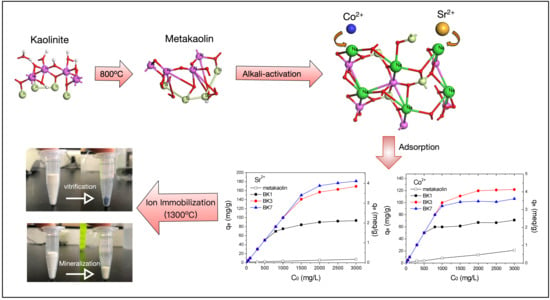
Highly Efficient Adsorption of Sr2+ and Co2+ Ions by Ambient Prepared Alkali Activated Metakaolin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Material Characterization
2.3. Adsorption Experiments
2.4. Preliminary Immobilization Test
3. Results and Discussion
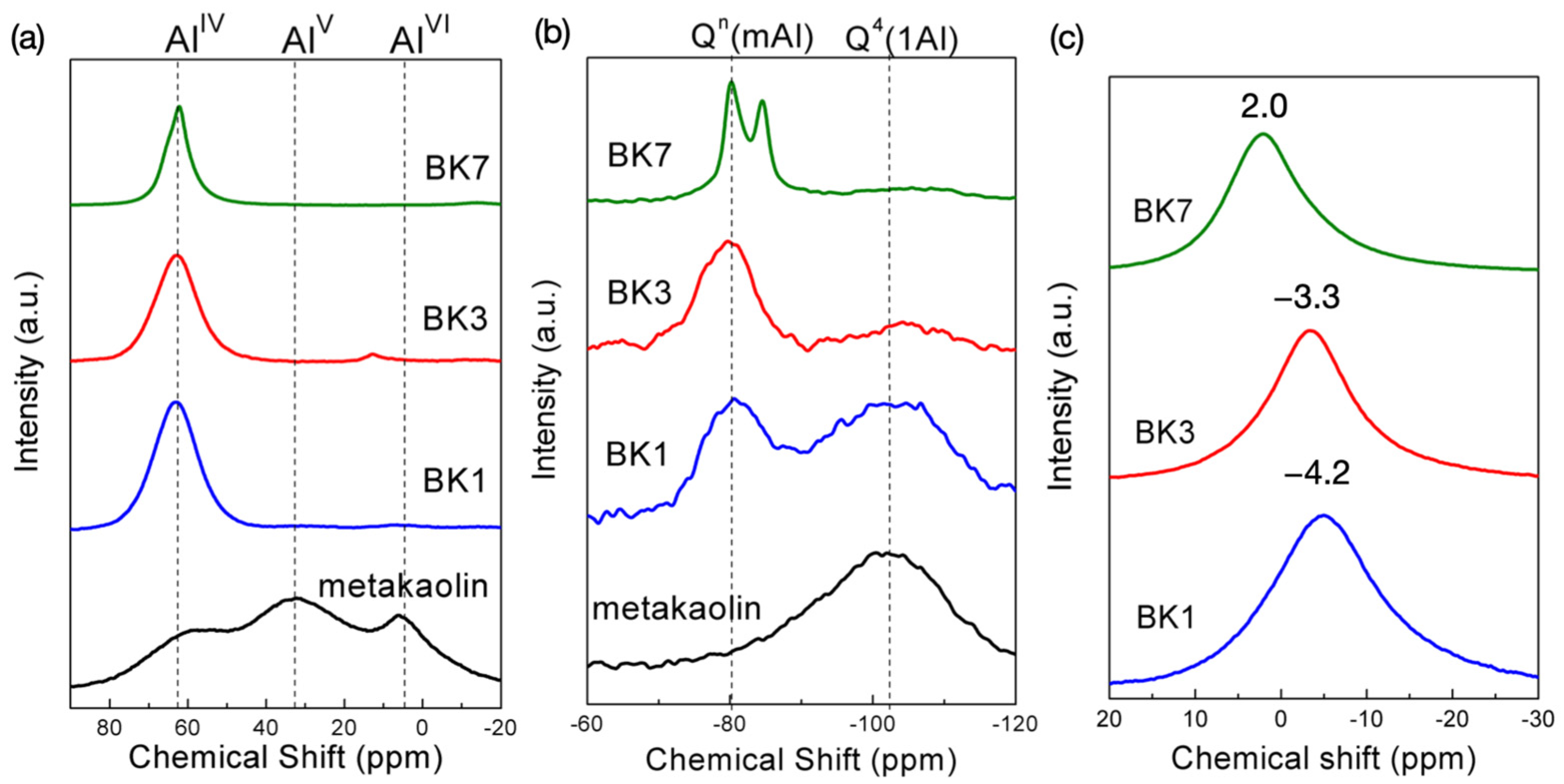
3.1. Material Characterizations
3.2. Polymerization Process
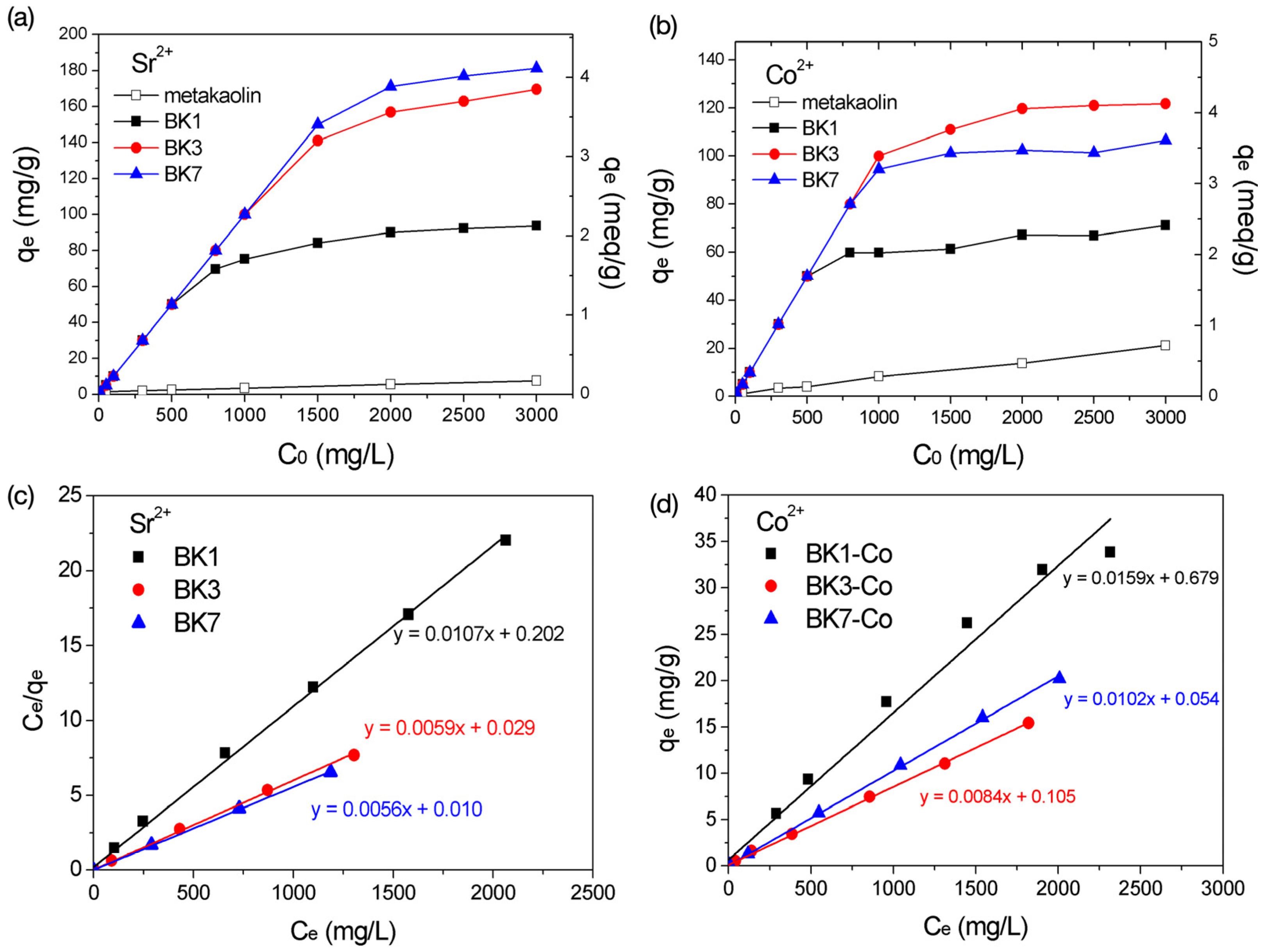
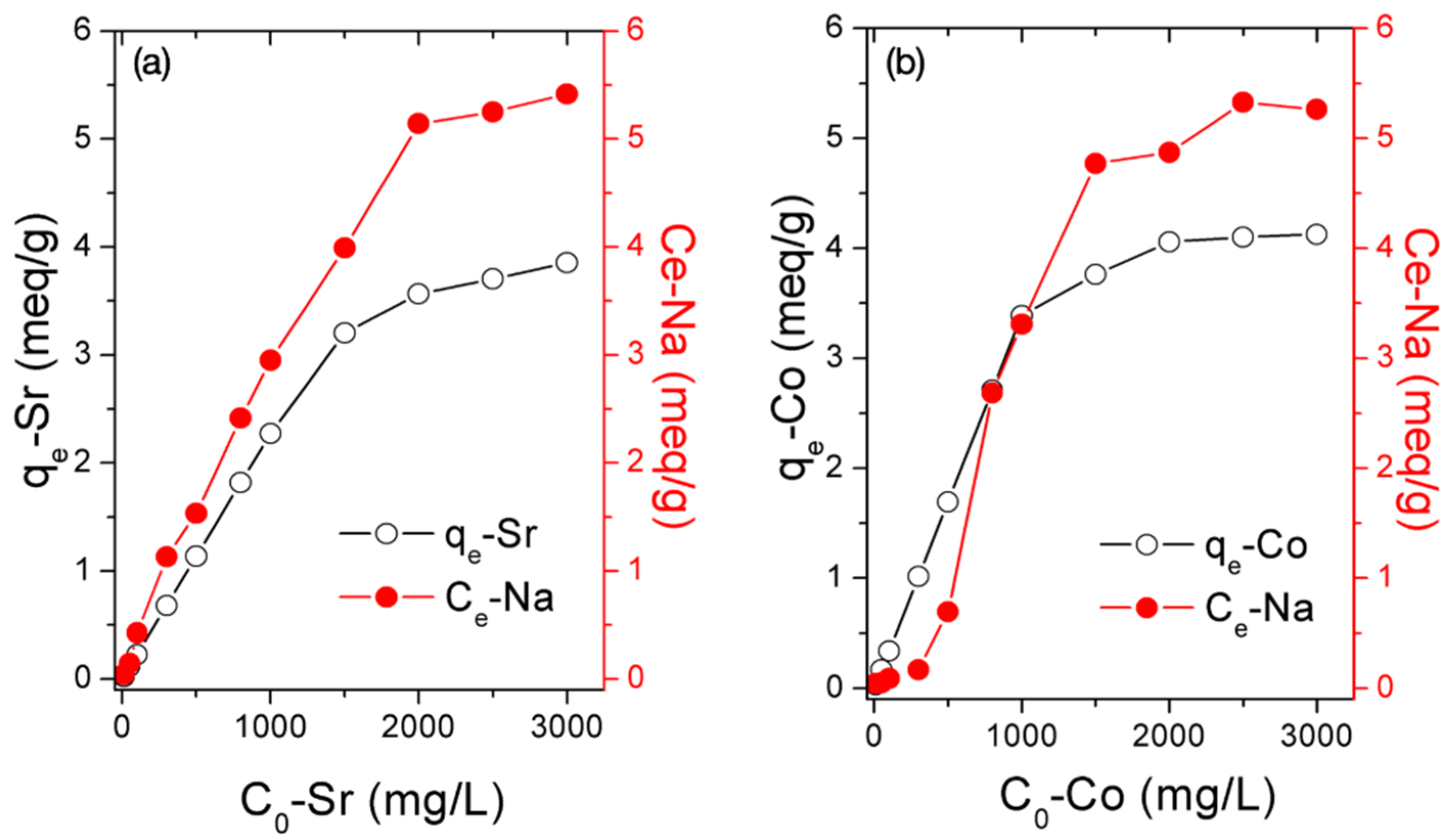
3.3. Adsorption Capacity of Sr2+ and Co2+
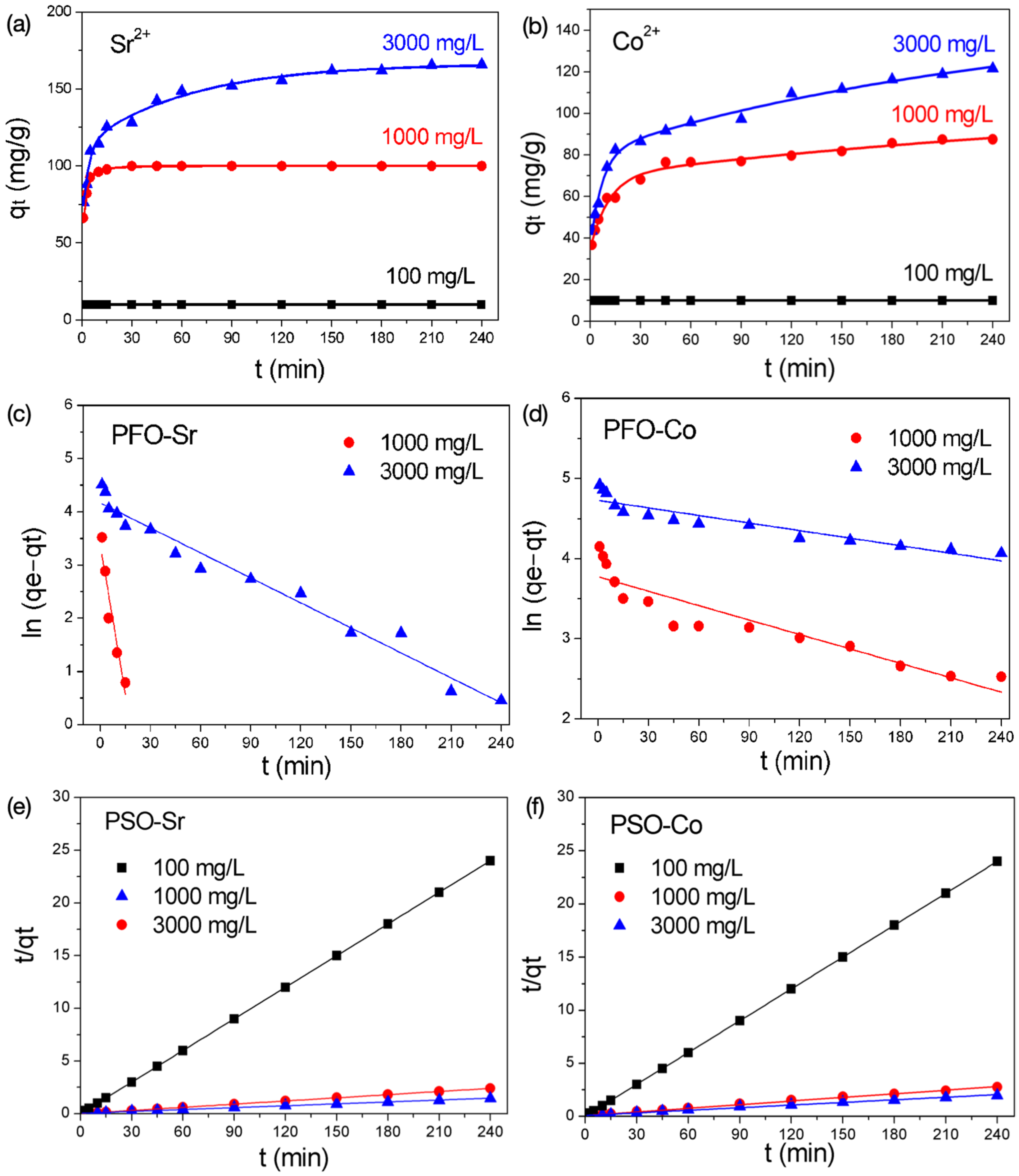
3.4. Adsorption Kinetics
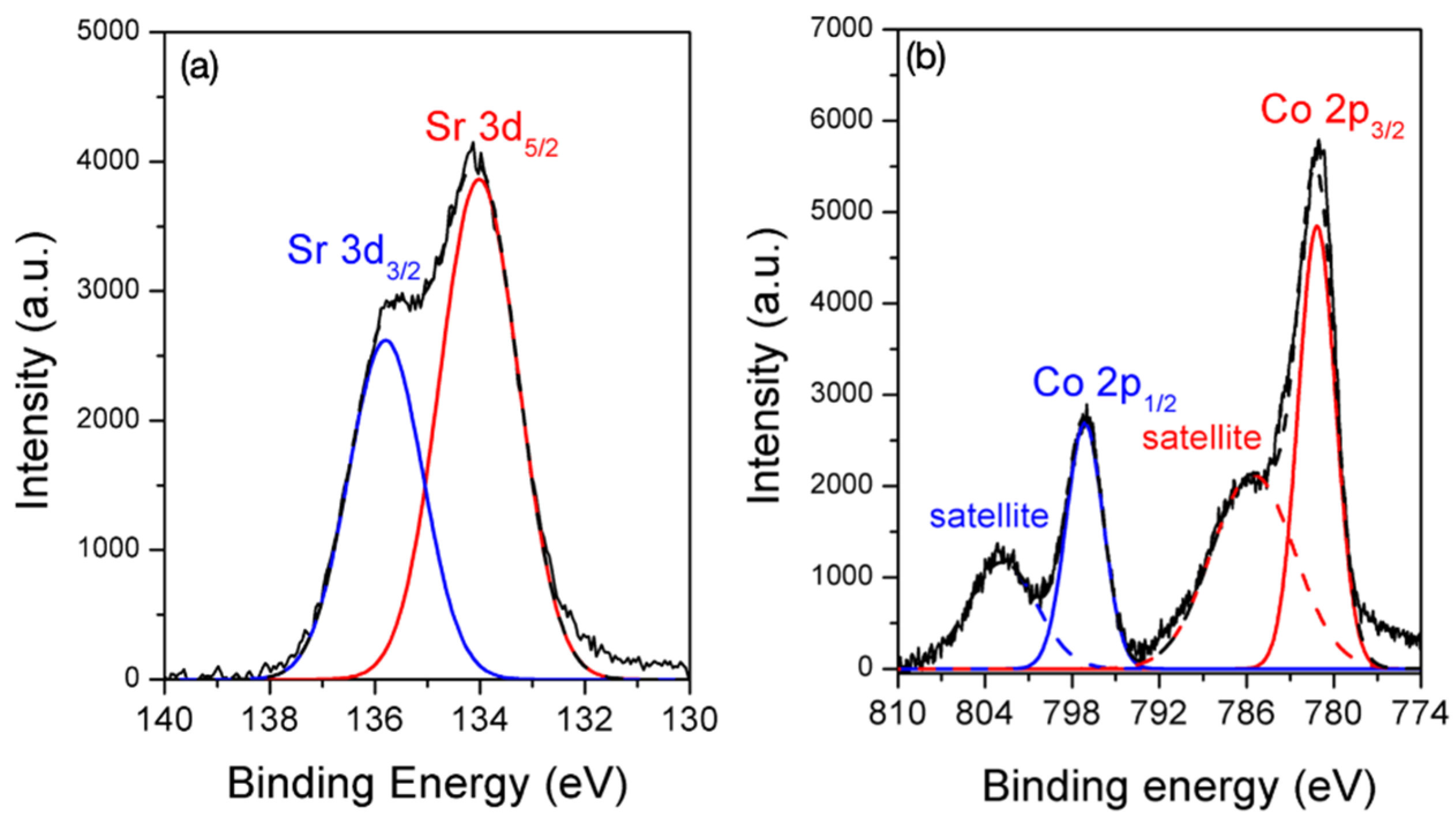
3.5. Sorption Mechanism
3.6. Competitive Adsorption of Sr2+, Co2+ and Cs+
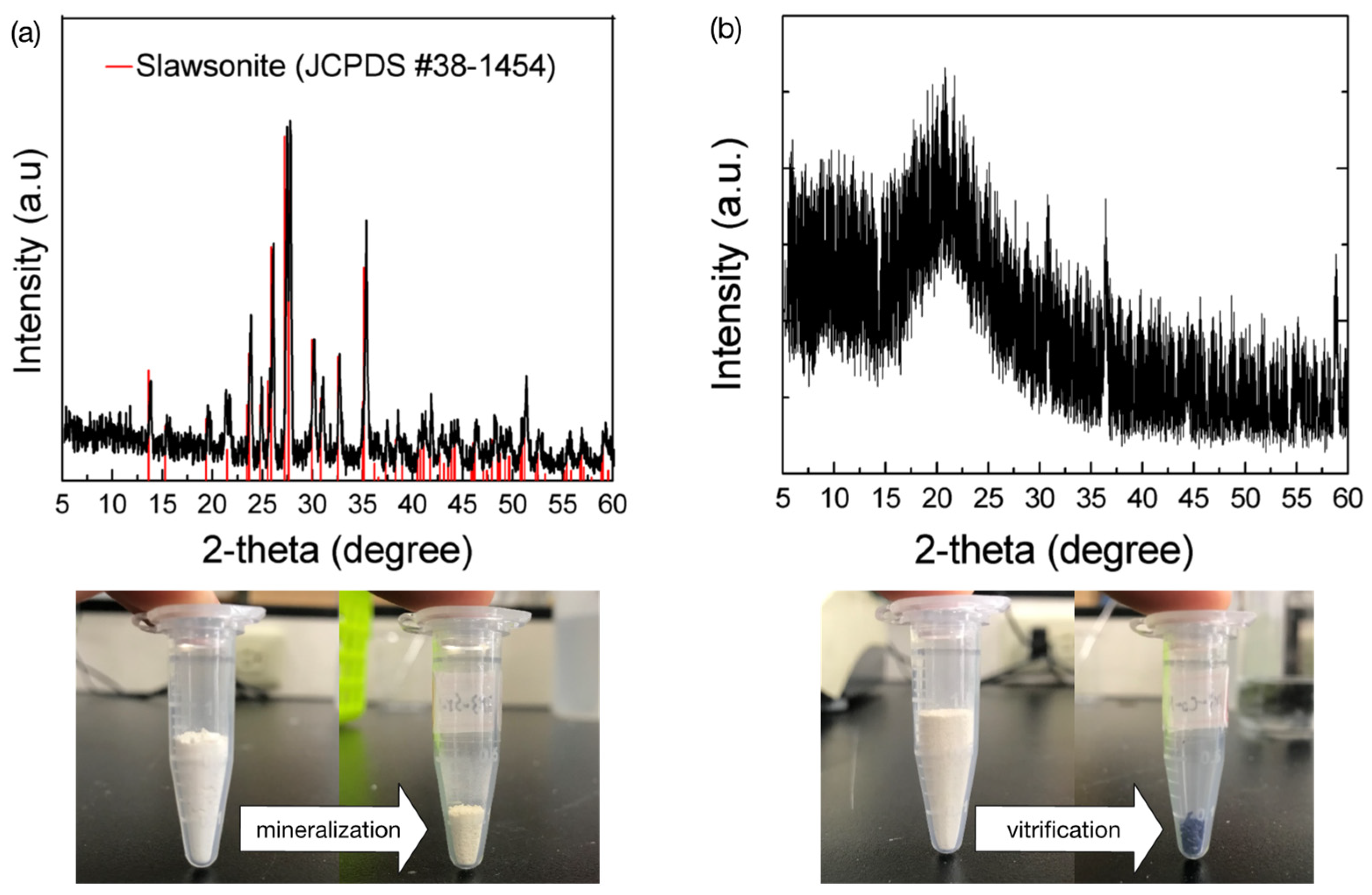
3.7. Mineralization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delacroix, D.; Guerre, J.P.; Leblanc, P.; Hickman, C. Radionuclide and radiation protection data handbook. Radiat. Prot. Dosim. 2002, 98, 1–168. [Google Scholar] [CrossRef]
- Munthali, M.W.; Johan, E.; Aono, H.; Matsue, N. Cs+ and Sr2+ adsorption selectivity of zeolites in relation to radioactive decontamination. J. Asian Ceram. Soc. 2015, 3, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Prajitno, M.Y.; Taufiqurrakhman, M.; Harbottle, D.; Hunter, T.N. Kinetic Studies of Cs+ and Sr2+ Ion Exchange Using Clinoptilolite in Static Columns and an Agitated Tubular Reactor (ATR). ChemEngineering 2021, 5, 9. [Google Scholar] [CrossRef]
- El-Kamash, A.M. Evaluation of zeolite A for the sorptive removal of Cs+ and Sr2+ ions from aqueous solutions using batch and fixed bed column operations. J. Hazard. Mater. 2008, 151, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Merceille, A.; Weinzaepfel, E.; Barré, Y.; Grandjean, A. The sorption behaviour of synthetic sodium nonatitanate and zeolite A for removing radioactive strontium from aqueous wastes. Sep. Purif. Technol. 2012, 96, 81–88. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, C.H. Adsorption and Desorption Characteristics of Sr, Cs, and Na Ions with Na-A Zeolite Synthesized from Coal Fly Ash in Low-Alkali Condition. J. Environ. Sci. Int. Korean Environ. Sci. Soc. 2019, 28, 561–570. [Google Scholar] [CrossRef]
- Hong, S.; Um, W. Top-Down Synthesis of NaP Zeolite from Natural Zeolite for the Higher Removal Efficiency of Cs, Sr, and Ni. Minerals 2021, 11, 252. [Google Scholar] [CrossRef]
- Chen, Y.L.; Tong, Y.Y.; Pan, R.W.; Tang, J. The Research on Adsorption Behaviors and Mechanisms of Geopolymers on Sr2+, Co2+ and Cs+. Adv. Mater. Res. 2013, 704, 313–318. [Google Scholar] [CrossRef]
- Król, M.; Rożek, P.; Chlebda, D.; Mozgawa, W. TR/FT-IR studies of zeolite formation during alkali-activation of metakaolin. Solid State Sci. 2019, 94, 114–119. [Google Scholar] [CrossRef]
- De Rossi, A.; Simão, L.; Ribeiro, M.J.; Novais, R.M.; Labrincha, J.A.; Hotza, D.; Moreira, R.F.P.M. In-situ synthesis of zeolites by geopolymerization of biomass fly ash and metakaolin. Mater. Lett. 2019, 236, 644–648. [Google Scholar] [CrossRef]
- Luukkonen, T.; Heponiemi, A.; Runtti, H.; Pesonen, J.; Yliniemi, J.; Lassi, U. Application of alkali-activated materials for water and wastewater treatment: A review. Rev. Environ. Sci. Bio/Technol. 2019, 18, 271–297. [Google Scholar] [CrossRef] [Green Version]
- Andrejkovičová, S.; Sudagar, A.; Rocha, J.; Patinha, C.; Hajjaji, W.; Silva, E.F.; Velosa, A.; Rocha, F. The effect of natural zeolite on microstructure, mechanical and heavy metals adsorption properties of metakaolin based geopolymers. Appl. Clay Sci. 2016, 126, 141–152. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Batyukhnova, O.G. Glasses for nuclear waste immobilization. WM 2007, 7, 15. [Google Scholar]
- Davidovits, J. Geopolymer Chemistry and Applications, 3rd ed.; Geopolymer Institute: Saint-Quentin, France, 2011. [Google Scholar]
- Lin, G.; Zhuang, Q.; Cui, Q.; Wang, H.; Yao, H. Synthesis and adsorption property of zeolite FAU/LTA from lithium slaf with utilization of mother liquid. Chin. J. Chem. Eng. 2015, 23, 1768–1773. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Pramada, P.N. Microwave assisted synthesis of zeolite A from metakaolin. Microporous Mesoporous Mater. 2008, 18, 152–161. [Google Scholar] [CrossRef]
- Fyfe, C.A.; Thomas, J.M.; Klinowski, J.; Gobbi, G.C. Magic-Angle-Spinning NMR (MAS-NMR) Spectroscopy and the Structure of Zeolites. Angew. Chem. Int. Ed. Engl. 1983, 22, 259–275. [Google Scholar] [CrossRef]
- Walkley, B.; Kashani, A.; Sani, M.A.; Ngo, T.D.; Mendis, P. Examination of alkali-activated material nanostructure during thermal treatment. J. Mater. Sci. 2018, 53, 9486–9503. [Google Scholar] [CrossRef] [Green Version]
- Antonijevic, S.; Ashbrook, S.E.; Walton, R.I.; Wimperis, S. A multiple-quantum 23Na MAS NMR study of amorphous sodium gallium silicate zeolite precursors. J. Mater. Chem. 2002, 12, 1469–1474. [Google Scholar] [CrossRef]
- Yang, H.; Walton, R.I.; Antonijevic, S.; Wimperis, S.; Hannon, A.C. Local Order of Amorphous Zeolite Precursors from 29Si{H} CPMAS and 27Al and 23Na MQMAS NMR and Evidence for the Nature of Medium-Range Order from Neutron Diffraction. J. Phys. Chem. B 2004, 108, 8208–8217. [Google Scholar] [CrossRef]
- Rowles, M.; Hanna, J.; Pike, K.; Smith, M. 29Si, 27Al, 1H and 23Na MAS NMR Study of the Bonding Character in Aluminosilicate Inorganic. Polymers 2007, 32, 663. [Google Scholar] [CrossRef]
- Walkley, B.; Provis, J.L. Solid-state nuclear magnetic resonance spectroscopy of cements. Mater. Adv. 2019, 1, 10007. [Google Scholar] [CrossRef]
- Hong, M.; Yu, L.; Wang, Y.; Zhang, J.; Chen, Z.; Dong, L.; Zan, Q.; Li, R. Heavy metal adsorption with zeolites: The role of hierarchical pore architecture. Chem. Eng. J. 2019, 359, 363–372. [Google Scholar] [CrossRef]
- Nightingale, E.R., Jr. Phenomenological theory of ion solvation. Effective radii of hydrated ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Opitz, A.K.; Rameshan, C.; Kubicek, M.; Rupp, G.M.; Nenning, A.; Götsch, T.; Blume, R.; Hävecker, M.; Knop-Gericke, A.; Rupprechter, G.; et al. The Chemical Evolution of the La0.6Sr0.4CoO3−δ Surface under SOFC Operating Conditions and Its Implications for Electrochemical Oxygen Exchange Activity. Top. Catal. 2018, 61, 2129–2141. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Cao, Y.; Pal, B.; Middey, S.; Kareev, M.; Choi, Y.; Chakhalian, J. Synthesis and electronic properties of Ruddlesden-Popper strontium iridate epitaxial thin films stabilized by control of growth kinetics. Phys. Rev. Mater. 2017, 1, 075004. [Google Scholar] [CrossRef]
- Vipin, A.K.; Ling, S.; Fugetsu, B. Removal of Cs+ and Sr2+ from water using MWCNT reinforced Zeolite-A beads. Microporous Mesoporous Mater. 2016, 224, 84–88. [Google Scholar] [CrossRef]
- Dillard, J.G.; Koppelman, M.H. X-ray Photoelectron Spectroscopic (XPS) Surface Characterization of Cobalt on the Surface of Kaolinite. J. Colloid Interface Sci. 1982, 87, 46–55. [Google Scholar] [CrossRef]
- Murray, J.W.; John, G.D. The oxidation of cobalt(II) adsorbed on manganese dioxide. Geochim. Cosmochim. Acta 1979, 43, 781–787. [Google Scholar] [CrossRef]
- Wang, J.; Xie, T.; Deng, Q.; Wang, Y.; Zhu, Q.; Liu, S. Three-dimensional interconnected Co(OH)2 nanosheets on Ti mesh as a highly sensitive electrochemical sensor for hydrazine detection. New J. Chem. 2019, 43, 3218–3225. [Google Scholar] [CrossRef]
- Ma, B.; Oh, S.; Shin, W.S.; Choi, S.J. Removal of Co2+, Sr2+ and Cs+ from aqueous solution by phosphate-modified montmorillonite (PMM). Desalination 2011, 276, 336–346. [Google Scholar] [CrossRef]
- Su, Q.; Deng, L.; Ye, Q.; He, Y.; Cui, X. KOH-Activated Geopolymer Microspheres Recycle Co(II) with Higher Adsorption Capacity than NaOH-Activated Ones. ACS Omega 2020, 5, 23898–23908. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.A.; Abdel Moamen, O.A.; Abdel Monem, N.; Ismail, I.M. Assessment of kinetic and isotherm models for competitive sorption of Cs+ and Sr2+ from binary metal solution onto nanosized zeolite. Chem. Eng. Commun. 2018, 205, 1274–1287. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, C.; Han, E.; Lee, H.; Cho, H.S.; Choi, M. Relationship between zeolite structure and capture capability for radioactive cesium and strontium. J. Hazard. Mater. 2021, 29, 124419. [Google Scholar] [CrossRef]
- Yuki, K.; Patcharaporn, W.; Yoshiya, H.; Makoto, S.; Toshimitsu, S.; Takanori, M. Highly effective K-Merlinoite adsorbent for removal of Cs+ and Sr2+ in aqueous solution. RSC Adv. 2017, 7, 30919–30928. [Google Scholar]
- Wang, J.M. Study on Competitive Sorption Capacity of Sr2+/Cs+ in Zeolite and Palygorskite. Adv. Mater. Res. 2012, 347–353, 2515–2518. [Google Scholar]
- Lee, M.G.; Kam, S.K.; Lee, C.H. Kinetic and isothermal adsorption properties of strontium and cesium ions by zeolitic materials synthesized from Jeju volcanic rocks. Environ. Eng. Res. 2021, 26, 200127. [Google Scholar] [CrossRef]
- Barrer, R.M.; Klinowski, J. Influence of framework charge density on ion-exchange properties of zeolites. J. Chem. Soc. 1972, 1, 1956–1963. [Google Scholar] [CrossRef]
- Coker, E.N. Ion exchange equilibria and kinetics in zeolites: Influences of framework flexibility and charge density. Stud. Surf. Sci. Catal. 2007, 170, 110–120. [Google Scholar]



| Content (At.%) | CEC (meq/g) | Al/Si | SBET (m2/g) | |||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Na2O | TiO2 | Other Oxides | ||||
| Metakaolin | 56.5 | 29.4 | - | 9.9 | 4.1 | - | 1.04 | 20.3 |
| BK1 | 58.9 | 27.9 | 9.5 | 3.1 | 0.5 | 3.91 | 0.95 | 56.4 |
| BK3 | 50.6 | 24.1 | 22.4 | 2.6 | 0.3 | 6.80 | 0.95 | 48.9 |
| BK7 | 49.8 | 23.4 | 24.7 | 1.7 | 0.4 | 6.99 | 0.94 | 454.3 |
| Sample | R2 | KL | qmax (mg/g) | qmax (meq/g) |
|---|---|---|---|---|
| Sr2+ | ||||
| BK1 | 0.998 | 0.053 | 93.5 | 2.13 |
| BK3 | 0.998 | 0.206 | 167.5 | 3.81 |
| BK7 | 0.999 | 0.555 | 180.2 | 4.10 |
| Co2+ | ||||
| BK1 | 0.984 | 0.023 | 63.1 | 2.14 |
| BK3 | 0.999 | 0.080 | 118.5 | 4.02 |
| BK7 | 0.999 | 0.189 | 98.0 | 3.30 |
| Concentration | Sr2+ | Co2+ | ||||
|---|---|---|---|---|---|---|
| k | qe | R2 | k | qe | R2 | |
| Pseudo-First-Order Model (PFO) | ||||||
| 1000 mg/L | 195.9 | 35.0 | 0.978 | 6 | 43.5 | 0.845 |
| 3000 mg/L | 156.6 | 64.6 | 0.970 | 3.14 | 113.0 | 0.861 |
| Pseudo-Second-Order Model (PSO) | ||||||
| 1000 mg/L | 30.46 | 100.0 | 0.999 | 1.71 | 88.3 | 0.997 |
| 3000 mg/L | 1.09 | 167.8 | 0.998 | 0.83 | 122.4 | 0.994 |
| Sample | R2 | KL | qmax (mg/g) | qmax (meq/g) |
|---|---|---|---|---|
| Single Solute | ||||
| Sr2+ | 0.999 | 0.206 | 167.5 | 3.82 |
| Co2+ | 0.999 | 0.080 | 118.5 | 4.02 |
| Cs+ | 0.949 | 0.555 | 190.2 | 1.43 |
| Ternary Solute | ||||
| Sr2+ | 0.998 | 0.019 | 76.6 | 1.74 |
| Co2+ | 0.999 | 0.083 | 68.4 | 2.32 |
| Cs+ | 0.996 | 0.014 | 61.2 | 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-H.; Wu, Y.-C. Highly Efficient Adsorption of Sr2+ and Co2+ Ions by Ambient Prepared Alkali Activated Metakaolin. Polymers 2022, 14, 992. https://doi.org/10.3390/polym14050992
Huang Y-H, Wu Y-C. Highly Efficient Adsorption of Sr2+ and Co2+ Ions by Ambient Prepared Alkali Activated Metakaolin. Polymers. 2022; 14(5):992. https://doi.org/10.3390/polym14050992
Chicago/Turabian StyleHuang, Yi-Hsuan, and Yu-Chun Wu. 2022. "Highly Efficient Adsorption of Sr2+ and Co2+ Ions by Ambient Prepared Alkali Activated Metakaolin" Polymers 14, no. 5: 992. https://doi.org/10.3390/polym14050992
APA StyleHuang, Y.-H., & Wu, Y.-C. (2022). Highly Efficient Adsorption of Sr2+ and Co2+ Ions by Ambient Prepared Alkali Activated Metakaolin. Polymers, 14(5), 992. https://doi.org/10.3390/polym14050992