Microscopic and Structural Studies of an Antimicrobial Polymer Film Modified with a Natural Filler Based on Triterpenoids
Abstract
:1. Introduction
- -
- For the first time, variants of a modified low-density polyethylene (LDPE) film with a natural hydrophobic filler based on triterpenoids were obtained by melt matching with confirmed antimicrobial activity;
- -
- New knowledge about the film surface morphology and the structure of its side section was obtained using methods not previously used in polymer research (histological);
- -
- It has been determined that the betulin distribution on the surface and in the bulk of the polymer is uneven; however, it has an antimicrobial effect in the case of forced contamination of the film by 2–3 orders of magnitude, depending on the selected microorganisms.
2. Materials and Methods
2.1. Research Objects
2.2. Research Methods
2.2.1. Optical Microscopy Methods
2.2.2. Microbiological Research
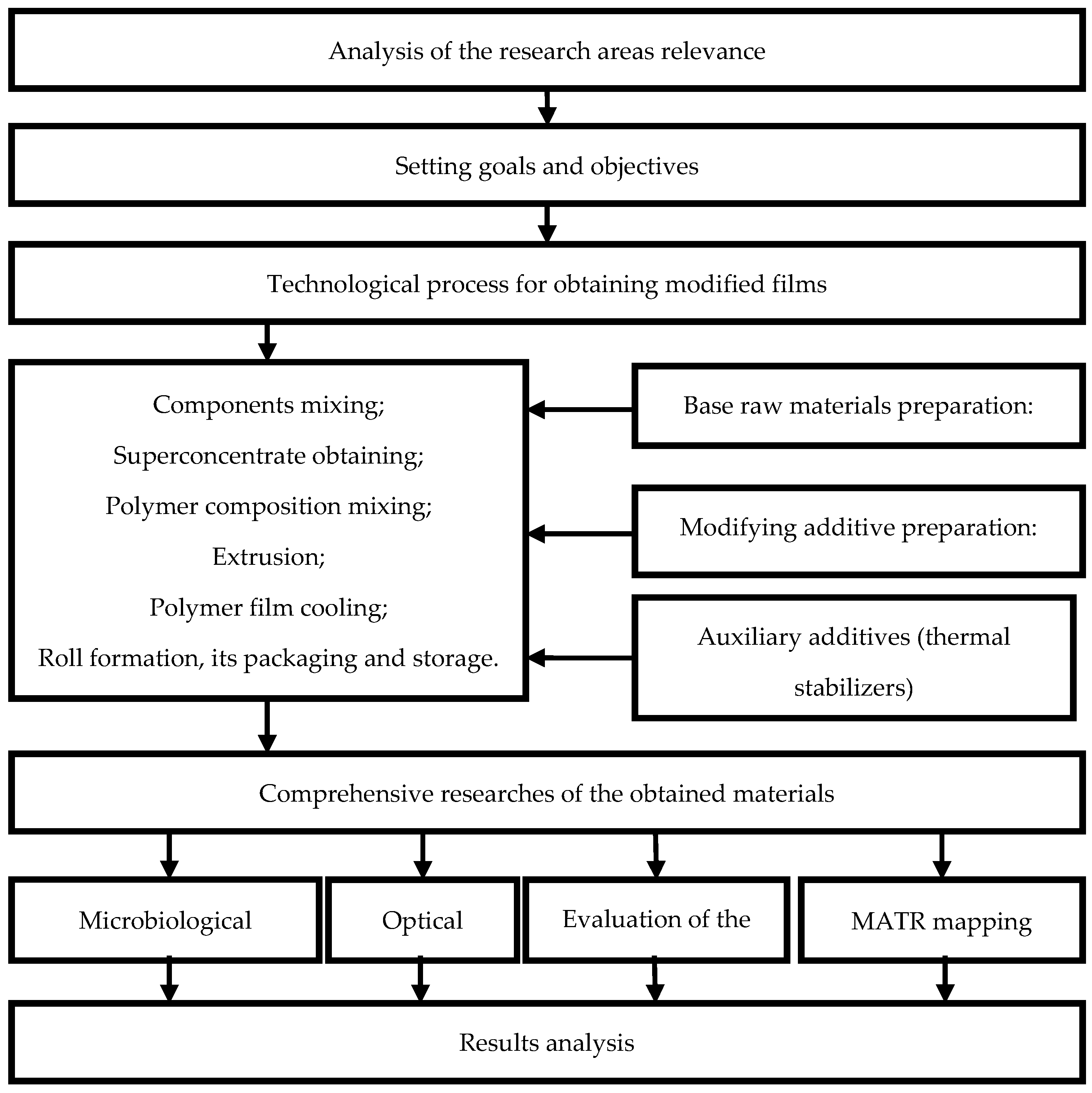
3. Results and Discussion
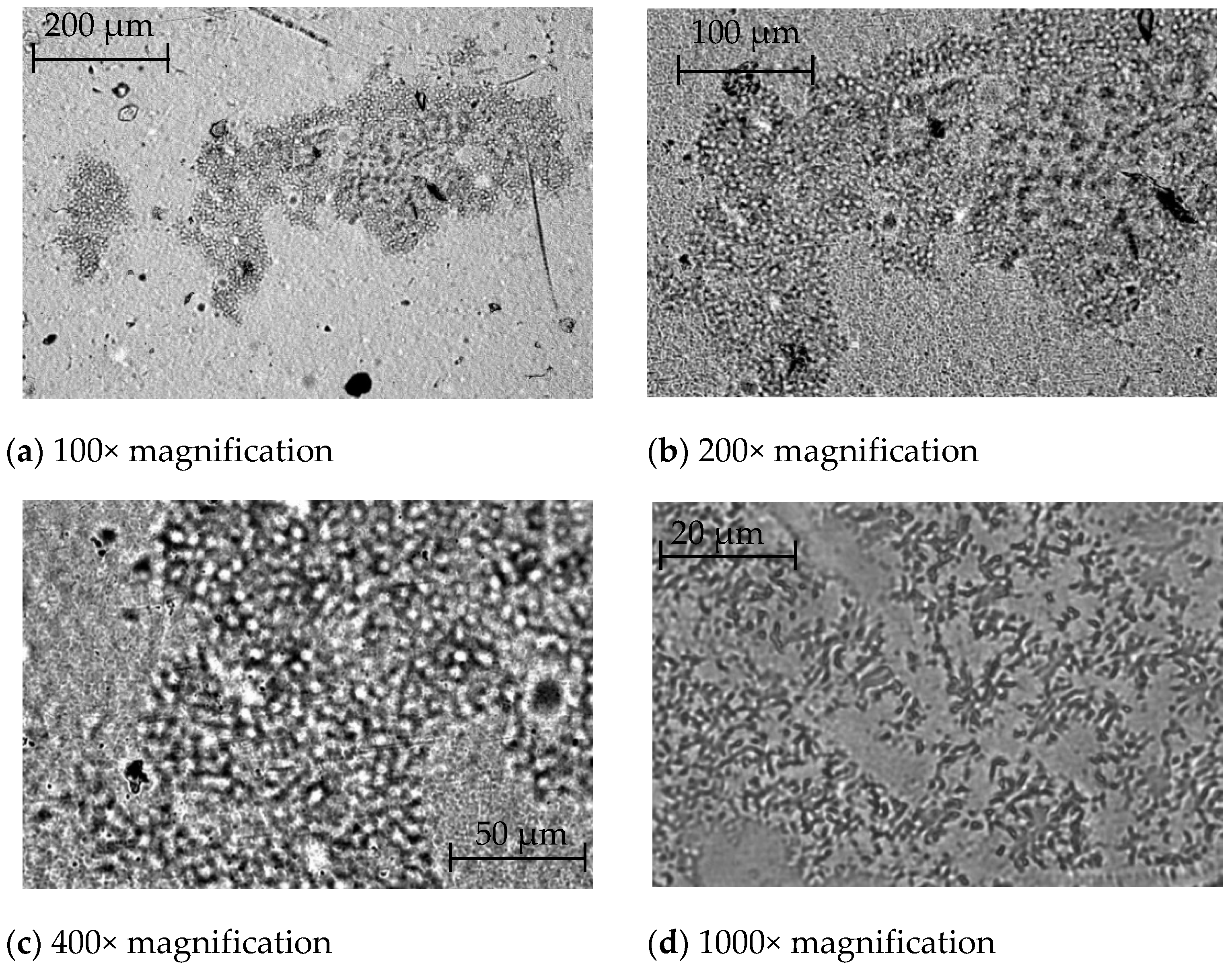
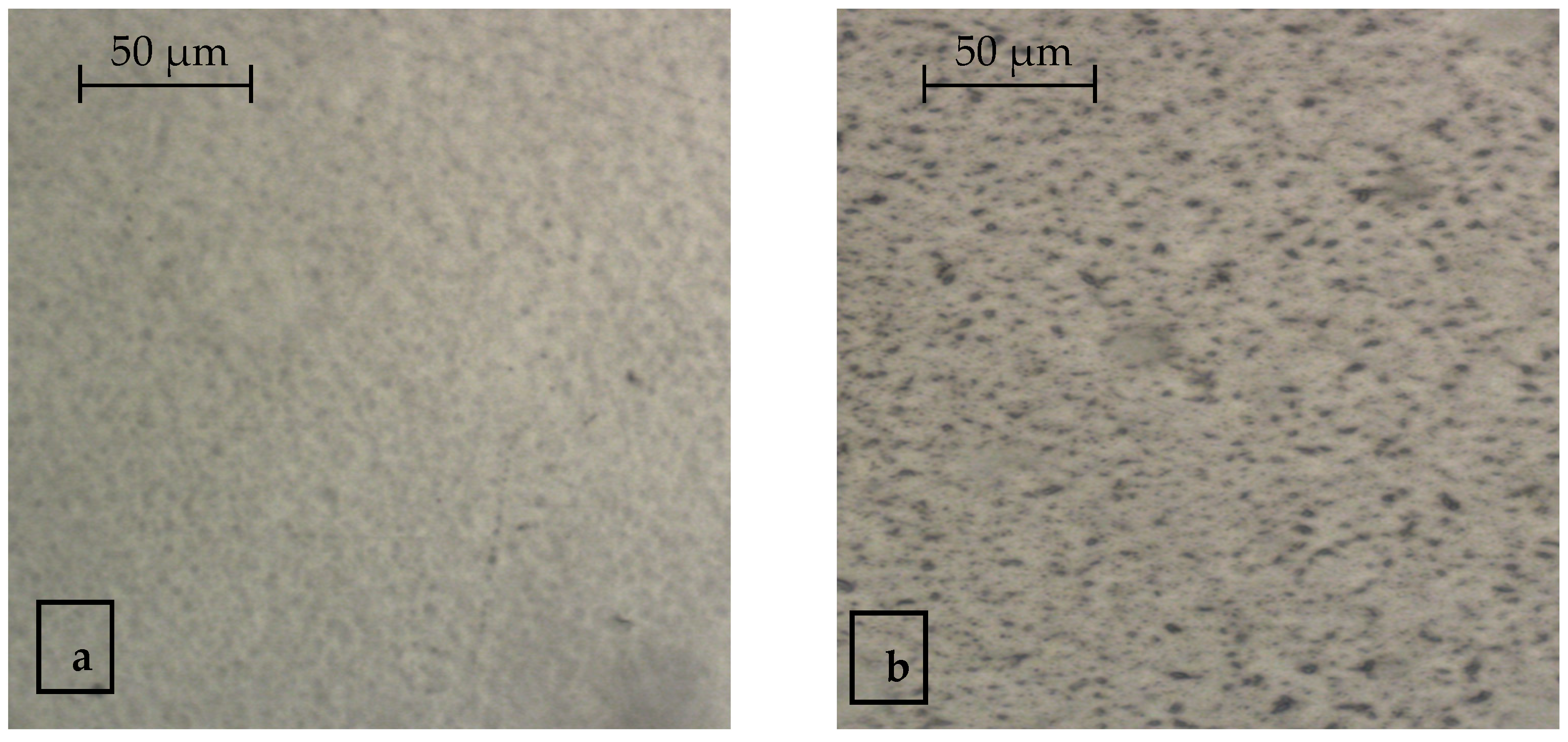
3.1. Visualization of Antimicrobial Additives on the Film Surface and in Polymer Mass by Optical Microscopy
3.2. Results of Microbiological Research
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maksanova, L.A. High-Molecular Compounds and Materials Based on Them, Used in the Food Industry. 2005. Available online: https://www.ozon.ru/product/vysokomolekulyarnye-soedineniya-i-materialy-na-ih-osnove-primenyaemye-v-pishchevoy-27905636/?sh=C3_drAAAAA (accessed on 11 January 2022).
- Galstyan, A.G.; Aksyonova, L.M.; Lisitsyn, A.B.; Oganesyants, L.A.; Petrov, A.N. Modern approaches to storage and efficient processing of agricultural products to obtain high quality food products. Bull. Russ. Acad. Sci. 2019, 89, 539–542. [Google Scholar]
- Yurova, E.A.; Kobzeva, T.V.; Polyakova, O.S. Development of assessing methods for the storage capacity of dairy products. Milk Processing 2016, 12, 38–41. [Google Scholar]
- Zobkova, Z.S.; Fursova, T.P.; Zenina, D.V. Selection of protein ingredients that enrich and modify the structure of fermented milk drinks. Curr. Issues Beverage Ind. 2018, 2, 64–69. [Google Scholar]
- Kruchinin, A.G.; Agarkova, E.Y. Various approaches to the functional properties of dairy products formation. Milk Processing 2018, 5, 36–39. [Google Scholar]
- Abdel-Bari, E.M.; Zaikova, G.E. Polymer Films; Profession: Saint Petersburg, Russia, 2006; p. 352. [Google Scholar]
- Aksenova, T.I.; Baburina, O.V.; Baburina, T.M.; Cheremnykh, E.G. Effect of physical surface modification on the properties of packaging materials. Technology of packaging production and food engineering in the light of the environmental safety of food raw materials and foodstuffs: A collection of materials of the second scientific and practical conference. 2011. Available online: https://cyberleninka.ru/article/n/vliyanie-fizicheskoy-modifikatsii-upakovki-na-razvitie-porchi-pischevyh-produktov/viewer (accessed on 11 January 2022).
- Mastalygina, E.E.; Kolesnikova, N.N.; Karpova, S.G.; Popov, A.A. Investigation of Polypropylene/Low-Density Polyethylene Blends. In A Systematic Approach to Experiments, Evaluation, and Modeling; Apple Academic Press Inc.: Berlin/Heidelberg, Germany, 2016; pp. 103–121. [Google Scholar]
- Mastalygina, E.E.; Varyan, I.A.; Kolesnikova, N.N.; Monakhova, T.V.; Karpova, S.G.; Popov, A.A. The role of the filler in the formation of the structure and properties of polyolefin composites. In Structure and Dynamics of Molecular Systems; IPChE RAS: Moscow, Russia, 2016; pp. 354–362. [Google Scholar]
- Anpilova, A.Y.; Mastalygina, E.E.; Khrameeva, N.P.; Popov, A.A. Methods for Cellulose Modification in the Development of Polymeric Composite Materials. Russ. J. Phys. Chem. B 2020, 14, 176–182. [Google Scholar] [CrossRef]
- Seyedeh, H.F.; Seyed, H.P.; Seyed, J.S.; Abdulrasoul, O. Development of novel active polypropylene-based packaging filmscontaining different concentrations of sorbic acid. Food Packag. Shelf Life 2018, 18, 87–94. [Google Scholar]
- Lord, A.W. Food and nutritional analysis. In Packaging Materials; Elsevier: Amsterdam, The Netherlands, 2005; Volume 3, pp. 341–352. [Google Scholar]
- Beigmohammadi, F.; Peighambardoust, S.H.; Hesari, J.; Azadmard-Damirchi, S.; Peighambardoust, S.J.; Khosrowshahi, N.K. Antibacterial properties of LDPE nanocomposite films in packaging of UF cheese. LWT-Food Sci. Technol. 2016, 65, 106–111. [Google Scholar] [CrossRef]
- Haghighi-Manesh, S.; Azizi, M.H. Active packaging systems with emphasis on its applications in dairy products. J. Food Process Eng. 2017, 40, 12542. [Google Scholar] [CrossRef]
- Han, J.H. Antimicrobial food packaging. Food Technol. 2000, 54, 56–65. [Google Scholar]
- Han, J.H.; Floros, J.D. Casting antimicrobial packaging films and measuring their physical properties and antimicrobial activity. J. Plast. Film. Sheeting 1997, 13, 287–298. [Google Scholar] [CrossRef]
- Han, C.; Wang, J.; Li, Y.; Lu, F.; Cui, Y. Antimicrobial-coated polypropylene films with polyvinyl alcohol in packaging of fresh beef. Meat Sci. 2014, 96, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.M.; Taylor, T.M.; Schmidt, S.E. Chemical preservatives and natural antimicrobial compounds. In Food Microbiology; American Society of Microbiology (ASM) Press: Vancouver, WA, USA, 2013; pp. 765–801. [Google Scholar]
- Degirmencioglu, N.; Göcmen, D.; Inkaya, A.N.; Aydin, E.; Guldas, M.; Gonenc, S. Influence of modified atmosphere packaging and potassium sorbate on microbiological characteristics of sliced bread. J. Food Sci. Technol. 2011, 48, 236–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guynot, M.E.; Marín, S.; Sanchis, V.; Ramos, A.J. An attempt to minimize potassium sorbate concentration in sponge cakes by modified atmosphere packaging combination to prevent fungal spoilage. Food Microbiol. 2004, 21, 449–457. [Google Scholar] [CrossRef]
- Kamarudin, S.H.; Rayung, M.; Abu, F.; Ahmad, S.; Fadil, F.; Karim, A.A.; Norizan, M.N.; Sarifuddin, N.; Mat Desa, M.S.Z.; Mohd Basri, M.S.; et al. A Review on Antimicrobial Packaging from Biodegradable Polymer Composites. Polymers 2022, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, U.K.; Skrivars, M.; Persson, A. A review of natural fibers used in biocomposites: Plant, animal, and regenerated cellulose fibers. Polymers 2015, 55, 107–162. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Dawoodpour, Y.; Islam, M.; Mustafa, A.; Sudesh, K.; Dungany, R.; Jawaid, M. Manufacture and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef]
- Jonubi, M.; Harun, J.; Tahir, P.M.; Shakeri, A.; Saifulazri, S.; Makinejad, M.D. Physicochemical characterization of cellulose and nanofibers from kenaf stem. Mater. Lat. 2011, 65, 1098–1100. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Liu, Y.; Hai, Y.; Zhang, M.; Chen, P. Isolation and characterization of cellulose nanofibers from four plant cellulose fibers using a chemo-ultrasonic process. Cellulose 2011, 18, 433–442. [Google Scholar] [CrossRef]
- Alemdar, A.; Sign, M. Isolation and characterization of nanofibers from agricultural waste—Wheat straw and soy husks. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Jonubi, M.; Harun, J.; Shakeri, A.; Mishra, M.; Oxmand, K. Chemical composition, crystallinity, and thermal degradation of bleached and unbleached kenaf (Hibiscus cannabinus) bast pulp and nanofibers. Bioresources 2009, 4, 626–639. [Google Scholar]
- Norrahim, M.N.F.; Ariffin, H.; Yasim-Anuar, T.A.T.; Gami, F.; Hassan, M.; Ibrahim, N.A.; Nji, J.L.H.; Yunus, W.M.Z.W. Superheated steam pretreatment of pulp affects its electrospinning ability to produce microfibrillated pulp. Cellulose 2018, 25, 3853–3859. [Google Scholar] [CrossRef]
- Fahma, F.; Iwamoto, S.; Hori, N.; Iwata, T.; Takemura, A. Isolation, preparation and characterization of oil palm empty fruit nanofibers (OPEFB). Cellulose 2010, 17, 977–985. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Gomez, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.; Galindo, R.; Cano, A.; Espina, M.; Etcheto, M.; Kamins, A.; et al. Metal-based nanoparticles as antimicrobial agents: A review. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, W.T.; Wu, V.T.; Nguyen, T.H.; Nguyen, T.A.; Tran, V.K.; Nguyen-Tri, P. Antibacterial activity of TiO2- and ZNO-decorated silver nanoparticles. J. Compos. Sci. 2019, 3, 61. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, H.; Abedi, B.; Bodagi, H.; Davarinejad, G.; Kharatizadeh, H.; Conte, A. Investigation of a developed clay-nanocomposite packaging film on the quality of peach fruits (Prunus persica Cv. Alberta) in refrigerated storage. J. Food Process Save 2018, 42, e13466. [Google Scholar] [CrossRef]
- Uz, M.; Altınkaya, S.A. Development of mono and multilayer antimicrobial food packaging materials for controlled release of potassium sorbate. LWT-Food Sci. Technol. 2011, 44, 2302–2309. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.C.; Manjeet, S.C. Biopolymer-based antimicrobial packaging: A review. Crit. Rev. Food Sci. Nutr. 2004, 44, 223–237. [Google Scholar]
- Baldevraj, R.M.; Jagadish, R.S. Incorporation of chemical antimicrobial agents into polymeric films for food packaging. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Woodhead Publishing: Sawston, UK, 2011; pp. 368–420. [Google Scholar]
- Cagri, A.; Ustunol, Z.; Ryser, E.T. Antimicrobial, mechanical, and moisture barrier properties of low pH whey protein-based edible films containing p-Aminobenzoic or sorbic acids. J. Food Sci. 2001, 66, 865–870. [Google Scholar] [CrossRef]
- Lee, J.W.; Son, S.M.; Hong, S.I. Characterization of protein-coated polypropylene films as a novel composite structure for active food packaging application. J. Food Eng. 2008, 86, 484–493. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Characterization and antimicrobial activity studies of polypropylene films with carvacrol and thymol for active packaging. J. Food Eng. 2012, 109, 513–519. [Google Scholar] [CrossRef]
- Torlak, E.; Sert, D. Antibacterial effectiveness of chitosan–propolis coated polypropylene films against foodborne pathogens. Int. J. Biol. Macromol. 2013, 60, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Meira, S.M.M.; Zehetmeyer, G.; Jardim, A.I.; Scheibel, J.M.; de Oliveira, R.V.B.; Brandelli, A. Polypropylene/montmorillonite nanocomposites containing nisin as antimicrobial food packaging. Food Bioprocess Technol. 2014, 7, 3349–3357. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Pichardo, S.; Bermudez, J.M.; Baños, A.; Ariza, J.J.; Guillamón, E.; Aucejo, S.; Cameán, A.M. Characterization and antimicrobial activity of active polypropylene films containing Oregano essential oil and Allium extract to be used in packaging for meat products. Food Addit. Contam. Part A 2017, 35, 782–791. [Google Scholar]
- Krepker, M.; Prinz-Setter, O.; Shemesh, R.; Vaxman, A.; Alperstein, D.; Segal, E. Antimicrobial carvacrol-containing polypropylene films: Composition, structure and function. Polymers 2018, 10, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedotova, O.B.; Myalenko, D.M. An unconventional approach to food packaging disinfection. Dairy Ind. 2016, 1, 25–27. [Google Scholar]
- Fedotova, O.B.; Myalenko, D.M.; Shalaeva, A.B. “Active packaging” made of polymeric materials. Food Ind. 2010, 1, 22–23. [Google Scholar]
- Cooksey, K. Utilization of antimicrobial packaging films for inhibition of selected microorganism. In Food Packaging Testing Methods and Applications; Risch, S.J., Ed.; American Chemical Society: Seattle, WA, USA, 2000; pp. 17–25. [Google Scholar]
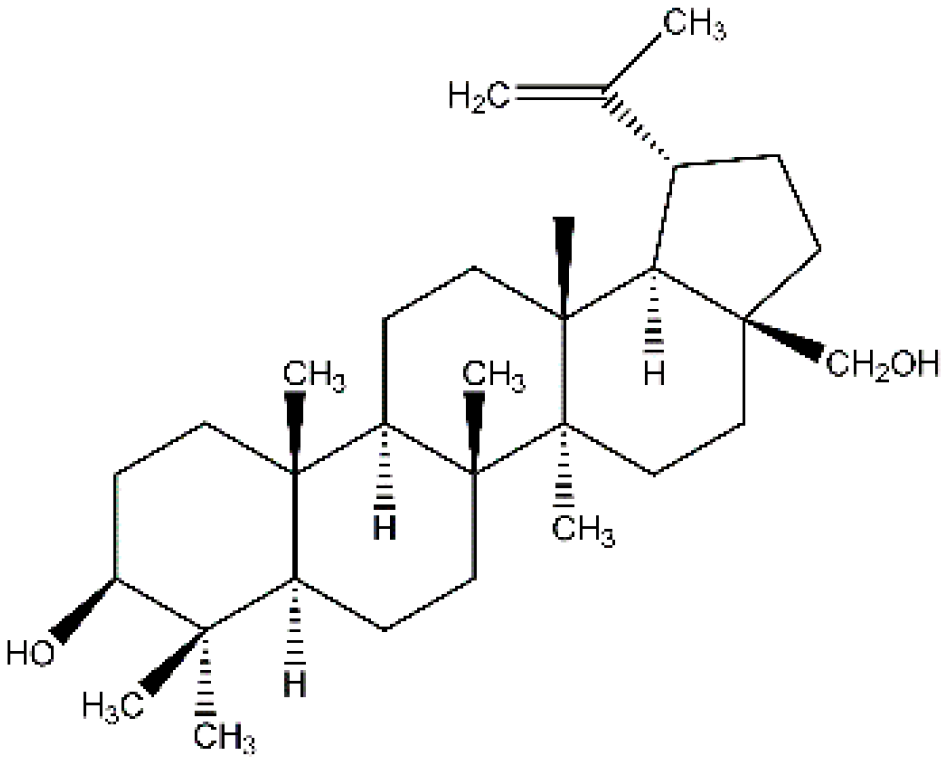
- Tolstikova, T.G.; Sorokina, I.V. Lupane series terpenoids—Biological activity and pharmacological prospects. Derivatives of the lupane series. Bioorganic Chem. 2006, 1, 42–55. [Google Scholar]
- Alakurtti, S.; Mäkelä, T.; Koskimies, S.; Yli-Kauhaluoma, J. Pharmacological properties of the ubiquitous natural product botulin. Eur. J. Pharm. Sci. 2006, 29, 1–13. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Kotsanopoulos, K.V. Migration phenomenon in food packaging. Food–Package interactions, mechanisms, types of migrants, testing and relative legislation—A review. Food Bioprocess Technol. 2014, 7, 21–36. [Google Scholar] [CrossRef]
- Blondeau, D.; St-Pierre, A.; Bourdeau, N.; Bley, J.; Lajeunesse, A.; Desgagné-Penix, I. Antimicrobial activity and chemical composition of white birch (Betula papyrifera Marshall) bark extracts. Microbiologyopen 2020, 9, e00944. [Google Scholar] [CrossRef] [Green Version]
- Khaitbaev, A.K.; Turgunbaev, S.S. Synthesis of betulinic acid. Bull. Fergu. 2019, 4, 24–28. [Google Scholar]
- Turgunbaev, S.S.; Khaitbaev, A.K. Obtaining extractive substances of birch. Univers. Chem. Biol. Electron. Sci. Mag. 2020, 8, 27–31. [Google Scholar]
- Zalesińska, M.D.; Borska, S. Betulin and its derivatives—Precursors of new drugs. World Sci. News 2019, 127, 123–138. [Google Scholar]
- Huang, T.; Chen, C.; Li, D.; Ek, M. Hydrophobic and antibacterial textile fibres prepared by covalently attaching betulin to cellulose. Cellulose 2019, 26, 665–677. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Li, D.; Ek, M. Water repellency improvement of cellulosic textile fibers by betulin and a betulin-based copolymer. Cellulose 2018, 25, 2115–2128. [Google Scholar] [CrossRef] [Green Version]
- Tolstikova, T.G.; Sorokina, I.V.; Tolstikov, G.A.; Tolstikov, A.G.; Flekhter, O.B. Biological activity and pharmacological prospects of lupane terpenoids: I. Natural lupane derivatives. Russ. J. Bioorganic Chem. 2006, 32, 37–49. [Google Scholar] [CrossRef]
- Alexandrovna, V.O. Development and Standardization of the Phytopreparation Betulin and Thymol Based on Pumpkin Seed Oil. Ph.D. Thesis, Samara State Medical University of the Ministry of Health of the Russian Federation, Samara, Russia, 2016; p. 120. [Google Scholar]
- Shachtschneider, T.P.; Kuznetsova, S.A.; Mikhailenko, M.A. Obtaining non-toxic composites of betulin with polyvinylpyrrolidone and polyethylene glycol. Chemistry 2012, 1, 52–60. [Google Scholar]
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol. Adv. 2020, 38, 107409. [Google Scholar] [CrossRef]
- Tuli, H.S.; Sak, K.; Gupta, D.S.; Kaur, G.; Aggarwal, D.; Chaturvedi Parashar, N.; Choudhary, R.; Yerer, M.B.; Kaur, J.; Kumar, M.; et al. Anti-Inflammatory and Anticancer Properties of Birch Bark-Derived Betulin: Recent Developments. Plants 2021, 10, 2663. [Google Scholar] [CrossRef]
- Nagorny, M.Y.; Fedotova, O.B. Inhibitory properties of a multilayer packaging material modified with an antimicrobial natural component. Food Industry 2013, 2, 32–33. [Google Scholar]
- Yuryevich, N.M. Development of a Modified Combined Material for Dairy Products Packaging. Ph.D. Thesis, V.M. Gorbatov Research Institute of Meat Industry, Moscow, Russia, 2013; p. 131. [Google Scholar]
- Fedotova, O.B.; Nagorny, M.Y.; Myalenko, D.M. Development of modified packaging material. Milk Processing 2014, 1, 6–7. [Google Scholar]
- GOST 31796-2012. Meat and Meat Products. Accelerated Histological Method for Determining the Structural Composition Formulation. 2019. Available online: https://www.turkmenistanlaws.com/p-56107-gost-31796-2012.aspx (accessed on 11 January 2022).
- Elizaquivel, P.; Aznar, R. A multiplex RTi-PCR reaction for simultaneous detection of Escherichia coli O157: H7, Salmonella spp. And Staphylococcus aureus on fresh, minimally processed vegetables. Food Microbiol. 2008, 25, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.A.; Karel, M. Microbial stabilization of intermediate moisture food surfaces III. Effects of surface preservative concentration and surface pH control on microbial stability of an intermediate moisture cheese analog. J. Food Processing Preserv. 1985, 9, 107–119. [Google Scholar] [CrossRef]
- Filchakova, S.A. Microbiological purity of packaging for dairy products. Dairy Ind. 2008, 7, 44–46. [Google Scholar]
- Filchakova, S.A.; Medvedev, E.V.; Tambovtsev, Y.N. Ecologically safe products with functional properties. Ecol. Syst. Devices 2006, 8, 42–44. [Google Scholar]
- Semenikhina, V.F.; Rozhkova, I.V. Microbiology in the dairy industry. Dairy Ind. 2004, 12, 22–24. [Google Scholar]
- Mialenko, D.M. Improving the Technology of Packaging Dairy Products by Disinfecting Consumer Containers with Pulsed Ultraviolet Radiation. Ph.D. Thesis, V.M. Gorbatov Research Institute of the Meat Industry, Moscow, Russia, 2009. [Google Scholar]
- Ovchinnikov, V.; Mastalygina, E.; Pantyukhov, P. Investigation of novel polymer composites based on recycled multilayer combined packaging materials. Solid State Phenom. 2020, 299, 94–99. [Google Scholar] [CrossRef]
- Nazarov, V.A.; Kondratov, A.P.; Stolyarov, V.P.; Evlampiev, L.A.; Baranov, V.A.; Gagarin, M.A. Surface morphology of a layer of polymers modified with gaseous fluorine. High-Mol. Compd. Ser. A 2006, 48, 1976–1984. [Google Scholar] [CrossRef]
- Bhargava, R.; Wang, S.-Q.; Koenig, J.L. FTIR Microspectroscopy of Polymeric Systems. Adv. Polym. Sci. 2003, 163, 137–191. [Google Scholar]






| № | Birch | Betulin Mass Fraction, % |
|---|---|---|
| 1 | Betula costata | 5 |
| 2 | Betula mandshurica | 27 |
| 3 | Betula pendula | 14 |
| 4 | Betula pubescens | 44 |
| No. | Indicator | Dimension | Value |
|---|---|---|---|
| 1 | Density | g/cm2 | 0.918–0.930 |
| 2 | Breaking stress in disease | kgf/cm2 | 100–170 |
| 3 | Breaking stress in static bending | kgf/cm2 | 120–170 |
| 4 | Breaking stress at shear | kgf/cm2 | 140–170 |
| 5 | Relative strength at break | % | 500–600 |
| 6 | Elasticity modulus in bending | kgf/cm2 | 1200–2600 |
| 7 | Hygiene yield strength | kgf/cm2 | 90–160 |
| 8 | Relative attachment at the beginning of the current | % | 15–20 |
| 9 | Brinell hardness | kgf/mm2 | 1.4–2.5 |
| No. | Indicator | Actual Result |
|---|---|---|
| Physicochemical indicators * | ||
| Water mass fraction, % | Cream-colored powder, with a specific characteristic odor | |
| 2 | Quantitative betulinol content, % | 1.0 |
| 3 | Water mass fraction, % | 70–98 |
| Toxic elements | ||
| 4 | Lead, mg/kg | Less than 0.15 |
| 5 | Arsenic, mg/kg | Less than 0.02 |
| 6 | Cadmium, mg/kg | Less than 0.015 |
| 7 | Mercury, mg/kg | Less than 0.01 |
| Pesticides | ||
| 8 | Hexachlorocyclohexane (α, β, γ-isomers), mg/kg | Not found |
| 9 | DDT and its metabolites, mg/kg | Not found |
| 10 | Heptachlor, mg/kg | Not found |
| 11 | Aldrin, mg/kg | Not found |
| Microbiological indicators | ||
| 12 | QMAFAnM, CFU/g | Less than 10 |
| 13 | CB (coliforms) in 0.1 g | NA |
| 14 | E. coli in 1.0 g | NA |
| 15 | Pathogenic, including salmonella in 10.0 g | NA |
| 16 | Yeast and mold, CFU/g | 20 |
| Base Polymer | Polymer: Extract Ratio | Extract Content in Concentrate, % | Extract Concentration, % | Active Betulin Content, % |
|---|---|---|---|---|
| LDPE | 9:1 | 10 | 70 | 4.90 |
| LDPE | 9:1 | 10 | 78 | 5.46 |
| LDPE | 9:1 | 20 | 78 | 5.46 |
| LDPE | 9:1 | 10 | 80 | 5.60 |
| LDPE | 9:1 | 10 | 90 | 6.30 |
| Additive Concentration (Betulin), % | Actual Result, Lg Nm/Nc | ||
|---|---|---|---|
| Yeasts | Mold Fungi | CB | |
| 70 | 1.00 ± 0.03 | 0.86 ± 0.04 | 1.00 ± 0.02 |
| 78 | 1.10 ± 0.04 | 0.96 ± 0.05 | 1.74 ± 0.02 |
| 80 | 1.18 ± 0.04 | 1.02 ± 0.04 | 1.76 ± 0.01 |
| 98 | 1.49 ± 0.05 | 1.00 ± 0.04 | 1.78 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedotova, O.; Myalenko, D.; Pryanichnikova, N.; Yurova, E.; Agarkova, E. Microscopic and Structural Studies of an Antimicrobial Polymer Film Modified with a Natural Filler Based on Triterpenoids. Polymers 2022, 14, 1097. https://doi.org/10.3390/polym14061097
Fedotova O, Myalenko D, Pryanichnikova N, Yurova E, Agarkova E. Microscopic and Structural Studies of an Antimicrobial Polymer Film Modified with a Natural Filler Based on Triterpenoids. Polymers. 2022; 14(6):1097. https://doi.org/10.3390/polym14061097
Chicago/Turabian StyleFedotova, Olga, Dmitry Myalenko, Nataliya Pryanichnikova, Elena Yurova, and Evgeniya Agarkova. 2022. "Microscopic and Structural Studies of an Antimicrobial Polymer Film Modified with a Natural Filler Based on Triterpenoids" Polymers 14, no. 6: 1097. https://doi.org/10.3390/polym14061097
APA StyleFedotova, O., Myalenko, D., Pryanichnikova, N., Yurova, E., & Agarkova, E. (2022). Microscopic and Structural Studies of an Antimicrobial Polymer Film Modified with a Natural Filler Based on Triterpenoids. Polymers, 14(6), 1097. https://doi.org/10.3390/polym14061097






