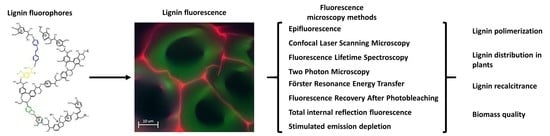
Fluorescence Microscopy Methods for the Analysis and Characterization of Lignin
Abstract
1. Introduction
2. Structure and Autofluorescence of Lignin
3. Fluorescence Microscopy Methods
3.1. Wield-Field Fluorescence or Epifluorescence (Epi)
3.2. Confocal Laser Scanning Microscopy (CLSM)
3.3. Fluorescence Lifetime Spectroscopy (FLIM)
3.4. Two-Photon Microscopy (TPM)
3.5. Förster Resonance Energy Transfer (FRET)
3.6. Fluorescence Recovery after Photobleaching (FRAP)
3.7. Total Internal Reflection Fluorescence (TIRF)
3.8. Stimulated Emission Depletion (STED)
4. Complementary Methods
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Method | Staining or Autofluorescence | Purpose | Species or Material Analyzed | Reference |
| CLSM | BODIPY, ovalbumin with fluorescein | Lignin peroxidase effectiveness | Lignin peroxidases | [79] |
| CLSM | Autofluorescence, monolignol fluorescence | Lignin transport and polymerization | Arabidopsis thaliana | [81,82,83] |
| CLSM | Monolignol fluorescence | Lignin polymerization | Arabidopsis thaliana, Pinus radiata | [85] |
| CLSM | Autofluorescence, monolignol fluorescence | Lignin polymerization | Linum usitatissimum | [86] |
| CLSM | Basic Fuchsin, Aramine O, Direct Yellow 96 | Protocol for staining cell walls | Arabidopsis thaliana | [73] |
| CLSM | Basic Fuchsin | Lignin distribution | Brachypodium distachyon | [74] |
| CLSM | Monolignol fluorescence | Lignin polymerization | Arabidopsis thaliana | [84] |
| CLSM | Autofluorescence | Recalcitrant biomass | Miscanthus, poplar, wheat straw | [77] |
| CLSM | Autofluorescence | Lignin distribution | Arabidopsis thaliana | [68] |
| CLSM | Autofluorescence | Protocol for lignin fluorescence intensity | Acacia melanoxylon, Eucalyptus nitens, Pinus radiata, Populus deltoides, P. heterophylla, Salix chilensis, and S. nigra | [39] |
| CLSM | Autofluorescence | Wood characterization and lignin distribution | Pinus radiata | [70] |
| CLSM | Safranin | Safranin staining protocol and lignin distribution | Pinus radiata, Populus deltoides, Pseudotsuga menziesii | [53] |
| CLSM | Autofluorescence | Lignin concentration | Arabidopsis thaliana, Linum usitatissimum, Populus tremula × alba, Zea mays | [69] |
| CLSM | Autofluorescence | Presence of lignin in cellulose nanofibers | Poplar powder | [75] |
| CLSM | Autofluorescence, phloroglucinol | Presence of fluorophores in pine leaves | Pinus radiata needles | [41] |
| CLSM | Autofluorescence | Archaeological wood degradation | Fir wood | [72] |
| CLSM | p-HTEA solution | New biomass staining protocol | Lignocellulosic biomass | [71] |
| CLSM | Autofluorescence, monolignol fluorescence | Dual labeling protocol for lignin | Linum usitatissimum | [83] |
| CLSM | Autofluorescence | Structural changes due to heat treatments | Populus cathayana | [78] |
| CLSM | Basic Fuchsin, Aramine O, calcofluor white | Laccases lignin polymerization | Arabidopsis thaliana | [87] |
| CLSM, FLIM | Autofluorescence | Comparison between normal and compression wood | Pinus radiata | [89] |
| CLSM, FLIM | Autofluorescence | Lignin characterization | Arabidopsis thaliana | [90] |
| CLSM, FLIM | Autofluorescence | Presence of lignin in cellulose nanofibers | Nanocellulose fibers | [38] |
| CLSM, FLIM, Epi | Acridine orange | Fungal oxidation of wood | White spruce sapwood | [94] |
| CLSM, FLIM, FRET | Autofluorescence, Rhodamine B | Saccharification treatments | Pine, poplar coppice | [25] |
| CLSM, FLIM, TPM | Autofluorescence | Saccharification treatments | Miscanthus × giganteus, poplar, wheat straw | [43] |
| CLSM, FLIM, TPM | Autofluorescence | Alkaline and acid treatments for lignin | Eucalyptus fibers, sugarcane bagasse | [92] |
| CLSM, FLIM, TPM | Autofluorescence | Characterization of lignocellulosic biomass | Sugarcane bagasse | [93] |
| CLSM, FRAP | Autofluorescence, PEG-rhodamine | Saccharification treatments | Populus nigra × deltoides | [25] |
| CLSM, FRAP | Autofluorescence and probes | Diffusion of probes and FRAP effectiveness | Feruloylated arabinoxylan gels | [106] |
| CLSM, FRET | FITC, Rhodamine B | Lignin nanoparticles with peroxidases and laccases | Lignin nanoparticles | [66] |
| CLSM, FRET | Autofluorescence and PEG-rhodamine | Interaction between PEG and recalcitrant lignin | Pinus radiata | [67] |
| CLSM, FRET | Autofluorescence and Congo red | Lignin recalcitrance | Pinus | [80] |
| CLSM, FRET, SLIM | Autofluorescence and Rhodamine B | Probe effectiveness | Wheat straw | [97] |
| CLSM, STED | PEG-rhodamine (PEG-R) | Method Comparison | Poplar | [109] |
| CLSM, TIRF, Epi | Calcofluor white | Fiber characterization | Agave fibers, garlic skin | [108] |
| CLSM, TPM | Autofluorescence | Changes in wood | Spruce | [101] |
| Epi | Autofluorescence, safranin | Lignin distribution | Fraxinus ornus, Myrtus communis, Olea europaea, Pistacia lentiscus, Rhamnus alaternus | [36] |
| Epi | Safranin/fast green | Characterization of secondary xylem lignin | 34 species of Cactaceae | [62] |
| Epi | Safranin/fast green | Characterization of primary xylem lignin | 8 species of Cactaceae | [115] |
| Epi | Autofluorescence | Lignin distribution | Pinus yunnanensis | [59] |
| Epi | Modified Mäule staining | Staining protocol | Betula platyphylla | [61] |
| Epi | Autofluorescence | Structural changes due to heat treatments | Fraxinus excelsior | [60] |
| Epi | Safranin | Fungal degradation of wood | Eucalyptus grandis | [64] |
| Epi | Acriflavine | Lignocellulose distribution | Cannabis sativa | [58] |
| Epi, CLSM | Autofluorescence, Filipin fluorescent antibiotic reacting | Biopulping with fungus | Eucalyptus globulus | [76] |
| Epi, CLSM | Autofluorescence, Congo red, Fluorol yellow | Protocol for analyzing cell walls | Bruguiera gymnorrhiza, Picea glauca, Pinus ponderosa, Populus tremuloides, Rhizophora apiculata | [57] |
| Epi, FLIM, TPM | Autofluorescence | Biomass morphological changes due to hydrolysis | Corn stover | [55] |
| Epi, FRAP | Autofluorescence, m-CHERRY red protein | Lignin polymerization | Arabidopsis | [104] |
| Epi, FRET | Synthesized fluorochromes | Lignin polymerization | Artificial lignified maize cell walls | [44] |
| FLIM | Autofluorescence | Protocol to identify timber species | Beech, maple, spruce, and walnut | [95] |
| FLIM | Autofluorescence | Lignin composition | Arabidopsis thaliana | [96] |
| TPM | Autofluorescence | Structure analysis of chitosan-lignin film | Chitosan-lignin composite film | [102] |
| TPM, FRAP, FRET, sFLIM | Autofluorescence | Protocol to analyze biomass | Wheat straw | [103] |
References
- Renault, H.; Werck-Reichhart, D.; Weng, J.-K. Harnessing lignin evolution for biotechnological applications. Curr. Opin. Biotechnol. 2018, 56, 105–111. [Google Scholar] [CrossRef]
- Liu, B.; Tang, L.; Chen, Q.; Zhu, L.; Zou, X.; Li, B.; Zhou, Q.; Fu, Y.; Lu, Y. Lignin Distribution on Cell Wall Micro-Morphological Regions of Fibre in Developmental Phyllostachys pubescens Culms. Polymers 2022, 14, 312. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, C.; Costa, L.T.; Abud, Y.; Biancatto, L.; Miguens, F.C.; de Souza, W. Sugarcane cell wall structure and lignin distribution investigated by confocal and electron microscopy. Microsc. Res. Tech. 2013, 76, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.V.; Rencoret, J.; Gutiérrez, A.; del Río, J.C.; Pereira, H. Ferulates and lignin structural composition in cork. Holzforschung 2015, 70, 275–289. [Google Scholar] [CrossRef]
- Goodell, B.; Winandy, J.E.; Morrell, J.J. Fungal Degradation of Wood: Emerging Data, New Insights and Charging Perceptions. Coatings 2020, 10, 1210. [Google Scholar] [CrossRef]
- Chauve, M.; Barre, L.; Tapin-Lingua, S.; Perez, D.D.S.; Decottignies, D.; Perez, S.; Ferreira, N.L. Evolution and impact of cellulose architecture during enzymatic hydrolysis by fungal cellulases. Adv. Biosci. Biotechnol. 2013, 04, 1095–1109. [Google Scholar] [CrossRef][Green Version]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part II: Fundamentals of different pre-treatments to increase the enzymatic digestibility of lignocellulose. Biofuels, Bioprod. Biorefining 2012, 6, 561–579. [Google Scholar] [CrossRef]
- Singh, H.; Tomar, S.; Qureshi, K.A.; Jaremko, M.; Rai, P.K. Recent Advances in Biomass Pretreatment Technologies for Biohydrogen Production. Energies 2022, 15, 999. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, S.; Yang, S.; Ding, S.-Y. Lignin plays a negative role in the biochemical process for producing lignocellulosic biofuels. Curr. Opin. Biotechnol. 2014, 27, 38–45. [Google Scholar] [CrossRef]
- Sekeri, S.H.; Ibrahim, M.N.M.; Umar, K.; Yaqoob, A.A.; Azmi, M.N.; Hussin, M.H.; Othman, M.B.H.; Malik, M.F.I.A. Preparation and characterization of nanosized lignin from oil palm (Elaeis guineensis) biomass as a novel emulsifying agent. Int. J. Biol. Macromol. 2020, 164, 3114–3124. [Google Scholar] [CrossRef]
- Vena, P.F.; Görgens, J.F.; Rypstra, T. Hemicelluloses extraction from giant bamboo prior to Kraft and soda AQ pulping to produce paper pulps, value-added biopolymers and bioethanol. Cellul. Chem. Technol. 2010, 44, 153–163. [Google Scholar]
- Megra, M.B.; Bachheti, R.K.; Tadesse, M.G.; Worku, L.A. Evaluation of Pulp and Papermaking Properties of Melia azedarach. Forests 2022, 13, 263. [Google Scholar] [CrossRef]
- Ekpo, I.; Ogali, R.; Ofodile, S.; Achugasim, O. Comparison of biomass content for the evaluation of cellulosic ethanol fuel production from predominant perennial grasses in south-south. Nigeria Int. J. Appl. Sci. Technol. 2016, 6, 38–46. [Google Scholar]
- Kumarasamy, R.; Uthandi, S.; Thangappan, S. Lignin Depolymerization Route Derived Commodities: Towards Tangible Bio-Economy. Madras Agric. J. 2017, 104, 207. [Google Scholar] [CrossRef]
- Maceda, A.; Soto-Hernández, M.; Peña-Valdivia, C.B.; Trejo, C.; Terrazas, T. Characterization of lignocellulose of Opuntia (Cactaceae) species using FTIR spectroscopy: Possible candidates for renewable raw material. Biomass Convers. Biorefinery 2020, 1–10. [Google Scholar] [CrossRef]
- Reyes-Rivera, J.; Soto-Hernández, M.; Canché-Escamilla, G.; Terrazas, T. Structural Characterization of Lignin in Four Cacti Wood: Implications of Lignification in the Growth Form and Succulence. Front. Plant Sci. 2018, 9, 1518. [Google Scholar] [CrossRef]
- Donaldson, L.A. Wood cell wall ultrastructure The key to understanding wood properties and behaviour. IAWA J. 2019, 40, 645–672. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Ghodake, G.; Cho, S.-K.; Kadam, A.; Kumar, G.; Jeon, B.-H.; Pant, D.; Bhatnagar, A.; Shin, H.S. Wheat straw extracted lignin in silver nanoparticles synthesis: Expanding its prophecy towards antineoplastic potency and hydrogen peroxide sensing ability. Int. J. Biol. Macromol. 2019, 128, 391–400. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.-K.; Saratale, G.D.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Bharagava, R.N.; Kumar, G.; Kim, D.S.; Mulla, S.I.; et al. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour. Technol. 2021, 325, 124685. [Google Scholar] [CrossRef]
- Welker, C.M.; Balasubramanian, V.K.; Petti, C.; Rai, K.M.; DeBolt, S.; Mendu, V. Engineering Plant Biomass Lignin Content and Composition for Biofuels and Bioproducts. Energies 2015, 8, 7654–7676. [Google Scholar] [CrossRef]
- Studer, M.; DeMartini, J.D.; Davis, M.; Sykes, R.W.; Davison, B.; Keller, M.; Tuskan, G.; Wyman, C.E. Lignin content in natural Populus variants affects sugar release. Proc. Natl. Acad. Sci. USA 2011, 108, 6300–6305. [Google Scholar] [CrossRef] [PubMed]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Ralph, S.A.; Christensen, J.H.; et al. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenyl- propanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Wagner, A.; Tobimatsu, Y.; Phillips, L.; Flint, H.; Geddes, B.; Lu, F.; Ralph, J. Syringyl lignin production in conifers: Proof of concept in a Pine tracheary element system. Proc. Natl. Acad. Sci. USA 2015, 112, 6218–6223. [Google Scholar] [CrossRef] [PubMed]
- Mottiar, Y.; Vanholme, R.; Boerjan, W.; Ralph, J.; Mansfield, S. Designer lignins: Harnessing the plasticity of lignification. Curr. Opin. Biotechnol. 2016, 37, 190–200. [Google Scholar] [CrossRef]
- Chabbert, B.; Terryn, C.; Herbaut, M.; Vaidya, A.; Habrant, A.; Paës, G.; Donaldson, L. Fluorescence techniques can reveal cell wall organization and predict saccharification in pretreated wood biomass. Ind. Crop. Prod. 2018, 123, 84–92. [Google Scholar] [CrossRef]
- Grabber, J.H. How Do Lignin Composition, Structure, and Cross-Linking Affect Degradability? A Review of Cell Wall Model Studies. Crop Sci. 2005, 45, 820–831. [Google Scholar] [CrossRef]
- Lu, F.; Ralph, J. Lignin. In Cereal Straw as a Resource for Sustainable Biomaterials and Biofuels; Run-Cang, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 169–207. ISBN 9780444532343. [Google Scholar]
- Chen, F.; Tobimatsu, Y.; Havkin-Frenkel, D.; Dixon, R.A.; Ralph, J. A polymer of caffeyl alcohol in plant seeds. Proc. Natl. Acad. Sci. USA 2012, 109, 1772–1777. [Google Scholar] [CrossRef]
- Barceló, A.R.; Ros, L.G.; Gabaldón, C.; López-Serrano, M.; Pomar, F.; Carrión, J.; Pedreño, M. Basic peroxidases: The gateway for lignin evolution? Phytochem. Rev. 2004, 3, 61–78. [Google Scholar] [CrossRef]
- Drummen, G.P.C. Fluorescent Probes and Fluorescence (Microscopy) Techniques—Illuminating Biological and Biomedical Research. Molecules 2012, 17, 14067–14090. [Google Scholar] [CrossRef]
- Paës, G. Fluorescent Probes for Exploring Plant Cell Wall Deconstruction: A Review. Molecules 2014, 19, 9380–9402. [Google Scholar] [CrossRef]
- Albinsson, B.; Li, S.; Lundquist, K.; Stomberg, R. The origin of lignin fluorescence. J. Mol. Struct. 1999, 508, 19–27. [Google Scholar] [CrossRef]
- Donaldson, L.; Radotić, K.; Kalauzi, A.; Djikanović, D.; Jeremić, M. Quantification of compression wood severity in tracheids of Pinus radiata D. Don using confocal fluorescence imaging and spectral deconvolution. J. Struct. Biol. 2010, 169, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.E.D.H.; De Paula, R.; Ruggiero, R.; Gardrat, C.; Castellan, A. Photophysics of dibenzodioxocins. J. Photochem. Photobiol. A Chem. 2006, 180, 165–174. [Google Scholar] [CrossRef]
- Djikanović, D.; Kalauzi, A.; Radotić, K.; Lapierre, C.; Jeremić, M. Deconvolution of lignin fluorescence spectra: A contribution to the comparative structural studies of lignins. Russ. J. Phys. Chem. A 2007, 81, 1425–1428. [Google Scholar] [CrossRef]
- Xue, Y.; Qiu, X.; Wu, Y.; Qian, Y.; Zhou, M.; Deng, Y.; Li, Y. Aggregation-induced emission: The origin of lignin fluorescence. Polym. Chem. 2016, 7, 3502–3508. [Google Scholar] [CrossRef]
- Radotić, K.; Kalauzi, A.; Djikanović, D.; Jeremić, M.; Leblanc, R.M.; Cerović, Z.G. Component analysis of the fluorescence spectra of a lignin model compound. J. Photochem. Photobiol. B Biol. 2006, 83, 1–10. [Google Scholar] [CrossRef]
- Patel, I.; Woodcock, J.; Beams, R.; Stranick, S.; Nieuwendaal, R.; Gilman, J.; Mulenos, M.; Sayes, C.; Salari, M.; DeLoid, G.; et al. Fluorescently Labeled Cellulose Nanofibers for Environmental Health and Safety Studies. Nanomaterials 2021, 11, 1015. [Google Scholar] [CrossRef]
- Donaldson, L. Softwood and Hardwood Lignin Fluorescence Spectra of Wood Cell Walls in Different Mounting Media. IAWA J. 2013, 34, 3–19. [Google Scholar] [CrossRef]
- Donaldson, L. Autofluorescence in Plants. Molecules 2020, 25, 2393. [Google Scholar] [CrossRef]
- Donaldson, L.; Williams, N. Imaging and Spectroscopy of Natural Fluorophores in Pine Needles. Plants 2018, 7, 10. [Google Scholar] [CrossRef]
- Donaldson, L.A.; Knox, J.P. Localization of Cell Wall Polysaccharides in Normal and Compression Wood of Radiata Pine: Relationships with Lignification and Microfibril Orientation. Plant Physiol. 2011, 158, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Auxenfans, T.; Terryn, C.; Paës, G. Seeing biomass recalcitrance through fluorescence. Sci. Rep. 2017, 7, 8838. [Google Scholar] [CrossRef] [PubMed]
- Tobimatsu, Y.; Davidson, C.L.; Grabber, J.H.; Ralph, J. Fluorescence-Tagged Monolignols: Synthesis, and Application to Studying In Vitro Lignification. Biomacromolecules 2011, 12, 1752–1761. [Google Scholar] [CrossRef]
- Ma, Y.; Sawhney, V.; Steeves, T. Staining of paraffin-embedded plant material in safranin and fast green without prior removal of the paraffin. Can. J. Bot. 1993, 71, 996–999. [Google Scholar] [CrossRef]
- Loza-Cornejo, S.; Terrazas, T. Anatomía del tallo y de la raíz de dos especies de Wilcoxia Britton & Rose (Cactaceae) del noreste de México. Bot. Sci. 2017, 13–23. [Google Scholar] [CrossRef]
- Kuroda, H.; Higuchi, T. O-Methyltransferase as a tool to evaluate the lignin evolution. Wood Res. 1982, 68, 53961233. [Google Scholar]
- Pomar, F.; Merino, F.; Barceló, A.R. O -4-Linked coniferyl and sinapyl aldehydes in lignifying cell walls are the main targets of the Wiesner (phloroglucinol-HCl) reaction. Protoplasma 2002, 220, 17–28. [Google Scholar] [CrossRef]
- Espiñeira, J.M.; Uzal, E.N.; Ros, L.V.G.; Carrión, J.S.; Merino, F.; Barceló, A.R.; Pomar, F. Distribution of lignin monomers and the evolution of lignification among lower plants. Plant Biol. 2010, 13, 59–68. [Google Scholar] [CrossRef]
- Ros-Barceló, A.; Gómez-Ros, L.V.; Ferrer, M.A.; Hernandez, J.A. The apoplastic antioxidant enzymatic system in the wood-forming tissues of trees. Trees 2006, 20, 145–156. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Enstone, D.E.; Peterson, C.A. A berberine-aniline blue fluorescent staining procedure for suberin, lignin, and callose in plant tissue. Protoplasma 1988, 146, 133–142. [Google Scholar] [CrossRef]
- Donaldson, L.A. Abnormal lignin distribution in wood from severely drought stressed pinus radiata trees. IAWA J. 2002, 23, 161–178. [Google Scholar] [CrossRef]
- Bond, J.; Donaldson, L.; Hill, S.; Hitchcock, K. Safranine fluorescent staining of wood cell walls. Biotech. Histochem. 2008, 83, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.D.; Kolesik, P.; Hoj, P.B.; Coombe, B.G. Confocal measurement of the three-dimensional size and shape of plant parenchyma cells in a developing fruit tissue. Plant J. 1999, 19, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Kapsokalyvas, D.; Loos, J.; Boogers, I.A.L.A.; Appeldoorn, M.M.; Kabel, M.; Van Zandvoort, M. Quantification of morphochemical changes during in situ enzymatic hydrolysis of individual biomass particles based on autofluorescence imaging. Biopolymers 2019, 111, e23347. [Google Scholar] [CrossRef] [PubMed]
- DeVree, B.T.; Steiner, L.M.; Głazowska, S.; Ruhnow, F.; Herburger, K.; Persson, S.; Mravec, J. Current and future advances in fluorescence-based visualization of plant cell wall components and cell wall biosynthetic machineries. Biotechnol. Biofuels 2021, 14, 1–26. [Google Scholar] [CrossRef]
- Kitin, P.; Nakaba, S.; Hunt, C.G.; Lim, S.; Funada, R. Direct fluorescence imaging of lignocellulosic and suberized cell walls in roots and stems. AoB Plants 2020, 12, plaa032. [Google Scholar] [CrossRef] [PubMed]
- Kiyoto, S.; Yoshinaga, A.; Fernandez-Tendero, E.; Day, A.; Chabbert, B.; Takabe, K. Distribution of Lignin, Hemicellulose, and Arabinogalactan Protein in Hemp Phloem Fibers. Microsc. Microanal. 2018, 24, 442–452. [Google Scholar] [CrossRef]
- Ji, Z.; Ma, J.-F.; Zhang, Z.-H.; Xu, F.; Sun, R.-C. Distribution of lignin and cellulose in compression wood tracheids of Pinus yunnanensis determined by fluorescence microscopy and confocal Raman microscopy. Ind. Crop. Prod. 2013, 47, 212–217. [Google Scholar] [CrossRef]
- Kim, J.S.; Gao, J.; Terziev, N.; Cuccui, I.; Daniel, G. Chemical and ultrastructural changes of ash wood thermally modified using the thermo-vacuum process: I. Histo/cytochemical studies on changes in the structure and lignin chemistry. Holzforschung 2015, 69, 603–613. [Google Scholar] [CrossRef]
- Yamashita, D.; Kimura, S.; Wada, M.; Takabe, K. Improved Mäule color reaction provides more detailed information on syringyl lignin distribution in hardwood. J. Wood Sci. 2016, 62, 131–137. [Google Scholar] [CrossRef]
- Maceda, A.; Reyes-Rivera, J.; Soto-Hernández, M.; Terrazas, T. Distribution and Chemical Composition of Lignin in Secondary Xylem of Cactaceae. Chem. Biodivers. 2021, 18, e2100431. [Google Scholar] [CrossRef] [PubMed]
- De Micco, V.; Aronne, G. Combined histochemistry and autofluorescence for identifying lignin distribution in cell walls. Biotech. Histochem. 2007, 82, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Benítez, V.; Franco, J.; Camargo, Á.; Raimonda, P.; Mantero, C.; Ibáñez, C.M. Influence of initial wood moisture on decay process by two brown-rot fungi. Maderas. Cienc. Tecnol. 2021, 23. [Google Scholar] [CrossRef]
- Guo, A.-Y.; Zhang, Y.-M.; Wang, L.; Bai, D.; Xu, Y.-P.; Wu, W.-Q. Single-Molecule Imaging in Living Plant Cells: A Methodological Review. Int. J. Mol. Sci. 2021, 22, 5071. [Google Scholar] [CrossRef] [PubMed]
- Capecchi, E.; Piccinino, D.; Tomaino, E.; Bizzarri, B.M.; Polli, F.; Antiochia, R.; Mazzei, F.; Saladino, R. Lignin nanoparticles are renewable and functional platforms for the concanavalin a oriented immobilization of glucose oxidase–peroxidase in cascade bio-sensing. RSC Adv. 2020, 10, 29031–29042. [Google Scholar] [CrossRef]
- Donaldson, L.A.; Newman, R.H.; Vaidya, A. Nanoscale interactions of polyethylene glycol with thermo-mechanically pre-treatedPinus radiatabiofuel substrate. Biotechnol. Bioeng. 2013, 111, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Decou, R.; Serk, H.; Ménard, D.; Pesquet, E. Analysis of Lignin Composition and Distribution Using Fluorescence Laser Confocal Microspectroscopy. Methods Mol. Biol. 2017, 1544, 233–247. [Google Scholar] [CrossRef]
- Baldacci-Cresp, F.; Spriet, C.; Twyffels, L.; Blervacq, A.; Neutelings, G.; Baucher, M.; Hawkins, S. A rapid and quantitative safranin-based fluorescent microscopy method to evaluate cell wall lignification. Plant J. 2020, 102, 1074–1089. [Google Scholar] [CrossRef]
- Donaldson, L.A.; Grace, J.; Downes, G.M. Within-tree variation in anatomical properties of compression wood in radiata pine. IAWA J. 2004, 25, 253–271. [Google Scholar] [CrossRef]
- Choong, F.X.; Bäck, M.; Steiner, S.; Melican, K.; Nilsson, K.P.R.; Edlund, U.; Richter-Dahlfors, A. Nondestructive, real-time determination and visualization of cellulose, hemicellulose and lignin by luminescent oligothiophenes. Sci. Rep. 2016, 6, 35578. [Google Scholar] [CrossRef]
- Huang, C.; Su, Y.; Shi, J.; Yuan, C.; Zhai, S.; Yong, Q. Revealing the effects of centuries of ageing on the chemical structural features of lignin in archaeological fir woods. New J. Chem. 2019, 43, 3520–3528. [Google Scholar] [CrossRef]
- Ursache, R.; Andersen, T.G.; Marhavý, P.; Geldner, N. A protocol for combining fluorescent proteins with histological stains for diverse cell wall components. Plant J. 2017, 93, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Kapp, N.; Barnes, W.; Richard, T.; Anderson, C.T. Imaging with the fluorogenic dye Basic Fuchsin reveals subcellular patterning and ecotype variation of lignification in Brachypodium distachyon. J. Exp. Bot. 2015, 66, 4295–4304. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fan, D.; Han, Y.; Lyu, S.; Lu, Y.; Li, G.; Jiang, F.; Wang, S. Effect of high residual lignin on the properties of cellulose nanofibrils/films. Cellulose 2018, 25, 6421–6431. [Google Scholar] [CrossRef]
- Speranza, M.; Gutiérrez, A.; Del Río, J.C.; Bettucci, L.; Martínez, A.T.; Martínez, M.J. Sterols and lignin in Eucalyptus globulus Labill. wood: Spatial distribution and fungal removal as revealed by microscopy and chemical analyses. Holzforschung 2009, 63, 362–370. [Google Scholar] [CrossRef]
- Herbaut, M.; Zoghlami, A.; Habrant, A.; Falourd, X.; Foucat, L.; Chabbert, B.; Paës, G. Multimodal analysis of pretreated biomass species highlights generic markers of lignocellulose recalcitrance. Biotechnol. Biofuels 2018, 11, 52. [Google Scholar] [CrossRef]
- Zhou, H.; Li, J.; Ma, E. Multiscale Modification of Populus cathayana by Alkali Lignin Combined with Heat Treatment. Polymers 2018, 10, 1240. [Google Scholar] [CrossRef]
- Houtman, C.; Maligaspe, E.; Hunt, C.; Fernández-Fueyo, E.; Martínez, A.T.; Hammel, K.E. Fungal lignin peroxidase does not produce the veratryl alcohol cation radical as a diffusible ligninolytic oxidant. J. Biol. Chem. 2018, 293, 4702–4712. [Google Scholar] [CrossRef]
- Donaldson, L.; Vaidya, A. Visualising recalcitrance by colocalisation of cellulase, lignin and cellulose in pretreated pine biomass using fluorescence microscopy. Sci. Rep. 2017, 7, 44386. [Google Scholar] [CrossRef]
- Bukowski, N.; Pandey, J.L.; Doyle, L.; Richard, T.L.; Anderson, C.T.; Zhu, Y. Development of a Clickable Designer Monolignol for Interrogation of Lignification in Plant Cell Walls. Bioconjugate Chem. 2014, 25, 2189–2196. [Google Scholar] [CrossRef]
- Pandey, J.L.; Wang, B.; Diehl, B.G.; Richard, T.; Chen, G.; Anderson, C.T. A Versatile Click-Compatible Monolignol Probe to Study Lignin Deposition in Plant Cell Walls. PLoS ONE 2015, 10, e0121334. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Lion, C.; Spriet, C.; Baldacci-Cresp, F.; Hawkins, S.; Biot, C. One, Two, Three: A Bioorthogonal Triple Labelling Strategy for Studying the Dynamics of Plant Cell Wall Formation In Vivo. Angew. Chem. Int. Ed. 2018, 57, 16665–16671. [Google Scholar] [CrossRef] [PubMed]
- Tobimatsu, Y.; Schuetz, M. Lignin polymerization: How do plants manage the chemistry so well? Curr. Opin. Biotechnol. 2018, 56, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Tobimatsu, Y.; Wagner, A.; Donaldson, L.; Mitra, P.; Niculaes, C.; Dima, O.; Kim, J.I.; Anderson, N.; Loque, D.; Boerjan, W.; et al. Visualization of plant cell wall lignification using fluorescence-tagged monolignols. Plant J. 2013, 76, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Lion, C.; Simon, C.; Huss, B.; Blervacq, A.-S.; Tirot, L.; Toybou, D.; Spriet, C.; Slomianny, C.; Guerardel, Y.; Hawkins, S.; et al. BLISS: A Bioorthogonal Dual-Labeling Strategy to Unravel Lignification Dynamics in Plants. Cell Chem. Biol. 2017, 24, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Zuo, D.; Tao, Y.; Cai, H.; Li, L. Laccase3-based extracellular domain provides possible positional information for directing Casparian strip formation inArabidopsis. Proc. Natl. Acad. Sci. USA 2020, 117, 15400–15402. [Google Scholar] [CrossRef]
- Simon, C.; Spriet, C.; Hawkins, S.; Lion, C. Visualizing Lignification Dynamics in Plants with Click Chemistry: Dual Labeling is BLISS! J. Vis. Exp. 2018, e56947. [Google Scholar] [CrossRef]
- Donaldson, L.; Radotic, K. Fluorescence lifetime imaging of lignin autofluorescence in normal and compression wood. J. Microsc. 2013, 251, 178–187. [Google Scholar] [CrossRef]
- Wightman, R.; Busse-Wicher, M.; Dupree, P. Correlative FLIM-confocal-Raman mapping applied to plant lignin composition and autofluorescence. Micron 2019, 126, 102733. [Google Scholar] [CrossRef]
- Berezin, M.Y.; Achilefu, S. Fluorescence Lifetime Measurements and Biological Imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef]
- Coletta, V.C.; Rezende, C.A.; da Conceição, F.R.; Polikarpov, I.; Guimarães, F.E.G. Mapping the lignin distribution in pretreated sugarcane bagasse by confocal and fluorescence lifetime imaging microscopy. Biotechnol. Biofuels 2013, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Chimenez, T.A.; Gehlen, M.H.; Marabezi, K.; Curvelo, A.A.S. Characterization of sugarcane bagasse by autofluorescence microscopy. Cellulose 2013, 21, 653–664. [Google Scholar] [CrossRef]
- Houtman, C.; Kitin, P.; Houtman, J.; Hammel, K.E.; Hunt, C. Acridine Orange Indicates Early Oxidation of Wood Cell Walls by Fungi. PLoS ONE 2016, 11, e0159715. [Google Scholar] [CrossRef]
- Leiter, N.; Wohlschläger, M.; Auer, V.; Versen, M.; Laforsch, C. B10.2 A Novel Approach to Identify Wood Species Optically Using Fluorescence Lifetime Imaging Microscopy; AMA: Berlin, Germany, 2021; pp. 169–170. [Google Scholar] [CrossRef]
- Escamez, S.; Terryn, C.; Gandla, M.L.; Yassin, Z.; Scheepers, G.; Näsholm, T.; Sundman, O.; Jönsson, L.J.; Lundberg-Felten, J.; Tuominen, H.; et al. Fluorescence Lifetime Imaging as an In Situ and Label-Free Readout for the Chemical Composition of Lignin. ACS Sustain. Chem. Eng. 2021. [Google Scholar] [CrossRef]
- Terryn, C.; Paës, G.; Spriet, C. FRET-SLiM on native autofluorescence: A fast and reliable method to study interactions between fluorescent probes and lignin in plant cell wall. Plant Methods 2018, 14, 74. [Google Scholar] [CrossRef]
- Benninger, R.; Piston, D.W. Two-Photon Excitation Microscopy for the Study of Living Cells and Tissues. Curr. Protoc. Cell Biol. 2013, 59, 4.11.1–4.11.24. [Google Scholar] [CrossRef]
- Nadiarnykh, O.; LaComb, R.B.; Campagnola, P.J.; Mohler, W.A. Coherent and incoherent SHG in fibrillar cellulose matrices. Opt. Express 2007, 15, 3348–3360. [Google Scholar] [CrossRef]
- Peciulyte, A.; Kiskis, J.; Larsson, P.T.; Olsson, L.; Enejder, A. Visualization of structural changes in cellulosic substrates during enzymatic hydrolysis using multimodal nonlinear microscopy. Cellulose 2016, 23, 1521–1536. [Google Scholar] [CrossRef]
- Tai, H.-C.; Chen, P.-L.; Xu, J.-W.; Chen, S.-Y. Two-photon fluorescence and second harmonic generation hyperspectral imaging of old and modern spruce woods. Opt. Express 2020, 28, 38831–38841. [Google Scholar] [CrossRef]
- Crouvisier-Urion, K.; Lagorce-Tachon, A.; Lauquin, C.; Winckler, P.; Tongdeesoontorn, W.; Domenek, S.; Debeaufort, F.; Karbowiak, T. Impact of the homogenization process on the structure and antioxidant properties of chitosan-lignin composite films. Food Chem. 2017, 236, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Terryn, C.; Habrant, A.; Paës, G.; Spriet, C. Measuring Interactions between Fluorescent Probes and Lignin in Plant Sections by sFLIM Based on Native Autofluorescence. J. Vis. Exp. 2020, e59925. [Google Scholar] [CrossRef] [PubMed]
- Chou, E.Y.; Schuetz, M.; Hoffmann, N.; Watanabe, Y.; Sibout, R.; Samuels, A.L. Distribution, mobility, and anchoring of lignin-related oxidative enzymes in Arabidopsis secondary cell walls. J. Exp. Bot. 2018, 69, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Kvist, P.; Schuster, E.; Lorén, N.; Rasmuson, A. Using fluorescent probes and FRAP to investigate macromolecule diffusion in steam-exploded wood. Wood Sci. Technol. 2018, 52, 1395–1410. [Google Scholar] [CrossRef]
- Paës, G.; Chabbert, B. Characterization of Arabinoxylan/Cellulose Nanocrystals Gels to Investigate Fluorescent Probes Mobility in Bioinspired Models of Plant Secondary Cell Wall. Biomacromolecules 2011, 13, 206–214. [Google Scholar] [CrossRef]
- Herbaut, M.; Zoghlami, A.; Paës, G. Dynamical assessment of fluorescent probes mobility in poplar cell walls reveals nanopores govern saccharification. Biotechnol. Biofuels 2018, 11, 271. [Google Scholar] [CrossRef]
- Hernández-Varela, J.; Chanona-Pérez, J.; Benavides, H.C.; Cerda, S.G.; Victoriano, L.G.; Flores, M.D.J.P.; López, M.C.; Candelas, L.E.R.; Tamato, B.A. CLSM and TIRF images from lignocellulosic materials: Garlic skin and agave fibers study. Microsc. Microanal. 2021, 27, 1730–1734. [Google Scholar] [CrossRef]
- Paës, G.; Habrant, A.; Terryn, C. Fluorescent Nano-Probes to Image Plant Cell Walls by Super-Resolution STED Microscopy. Plants 2018, 7, 11. [Google Scholar] [CrossRef]
- Blom, H.; Brismar, H. STED microscopy: Increased resolution for medical research? J. Intern. Med. 2014, 276, 560–578. [Google Scholar] [CrossRef]
- Schubert, V. Super-resolution Microscopy—Applications in Plant Cell Research. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Donaldson, L.A. Lignification and lignin topochemistry—An ultrastructural view. Phytochemistry 2001, 57, 859–873. [Google Scholar] [CrossRef]
- Menezes, F.; Rocha, G.; Maciel Filho, R. Obtainment and characterization of lignin from enzymatic hydrolysis of sugarcane bagasse of e2g process in pilot scale. Chem. Eng. Trans. 2016; 50, 397–402. [Google Scholar] [CrossRef]
- Cabrales, L.; Abidi, N.; Manciu, F. Characterization of Developing Cotton Fibers by Confocal Raman Microscopy. Fibers 2014, 2, 285–294. [Google Scholar] [CrossRef]
- Maceda, A.; Soto-Hernández, M.; Peña-Valdivia, C.B.; Trejo, C.; Terrazas, T. Differences in the Structural Chemical Composition of the Primary Xylem of Cactaceae: A Topochemical Perspective. Front. Plant Sci. 2019, 10, 1497. [Google Scholar] [CrossRef] [PubMed]
- Vítek, P.; Klem, K.; Urban, O. Application of Raman spectroscopy to analyse lignin/cellulose ratio in Norway spruce tree rings. Beskydy 2017, 10, 41–48. [Google Scholar] [CrossRef][Green Version]
- Rashid, T.; Kait, C.F.; Murugesan, T. A “Fourier Transformed Infrared” Compound Study of Lignin Recovered from a Formic Acid Process. Procedia Eng. 2016, 148, 1312–1319. [Google Scholar] [CrossRef]
- Ai, N.; Jiang, Y.; Omar, S.; Wang, J.; Xia, L.; Ren, J. Rapid Measurement of Cellulose, Hemicellulose, and Lignin Content in Sargassum horneri by Near-Infrared Spectroscopy and Characteristic Variables Selection Methods. Molecules 2022, 27, 335. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-L.; Xue, B.-L.; Sun, R.-C. Recent Advances in Characterization of Lignin Polymer by Solution-State Nuclear Magnetic Resonance (NMR) Methodology. Materials 2013, 6, 359–391. [Google Scholar] [CrossRef]
- Reyes-Rivera, J.; Canché-Escamilla, G.; Soto-Hernandez, M.; Terrazas, T. Wood Chemical Composition in Species of Cactaceae: The Relationship between Lignification and Stem Morphology. PLoS ONE 2015, 10, e0123919. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maceda, A.; Terrazas, T. Fluorescence Microscopy Methods for the Analysis and Characterization of Lignin. Polymers 2022, 14, 961. https://doi.org/10.3390/polym14050961
Maceda A, Terrazas T. Fluorescence Microscopy Methods for the Analysis and Characterization of Lignin. Polymers. 2022; 14(5):961. https://doi.org/10.3390/polym14050961
Chicago/Turabian StyleMaceda, Agustín, and Teresa Terrazas. 2022. "Fluorescence Microscopy Methods for the Analysis and Characterization of Lignin" Polymers 14, no. 5: 961. https://doi.org/10.3390/polym14050961
APA StyleMaceda, A., & Terrazas, T. (2022). Fluorescence Microscopy Methods for the Analysis and Characterization of Lignin. Polymers, 14(5), 961. https://doi.org/10.3390/polym14050961