef]
- Piyada, K.; Waranyou, S.; Thawien, W. Mechanical, thermal and structural properties of rice starch films reinforced with rice starch nanocrystals. Int. Food Res. J. 2013, 20, 439–449. [Google Scholar]
- Tian, H.; Xu, G. Processing and characterization of glycerol-plasticized soy protein plastics reinforced with citric acid-modified starch nanoparticles. J. Polym. Environ. 2011, 19, 582–588. [Google Scholar] [CrossRef]
- McGlashan, S.A.; Halley, P.J. Preparation and characterization of biodegradable starch-based nanocomposite materials. Polym. Int. 2003, 52, 1767–1773. [Google Scholar] [CrossRef]
- Earle, M.; Earle, R. Case Studies in Food Product Development Effect of Starch Film Incorporated with Multi-Walled and Hydroxylated Multi-Walled Carbon Nanotubes; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Steele, R. Understanding and Measuring the Shelf-Life of Food; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Man, D. Shelf Life; Blackwell Science: Oxford, UK, 2002. [Google Scholar]
- Phimolsiripol, Y.; Suppakul, P. Techniques in Shelf Life Evaluation of Food Products. Ref. Modul. Food Sci. 2016, 1, 1–8. [Google Scholar]
- Gaikwad, K.K.; Singh, S.; Lee, Y.S. Oxygen scavenging films in food packaging. Environ. Chem. Lett. 2018, 16, 523–538. [Google Scholar] [CrossRef]
- Sadeghi, K.; Lee, Y.; Seo, J. Ethylene scavenging systems in packaging of fresh produce: A review. Food Rev. Int. 2019, 37, 155–176. [Google Scholar] [CrossRef]
- Zhou, L.; Lv, S.; He, G.; He, Q.; Shi, B. Effect of Pe/Ag2o Nano-packaging on the quality of apple slices. J. Food Qual. 2011, 34, 171–176. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Ajji, A. Moisture absorbers for food packaging applications. Environ. Chem. Lett. 2019, 17, 609–628. [Google Scholar] [CrossRef]
- Han, J.W.; Ruiz-Garcia, L.; Qian, J.P.; Yang, X.T. Food packaging: A comprehensive review and future trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Kilcast, D.; Subramaniam, P. The Stability and Shelf-Life of Food; Woodhead Publishing Ltd.: Cambridge, UK, 2000. [Google Scholar]
- Hough, G. Sensory Shelf Life Estimation of Food Products; Taylor & Francis: Boca Raton, FL, USA, 2010. [Google Scholar]
- Ribba, L.; Garcia, N.; Accorso, N.D.; Goyanes, S. Disadvantages of starch-based materials, feasible alternatives in order to overcome these limitation. In Starch-Based Materials in Food Packaging; Springer: Singapore, 2017; pp. 37–76, Chapter 3. [Google Scholar]
- Cao, T.L.; Song, K.B. Effects of gum karaya addition on the characteristics of loquat seed starch films containing oregano essential oil. Food Hydrocoll. 2019, 97, 105198. [Google Scholar] [CrossRef]
- Do Evangelho, J.A.; da Silva Dannenberg, G.; Biduski, B.; El Halal, S.L.M.; Kringel, D.H.; Gularte, M.A.; Fiorentini, A.M.; da Rosa Zavareze, E. Antibacterial activity, optical, mechanical, and barrier properties of corn starch films containing orange essential oil. Carbohydr. Polym. 2019, 222, 114981. [Google Scholar] [CrossRef]
- Kang, J.-H.; Song, K.B. Characterization of Job’s tears (Coix lachryma-jobi L.) starch films incorporated with clove bud essential oil and their antioxidant effects on pork belly during storage. LWT-Food Sci. Technol. 2019, 111, 711–718. [Google Scholar] [CrossRef]
- Hasan, M.; Rusman, R.; Khaldun, I.; Ardana, L.; Mudatsir, M.; Fansuri, H. Active edible sugar palm starch-chitosan films carrying extra virgin olive oil: Barrier, thermo-mechanical, antioxidant, and antimicrobial properties. Int. J. Biol. Macromol. 2020, 163, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Domene-López, D.; Delgado-Marín, J.J.; Martin-Gullon, I.; García-Quesada, J.C.; Montalbán, M.G. Comparative study on properties of starch films obtained from potato, corn and wheat using 1-ethyl-3-methylimidazolium acetate as plasticizer. Int. J. Biol. Macromol. 2019, 135, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Sudheesh, C.; Sunooj, K.V.; Sasidharan, A.; Sabu, S.; Basheer, A.; M, M.N.; Raghavender, C.; Sinha, S.; George, J. Energetic neutral N2 atoms treatment on the kithul (Caryota urens) starch biodegradable film: Physico-chemical characterization. Food Hydrocoll. 2020, 103, 105650. [Google Scholar] [CrossRef]
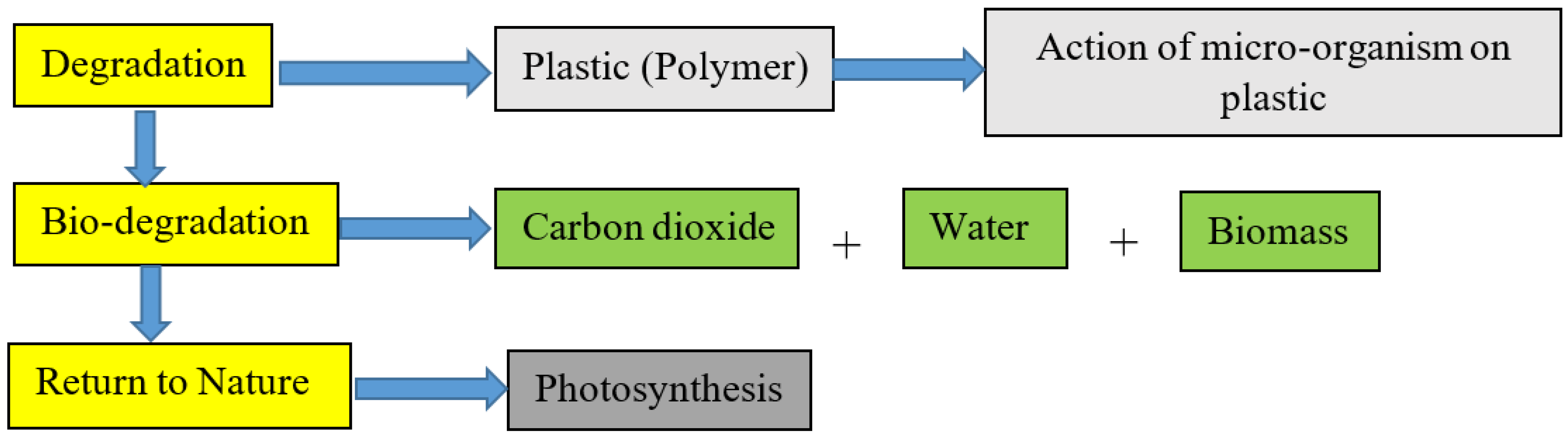
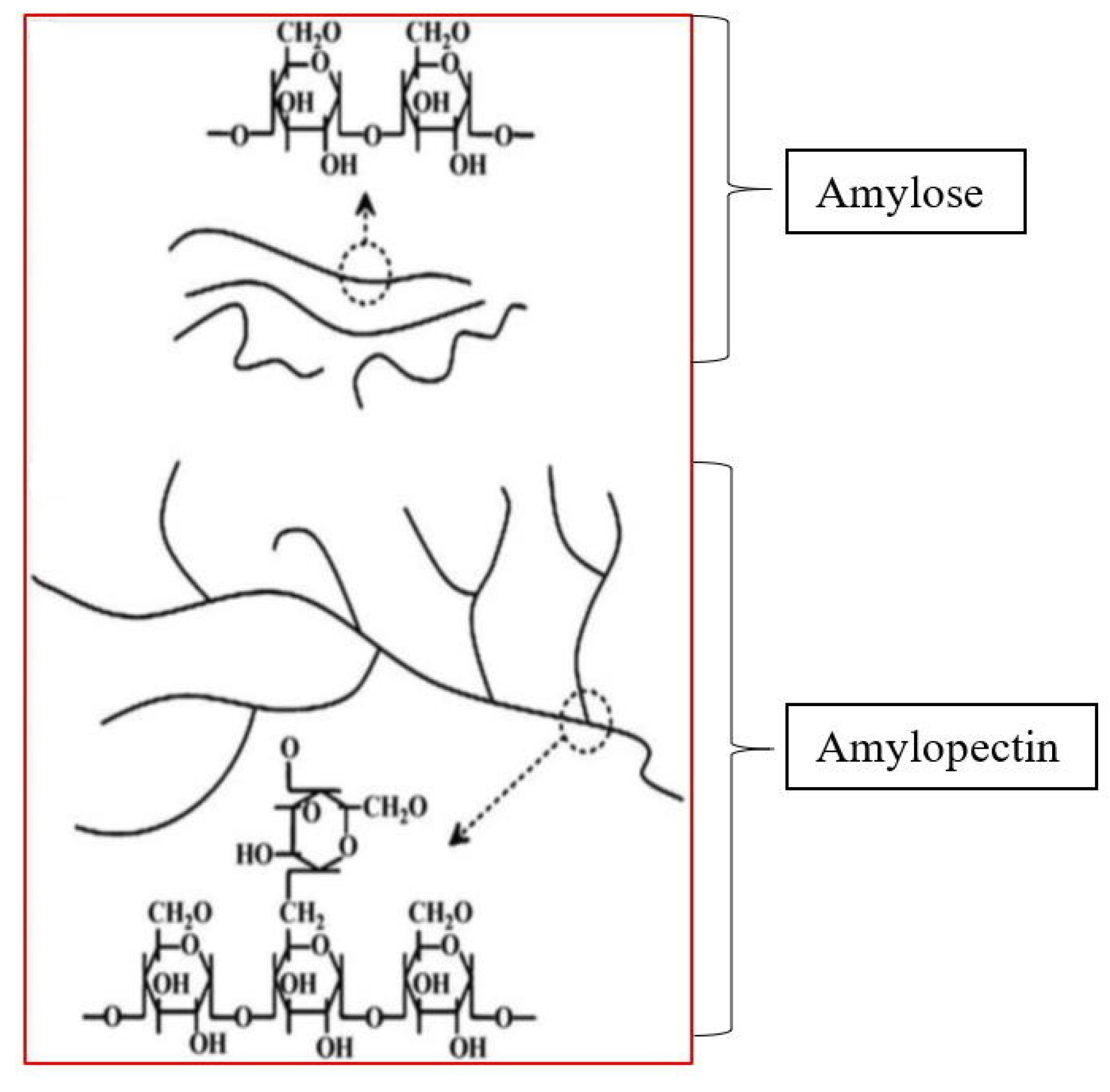
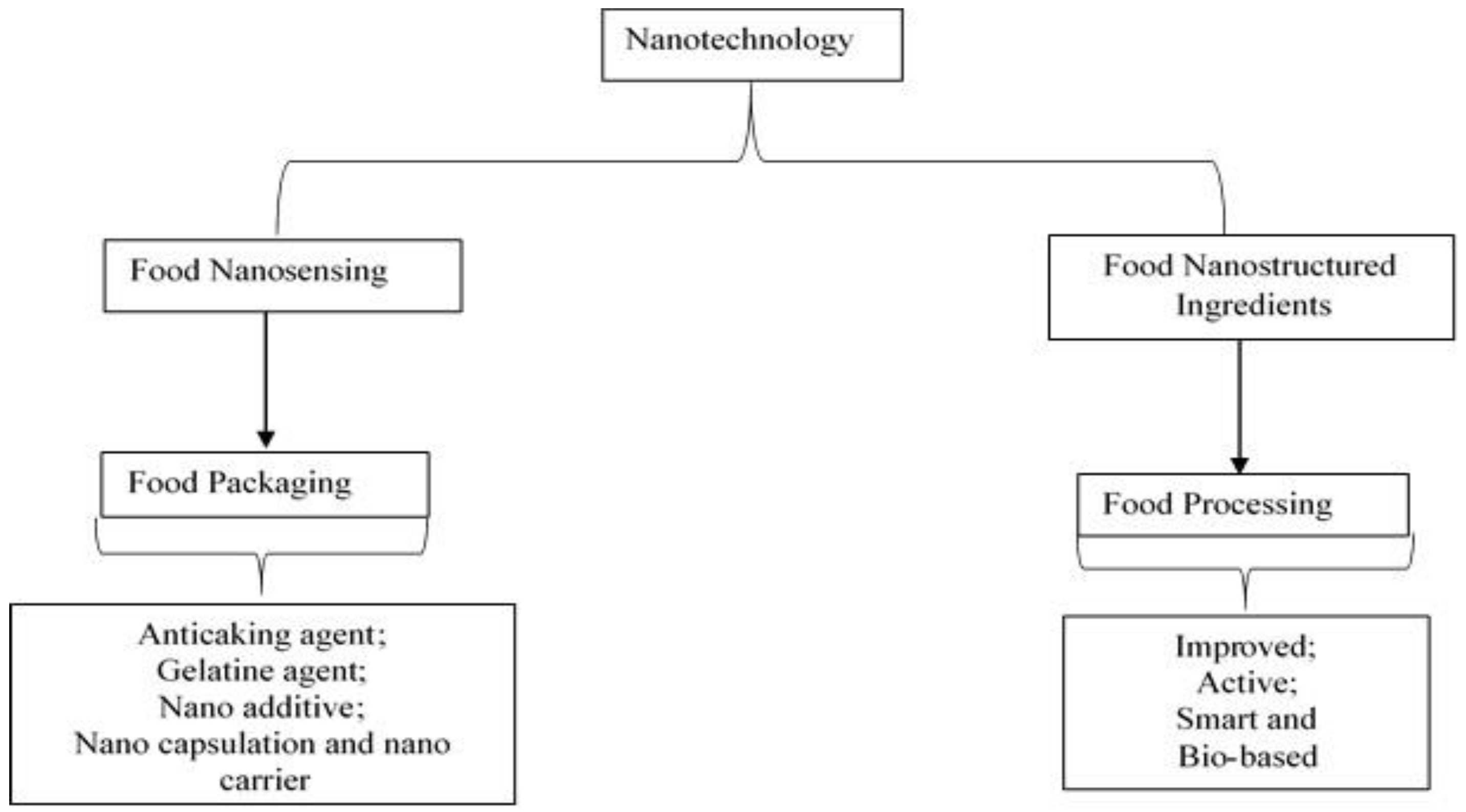
| Types of Starch | Sources | References |
|---|---|---|
| Corn | Soya flour, cassava starch, and corn | [33] |
| Potato | Rice flour, potato, white rice flour, soya flour, and egg powder | [34] |
| Cassava | Sorghum flour | [35] |
| Wheat | D-glucose, bakery yeast, locust bean gum, and wheat starch | [36] |
| Tapioca | Corn flour, soya bean flour, and cornflour | [37] |
| Biological Source | Geographical Source | Macroscopically Characters | Microscopy of Some Starch | Chemical Constituents |
|---|---|---|---|---|
| Starch consists of polysaccharides granules from the grains of Maize Zea mays L., rice Orza sativa L., wheat Triticum aestivum, or from the tubers of the potato Solarium tuberosum L. | Starch is produced in tropical and subtropical countries, such as Argentina, the USA, China, and India. However, Japan is regarded as the main starch-producing country globally. | It is found in irregular, angular masses or white powder. Insoluble in cold water and forms colloidal solution on boiling. Starch solution becomes a translucent jelly after cooling. | Germs are continuously separated from the suspension by liquid cyclones and used in the preparation of germ oils. The germs oil is characterized to be rich in vitamins. | Starch contains a mixture of two polysaccharides—80% amylopectin and 20% amylose. Amylopectin is insoluble in water, while amylose is soluble in water. |
| Properties | Description of the Properties |
|---|---|
| Structural properties | To examine the chemical structure and composition of packaging material, atomic force microscopy and Fourier transform infrared (FR-IR) spectroscopy were used [38]. Starch and PVA films exhibit homogenous and smooth surfaces. One factor influencing the structural properties is phase separation, which only occurs in the amylopectin type of starch. The phase separation is due to the amount of starch and phosphate groups. Recommendation indicated that the thickness of biodegradable packaging material should be less than 254 µm [39]. |
| Solubility properties | The solubility properties are directly proportional to the hydrophilic nature of polymers. For example, starch film and PVA solubility are reported to be 0.208 g dissolved/g dry films and 0.19 g dissolved/g dry films, respectively [40]. The recommendation of aqueous medium for packaging and storage is its low solubility values, which shows good stability [34]. |
| Mechanical properties | Biodegradable polylactic films exhibit poor mechanical properties compared with polylactic petroleum films [41,42,43]. Mechanical properties are associated with the crystallinity of polymer and content of amylose [44], the weight of properties, additive concentration, and distribution. The high tensile strength and elongation break experience in starch films result from the low molecular weight. |
| Optical properties | The decolourisation and deterioration of packaged food products are caused by overexposure to ultraviolet (UV) and visible radiations. To carry out quality control in packaged food products, transparency and UV screening are essential. Based on Vaezi et al. [45] study, nanocomposites increase the non-transparency of starch films, which suggests that nanoparticles are UV blockers, thereby minimizing the passage of light. |
| Permeability properties | The polymer matrix exhibits effective permeability of gases, which increases the shelf life of food products [46]. The shelf life and freshness of food are directly proportional to water transfer between the product and its surroundings. Hence, the main function of packaging has to deal with reducing the transfer of water. According to Yu et al. [47], silica nanoparticles’ presence in biodegradable films decreases moisture permeability. |
| Classification of Foods | Duration of Measurement |
|---|---|
| Highly perishable | Every day |
| Semi-perishable | Every two weeks |
| Highly stable | Every week or monthly |
| Types of Food Packaging | Characteristics | Medium/Agent | References |
|---|---|---|---|
| Oxygen scavengers | Oxidation of fat is prevented and avoided. | Metallic iron powder; organic (ascorbic acid); inorganic (ZnO); polymer- and enzyme-based agents (glucose). | [96] |
| Ethylene scavengers | Reduces the ripening of fruit and vegetables. | PdCl2; Pd-impregnated zeolite; polyvinyl chloride film containing ZnO nanoparticles; inorganic (silica gel); inorganic (xylitol, fructose). | [97,98] |
| Moisture absorber | Reduces the growth of microorganisms. | Polymer-based agents (starch). | [99] |
| Carbon dioxide | Inhibit food spoilage by the action of microorganism. | Citric acid, bicarbonate and ascorbate, and sodium. | [100,101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onyeaka, H.; Obileke, K.; Makaka, G.; Nwokolo, N. Current Research and Applications of Starch-Based Biodegradable Films for Food Packaging. Polymers 2022, 14, 1126. https://doi.org/10.3390/polym14061126
Onyeaka H, Obileke K, Makaka G, Nwokolo N. Current Research and Applications of Starch-Based Biodegradable Films for Food Packaging. Polymers. 2022; 14(6):1126. https://doi.org/10.3390/polym14061126
Chicago/Turabian StyleOnyeaka, Helen, KeChrist Obileke, Golden Makaka, and Nwabunwanne Nwokolo. 2022. "Current Research and Applications of Starch-Based Biodegradable Films for Food Packaging" Polymers 14, no. 6: 1126. https://doi.org/10.3390/polym14061126
APA StyleOnyeaka, H., Obileke, K., Makaka, G., & Nwokolo, N. (2022). Current Research and Applications of Starch-Based Biodegradable Films for Food Packaging. Polymers, 14(6), 1126. https://doi.org/10.3390/polym14061126








