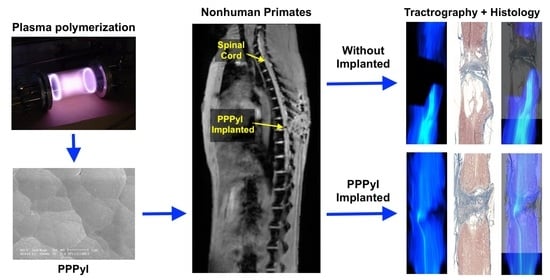
Evolution of Spinal Cord Transection of Rhesus Monkey Implanted with Polymer Synthesized by Plasma Evaluated by Diffusion Tensor Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Polypyrrole Iodine by Plasma
2.2. Polymer Characterization
2.3. Animal Grouping
2.4. Experimental Treatment
2.5. MRI and DTI Scan
2.6. Obtaining Tissue
3. Results
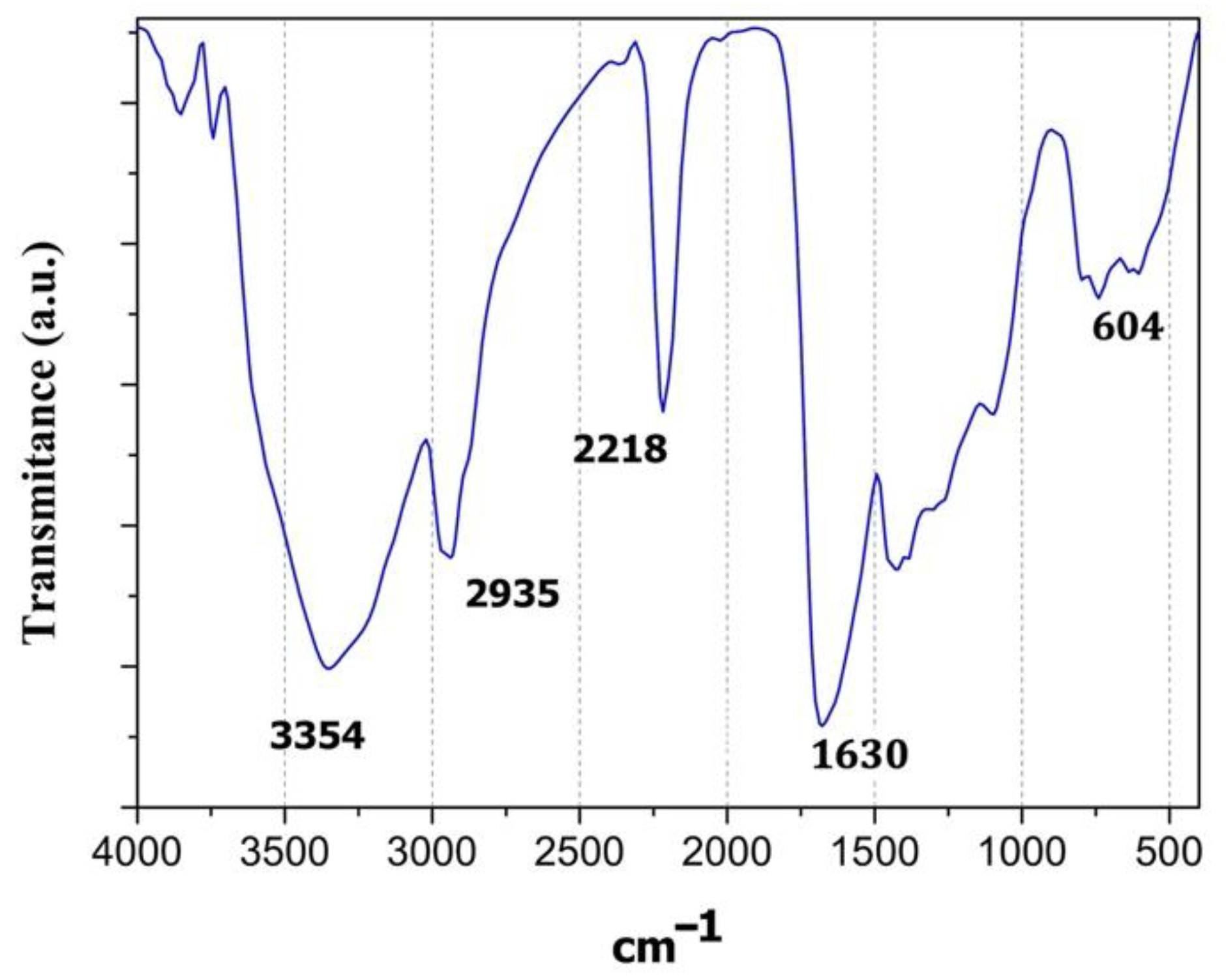
3.1. FT-IR Spectcroscopy
3.2. Elemental Analysis by XPS
3.3. Electric Conductivity
3.4. Implant Evolution
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strauss, D.; DeVivo, M.; Shavelle, R.; Brooks, J.; Paculdo, D. Economic factors and longevity in spinal cord injury: A reappraisal. Arch. Phys. Med. Rehabil. 2008, 89, 532. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 1–21. [Google Scholar] [CrossRef]
- National Spinal Cord Injury Statistical Center. 2020 Annual Statistical Report for the Spinal Cord Injury Model Systems; University of Alabama at Birmingham: Birmingham, AL, USA, 2021; pp. 1–162. [Google Scholar]
- Guízar-Sahagún, G.; Grijalva, I.; Hernández-Godínez, B.; Franco-Bourland, R.E.; Cruz-Antonio, L.; Martínez-Cruz, A.; Ibañez-Contreras, A. New approach for graded compression spinal cord injuries in Rhesus macaque: Method feasibility and preliminary observations. J. Med. Primatol. 2011, 40, 401–413. [Google Scholar] [CrossRef]
- Jannesar, S.; Salegio, E.A.; Beattie, M.S.; Bresnahan, J.C.; Sparrey, C.J. Correlating Tissue Mechanics and Spinal Cord Injury: Patient- Specific Finite Element Models of Unilateral Cervical Contusion Spinal Cord Injury in Non-Human Primates. J. Neurotrauma 2021, 38, 698–717. [Google Scholar] [CrossRef]
- Courtine, G.; Bunge, M.B.; Fawcett, J.W.; Hodgson, J.; McKay, H.; Yang, H.; Zhong, H.; Tuszynski, M.H.; Edgerton, V.R. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat. Med. 2007, 13, 561. [Google Scholar] [CrossRef]
- Festing, S.; Wilkinson, R. The ethics of animal research. EMBO Rep. 2007, 8, 1–5. [Google Scholar] [CrossRef]
- Li, X.-H.; Zhu, X.; Liu, X.-Y.; Xu, H.-H.; Jiang, W.; Wang, J.-J.; Chen, F.; Zhang, S.; Li, R.-X.; Chen, X.-Y.; et al. The corticospinal tract structure of collagen/silk fibroin scaffold implants using 3D printing promotes functional recovery after complete spinal cord transection in rats. J. Mater. Sci. Mater. Med. 2021, 32, 31. [Google Scholar] [CrossRef]
- Estrada, V.; Brazda, N.; Schmitz, C.; Heller, S.; Blazyca, H.; Martini, R.; Müller, H.W. Long-lasting significant functional improvement in chronic severe spinal cord injury following scar resection and polyethylene glycol implantation. Neurobiol. Dis. 2014, 67, 165–179. [Google Scholar] [CrossRef]
- Novikov, L.N.; Novikova, L.N.; Mosahebi, A.; Wiberg, M.; Terenghi, G.; Kellerth, J.-O. A novel biodegradable implant for neuronal rescue and regeneration after spinal cord injury. Biomaterials 2002, 23, 3369–3376. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; Zhang, A.; Wang, T.; Chen, W. Repair of thoracic spinal cord injury by chitosan tube implantation in adult rats. Biomaterials 2009, 30, 1121–1132. [Google Scholar] [CrossRef]
- Wong, D.Y.; Leveque, J.-C.; Brumblay, H.; Krebsbach, P.H.; LaMarca, F. Macro-Architectures in Spinal Cord Scaffold Implants Influence Regeneration. J. Neurotrauma 2008, 25, 1027–1037. [Google Scholar] [CrossRef]
- Gelain, F.; Panseri, S.; Antonini, S.; Cunha, C.; Donega, M.; Lowery, J.; Taraballi, F.; Cerri, G.; Montagna, M.; Baldissera, F.; et al. Transplantation of Nanostructured Composite Scaffolds Results in the Regeneration of Chronically Injured Spinal Cords. ACS NANO 2010, 5, 227–236. [Google Scholar] [CrossRef]
- Olayo, R.; Ríos, C.; Salgado-Ceballos, H.; Cruz, G.J.; Morales, J.; Olayo, M.-G.; Alcaraz-Zubeldia, M.; Alvarez, A.L.; Mondragon, R.; Morales, A.; et al. Tissue spinal cord response in rats after implants of polypyrrole and polyethylene glycol obtained by plasma. J. Mater. Sci. Mater. Med. 2007, 19, 817–826. [Google Scholar] [CrossRef]
- Alvarez-Mejía, L.; Morales, J.; Cruz, G.J.; Olayo, M.-G.; Olayo, R.; Diaz-Ruiz, A.; Ríos, C.; Mondragón-Lozano, R.; Sánchez-Torres, S.; Morales-Guadarrama, A.; et al. Functional recovery in spinal cord injured rats using polypyrrole/iodine implants and treadmill training. J. Mater. Sci. Mater. Med. 2015, 26, 209. [Google Scholar] [CrossRef]
- Sánchez-Torres, S.; Diaz-Ruiz, A.; Ríos, C.; Olayo, M.G.; Cruz, G.J.; Olayo, R.; Morales, J.; Mondragón-Lozano, R.; Fabela-Sánchez, O.; Orozco-Barrios, C.; et al. Recovery of motor function after traumatic spinal cord injury by using plasma-synthesized polypyrrole/iodine application in combination with a mixed rehabilitation scheme. J. Mater. Sci. Mater. Med. 2020, 31, 58. [Google Scholar] [CrossRef]
- Álvarez-Mejía, L.; Salgado-Ceballos, H.; Olayo, R.; Cruz, G.J.; Olayo, M.G.; Díaz-Ruiz, A.; Ríos, C.; Mondragón-Lozano, R.; Morales-Guadarrama, A.; Sánchez-Torres, S.; et al. Effect of Pyrrole Implants Synthesized by Different Methods on Spinal Cord Injuries of Rats. Rev. Mex. Ing. Biomédica 2015, 36, 7–21. [Google Scholar]
- Kozlowski, P.; Raj, D.; Liu, J.; Lam, C.; Yung, C.A.; Tetzlaff, W. Characterizing white matter damage in rat spinal cord with quantitative MRI and histology. J. Neurotrauma 2008, 25, 653–676. [Google Scholar] [CrossRef]
- Freund, P.; Seif, M.; Weiskopf, N.; Friston, K.; Fehlings, M.G.; Thompson, A.J.; Curt, A. MRI in traumatic spinal cord injury: From clinical assessment to neuroimaging biomarkers. Lancet Neurol. 2019, 18, 1123–1135. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, N.; Seo, J. Morphometric Magnetic Resonance Imaging Evaluation of Cervical Spinal Canal and Cord in Normal Small-Breed Dogs. Front. Vet. Sci. 2021, 8, 732953. [Google Scholar]
- Li, X.-H.; Li, J.-B.; He, X.-J.; Wang, F.; Huang, S.-L.; Bai, Z.-L. Timing of diffusion tensor imaging in the acute spinal cord injury of rats. Sci. Rep. 2015, 5, 12639. [Google Scholar] [CrossRef]
- Budde, M.D.; Skinner, N.P. Diffusion MRI in acute nervous system injury. J. Magn. Reson. 2018, 292, 137–148. [Google Scholar] [CrossRef]
- Beaulieu, C. The basis of anisotropic water diffusion in the nervous system a technical review. NMR Biomed. Int. J. Devoted Dev. Appl. Magn. Reson. In Vivo 2002, 15, 435–455. [Google Scholar] [CrossRef]
- Basser, J.P.; Mattiello, J.; Lebihan, D. Estimation of the effective selfdiffusion tensor from the NMR spin echo. J. Magn. Reson. Ser. B 1994, 103, 247–254. [Google Scholar] [CrossRef]
- Basser, P.J.; Pajevic, S.; Pierpaoli, C.; Duda, J.; Aldroubi, A. In vivo fiber tractography using Dt-MRI Data. Magn. Reson. Med. 2000, 625–632. [Google Scholar] [CrossRef]
- Shi, Y.; Short, S.J.; Knickmeyer, R.C.; Wang, J.; Coe, C.L.; Niethammer, M.; Gilmore, J.H.; Zhu, H.; Styner, M.A. Diffusion Tensor Imaging-Based Characterization of Brain Neurodevelopment in Primates. Cereb. Cortex 2012, 23, 36–48. [Google Scholar] [CrossRef]
- Manzanera Esteve, I.V.; Farinas, A.F.; Pollins, A.C.; Nussenbaum, M.E.; Cardwell, N.L.; Kahn, H.; Does, M.D.; Dortch, R.D.; Thayer, W.P. Noninvasive diffusion MRI to determine the severity of peripheral nerve injury. Magn. Reson. Imaging 2021, 83, 96–106. [Google Scholar] [CrossRef]
- Cruz, G.J.; Morales, J.; Olayo, R. Films obtained by plasma polymerization of pyrrole. Thin Solid Film. 1999, 342, 119–126. [Google Scholar] [CrossRef]
- Osorio-Londoño, D.M.; Godínez-Fernández, J.R.; Acosta-García, M.C.; Morales-Corona, J.; Olayo Gonzalez, R.; Morales-Guadarrama, A. Pyrrole Plasma Polymer-Coated Electrospun Scaffolds for Neural Tissue Engineering. Polymers 2021, 13, 3876. [Google Scholar] [CrossRef]
- Yeh, F.-C.; Verstynen, T.D.; Wang, Y.; Fernández-Miranda, J.C.; Tseng, W.-Y.I. Deterministic Diffusion Fiber Tracking Improved by Quantitative Anisotropy. PLoS ONE 2013, 8, e80713. [Google Scholar] [CrossRef]
- Kelley, B.J.; Harel, N.Y.; Kim, C.-Y.; Papademetris, X.; Coman, D.; Wang, X.; Hasan, O.; Kaufman, A.; Globinsky, R.; Staib, L.H.; et al. Diffusion Tensor Imaging as a Predictor of Locomotor Function after Experimental Spinal Cord Injury and Recovery. J. Neurotrauma 2014, 31, 1362–1373. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, S.; Xie, S.; Wang, X.; Chang, F.; Luo, J.; Wang, J.; Fu, J. Effect of hierarchically aligned fibrin hydrogel in regeneration of spinal cord injury demonstrated by tractography: A pilot study. Sci. Rep. 2016, 7, 40017. [Google Scholar] [CrossRef]
- Sasiadek, M.J.; Szewczyk, P.; Bladowska, J. Application of diffusion tensor imaging (DTI) in pathological changes of the spinal cord. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2012, 18, 73–79. [Google Scholar] [CrossRef]
- Vedantam, A.; Jirjis, M.; Eckhardt, G.; Sharma, A.; Schmit, B.D.; Wang, M.C.; Ulmer, J.L.; Kurpad, S. Diffusion tensor imaging of the spinal cord: A review. Coluna/Columna 2013, 12, 64–69. [Google Scholar] [CrossRef][Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Guadarrama, A.; Salgado-Ceballos, H.; Grijalva, I.; Morales-Corona, J.; Hernández-Godínez, B.; Ibáñez-Contreras, A.; Ríos, C.; Diaz-Ruiz, A.; Cruz, G.J.; Olayo, M.G.; et al. Evolution of Spinal Cord Transection of Rhesus Monkey Implanted with Polymer Synthesized by Plasma Evaluated by Diffusion Tensor Imaging. Polymers 2022, 14, 962. https://doi.org/10.3390/polym14050962
Morales-Guadarrama A, Salgado-Ceballos H, Grijalva I, Morales-Corona J, Hernández-Godínez B, Ibáñez-Contreras A, Ríos C, Diaz-Ruiz A, Cruz GJ, Olayo MG, et al. Evolution of Spinal Cord Transection of Rhesus Monkey Implanted with Polymer Synthesized by Plasma Evaluated by Diffusion Tensor Imaging. Polymers. 2022; 14(5):962. https://doi.org/10.3390/polym14050962
Chicago/Turabian StyleMorales-Guadarrama, Axayacatl, Hermelinda Salgado-Ceballos, Israel Grijalva, Juan Morales-Corona, Braulio Hernández-Godínez, Alejandra Ibáñez-Contreras, Camilo Ríos, Araceli Diaz-Ruiz, Guillermo Jesus Cruz, María Guadalupe Olayo, and et al. 2022. "Evolution of Spinal Cord Transection of Rhesus Monkey Implanted with Polymer Synthesized by Plasma Evaluated by Diffusion Tensor Imaging" Polymers 14, no. 5: 962. https://doi.org/10.3390/polym14050962
APA StyleMorales-Guadarrama, A., Salgado-Ceballos, H., Grijalva, I., Morales-Corona, J., Hernández-Godínez, B., Ibáñez-Contreras, A., Ríos, C., Diaz-Ruiz, A., Cruz, G. J., Olayo, M. G., Sánchez-Torres, S., Mondragón-Lozano, R., Alvarez-Mejia, L., Fabela-Sánchez, O., & Olayo, R. (2022). Evolution of Spinal Cord Transection of Rhesus Monkey Implanted with Polymer Synthesized by Plasma Evaluated by Diffusion Tensor Imaging. Polymers, 14(5), 962. https://doi.org/10.3390/polym14050962