Hair Growth-Promoting Activities of Glycosaminoglycans Extracted from the Tunics of Ascidian (Halocynthia roretzi)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Extraction and Proximate Analysis of Ascidian Tunic Mucopolysaccharides
2.3. Separation of Glycosaminoglycans Fractions Using Ion-Exchange Chromatography
2.4. Analysis of Neutral and Amino Sugar Contents by High-Performance Anion Exchange Chromatography-Pulsed Amperometric Detector (HPAEC-PAD)
2.5. HPLC Analysis for Disaccharide Composition
2.6. HFDP Cell Culture and Viability Test
2.7. Inhibition of Dihydrotestosterone (DHT) Production
2.8. Hair Regrowth Activity in C57BL/6 Mice
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of the Ascidian Tunic
3.2. Purification of Ascidian Tunic GAGs Extracts by Ion-Exchange Chromatography
3.3. Sugar Compositions of Ascidian Tunic GAGs Fractions
3.4. Disaccharide Composition of Ascidian GAGs as Determined by HPLC
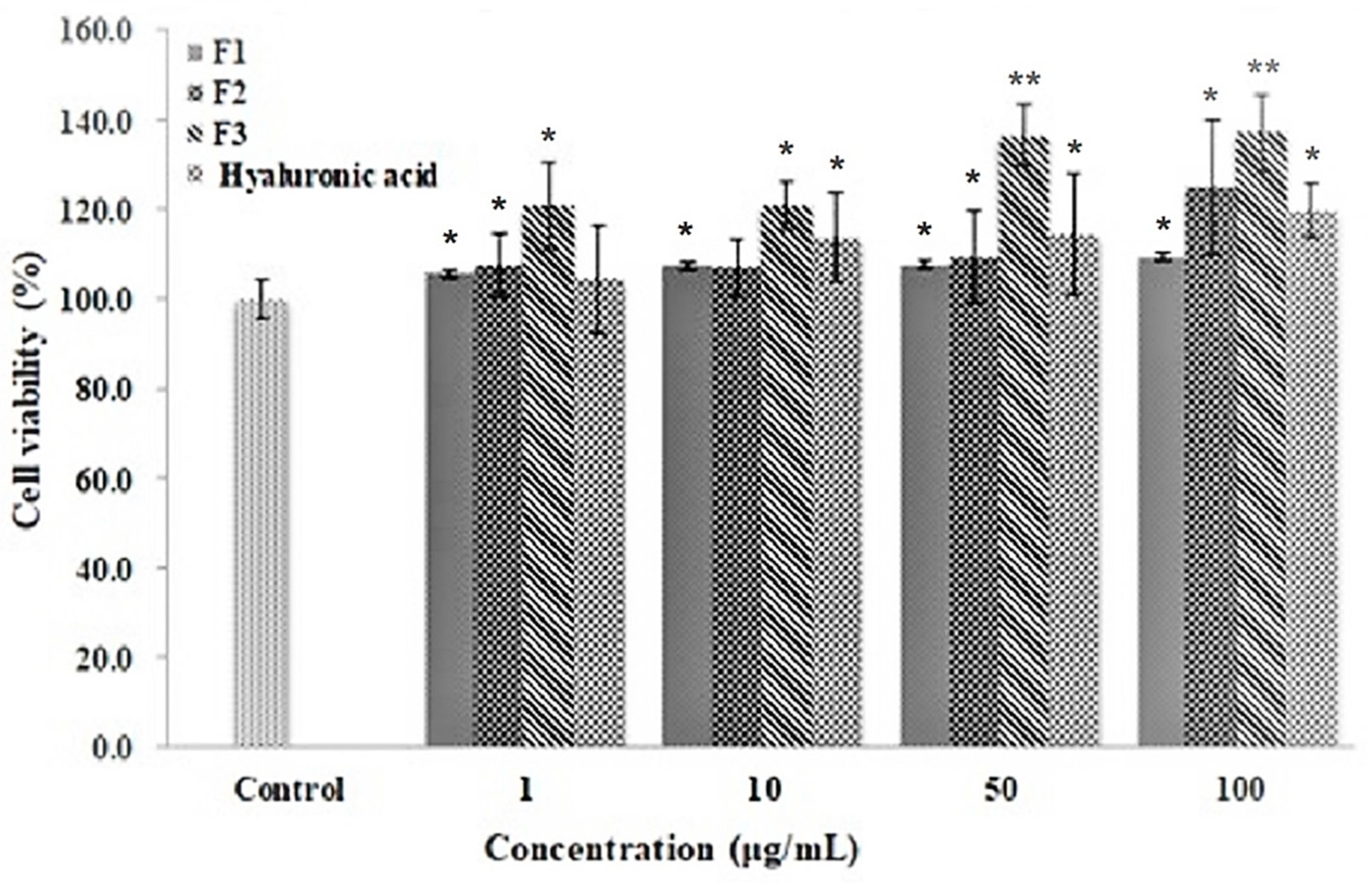
3.5. Cell Proliferation Activity of Ascidian Tunic GAGs on Human Follicle Dermal Papilla (HFDP)
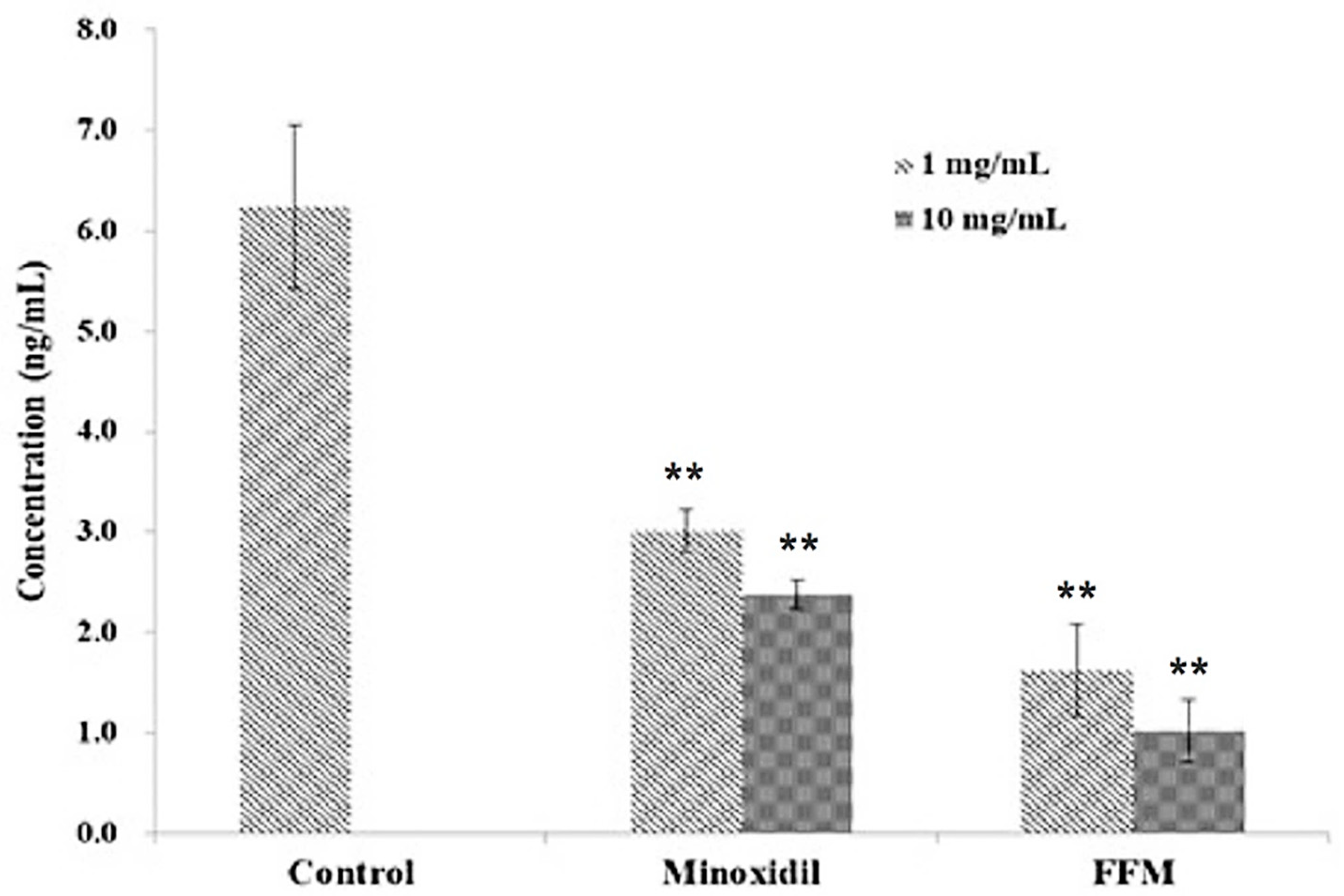
3.6. Inhibitory Effects of the FFM Fraction on Hormonal Factor Dihydrotestosterone (DHT)
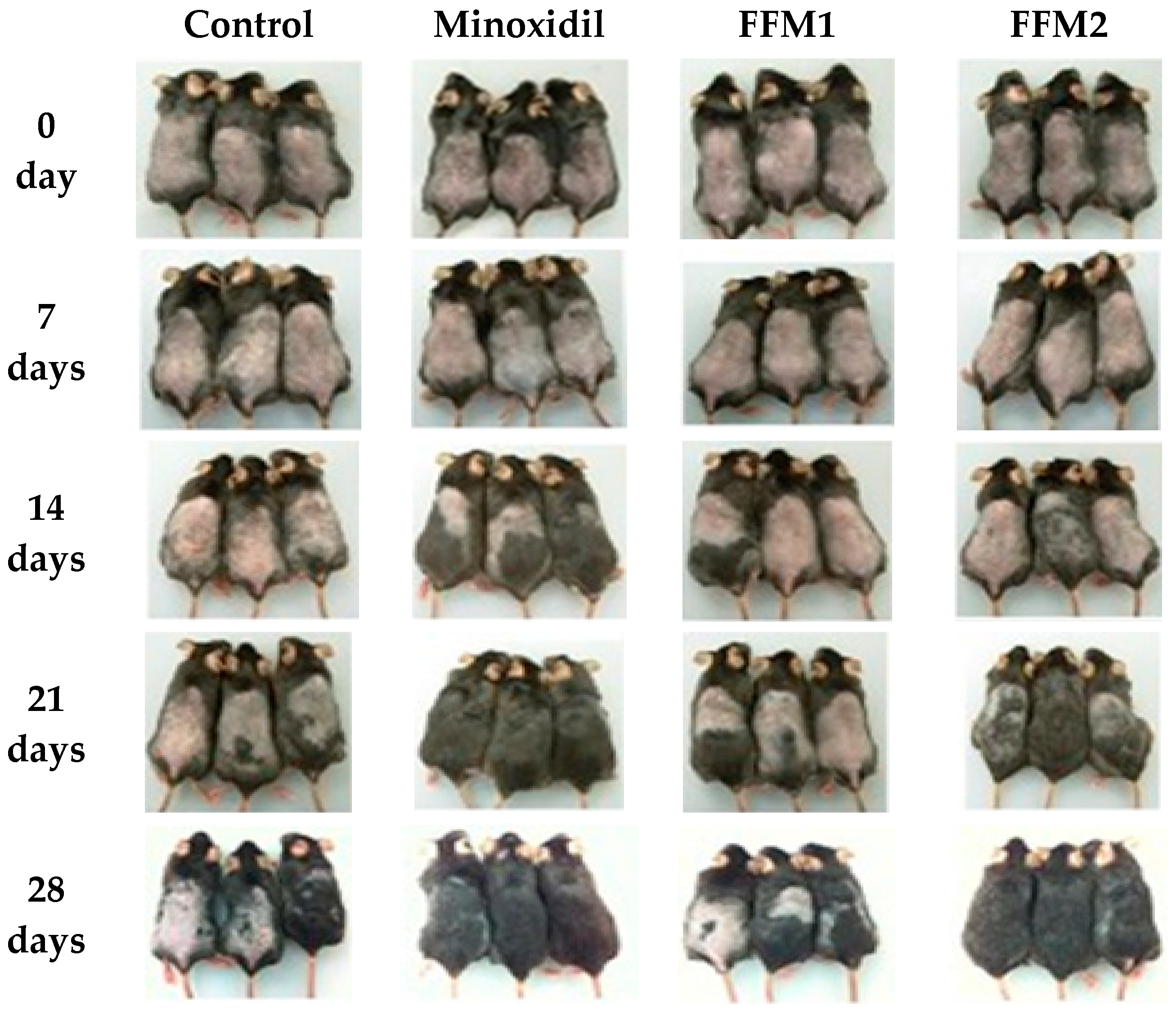
3.7. Hair Growth in C57BL/6
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Rogers, G.E. Hair follicle differentiation and regulation. Int. J. Dev. Biol. 2004, 48, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Stenn, K.S.; Paus, R. Controls of hair follicle cycling. Physiol. Rev. 2001, 81, 449–494. [Google Scholar] [CrossRef] [PubMed]
- Purba, T.S.; Brunken, L.; Peake, M.; Shahmalak, A.; Chaves, A.; Poblet, E.; Ceballos, L.; Gandarillas, A.; Paus, R. Characterizations of cell cycle arrest and terminal differentiation in a maximally proliferative human epithelial tissue: Lessons from the human hair follicle matrix. Eur. J. Cell Biol. 2017, 96, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Madaan, A.; Joshi, V.; Kishore, A.; Verma, R.; Singh, A.T.; Jaggi, M.; Sung, Y.K. In vitro hair growth promoting effects of naringenin and hesperetin on human dermal papilla cells and keratinocytes. Am. J. Dermatol. Venereol. 2017, 6, 51–57. [Google Scholar]
- Jung, M.H. Evaluation of antioxidant fractions and hair loss prevention effects of Platycodon grandiflorum. J. Life Sci. 2019, 29, 779–784. [Google Scholar]
- Lee, B.H.; Lee, J.S.; Kim, Y.C. Hair growth-promoting effects of lavender oil in C57BL/6 mice. Toxicol. Res. 2016, 32, 103–108. [Google Scholar] [CrossRef]
- Shah, K.B.; Shah, A.N.; Solanki, R.B.; Raval, R.C. A comparative study of microneedling with platelet-rich plasma plus topical minoxidil (5%) and topical minoxidil (5%) alone in androgenetic alopecia. Int. J. Trichol. 2017, 9, 14–18. [Google Scholar] [CrossRef]
- Lee, K.H.; Choi, D.B.; Jeong, S.I.; Kim, S.J.; Lee, C.H.; Seo, H.S.; Jeong, H.S. Eclipta prostrata promotes the induction of anagen, sustains the anagen phase through regulation of FGF-7 and FGF-5. Pharm. Biol. 2019, 57, 105–111. [Google Scholar] [CrossRef]
- Price, V.H. Treatment of hair loss. N. Engl. J. Med. 1999, 341, 964–973. [Google Scholar] [CrossRef]
- Rogers, N.E.; Avram, M.R. Medical treatments for male and female pattern hair loss. J. Am. Acad. Dermatol. 2008, 59, 547–566. [Google Scholar] [CrossRef]
- Varki, A.; Sharon, N. Historical background and overview. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2009; pp. 1–22. [Google Scholar]
- Korean Statistics Information Service (KOSIS). Available online: http://kosis.kr/statHtml/statHtml.do?orgId=146&tblId=DT_114_2013_S0015&vw_cd=MT_ZTITLE&list_id=114_12304&seqNo=&lang_mode=ko&language=kor&obj_var_id=&itm_id=&conn_path=MT_ZTITLE (accessed on 10 December 2021).
- Lambert, G.; Karney, R.C.; Rhee, W.Y.; Carman, M.R. Wild and cultured edible tunics: A review. Manag. Biol. Invasion 2016, 7, 59–66. [Google Scholar] [CrossRef]
- Park, S.H.; Jeong, S.B.; Choi, B.D. Immuno- and anti-cancer activities of Halocynthia roretzi tunic extracts on cells. J. Agric. Life Sci. 2013, 47, 163–170. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 7, 911–917. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Robers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Handley, C.J.; Buttle, D.J. Assay of proteoglycan degradation. Methods Enzymol. 1995, 248, 47–58. [Google Scholar]
- Bitter, T.; Muir, H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef]
- Cataldi, T.R.I.; Campa, C.; De Benedetto, G.E. Analysis of carbohydrates by high-performance anion-exchange chromatography with pulsed amperometric detection: The potential is still growing. Fresenius J. Anal. Chem. 2000, 368, 739–758. [Google Scholar] [CrossRef]
- Krichen, F.; Bougatef, H.; Sayari, N.; Capitani, F.; Amor, I.B.; Koubaa, I.; Maccari, F.; Mantovani, V.; Galeotti, F.; Volpi, N.; et al. Isolation, purification and structural characteristics of chondroitin sulfate from smooth hound cartilage: In vitro anticoagulant and antiproliferative properties. Carbohydr. Polym. 2018, 197, 451–459. [Google Scholar] [CrossRef]
- Lee, J.J.; Ticar, B.F.; Rohmah, Z.; Park, S.H.; Kang, D.; Kang, S.J.; Choi, B.D. Skin regeneration effect of the glycosaminoglycans from Liparis tessellatus eggs. Int. J. Biol. Macromol. 2017, 105, 1369–1374. [Google Scholar] [CrossRef]
- Matsuda, H.; Yamazaki, M.; Naruo, S.; Asanuma, Y.; Kubo, M. Anti-androgenic and hair growth promoting activities of Lygodii spora (Spore of Lygodium japonicum) I. Active constituents inhibiting testosterone 5α-reductase. Biol. Pharm. Bull. 2002, 25, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Handel, T.M.; Johnson, Z.; Crown, S.E.; Lau, E.K.; Proudfoot, A.E. Regulation of protein function by glycosaminoglycans-as exemplified by chemokines. Annu. Rev. Biochem. 2005, 74, 385–410. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.; Ojeda, R.; de Paz, J.L.; Lucas, R.; Nieto, P.M.; Lozano, R.M.; Redondo-Horcajo, M.; Giménez-Gallego, G.; Martín-Lomas, M. The activation of fibroblast growth factors (FGFs) by glycosaminoglycans: Influence of the sulfation pattern on the biological activity of FGF-1. ChemBioChem 2004, 5, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M. Heparin and angiogenic therapy. Eur. Heart J. 2000, 21, 270–274. [Google Scholar] [CrossRef][Green Version]
- Hong, B.I.; Hong, B.C.; Jung, W.J.; Ruck, J.H.; Choi, B.D.; Kee, K.H. Chemical properties of sulfated polysaccharides in ascidian (Halocynthia roretzi) tunic. J. Korean Fish. Soc. 2001, 34, 632–637. [Google Scholar]
- Nacional, L.M.; Lee, J.S.; Kang, S.J.; Choi, B.D. Seasonal variation in the nutritional content of mideodeok Styela clava. J. Fish. Sci. Technol. 2006, 9, 49–56. [Google Scholar]
- Garnjanagoonhorn, W.; Wongekalak, L.; Engkagul, A. Determination of chondroitin sulfate from different sources of cartilage. Chem. Eng. Process. 2007, 46, 465–471. [Google Scholar] [CrossRef]
- Guilloux, K.; Gaillard, I.; Courtois, J.; Courtois, B.; Petit, E. Production of arabinoxylan-oligosaccharides from flaxseed (Linum usitatissimum). J. Agric. Food Chem. 2009, 57, 11308–11313. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef]
- Dry, E. The coat of the mouse (Mus musculus). J. Genet. 1926, 16, 287–340. [Google Scholar] [CrossRef]
- Randall, V.A. Androgens are the main regulator of human hair growth. In Hair and Its Disorders, Biology, Pathology and Management; Camacho, F., Randall, V., Price, V.A., Eds.; Martin Dunitz Ltd.: London, UK, 2000; pp. 121–136. [Google Scholar]
- Pomin, V.H. NMR structural determination of unique invertebrate glycosaminoglycans endowed with medical properties. Carbohydr. Res. 2015, 413, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Malgouries, S.; Thibaut, S.; Bernard, B.A. Proteoglycan expression patterns in human hair follicle. Br. J. Dermatol. 2008, 158, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Couchman, J.R.; Caterson, B.; Christner, J.E.; Baker, J.R. Mapping by monoclonal antibody detection of glycosaminoglycans in connective tissues. Nature 1984, 307, 650–652. [Google Scholar] [CrossRef] [PubMed]
- Westgate, G.E.; Messenger, A.G.; Watson, L.P.; Gibson, W.T. Distribution of proteoglycans during the hair growth cycle in human skin. J. Investig. Dermatol. 1991, 96, 191–195. [Google Scholar] [CrossRef]
- Urysiak-Czubatka, I.; Kmiec, M.L.; Broniarczyk-Dyła, G. Assessment of the usefulness of dihydrotestosterone in the diagnostics of patients with androgenetic alopecia. Postep. Dermatol. Alergol. 2014, 31, 207–215. [Google Scholar] [CrossRef]
- Semalty, M.; Semalty, A.; Joshi, G.P.; Rawat, M.S.M. Hair growth and rejuvenation: An overview. J. Dermatol. Treat. 2011, 22, 123–132. [Google Scholar] [CrossRef]
- Mella, J.M.; Perret, M.C.; Manzotti, M.; Catalano, H.N. Efficacy and safety of finasteride therapy for androgenetic alopecia: A systematic review. Arch. Dermatol. 2010, 146, 1141–1150. [Google Scholar] [CrossRef]
- Botchkarev, V.A.; Kishimoto, J. Molecular control of epithelial-mesenchymal interactions during hair follicle cycling. J. Investig. Dermatol. Symp. Proc. 2003, 8, 46–55. [Google Scholar] [CrossRef]


| Components | Percentage 1 (%) |
|---|---|
| Yield | 5.3 ± 0.3 |
| Moisture | 3.1 ± 0.4 c |
| Crude protein | 35.9 ± 2.3 a |
| Lipid | 1.2 ± 0.1 c |
| Ash | 32.0 ± 1.2 a |
| Carbohydrates | 27.8 ± 2.9 a,b |
| Mucopolysaccharides | 57.1 ± 1.5 |
| Sulfate | 3.6 ± 0.3 |
| Fractions | Yields (mg%) | Sulfated GAGs (%) |
|---|---|---|
| F1 | 15.7 ± 0.2 c | - |
| F2 | 29.0 ± 0.4 b | 7.7 ± 0.6 b |
| F3 | 36.0 ± 0.7 a | 18.2 ± 1.5 a |
| Sugar Components | Fractions | ||
|---|---|---|---|
| F1 | F2 | F3 | |
| Fucose | ND | 0.19 | ND |
| Rhamnose | ND | 0.28 | ND |
| Arabinose | ND | ND | ND |
| Galactose | ND | 3.19 | 1.60 |
| Glucose | 1.00 | 1.00 | 1.00 |
| Mannose | ND | 0.72 | 0.87 |
| Amino sugars | |||
| N-acetyl galactosamine | 0.01 | 2.23 | 1.48 |
| N-acetyl glucosamine | 0.01 | 4.16 | 6.56 |
| Peak No. a | Disaccharide | tR(min) b | Proportion of the Disaccharides c | |||
|---|---|---|---|---|---|---|
| F2abc d | F2ac e | F3abc f | F3ac g | |||
| 1 | Di-OS | 27.35 | ND | ND | ND | ND |
| 2 | Di-6S | 47.79 | 47.26 | 49.32 | 49.32 | 43.10 |
| 3 | Di-4S | 51.40 | 52.74 | 50.68 | 50.68 | 56.90 |
| 4 | Di-SD | 74.93 | ND | ND | ND | ND |
| 5 | Di-SE | 77.95 | ND | ND | ND | ND |
| 6 | Di-SB | 78.49 | ND | ND | ND | ND |
| 7 | Di-TriS | 85.66 | ND | ND | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neri, T.A.N.; Palmos, G.N.; Park, S.Y.; Jung, T.S.; Choi, B.-D. Hair Growth-Promoting Activities of Glycosaminoglycans Extracted from the Tunics of Ascidian (Halocynthia roretzi). Polymers 2022, 14, 1096. https://doi.org/10.3390/polym14061096
Neri TAN, Palmos GN, Park SY, Jung TS, Choi B-D. Hair Growth-Promoting Activities of Glycosaminoglycans Extracted from the Tunics of Ascidian (Halocynthia roretzi). Polymers. 2022; 14(6):1096. https://doi.org/10.3390/polym14061096
Chicago/Turabian StyleNeri, Therese Ariane N., Grace N. Palmos, Shin Young Park, Tae Sung Jung, and Byeong-Dae Choi. 2022. "Hair Growth-Promoting Activities of Glycosaminoglycans Extracted from the Tunics of Ascidian (Halocynthia roretzi)" Polymers 14, no. 6: 1096. https://doi.org/10.3390/polym14061096
APA StyleNeri, T. A. N., Palmos, G. N., Park, S. Y., Jung, T. S., & Choi, B.-D. (2022). Hair Growth-Promoting Activities of Glycosaminoglycans Extracted from the Tunics of Ascidian (Halocynthia roretzi). Polymers, 14(6), 1096. https://doi.org/10.3390/polym14061096






