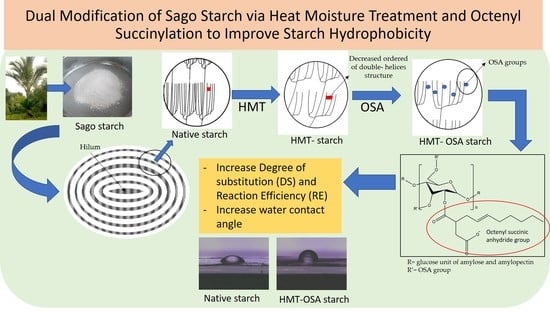
Dual Modification of Sago Starch via Heat Moisture Treatment and Octenyl Succinylation to Improve Starch Hydrophobicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Starch Modification
2.2.1. Heat Moisture Treatment (HMT) Modified Sago Starch
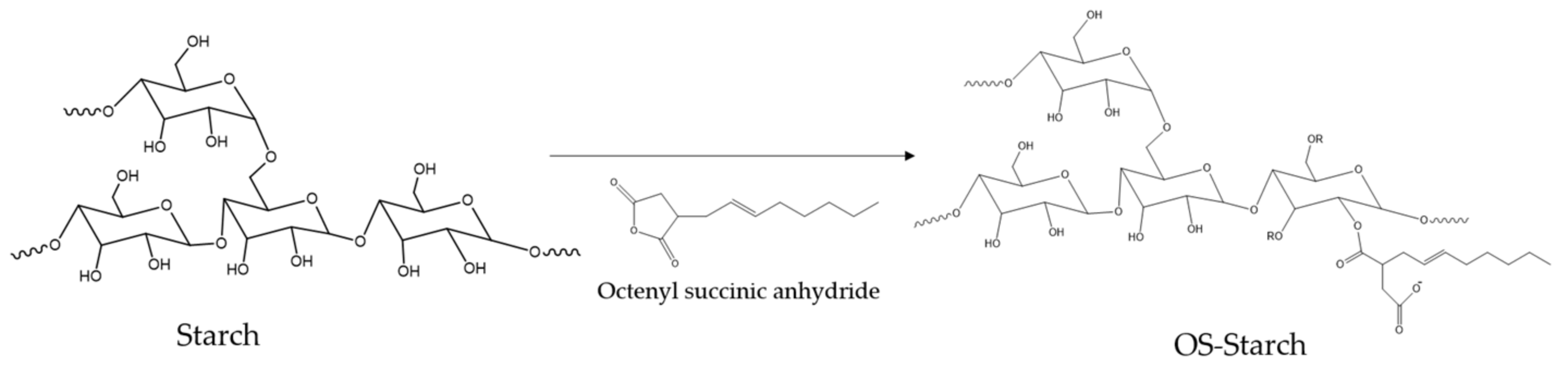
2.2.2. OSA Modified Sago Starch
2.3. Physicochemical Properties of Native and Modified Sago Starch Determination
2.4. Pasting Properties Analysis
2.5. Paste Clarity Determination
2.6. Analysis of the Thermal Properties
2.7. X-ray Diffraction Analysis
2.8. Morphology Granules Analysis
2.9. Degree of Substitution and Reaction Efficiency Determination
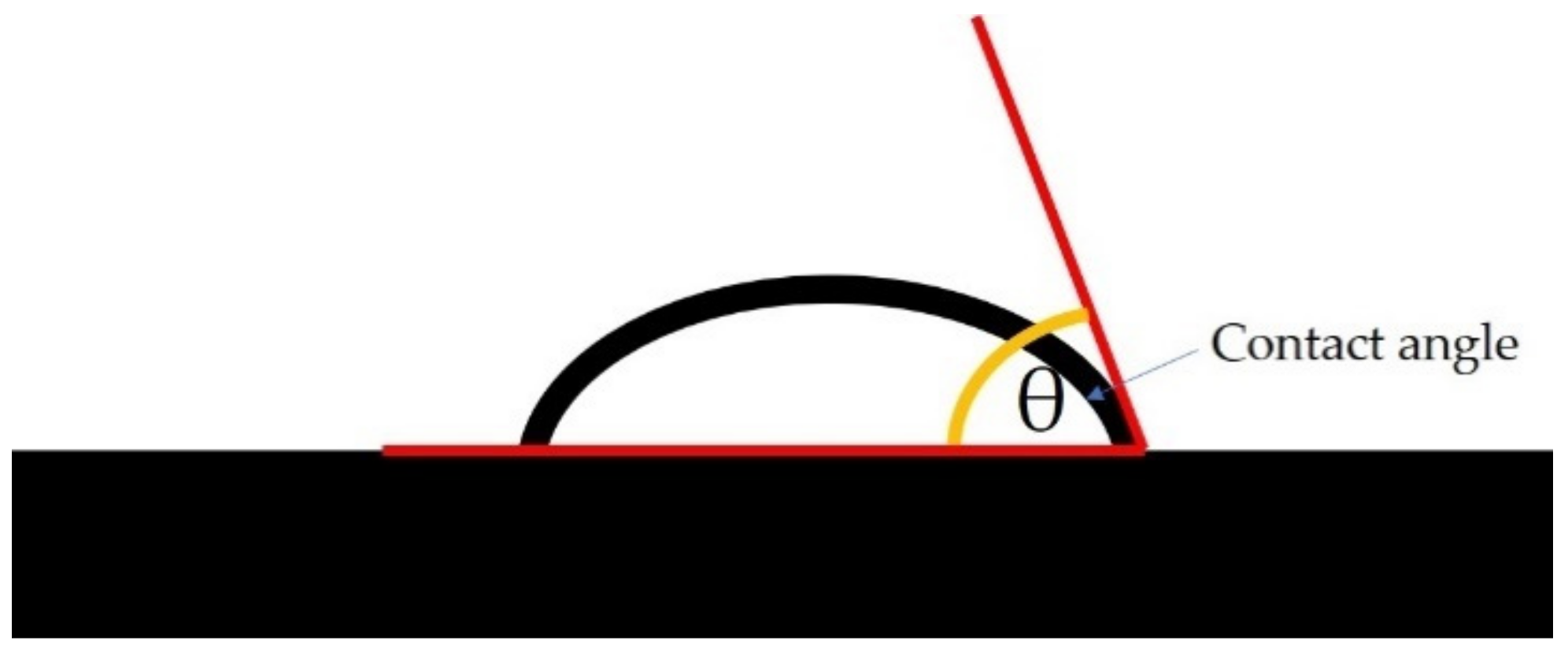
2.10. Water Contact Angle Measurement
2.11. Water Solubility Test
2.12. Water Vapor Permeability Test
2.13. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3. Results
3.1. Physicochemical Properties
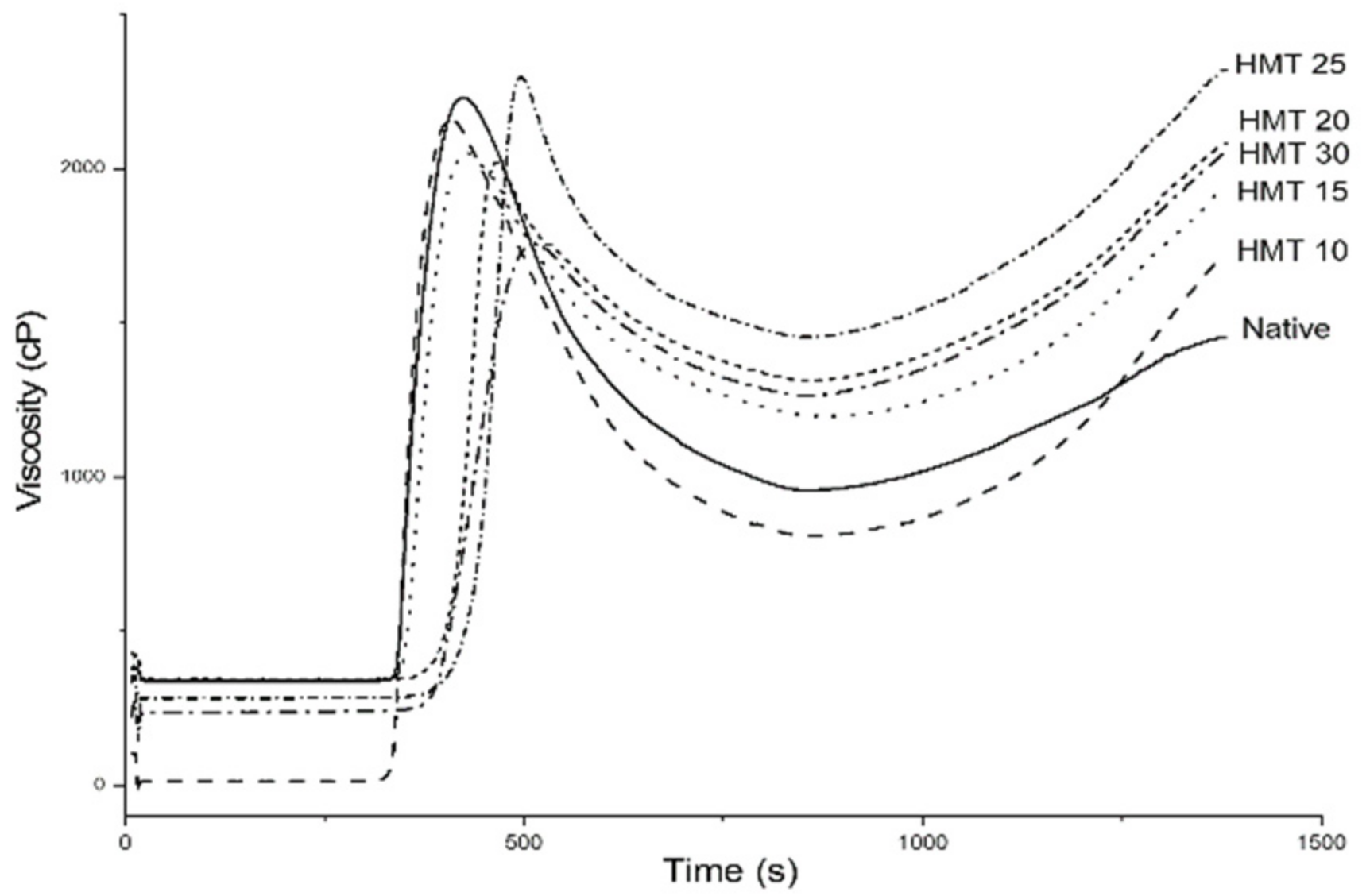
3.2. Pasting Properties
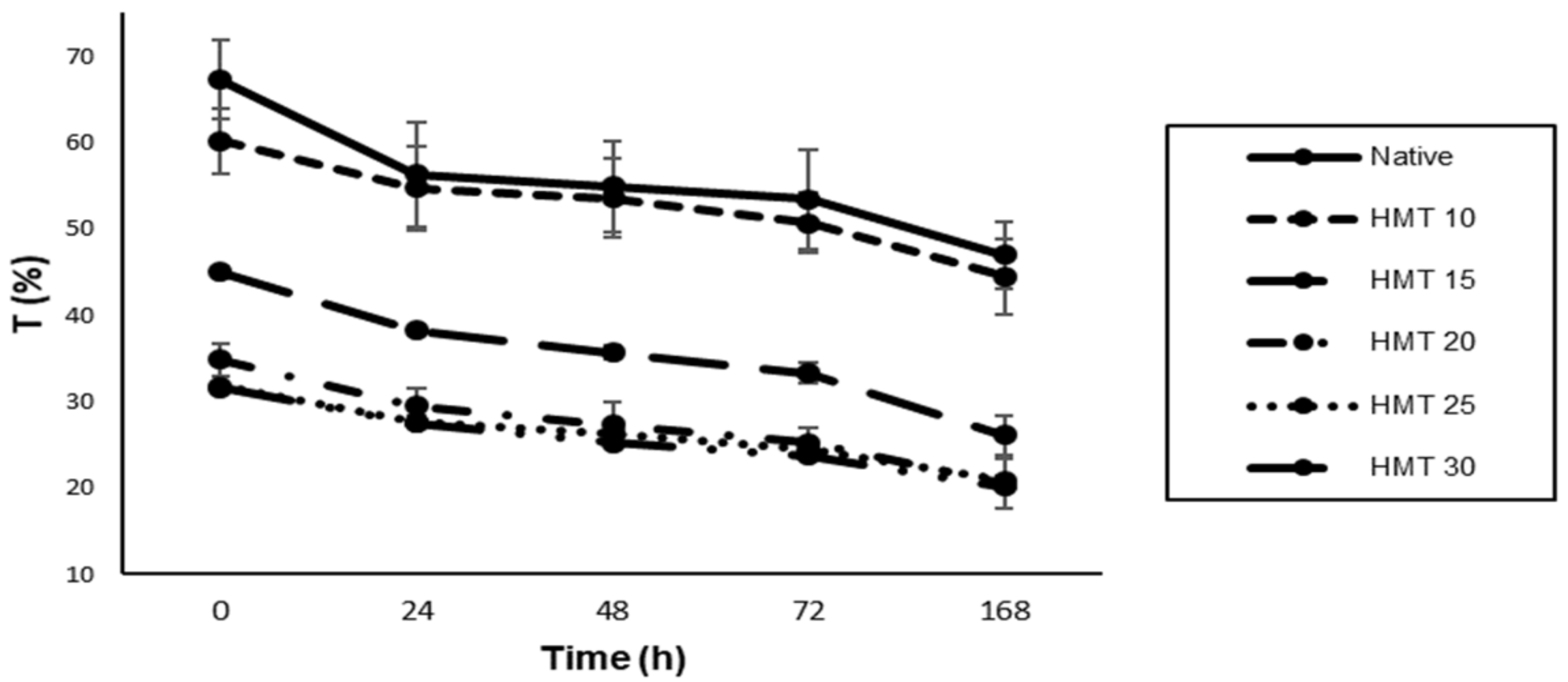
3.3. Paste Clarity
3.4. Thermal Properties
3.5. X-ray Diffraction Pattern and Relative Crystallinity
3.6. Granule Morphology
3.7. Degree of Substitution and Reaction Efficiency
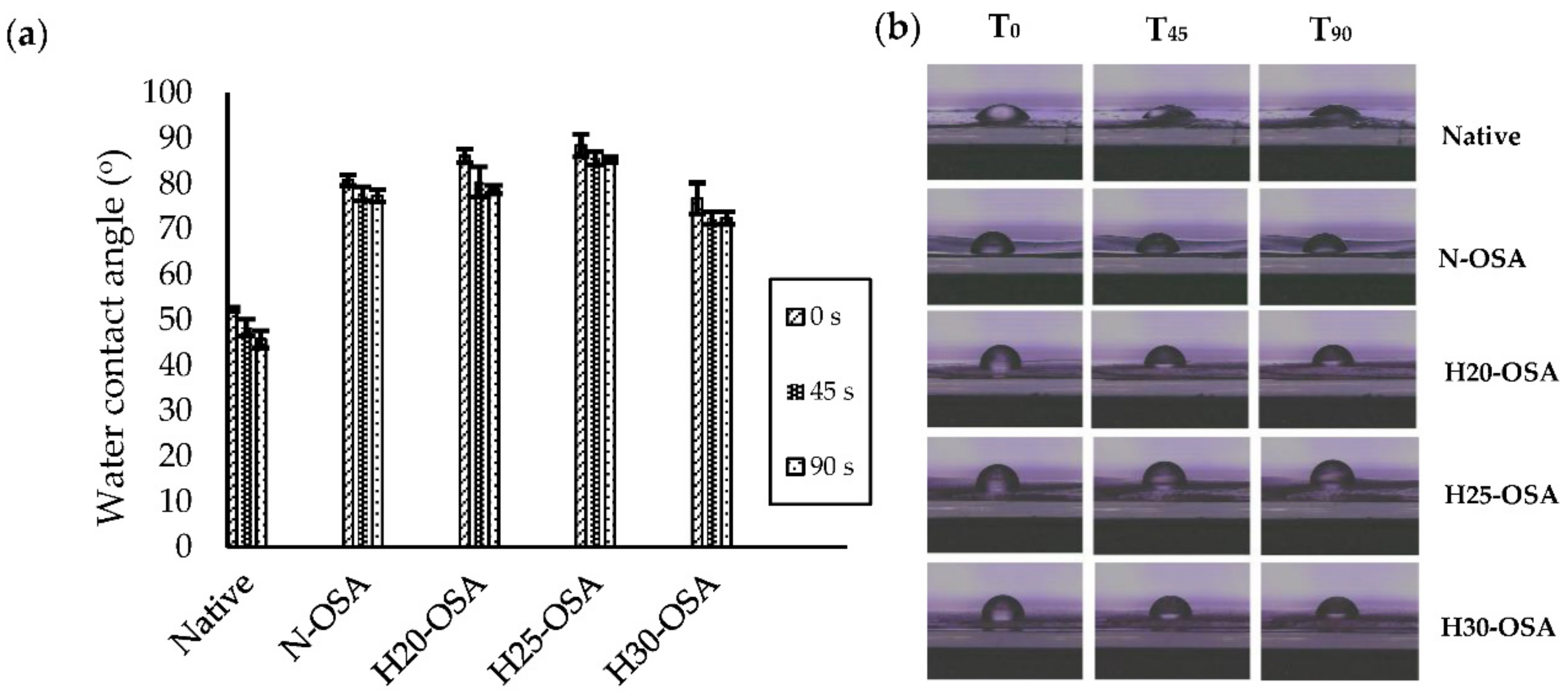
3.8. Water Contact Angle
3.9. Oil Absorption Capacity, Water Solubility, and Water Vapor Permeability
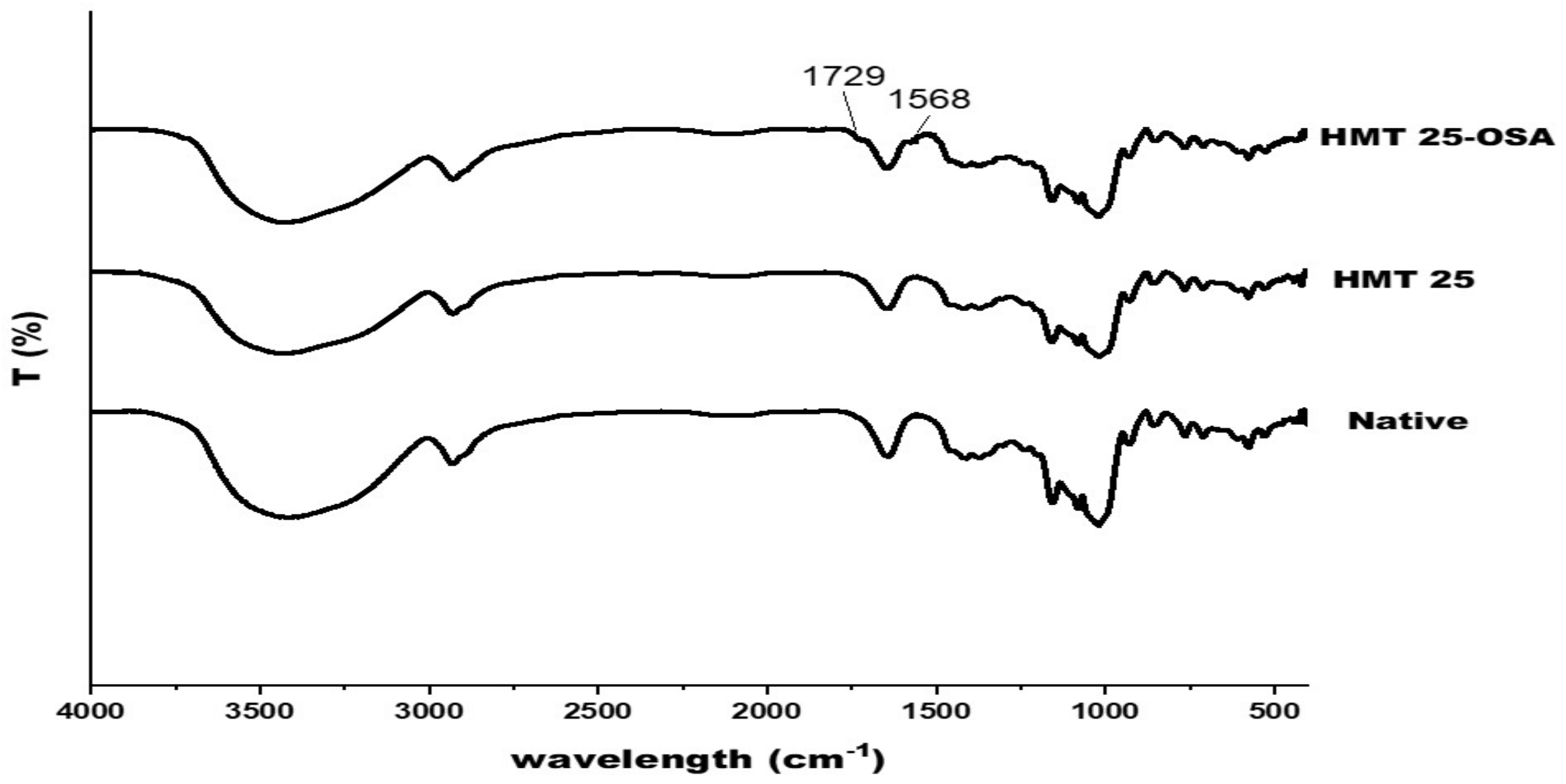
3.10. FTIR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abiddin, N.Z.; Yusoff, A.; Ahmad, N. Effect of octenylsuccinylation on physicochemical, thermal, morphological, and stability of octenyl succinic anhydride (OSA) modified sago starch. Food Hydrocoll. 2018, 75, 138–146. [Google Scholar] [CrossRef]
- Zhu, F. Recent advances in modifications and applications of sago starch. Food Hydrocoll. 2019, 96, 412–423. [Google Scholar] [CrossRef]
- Polnaya, F.J.; Talahatu, J.; Marseno, D.W. Properties of biodegradable films from hydroxypropyl sago starches. Asian J. Food Agro-Ind. 2012, 5, 183–192. [Google Scholar]
- Zhang, Y.R.; Zhang, S.D.; Wang, X.L.; Chen, R.Y.; Wang, Y.Z. Effect of carbonyl content on the properties of thermoplastic oxidized starch. Carbohydr. Polym. 2009, 78, 157–161. [Google Scholar] [CrossRef]
- da Rosa Zavareze, E.; Pinto, V.Z.; Klein, B.; El Halal, S.L.M.; Elias, M.C.; Prentice-Hernández, C.; Dias, A.R.G. Development of oxidized and heat-moisture treated potato starch film. Food Chem. 2012, 132, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Ye, F.; Liu, J.; Zhao, G. Effects of octenylsuccination on physical, mechanical, and moisture-proof properties of stretchable sweet potato starch film. Food Hydrocoll. 2015, 46, 226–232. [Google Scholar] [CrossRef]
- Panrong, T.; Karbowiak, T.; Harnkarnsujari, N. Effects of acetylated and octenyl-succi starch on properties and release of green tea compounded starch/LLDPE blend films. J. Food Eng. 2020, 284, 110057. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, B.; Li, J.; Shu, Z.; Wang, P.; Zeng, X. Structure and properties of octenyl succinic anhydride-modified high-amylose japonica rice starches. Polymers 2021, 13, 1325. [Google Scholar] [CrossRef]
- Jiranuntakul, W.; Puncha-arnon, S.; Uttapap, D. Enhancement of octenyl succinylation of cassava starch by prior modification with heat-moisture treatment. Starch/Starke 2014, 66, 1071–1078. [Google Scholar] [CrossRef]
- Han, S.; Zhu, X.; Zhang, B. Optimization of Reaction Conditions of Octenyl Succinic Anhydride Potato Starch and Its Morphology, Crystalline Structure and Thermal Characterization. Adv. Mater. Res. 2011, 238, 2279–2289. [Google Scholar] [CrossRef]
- Altuna, L.; Lidia, M.; Laura, M. Synthesis and characterization of octenyl succinic anhydride modified starches for food applications. A review of recent literature. Food Hydrocoll. 2018, 80, 97–110. [Google Scholar] [CrossRef]
- Abiddin, Z.; Yussof, A.; Ahmad, N. Optimisation of reaction conditions of octenyl succinic anhydride (OSA) modified sago starch using response surface methodology (RSM). Int. Food Res. J. 2015, 22, 930–935. [Google Scholar]
- Saw, S.S.; Shariffa, N.Y.; Rusi, A.S.; Uthumporn, U. Physicochemical and emulsifying properties of pre-treated octenyl succinic anhydride (OSA) sago starch in a simple emulsion system. Food Res. 2020, 4, 1326–1332. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, S.; Xiong, S. Molecular properties of octenyl succinic esters of mechanically activated Indica rice starch. Starch/Starke 2013, 65, 453–460. [Google Scholar] [CrossRef]
- Sharma, M.; Singh, A.K.; Yadav, D.N.; Arora, S.; Vishwakarma, R.K. Impact of octenyl succinylation on rheological, pasting, thermal, and physicochemical properties of pearl millet (Pennisetum typhoides) starch. LWT-Food Sci. Technol. 2016, 73, 52–59. [Google Scholar] [CrossRef]
- Bhosale, R.; Singhal, R. Process optimization for the synthesis of octenyl succinyl derivative of waxy corn and amaranth starches. Carbohydr. Polym. 2006, 66, 521–527. [Google Scholar] [CrossRef]
- Ruan, H.; Chen, Q.; Fu, M.; Xu, Q.; He, G. Preparation and properties of octenyl succinic anhydride modified potato starch. Food Chem. 2009, 114, 81–86. [Google Scholar]
- Lv, Q.-Q.; Li, G.-Y.; Xie, Q.-T.; Zhang, B.; Li, X.-M.; Pan, Y.; Chen, H.-Q. Evaluation studies on the combined effect of hydrothermal treatment and octenyl succinylation on the physicochemical, structural, and digestibility characteristics of sweet potato starch. Food Chem. 2018, 256, 413–418. [Google Scholar] [CrossRef]
- Sweedman, M.C.; Tizzotti, M.J.; Schäfer, C.; Gilbert, R. Structure and physicochemical properties of octenyl succinic anhydride modified starches: A review. Carbohydr. Polym. 2013, 92, 905–992. [Google Scholar] [CrossRef]
- Hu, H.; Liu, W.; Shi, J.; Huang, Z.; Zhang, Y.; Huang, A.; Yang, M.; Qin, X.; Shen, F. Structure and functional properties of octenyl succinic anhydride modified starch prepared by a non-conventional technology. Starch/Staerke 2016, 68, 151–159. [Google Scholar] [CrossRef]
- Zavareze, E.D.R.; Dias, A.R.G. Impact of heat-moisture treatment and annealing in starches: A review. Carbohydr. Polym. 2011, 83, 317–328. [Google Scholar] [CrossRef]
- Bharti, I.; Singh, S.; Saxena, D.C. Exploring the influence of heat moisture treatment on physicochemical, pasting, structural and morphological properties of mango kernel starches from Indian cultivars. LWT-Food Sci. Technol. 2018, 110, 197–206. [Google Scholar] [CrossRef]
- Huang, T.T.; Zhou, D.N.; Jin, Z.Y.; Xu, X.M.; Chen, H.Q. Effect of repeated heat-moisture treatments on digestibility, physicochemical and structural properties of sweet potato starch. Food Hydrocoll. 2016, 54, 202–210. [Google Scholar] [CrossRef]
- Khunae, P.; Tran, T.; Sirivongpaisal, P. Effect of heat-moisture treatment on structural and thermal properties of rice starches differing in amylose content. Starch/Starke 2007, 59, 593–599. [Google Scholar] [CrossRef]
- Chen, X.; He, X.; Huan, Q. Effects of hydrothermal pretreatment on subsequent octenyl succinic anhydride (OSA) modification of cornstarch. Carbohydr. Polym 2014, 101, 493–498. [Google Scholar] [CrossRef]
- Pukkahuta, C.; Varavinit, S. Structural transformation of sago starch by heat-moisture and osmotic-pressure treatment. Starch/Starke 2007, 59, 624–631. [Google Scholar] [CrossRef]
- Zavareze, E.D.R.; Storck, C.R.; de Castro, L.A.S.; Schirmer, M.A.; Dias, A.R.G. Effect of heat-moisture treatment on rice starch of varying amylose content. Food Chem. 2009, 121, 358–365. [Google Scholar] [CrossRef]
- Uzomah, A.; Ibe, C. The functional properties, pasting and baking behavior of chemically modified sour cassava starches. Afr. J. Food Sci. 2011, 5, 686–694. [Google Scholar]
- Mehboob, S.; Ali, T.M.; Alam, F.; Hasnain, A. Dual modification of native white sorghum (Sorghum bicolor) starch via acid hydrolysis and succinylation. LWT-Food Sci. Technol. 2015, 64, 459–467. [Google Scholar] [CrossRef]
- Rafiq, S.I.; Singh, S.; Saxena, D.C. Effect of alkali-treatment on physicochemical, pasting, thermal, morphological and structural properties of Horse Chestnut (Aesculus indica) starch. Food Hydrocoll. 2016, 57, 103–113. [Google Scholar] [CrossRef]
- Lu, X.; Liu, H.; Huang, Q. Fabrication and characterization of resistant starch stabilized Pickering emulsions. Food Hydrocoll. 2020, 103, 105703. [Google Scholar] [CrossRef]
- Herniou, C.; Mendieta, J.R.; Gutiérrez, T.J. Characterization of biodegradable/non-compostable films made from cellulose acetate/corn starch blends processed under reactive extrusion conditions. Food Hydrocoll. 2019, 89, 67–79. [Google Scholar] [CrossRef]
- Fang, C.; Huang, J.; Pu, H.; Yang, Q.; Chen, Z. Cold-water solubility, oil-adsorption, and enzymolysis properties of amorphous granular starches. Food Hydrocoll. 2021, 117, 106669. [Google Scholar] [CrossRef]
- Detduangchan, N.; Wittaya, T. Effect of UV-Treatment on Properties of Biodegradable Film from Rice Starch. World Acad. Sci. Eng. Technol. 2011, 57, 464–469. [Google Scholar]
- Du, C.; Jiang, F.; Jiang, W.; Ge, W.; Du, S.K. Physicochemical and structural properties of sago starch. Int. J. Biol. Macromol. 2020, 164, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Adawiyah, D.R.; Sasaki, T.; Kohyama, K. Characterization of arenga starch in comparison with sago starch. Carbohydr. Polym. 2013, 92, 2306–2313. [Google Scholar] [CrossRef] [PubMed]
- Pranoto, Y.; Rahmayuni; Rakshit, S.K.; Haryadi. Physicochemical properties of heat moisture treated sweet potato starches of selected Indonesian varieties. Int. Food Res. J. 2015, 21, 2031–2038. [Google Scholar]
- Sukhija, S.; Singh, S.; Riar, C.S. Effect of oxidation, cross-linking, and dual modification on physicochemical, crystallinity, morphological, pasting, and thermal characteristics of elephant foot yam (Amorphophallus paeoniifolius) starch. Food Hydrocoll. 2015, 55, 56–64. [Google Scholar] [CrossRef]
- Adebowale, K.O.; Henle, T.; Schwarzenbolz, U.; Doert, T. Modification and properties of African yam bean (Sphenostylis stenocarpa Hochst. Ex A. Rich.) Harms starch I: Heat moisture treatments and annealing. Food Hydrocoll. 2009, 23, 1947–1957. [Google Scholar] [CrossRef]
- Paul, A.; Tuaño, P.; Clomer, E.; Barcellano, G.; Rodriguez, M.S. Resistant starch levels and in vitro starch digestibility of selected cooked Philippine brown and milled rices varying in apparent amylose content and glycemic index. Food Chem. 2021, 2, 100010. [Google Scholar]
- Santoso, B.; Sarungallo, Z.L.; Puspita, A.M. Physicochemical and functional properties of spineless, short-spines, and long-spines sago starch. Biodiversitas 2021, 22, 137–143. [Google Scholar] [CrossRef]
- Singh, G.D.; Bawa, A.S.; Riar, C.S.; Saxena, D.C. Influence of heat-moisture treatment and acid modifications on physicochemical, rheological, thermal, and morphological characteristics of Ind water chestnut (Trapa natans) starch and its application in biodegradable films. Starch/Starke 2009, 61, 503–513. [Google Scholar] [CrossRef]
- Sun, Q.; Han, Z.; Wang, L.; Xiong, L. Physicochemical differences between sorghum starch and sorghum flour modified by heat-moisture treatment. Food Chem. 2014, 145, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Cao, S.; Yu, Y.; Xu, X.; Cao, X.; Chen, W. Modification in physicochemical, structural, and digestive properties of pea starch during heat-moisture process assisted by pre- and post-treatment of ultrasound. Food Chem. 2021, 360, 129929. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, X.; Li, W.; Wang, X.; lv, M.; Peng, Q.; Wang, M. Changes in physicochemical properties and in vitro digestibility of common buckwheat starch by heat-moisture treatment and annealing. Carbohydr. Polym. 2015, 132, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Uzizerimana, F.; Dang, K.; Yang, Q.; Hossain, S.; Gao, S.; Bahati, P.; Mugiraneza, N.G.; Yang, P.; Feng, B. Physicochemical properties and in vitro digestibility of Tartary buckwheat starch modified by heat moisture treatment: A comparative study. NFS J. 2021, 25, 12–20. [Google Scholar] [CrossRef]
- Adawiyah, D.R.; Akuzawa, S.; Sasaki, T.; Kohyama, K. A comparison of the effects of heat moisture treatment (HMT) on rheological properties and amylopectin structure in sago (Metroxylon sago) and arenga (Arenga pinnata) starches. J. Food Sci. Technol. 2017, 54, 3404–3410. [Google Scholar] [CrossRef]
- Schafranski, K.; Ito, V.C.; Lacerda, L.G. Impacts and potential applications: A review of the modification of starches by heat-moisture treatment (HMT). Food Hydrocoll. 2021, 117, 10669. [Google Scholar] [CrossRef]
- Liu, C.; Song, M.; Liu, L.; Hong, J.; Guan, E.; Bian, K.; Zheng, X. Effect of heat-moisture treatment on the structure and physicochemical properties of ball mill damaged starches from different botanical sources. Int. J. Biol. Macromol. 2020, 156, 403–410. [Google Scholar] [CrossRef]
- Naseri, A.; Shekarchizadeh, H.; Kadivar, M. Octenylsuccination of sago starch and investigation of the effect of calcium chloride and ferulic acid on physicochemical and functional properties of the modified starch film. Food Process. Preserv. 2019, 1, e13898. [Google Scholar] [CrossRef]
- Trung, P.T.B.; Ngoc, L.B.B.; Hoa, P.N.; Tien, N.N.T.; van Hun, P. Impact of heat-moisture and annealing treatments on physicochemical properties and digestibility of starches from different colored sweet potato varieties. Int. J. Biol. Macromol. 2017, 105, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Vermeylen, R.; Goderis, B.; Delcour, J.A. An X-ray study of hydrothermally treated potato starch. Carbohydr. Polym. 2006, 64, 364–375. [Google Scholar] [CrossRef]
- Tan, X.; Li, X.; Chen, L.; Xie, F.; Li, L.; Huang, J. Effect of heat-moisture treatment on multi-scale structures and physicochemical properties of breadfruit starch. Carbohydr. Polym. 2017, 161, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.M.L.; Ciacco, C.F.; Tavares, D.Q. Effect of the Heat-Moisture Treatment on the Enzymatic Susceptibility of Corn Starch Granules. Starch/Starke 1995, 47, 223–228. [Google Scholar] [CrossRef]
- Uthumporn, U.; Wahidah, N.; Karim, A.A. Physicochemical properties of starch from sago (Metroxylon sagu) palm grown in mineral soil at different growth stages. In Proceedings of the 2014 Global Conference on Polymer and Composite Materials (PCM 2014), Ningbo, China, 27–29 May 2014. [Google Scholar]
- Lacerda, L.G.; da Silva Carvalho Filho, M.A.; Bauab, T.; Demiate, I.M.; Colman, T.A.D.; Andrade, M.M.P.; Schnitzler, E. The effects of heat-moisture treatment on avocado starch granules: Thermoanalytical and structural analysis. J. Therm. Anal. Calorim. 2015, 120, 387–393. [Google Scholar] [CrossRef]
- Ojogbo, E.; Blanchard, R.; Mekonnen, T. Hydrophobic and Melt Processable Starch-Laurate Esters: Synthesis, Structure-Property Correlations. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2611–2622. [Google Scholar] [CrossRef]
- Ren, L.; Wang, Q.; Yan, X.; Tong, J.; Zhou, J.; Su, X. Dual modification of starch nanocrystals via crosslinking and esterification for enhancing their hydrophobicity. Food Res. Int. 2016, 87, 180–188. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W.; Dufresne, A. Polymer Grafting onto Starch Nanocrystals. Biomacromolecules 2007, 8, 2916–2927. [Google Scholar] [CrossRef]
- Wang, Q.; Li, L.; Zheng, X. Recent advances in heat-moisture modified cereal starch: Structure, functionality and its applications in starchy food systems. Food Chem. 2021, 344, 128700. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, G.; Gao, Q. Preparation and properties of granular cold-water-soluble porous starch. Int. J. Biol. Macromol. 2020, 144, 656–662. [Google Scholar] [CrossRef]
- Kurdziel, M.; Królikowska, K.; Łabanowska, M.; Pietrzyk, S.; Michalec, M. The effect of thermal and irradiation treatments on structural and physicochemical properties of octenyl succinate maize starches. Food Chem. 2020, 330, 127242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ran, C.; Jiang, X.; Dou, J. Impact of octenyl succinic anhydride (OSA) esterification on microstructure and physicochemical properties of sorghum starch. LWT-Food Sci. Technol. 2021, 152, 112320. [Google Scholar] [CrossRef]
- Xie, Y.; Li, M.N.; Chen, H.Q.; Zhang, B. Effects of the combination of repeated heat-moisture treatment and compound enzymes hydrolysis on the structural and physicochemical properties of porous wheat starch. Food Chem. 2018, 274, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Goff, H.D.; Nsor-Atindana, J.; Zhong, F. Distribution of octenyl succinic groups in modified waxy maize starch: An analysis at granular level. Food Hydrocoll. 2018, 84, 210–218. [Google Scholar] [CrossRef]
- Bai, Y.; Shi, Y.C.; Herrera, A.; Prakash, O. Study of octenyl succinic anhydride-modified waxy maize starch by nuclear magnetic resonance spectroscopy. Carbohydr. Polym. 2011, 83, 407–413. [Google Scholar] [CrossRef]
- Wang, P.-P.; Luo, Z.-G.; Chen, C.; Fu, X.; Tamer, T.M. Effects of octenyl succinic anhydride groups distribution on the storage and shear stability of Pickering emulsions formulated by modified rice starch. Carbohydr. Polym. 2020, 228, 115389. [Google Scholar] [CrossRef]
- Wang, C.; He, X.; Fu, X.; Huang, Q.; Zhang, B. Substituent distribution changes the pasting and emulsion properties of octenyl succinate starch. Carbohydr. Polym. 2016, 135, 64–71. [Google Scholar] [CrossRef]


| Swelling Power (g/g) | Solubility (%) | WAC (g/g) | Amylose Content (%) | |
|---|---|---|---|---|
| Native | 20.38 ± 0.29 a | 38.52 ± 1.10 a | 0.90 ± 0.01 a | 36.18 ± 0.26 a |
| HMT 10 | 17.10 ± 0.05 b | 37.87 ± 0.27 a | 1.03 ± 0.03 a | 35.62 ± 0.58 a |
| HMT 15 | 15.24 ± 0.24 c | 25.12 ± 1.18 b | 1.01 ± 0.05 a | 33.76 ± 1.72 a |
| HMT 20 | 15.24 ± 0.48 c | 25.17 ± 0.77 b | 1.12 ± 0.14 a | 35.21 ± 0.20 a |
| HMT 25 | 15.32 ± 0.36 c | 22.49 ± 0.64 c | 1.80 ± 0.13 b | 36.31 ± 0.36 a |
| HMT 30 | 15.71 ± 0.04 c | 23.23 ± 0.15 bc | 1.93 ± 0.18 b | 35.32 ± 0.03 a |
| To (°C) | Tp (°C) | Tc (°C) | Tc–To (°C) | ∆H (J/g) | |
|---|---|---|---|---|---|
| Native | 72.75 ± 0.03 a | 76.70 ± 0.23 a | 82.18 ± 2.52 a | 9.43 ± 2.55 bc | 11.83 ± 0.01 a |
| HMT 10 | 71.60 ± 0.13 a | 76.14 ± 0.67 a | 82.95 ± 0.68 a | 11.35 ± 0.81 ab | 11.77 ± 1.32 a |
| HMT 15 | 73.39 ± 0.29 a | 80.73 ± 1.40 b | 87.23 ± 2.00 b | 13.84 ± 1.71 b | 10.09 ± 1.53 a |
| HMT 20 | 82.88 ± 1.16 b | 87.18 ± 1.21 c | 90.06 ± 0.45 bc | 7.18 ± 0.71 c | 1.88 ± 0.16 b |
| HMT 25 | 85.93 ± 1.46 c | 90.98 ± 0.21 d | 93.18 ± 0.11 c | 7.25 ± 1.56 c | 1.56 ± 0.43 b |
| HMT 30 | 82.68 ± 0.56 b | 86.33 ± 0.64 c | 88.48 ± 1.56 b | 5.80 ± 1.00 c | 1.50 ± 0.49 b |
| DS | RE (%) | |
|---|---|---|
| N-OSA | 0.0009 ± 0.0000 a | 3.76 ± 0.00 a |
| H20-OSA | 0.0053 ± 0.0001 b | 22.06 ± 0.44 b |
| H25-OSA | 0.0086 ± 0.0006 c | 35.86 ± 0.25 c |
| H30-OSA | 0.0049 ± 0.0007 b | 20.69 ± 1.27 b |
| OAC (%) | Water Solubility (%) | WVP (g·s−1·m−1·Pa−1 × 10−5) | R1022/995 | |
|---|---|---|---|---|
| Native | 0.65 ± 0.01 a | 4.14 ± 0.11 a | 6.41 ± 0.55 a | 1.17 ± 0.00 a |
| N-OSA | 0.85 ± 0.01 b | 9.04 ± 1.07 ab | 7.54 ± 0.19 a | 1.12 ± 0.00 b |
| HMT 25 | 0.78 ± 0.00 c | 6.28 ± 1.67 b | 7.43 ± 0.14 a | 1.07 ± 0.00 c |
| H25-OSA | 1.03 ± 0.03 d | 8.44 ± 0.15 b | 4.92 ± 0.79 b | 1.14 ± 0.00 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewi, A.M.P.; Santoso, U.; Pranoto, Y.; Marseno, D.W. Dual Modification of Sago Starch via Heat Moisture Treatment and Octenyl Succinylation to Improve Starch Hydrophobicity. Polymers 2022, 14, 1086. https://doi.org/10.3390/polym14061086
Dewi AMP, Santoso U, Pranoto Y, Marseno DW. Dual Modification of Sago Starch via Heat Moisture Treatment and Octenyl Succinylation to Improve Starch Hydrophobicity. Polymers. 2022; 14(6):1086. https://doi.org/10.3390/polym14061086
Chicago/Turabian StyleDewi, Angela Myrra Puspita, Umar Santoso, Yudi Pranoto, and Djagal W. Marseno. 2022. "Dual Modification of Sago Starch via Heat Moisture Treatment and Octenyl Succinylation to Improve Starch Hydrophobicity" Polymers 14, no. 6: 1086. https://doi.org/10.3390/polym14061086
APA StyleDewi, A. M. P., Santoso, U., Pranoto, Y., & Marseno, D. W. (2022). Dual Modification of Sago Starch via Heat Moisture Treatment and Octenyl Succinylation to Improve Starch Hydrophobicity. Polymers, 14(6), 1086. https://doi.org/10.3390/polym14061086